Abstract
Human-mediated dispersal has reshaped distribution patterns and biogeographic relationships for many taxa. Long-distance and over-water dispersal were historically rare events for most species, but now human activities can move organisms quickly over long distances to new places. A potential consequence of human-mediated dispersal is the eventual reintroduction of individuals from an invasive population back into their native range; a dimension of biological invasion termed “cryptic back-introduction.” We investigated whether this phenomenon was occurring in the Cayman Islands where brown anole lizards (Anolis sagrei) with red dewlaps (i.e., throat fans), either native to Little Cayman or invasive on Grand Cayman, have been found on Cayman Brac where the native A. sagrei have yellow dewlaps. Our analysis of microsatellite data shows strong population-genetic structure among the three Cayman Islands, but also evidence for non-equilibrium. We found some instances of intermediate multilocus genotypes (possibly 3–9% of individuals), particularly between Grand Cayman and Cayman Brac. Furthermore, analysis of dewlap reflectance data classified six males sampled on Cayman Brac as having red dewlaps similar to lizards from Grand Cayman and Little Cayman. Lastly, one individual from Cayman Brac had an intermediate microsatellite genotype, a red dewlap, and a mtDNA haplotype from Grand Cayman. This mismatch among genetic and phenotypic data strongly suggests that invasive A. sagrei from Grand Cayman are interbreeding with native A. sagrei on Cayman Brac. To our knowledge, this is the first evidence of cryptic back-introduction. Although we demonstrate this phenomenon is occurring in the Cayman Islands, assessing its frequency there and prevalence in other systems may prove difficult due to the need for genetic data in most instances. Cryptic back-introductions may eventually provide some insight into how lineages are changed by the invasion process and may be an underappreciated way in which invasive species impact native biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In this era of widespread human-mediated dispersal, geographic distance is no longer a barrier to movement for many species. Exotic species introductions have altered fundamental biogeographic patterns such as species-area and species-isolation relationships (MacArthur and Wilson 1963; Sax et al. 2005). For example, colonization by exotic Anolis lizards in the Caribbean has altered pre-invasion biogeographic patterns (Losos and Schluter 2000), effectively reducing geographic isolation among islands and flattening the negative species-isolation relationship for anoles (Helmus et al. 2014). Long-distance colonization events due to human activity can bring together evolutionarily distinct lineages that in some instances have been separated for millions of years (e.g., Kolbe et al. 2004). Novel interactions among these lineages could reshape existing ecological communities and alter patterns of species diversity (e.g., Stuart et al. 2014; Liu et al. 2014). Furthermore, hybridization or admixture between introduced and native taxa (e.g., Fitzpatrick and Shaffer 2007) compromises the genetic distinctiveness of taxa, including endemics, unique phenotypes, and evolutionarily significant units (Moritz 1994). A previously undocumented dimension of biological invasion occurs when invasive populations from outside of their native range are reintroduced into native-range populations; either back into their original source population or, more generally, to any part of their native range. Guo (2005) proposed the term “cryptic back-introduction” for this phenomenon, but to our knowledge it has never been demonstrated. Here we explore whether this phenomenon is occurring in the brown anole lizard (A. sagrei) and consider the implications of this type of invasion for native populations.
Anolis sagrei is a good natural colonizer, reaching many islands and mainland areas in the Caribbean by overwater dispersal (Williams 1969). During its up to 6.5 million year evolutionary history (based on coalescent dates from a molecular-clock calibrated mtDNA gene tree provided by R.G. Reynolds, pers. comm.), this species colonized all of Cuba, most islands in the Bahamas, the Atlantic coast of Mexico and Belize, and Swan Island (Schwartz and Henderson 1991). As long ago as 2.5 million years this species also colonized Cayman Brac and Little Cayman (R.G. Reynolds, pers. comm.), two small and relatively remote islands in the western Caribbean. Subsequently, A. sagrei differentiated into the yellow-dewlapped endemic subspecies A. s. luteosignifer on Cayman Brac and the red-dewlapped A. s. sagrei on Little Cayman (Schwartz and Henderson 1991); the dewlap is an extendable throat fan used for communication in anoles (Losos 2009). However, A. sagrei failed to colonize the third of the Cayman Islands, Grand Cayman, which is located ~100 km farther to the southwest of the other Cayman Islands from Cuba. That is, until the early 1980s when a red-dewlapped form of A. sagrei became established on Grand Cayman, thereby accomplishing a long-distance dispersal event that failed to occur naturally. Grand Cayman populations resulted from a human-mediated introduction of genetically admixed lizards, not from its native range, but from established non-native populations in south Florida (Minton and Minton 1984; Kolbe et al. 2004, 2008; Fig. 1). Since this time, inter-island supply shipments by air and sea within the Caymans—primarily from the capital and largest port on Grand Cayman (196 km2) to the much smaller Cayman Brac (~38 km2) and Little Cayman (<10 km2)—could have transported invasive and native A. sagrei among this trio of islands. Indeed, in 2010, a single A. sagrei individual with a red dewlap was observed on Cayman Brac (T. Sanger, pers. comm.) where only yellow dewlaps have been observed previously, suggesting an introduction to Cayman Brac of invasive A. sagrei from Grand Cayman or native A. s. sagrei from Little Cayman.
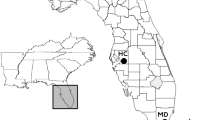
Map showing portions of the native and non-native ranges of Anolis sagrei relevant to this study: a Arrows indicate routes of primary invasion from multiple native-range source populations in Cuba to the non-native range in Florida and a secondary invasion from non-native populations in south Florida to Grand Cayman; b the arrow indicates a cryptic back-introduction of invasive brown anoles from Grand Cayman into the native range on Cayman Brac; and b, c circles show collection localities for genetic, ecological, morphological, and dewlap data on Grand Cayman, Little Cayman, and Cayman Brac with site numbers corresponding to those listed in Table S1
In this study, we surveyed A. sagrei populations across Cayman Brac looking for red-dewlapped lizards to determine whether invasive A. sagrei from Grand Cayman have in fact invaded Cayman Brac. We also collected lizards on Grand Cayman and Little Cayman to discriminate between these two potential sources of red-dewlapped A. sagrei. For lizards from all three islands, we quantified dewlap phenotypes using spectrophotometric methods, measured structural habitat use and body size, and genotyped ten nuclear microsatellite loci. For individuals with intermediate multilocus genotypes or a genotype that did not match their island, we sequenced mtDNA haplotypes (ND2) to test for nuclear-mitochondrial mismatches. Using these data, we evaluated whether invasive A. sagrei from Grand Cayman have been introduced to native populations on Cayman Brac, and if so, whether invasive lizards have interbred with native lizards.
Methods
We sampled lizards in the Cayman Islands in January 2011, focusing the majority of our effort on Cayman Brac due to the previous observation of a red-dewlapped A. sagrei there (Fig. 1; Table S1). On all three islands, for each undisturbed lizard encountered, we measured perch height (cm) and perch diameter (cm), two key aspects of the structural habitat niche in anoles (Losos 2009). We also measured two aspects of body size, snout-vent length (SVL, mm) and mass (g), for all lizards captured. We caught only males because females have very small dewlaps. We tested for differences among islands in lizard structural habitat use and body size using analysis of variance (ANOVA) in JMP (SAS 2014). Perch diameter values were log-transformed to achieve a normal distribution.
To quantify dewlap color, we measured reflectance spectra from 300 to 700 nm in wavelength (i.e., visible plus ultraviolet light) using an Ocean Optics USB4000 spectrophotometer with a pulsed Xenon light source (PX-2, Ocean Optics), calibrated regularly against a white reflectance standard (Labsphere, Inc.). On live lizards, we measured reflectance at nine points distributed across the dewlap by pressing the dewlap against a black background with the reflectance probe containing a black, 45°-angled tip used to prevent specular glare. The Ocean Optics software was set to a boxcar width of five and an integration time of 10 ms. We averaged ten scans to generate spectra for each point on the dewlap.
We used the R-package pavo (Maia et al. 2013) to manipulate spectrophotometric data. Briefly, we used the functions getspec to import our spectra within the 300–700 nm wavelength window, aggspec to generate a mean spectrum for each individual, and procspec to bin those spectra into 10-nm windows. With the values from these 10-nm bins, we used the base package R-function prcomp to conduct a principal component analysis (PCA) in R (R Core Team 2014). We tested for differences among islands on PC axes representing dewlap reflectance using ANOVA and Tukey’s Honestly Significant Difference (HSD) post hoc tests using JMP (SAS 2014). We then used discriminant function analysis (DFA) to predict group membership based on dewlap reflectance data from PC axes using JMP (SAS 2014). For the DFA, lizards from Grand Cayman and Little Cayman were combined a priori into a red dewlap group and Cayman Brac lizards were in the yellow dewlap group.
After dewlap data were collected, we removed ~15 mm of tail tissue for genetic analysis and released lizards at the point of capture. The tissues were preserved in 100% ethanol and stored at −20 °C. We extracted genomic DNA following a standard protocol using the AutoGenprep 965 machine (Autogen, Inc.) in the Bauer Core Facility at Harvard University. We amplified 10 microsatellite loci using PCR (Bardeleben et al. 2004; Wordley et al. 2011) and fluorescently labeled primers following standard protocols at the University of Rhode Island. Samples were genotyped at the DNA Analysis Facility at Yale University. Markers for all samples were analyzed with the software GeneMapper® v4.1 and visually inspected for accuracy. For a subset of individuals (n = 16), we sequenced the mtDNA marker ND2. Sequences were assembled and aligned in Geneious Pro v5.6.3 (Kearse et al. 2012). Data were combined with previously published microsatellite genotypes (Kolbe et al. 2008) and mtDNA (ND2) sequences for the Cayman Islands (Kolbe et al. 2004, 2007).
Using these multilocus microsatellite data, we estimated standard population genetic statistics for diversity within islands and tested for Hardy–Weinberg equilibrium for each locus on each island. To evaluate population differentiation among the three islands under the assumption of Hardy–Weinberg equilibrium, we calculated pairwise FST values using GenAlEx (Peakall and Smouse 2012) and conducted a Bayesian cluster analysis using STRUCTURE (Pritchard et al. 2000; Earl and Vonholdt 2012). We also assessed population differentiation among islands with methods that do not rely on assumptions of equilibrium, including principal coordinates analysis (PCoA) of genetic distances using the covariance matrix with data standardization in GenAlEx and discriminant analysis of principal components (DAPC) using the adegenet package in R (Jombart and Ahmed 2012). To determine the relationships among ND2 haplotypes, we constructed a maximum likelihood phylogeny using RAxML (Stamatakis et al. 2008; Silvestro and Michalak 2012). The final alignment consisted 540 bp of ND2 for 63 individuals, including samples from the Cayman Islands, Cuba, south Florida and A. bremerei as the outgroup (Kolbe et al. 2007). We used GTR + G, the best fitting available model for nucleotide substitution based on Modeltest 3.7 (Posada and Crandall 1998). For the ML search, we used the rapid bootstrapping algorithm with 1000 non-parametric replicates.
Results
We sampled a total of 280 male A. sagrei from 18 sites on Cayman Brac (n = 195), two sites on Little Cayman (n = 26), and six sites on Grand Cayman (n = 59) (Fig. 1; Table S1). We found no differences among islands in structural habitat use (perch height: F2,201 = 1.32, P = 0.27 and log-perch diameter: F2,173 = 0.32, P = 0.73) or body size (SVL: F2,254 = 0.02, P = 0.98 and mass: F2,241 = 2.38, P = 0.10; Table S2). When comparing PC axes, dewlap reflectance showed strong differentiation between yellow-dewlapped lizards on Cayman Brac and the red-dewlapped lizards on the other two islands (Fig. 2; Table 1a). DFA based on PC axes 1–4, which represented 98% of variation, classified six lizards sampled on Cayman Brac (CB008, CB026, CB046, CB107, CB170, CB178) as having red dewlaps (Figs. 2, 3). This supports our field observations of seemingly red-dewlapped lizards occurring on Cayman Brac (Fig. 3) and suggests the introduction of invasive A. sagrei from Grand Cayman or native A. s. sagrei from Little Cayman. One lizard from Little Cayman was classified as having a yellow dewlap (LC015).
Results of a PCA for dewlap reflectance using the average wavelength from the dewlap of each male lizard. Photos of lizards with their dewlaps extended represent the color variation along each PC axis. The six Cayman Brac lizards classified as having red dewlaps are labeled along with CB101 and LC015
Examples of Anolis sagrei dewlaps from a Grand Cayman, b Cayman Brac with red dewlaps, c Cayman Brac with intermediate genotypes, d Cayman Brac with native genotypes, and e Little Cayman. Genetically intermediate individuals were outside of the 95% confidence ellipse for Cayman Brac and intermediate between Cayman Brac and Grand Cayman based on the PCoA of multilocus microsatellite genotypes (see Fig. 4). Cayman Brac lizards with red dewlaps were based on the DFA (see Fig. 2; Supplementary Materials for photographic methods)
Cayman Brac had higher allelic diversity compared to the other two islands and Hardy–Weinberg equilibrium was rejected for most loci on this island (Table 2); both results are consistent with an influx of alleles to Cayman Brac. FST values showed greater differentiation between Grand Cayman and the other two islands (FST = 0.209–0.235) compared to that between Cayman Brac and Little Cayman (FST = 0.161; Table S3). Moreover, STRUCTURE identified two genetic clusters corresponding to (1) Grand Cayman and (2) Cayman Brac and Little Cayman (Fig. S1). However, rejection of HWE for half the loci across islands (Table 2) violates the assumption of equilibrium for FST and STRUCTURE analyses. Avoiding this assumption, comparison of PCo axes from multilocus microsatellite genotypes and genetic differentiation based on DAPC analysis showed similarly strong differentiation among islands (Figs. 4, S2; Table 1B), particularly Grand Cayman compared to the other two islands.
Results of a PCoA using multilocus genotypes from ten microsatellite loci. Confidence ellipses are based on 2SD (95%) and 3SD (99.7%) from the mean. Outliers beyond confidence limits are interpreted as individuals that are genetically intermediate between islands (see Fig. S1). Cayman Brac lizards classified as having red dewlaps are labeled including CB008, which had a mtDNA haplotype from Grand Cayman. Lizard CB101 sampled on Cayman Brac had a microsatellite genotype that clustered with Little Cayman, and two lizards sampled on Grand Cayman shared genetic affinities with Cayman Brac (GC066) and Little Cayman (JJK1128)
We identified genetically intermediate individuals on the PCo 1 v. PCo 2 plot (Fig. 4) as those outside of the 99.7% confidence ellipse of an island (i.e., 7 of 280 individuals, or 3%; CB007, CB101, CB104, CB109, CB118, CB144, GC066) or the 95% confidence ellipse (i.e., 25 of 280 individuals, or 9%). Most lizards with intermediate microsatellite genotypes were sampled on Cayman Brac and all but one of these lizards had mtDNA haplotypes matching their island. One lizard sampled on Cayman Brac (CB008) had a Grand Cayman mtDNA haplotype (see phylogenetic tree in Fig. S3), an intermediate genotype outside 95% confidence ellipse for Cayman Brac, and a red dewlap as classified by the DFA (Figs. 2, 3, 4). Moreover, its genotype included one allele that was rare on Cayman Brac (2%), but at a moderate frequency on Grand Cayman (36%). Another individual sampled on Cayman Brac (CB101) had a microsatellite genotype similar to Little Cayman lizards, yet it had a Cayman Brac mtDNA haplotype and a yellow dewlap (Figs. 2, 3, 4). This individual had two alleles found on Cayman Brac, but not sampled on Little Cayman. These two examples demonstrate that red-dewlapped lizards from both Grand Cayman and Little Cayman have been introduced to Cayman Brac and have subsequently interbred with endemic A. s. luteosignifer. Moreover, it shows that a lizard’s dewlap color can be dissociated from its microsatellite genotype and mtDNA haplotype. In addition to these definitive examples, numerous individuals show some mismatch among their microsatellite genotype, mtDNA haplotype, and dewlap phenotype, which could indicate some level of past interbreeding. For instance, most lizards from Cayman Brac with dewlaps classified as red had unambiguously Cayman Brac microsatellite genotypes and mtDNA haplotypes (Fig. 4). Forcing K = 3 clusters in the STRUCTURE analysis showed substantial mixed ancestry for numerous Cayman Brac lizards, primarily with Little Cayman but also a lower percentage shared with Grand Cayman (Fig. S1). DAPC analysis also showed some Cayman Brac lizards clustering with the predominately Little Cayman genetic group (Fig. S2).
Discussion
Our genetic and phenotypic data document for the first time the reintroduction of individuals from an invasive population back into their native range, termed cryptic back-introduction (Guo 2005). The two nuclear-mitochondrial mismatches clearly demonstrate that red-dewlapped A. sagrei from both Grand Cayman and Little Cayman have interbred with endemic, yellow-dewlapped A. s. luteosignifer on Cayman Brac. This supports human-mediated gene flow among islands in the Caymans, including transfer of invasive A. sagrei from Grand Cayman to native populations on Cayman Brac (i.e., cryptic back-introduction). Although demonstrative, the two lizards with nuclear-mitochondrial mismatches (<1%) could represent merely a low incidence of invasion. However, when including genetic intermediates and lizards with dewlaps not matching their island, 4–11% of lizards may be the product of admixture between invasive and native populations. Less clear is when and how many times A. sagrei has arrived on Cayman Brac over the past ~30 years since it invaded Grand Cayman, making it difficult to assess whether invasive alleles are persisting on Cayman Brac or being introduced repeatedly.
When invasive individuals from outside of their native range are introduced into a native population at least two outcomes are possible. First, invasive and native lineages may fail to interbreed and therefore represent cryptic species. Given that the two species will overlap substantially in their ecological niches (i.e., similar structural habitat use and body size in our system; Table S2), interspecific interactions such as competition may be important. If the invader is favored, then the persistence of the native species may be in jeopardy. Alternatively, if the native lineage is favored in interspecific interactions, then the native range may be a sink for propagules that arrive but whose alleles do not persist. A second possibility is that the lineages interbreed, altering the genetic distinctiveness of the native populations. This occurred when introduced tiger salamanders (Ambystoma tigrinum) hybridized with threatened California tiger salamanders (Ambystoma californiense), leading to changes in fitness, population viability, and patterns of natural selection that favored some invasive alleles (Fitzpatrick and Shaffer 2007; Fitzpatrick et al. 2010; Searcy et al. 2016). Given genetic admixture of A. sagrei among multiple geographically and genetically distinct native-range lineages during its invasion of Florida (Kolbe et al. 2004, 2008), successful interbreeding among invasive and native lineages in the Caymans is not surprising. In this instance, however, interbreeding occurred despite clear differences in dewlap color, a trait used for species recognition in anoles (Losos 2009) that could have served as a reproductive barrier between lineages. The genetic mixing of these lineages raises the pressing issue of how invasive A. sagrei, which are genetically and phenotypically divergent from native lineages (Kolbe et al. 2004, 2007, 2014), will alter the previously isolated populations on Cayman Brac.
The very nature of cryptic back-introductions makes it difficult to identify this phenomenon based on morphology alone, requiring genetic analyses in most instances. Invaders may harbor other types of phenotypic variation, such as in behavior or physiology, relevant for invasion success. A classic example of a cryptic invasion (though not cryptic back-introduction) is the replacement of native common reed (Phragmites australis) haplotypes in New England by a non-native haplotype over the past 100 years (Saltonstall 2002). The spread of this invasive haplotype is at least in part due to greater salinity tolerance and higher relative growth rates compared to native haplotypes (Vasquez et al. 2005), providing a mechanistic explanation for this invasion. Some aspects of phenotypic variation in invasive A. sagrei populations in Florida, including body size, head and limb proportions, and perhaps metabolic rate, are related to the invasion history and source populations (Kolbe et al. 2007, 2014). Other phenotypes like low-temperature tolerance and water loss rate, which follow clines in temperature and moisture in Florida, are suggestive of adaptive responses (Kolbe et al. 2012, 2014). Whether these traits or others differ between invasive Grand Cayman A. sagrei, which are derived from non-native populations in south Florida (Kolbe et al. 2004), and native lizards on Cayman Brac remains to be seen.
Our findings show that lizards with different colored dewlaps can interbreed; however, our data are insufficient to determine confidently whether assortative mating and asymmetric introgression are occurring or if mating is random. Females of this species have very small dewlaps, thus males are unlikely to use this trait for mate choice. Unlike other Anolis species that have stereotyped display patterns used during courtship (e.g., Anolis carolinensis; DeCourcy and Jenssen 1994; Losos 2009), male A. sagrei appear to lack courtship-specific displays on which females could base mate selection (Scott 1984). Variation in dewlap color and other courtship behaviors could faithfully signal the origin or genetic identity of a male lizard, but little is known about mate selection based on intraspecific variation in anoles. Our preliminary assessment of cyto-nuclear disequilibrium in genetically and phenotypically intermediate lizards from Cayman Brac shows only one case of a lizard with a Grand Cayman mtDNA haplotype (which is maternally derived), suggesting a higher frequency of mating between red-dewlapped males from Grand Cayman or Little Cayman and females from Cayman Brac.
The evolutionary history of isolated island groups has been largely contingent upon rare, long-distance dispersal events (Gillespie and Clague 2009). Periods of isolation promote divergence that leads to reproductive isolation and eventually speciation. It took A. sagrei millions of years to colonize Cayman Brac and Little Cayman naturally. In contrast, after finally invading Grand Cayman via human-mediated dispersal, invasive A. sagrei spread via human activity to native populations on Cayman Brac within just 30 years. As human commerce in the Anthropocene decreases the “distances” among islands (Helmus et al. 2014), these types of cryptic back-introductions are expected to increase in frequency. If inter-island introductions in the Caymans continue, or even increase, we predict the Cayman Brac lineage will lose its unique genetic and phenotypic identity. Unfortunately, the cryptic nature of back-introductions makes them difficult to monitor without genetic analyses. We therefore do not yet know the frequency with which cryptic back-introductions are occurring. Interbreeding will blend continuous traits, like dewlap color, that could contribute to species recognition, potentially derailing speciation and contributing to homogenization (McKinney and Lockwood 1999). Cryptic back-introductions may eventually provide some insight into whether the process of invasion (and its relatively brief period of isolation from native populations) changes lineages in ways that make them distinct from and potentially incompatible with conspecific native populations. For brown anoles in the Caymans, phenotypic divergence between invaders and native lizards has not led to reproductive isolation, but whether phenotypic variation in other traits has consequences for ecological interactions with native species on Cayman Brac awaits future study.
References
Bardeleben C, Palchevskiy V, Calsbeek R, Wayne RK (2004) Isolation of polymorphic tetranucleotide microsatellite markers for the brown anole (Anolis sagrei). Mol Ecol Notes 4:176–178
DeCourcy KR, Jenssen TA (1994) Structure and use of male territorial headbob signals by the lizard, Anolis carolinensis. Anim Behav 47:251–262
Earl DA, Vonholdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res 4:359–361
Fitzpatrick BM, Shaffer HB (2007) Hybrid vigor between native and introduced salamanders raises new challenges for conservation. Proc Natl Acad Sci 104:15793–15798
Fitzpatrick BM, Johnson JR, Kump DK, Smith JJ, Voss SR, Shaffer HB (2010) Rapid spread of invasive genes into a threatened native species. Proc Natl Acad Sci 107:3606–3610
Gillespie R, Clague D (eds) (2009) Encyclopedia of Islands. University of California Press, Berkelely
Guo Q (2005) Possible cryptic invasion through “back introduction”? Front Ecol Environ 3:470–471
Helmus MR, Mahler DL, Losos JB (2014) Island biogeography of the Anthropocene. Nature 513:543–546
Jombart T, Ahmed I (2012) Adegenet: an R package for the exploratory analysis of genetic and genomic data. http://adegenet.r-forge.r-project.org. R package version 1.3.4
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Kolbe JJ, Glor RE, Rodriguez-Schettino L, Chamizo-Lara A, Larson A, Losos JB (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181
Kolbe JJ, Larson A, Losos JB (2007) Differential admixture shapes morphological variation among invasive populations of the lizard Anolis sagrei. Mol Ecol 16:1579–1591
Kolbe JJ, Larson A, Losos JB, de Queiroz K (2008) Admixture determines genetic diversity and population differentiation in the biological invasion of a lizard species. Biol Lett 4:434–437
Kolbe JJ, VanMiddlesworth PS, Losin N, Dappen N, Losos JB (2012) Climatic niche shift predicts thermal trait response in one but not both introductions of the Puerto Rican lizard Anolis cristatellus to Miami, Florida, USA. Ecol Evol 2:1503–1516
Kolbe JJ, Ehrenberger JC, Moniz HA, Angilletta MJ (2014) Physiological variation among invasive populations of the Brown Anole (Anolis sagrei). Physiol Biochem Zool 87:92–104
Liu X, Li X, Liu Z, Tingley R, Kraus F, Guo Z, Li Y (2014) Congener diversity, topographic heterogeneity and human-assisted dispersal predict spread rates of alien herpetofauna at a global scale. Ecol Lett 17:821–829
Losos JB (2009) Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. University of California Press, Berkelely
Losos JB, Schluter D (2000) Analysis of an evolutionary species-area relationship. Nature 408:847–850
MacArthur RH, Wilson EO (1963) An equilibrium theory of insular zoogeography. Evolution 17:373–387
Maia R, Eliason CM, Bitton P-P, Doucet SM, Shawkey MD (2013) pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol Evol 4:906–913
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453
Minton SA, Minton MR (1984) Anolis sagrei (brown anole). Herpetol Rev 15:77
Moritz C (1994) Defining ‘evolutionary significant units’ for conservation. Trends Ecol Evol 9:373–375
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci 99:2445–2449
SAS Institute, Inc. (2014) JMP 11.1 Cary, NC
Sax DF, Brown JH, White EP, Gaines SD (2005) The dynamics of species invasions: insights into the mechanisms that limit species diversity. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasion: insights into ecology, evolution, and biogeography. Sinauer Associates, Sunderland, pp. 447–465
Schwartz A, Henderson RW (1991) Amphibians and reptiles of the West Indies: descriptions, distributions, and natural history. University of Florida Press, Gainesville
Scott MP (1984) Agonistic and courtship displays of male Anolis sagrei. Breviora 479:1–22
Searcy CA, Rollins HB, Shaffer HB (2016) Ecological equivalency as a tool for endangered species management. Ecol Appl 26:94–103
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Stuart YE, Campbell TS, Hohenlohe PA, Reynolds RG, Revell LJ, Losos JB (2014) Rapid evolution of a native species following invasion by a congener. Science 346:463–466
Vasquez EA, Glenn EP, Brown JJ, Guntenspergen GR, Nelson SG (2005) Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Mar Ecol Prog Ser 298:1–8
Williams EE (1969) The ecology of colonization as seen in the zoogeography of anoline lizards on small islands. Q Rev Biol 44:345–389
Wordley C, Slate J, Stapley J (2011) Mining online genomic resources in Anolis carolinensis facilitates rapid and inexpensive development of cross-species microsatellite markers for the Anolis lizard genus. Mol Ecol Resources 11:126–133
Acknowledgements
We thank Haley Moniz, Vikram Chhatre, Brianne Neptin, Melissa Losos, Bonnie Edwards, Thom Sanger, and the Rhode Island Genomics and Sequencing Center staff for assistance. This material is based in part upon work conducted at a Rhode Island NSF EPSCoR research facility, the Genomics and Sequencing Center, supported in part by the National Science Foundation EPSCoR Cooperative Agreement #EPS-1004057. A portion of this project was conducted during the summer Coastal Fellows Program, sponsored by the College of the Environment and Life Sciences at the University of Rhode Island. Permits were graciously provided by Matthew Cottam and the Department of Environment of the Cayman Islands. Funding was provided by a Putnam Expeditionary Grant from the Museum of Comparative Zoology and the University of Rhode Island. We thank Graham Reynolds for providing coalescent dates for A. sagrei and Sozos Michaelides for help with some population genetic analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolbe, J.J., Wegener, J.E., Stuart, Y.E. et al. An incipient invasion of brown anole lizards (Anolis sagrei) into their own native range in the Cayman Islands: a case of cryptic back-introduction. Biol Invasions 19, 1989–1998 (2017). https://doi.org/10.1007/s10530-017-1432-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1432-2