Abstract
Trout species often segregate along elevational gradients, yet the mechanisms driving this pattern are not fully understood. On the Logan River, Utah, USA, exotic brown trout (Salmo trutta) dominate at low elevations but are near-absent from high elevations with native Bonneville cutthroat trout (Onchorhynchus clarkii utah). We used a spatially-explicit Bayesian modeling approach to evaluate how abiotic conditions (describing mechanisms related to temperature and physical habitat) as well as propagule pressure explained the distribution of brown trout in this system. Many covariates strongly explained redd abundance based on model performance and coefficient strength, including average annual temperature, average summer temperature, gravel availability, distance from a concentrated stocking area, and anchor ice-impeded distance from a concentrated stocking area. In contrast, covariates that exhibited low performance in models and/or a weak relationship to redd abundance included reach-average water depth, stocking intensity to the reach, average winter temperature, and number of days with anchor ice. Even if climate change creates more suitable summer temperature conditions for brown trout at high elevations, our findings suggest their success may be limited by other conditions. The potential role of anchor ice in limiting movement upstream is compelling considering evidence suggesting anchor ice prevalence on the Logan River has decreased significantly over the last several decades, likely in response to climatic changes. Further experimental and field research is needed to explore the role of anchor ice, spawning gravel availability, and locations of historical stocking in structuring brown trout distributions on the Logan River and elsewhere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The availability of suitable abiotic conditions is one of the most important factors influencing the establishment and spread of invasive species (Baltz and Moyle 1993; Kondolf 1997). Habitat alteration and climate change could increase the availability of suitable habitat for many invaders, causing them to move from introduction areas and establish in habitats currently occupied by native species (Gesch et al. 2002; Sepulveda et al. 2009). In understanding how invasive species’ distributions are limited by the current distribution of suitable abiotic conditions, we can inform strategies to monitor and limit their expansion.
A combination of experimental, field, and statistical modeling approaches may be used to discern what abiotic factors limit the distribution of brown trout (Salmo trutta), one of the world’s most successful invasive species, within its introduced range (Lowe et al. 2000; McIntosh et al. 2012). In the Eastern USA, brown trout dominate at lower-elevation downstream reaches while native brook trout (Salvelinus fontinalis) thrive at higher-elevation upstream reaches (Weigel and Sorensen 2001). Similar patterns occur between brown trout and native cutthroat trout (Oncorhynchus clarkii) in the Western USA (de la Hoz Franco and Budy 2005). The lack of native trout (e.g., brook trout or cutthroat trout) at low elevations is often attributed to competitive interactions with brown trout, while the absence of brown trout from high elevations is often attributed to lack of suitable abiotic habitat conditions (Budy et al. 2008). Yet, despite the large amount of research conducted on brown trout habitat-relationships (Lowe et al. 2000; Sakai et al. 2001), the environmental conditions limiting the upstream expansion of brown trout have not been definitively determined. While a number of abiotic conditions are often correlated with patterns of brown trout abundance (e.g., temperature, substrate, and water depth) many studies only consider one or a few of these potential drivers and not within an experimental or broader modeling framework (Rahel and Nibbelink 1999; Weigel and Sorensen 2001), making it difficult to discern which are most influential.
The Logan River, Utah (USA) is an ideal setting to explore how multiple factors can influence the longitudinal distribution of brown trout. Brown trout have largely displaced native Bonneville cutthroat trout, a species of concern, in downstream sections. The abundance of Bonneville cutthroat trout is still high in upstream, higher-elevation sections where brown trout consistently exhibit low abundance (de la Hoz Franco and Budy 2005; Budy et al. 2008). A series of experimental and observational studies have already provided insight into potential mechanisms driving this distribution. For instance, to investigate the potentially interacting roles of interspecific competition and water temperature, brown trout and cutthroat trout were placed in enclosures located along an elevational gradient of the Logan River during the summer (McHugh and Budy 2005). Brown trout exhibited higher growth rates and condition than cutthroat trout at all reaches, suggesting that temperature-mediated competition does not explain the lack of brown trout at high elevations. These competition experiments then led to a series of field studies and experiments studying the potential influence of abiotic factors. The survival of brown trout embryos during the winter developmental period was assessed at multiple positions along an elevational gradient (Wood and Budy 2009). Although survival was lower at high-elevations, harsh winter conditions did not preclude hatching success (≥36%), suggesting that cold winter effects on early life-stages are also likely not responsible for the near-absence of brown trout from high elevations. Next, the influence of channel type and stream gradient on streambed scour and early life-stage mortality of brown trout was similarly assessed at multiple reaches along an elevational gradient (Meredith 2012). Scour depths tended to be low across all elevations, suggesting that streambed scour also does not play a strong role. Finally, a series of experiments investigated whether high native cutthroat densities could limit the spread of brown trout through biotic resistance. The performance and competitive interactions of individuals of both species were monitored within tanks containing both brown trout and cutthroat trout. They found that while brown trout were unaffected by varying densities of cutthroat trout, cutthroat performed much better when densities of conspecifics were high. These results provided some evidence that biotic resistance may lower the negative effects of brown trout on cutthroat trout, but still do not completely explain the lack of brown trout at high elevations (Saunders and Budy, in review).
We sought to complement this body of experimental research by exploring multiple abiotic conditions potentially limiting brown trout abundance on the Logan River within a statistical modeling framework. Considerable variation in environmental and stream conditions occurs along the longitudinal/elevational gradient of the Logan River, due to changing in geology and hydrology, which could influence brown trout abundance (Budy et al. 2008). For instance, the high brown trout abundance in low-gradient sections of river could be related to higher gravel availability for spawning (Beard and Carline 1991). Spatial changes in temperature due to the presence of springs could also influence abundance of brown trout through effects on growth, winter survival, and reproduction (Stonecypher et al. 1994; Baxter and McPhail 1999). However, abundance has only been estimated for a small subset of reaches albeit over a long time period (de la Hoz Franco and Budy 2005). In contrast, our modeling effort considered multiple abiotic conditions and many reaches along the longitudinal gradient.
We developed models relating reach-scale brown trout abundance to a suite of abiotic conditions we considered to be representative of potential mechanisms driving the brown trout distribution. Given that propagule pressure (or the number of individuals introduced) can outweigh the importance of abiotic factors in invasion success (Williamson 1996; Westley and Fleming 2011), we also considered covariates representing the intensity of past stocking to a reach and distance to a concentrated stocking area. Inclusion of this stocking-related covariate was based on principle of invasion ecology: the closer an area with a large number of propagules (e.g., “source area”) is to a given area, the more likely it contributes to the abundance of that area (Lockwood et al. 2005). We additionally incorporated a Bayesian conditional auto-regressive (CAR) modeling framework into our analysis, which allowed us to account for spatial auto-correlation that could not be explained by abiotic conditions or stocking.
Methods
Study area description and design
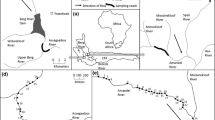
The Logan River, USA is a tributary of the Bear River, which terminates in the Great Salt Lake. The river is a snowmelt flood dominated system, and flood events typically occur in May and June. Average discharge varies from 2.16 m3/s at the most upstream reach in the study area to 4.76 m3/s at the most downstream reach. Three major tributaries flow into the Logan River within the study area, including Right Hand Fork, Temple Fork, and Beaver Creek (Fig. 1).
We selected a portion of the mainstem of the Logan River to represent the transition from high to low abundance of brown trout that occurs longitudinally on the river. The study area extended from upstream the impoundment of Third Dam to three km upstream of the confluence with Beaver Creek (Fig. 1). Third Dam is the most upstream of a series of three dams on the river, and the impoundment upstream supports some of the highest densities of mature brown trout adults in the system (Carl Saunders, personal communication, July 2013). Few cutthroat trout occur downstream from Third Dam, and the hydrology downstream from the dam is highly altered by diversions. In contrast, Beaver Creek represents the upper extent of the brown trout population, as no spawning or adult brown trout have been documented above the confluence with Beaver Creek. A more detailed description of the study area is available in Budy et al. (2008).
Estimating brown trout abundance
Although redd abundance may vary spatially due to the quality of spawning habitat and other factors, redd abundance is strongly correlated with trout abundance in cases where salmonids are relatively sedentary (Al-Chokhachy et al. 2005; Budy et al. 2008). In addition, we were most interested in relative differences in abundance along the elevational gradient and not necessarily absolute values. Accordingly, we used easily-measured redd abundance as our measure of the brown trout distribution. We counted redds in the Logan River at the end of the fall spawning season (December and January) for the years 2006–2011. Observers walked upstream, recording the presence of a redd feature. A feature was considered a redd only if it contained an extremely defined pit and tailspill (Burner 1951), or if it contained a spawning pair of brown trout. Logistical constraints (e.g., snow depth) prevented the sampling of all reaches during every year. In approximately 70% of reaches, we performed at least 2 years of redd surveys. In the remainder of reaches, we conducted surveys during only 1 year. However, for reaches sampled in multiple years, redd abundance varied by <20% across years; therefore, we had confidence in our redd abundance estimates across reaches. As response variable in our statistical model, we used the mean number of redds across years within 2000 m2, the typical evaluated reach area.
We verified that the relative abundance of brown trout (juvenile and adult combined) could be explained by brown trout redd abundance (Fig. 2). Abundance of fish at each index reach was estimated via snorkeling surveys in which two snorkelers swam adjacent to each other in a zigzag pattern, from the bottom to top of the reach (Thurow et al. 2006). Each snorkeler recorded the number of each species of fish observed, communicating with his/her partner to make sure fish were not double-counted. We used linear regression analysis to confirm a significant, positive relationship between redd abundance and total brown trout abundance observed during snorkeling. We also confirmed that cutthroat trout abundance increased with distance upstream. Our analysis indicated that 76% of the variation in brown trout abundance observed during snorkel surveys could be explained by redd abundance (p < 0.0001). This explained variation decreased to 42% when the high-abundance site immediately above the Third Dam Impoundment and concentrated stocking area was removed (p = 0.002), but was 65% when we removed both the high-abundance site and an outlier site sampled when visibility was low (p < 0.0001). Despite some variability in the relationship between redd and trout abundance, these results supported the use of redd counts as an estimate of relative abundance of brown trout.
Estimating environmental covariates
Our covariates reflected abiotic conditions and potential driving mechanisms that could influence brown trout abundance based on the literature (Table 1). We initially wanted to focus on conditions influencing brown trout in the species’ native range. However, because such studies are limited, we also used examples from research on brown trout in the species introduced range. In addition, because we did not find any studies of effects of anchor ice conditions on brown trout abundance (which we hypothesized is a potential driver), we use examples from studies on other trout species to corroborate that anchor ice could affect brown trout.
We used unit stream power, a measure of transport capacity, to estimate an index of spawning gravel availability at each reach. As a measure of energy associated with the ability to move sediments, unit stream power has successfully predicted median substrate size within regional areas of similar lithology (Golden and Springer 2006). We hypothesized that unit stream power could be used to estimate general availability of smaller, spawning gravel substrates on the Logan River. Other research has shown significant correlations between unit stream power and spawning activity (Moir et al. 2009). During the years 2008–2010, we used a Topcon Hiper Pro GPS Unit (Topcon, USA) to perform longitudinal profile surveys at each of the reaches. We collected elevation measurements at the bed and water surface, occurring at each break in slope (horizontal accuracy: 10 mm; vertical accuracy: 25 mm). For each of the 82 study reaches, we estimated unit stream power (ω) at bankfull. We calculated unit stream power (N/s m) as follows:
where \(\gamma\) is the unit weight of water (9800 N/m3), Q is Bankfull discharge (m3/s), s is slope (m), and w is bankfull width in meters (Golden and Springer 2006). We estimated water surface slope from a best fit line through the water surface longitudinal profile (Montgomery et al. 1999) at near-baseflow conditions, making the assumption that the water surface slope at bankfull discharge is similar to the water surface slope at the time of our longitudinal profile measurements. We estimated bankfull discharge (Q) from stage height-contributing area relationships developed from three pressure transducers located in the study portion of the Logan River. We measured bankfull width at multiple locations in each reach and averaged to obtain a representative estimate of bankfull width (w) for each reach.
To verify that unit stream power could be used as an appropriate estimate of spawning gravel availability, we determined that there was a significant negative relationship between unit stream power and the proportion of a reach in spawning gravel patches. We divided each reach into unique morphological units (run, riffle, pool, cascade) and performed 100-point counts of bed material in each unit (Wolman 1954). We quantified the number of particles between the sizes of 5.7 and 45 mm for each unit, and estimated the proportion gravel for each reach based on the percent comprised of each unit (Kondolf 1997). Because smaller values are generally underestimated in pebble counts, we used a lower value of 5.7 mm, which is in the range of “fine gravel” (Kondolf 1997). We used an upper value of 45 mm, because previous surveys of redds showed that substrates chosen for spawning on the Logan River were consistently 45 mm or less in size. We used linear regression to verify the positive, significant relationship between unit stream power and percent of reach in spawning gravels (Fig. 3; R2 = 0.35; p = 0.005). Much of the deviation from the regression was from two reaches with higher gravel than expected. These reaches were generally characterized by high confinement and unit stream power but also contained shorter, unconfined sections where the Logan River had a broad floodplain. Our reach-scale measure of unit stream power was not adequate at capturing these subtleties in geomorphology which can influence spawning gravel availability. However, we concluded that it was still adequate for estimating broad trends, which was our goal. We developed a spawning gravel availability index, in which we transformed unit stream power to a 0–1 scale (with 0 being the site with the fewest spawning gravels in the study area, and 1 being the site with the most spawning gravels) using the following equation:
where ω was indicated by unit stream power.
We observed a negative relationship between unit stream power and the proportion of particles in the spawning gravel size range (R2 = 0.35; p = 0.005). Much of the deviance from the regression line was from two reaches. Although characterized by high confinement and high unit stream power, sections of these reaches had broad floodplains characterized by a high availability of spawning gravel
We estimated reach-average baseflow depth (m) as another indicator of physical habitat suitability for spawning and adult brown trout (Shirvell and Dungey 1983; Ayllon et al. 2010) based on baseflow depth at the centerline of each reach, from longitudinal survey data. Baseflow estimates are appropriate because near-baseflow conditions are present during much of the year including during spawning. We calculated mean reach depth by averaging depth measurements across the reach.
We used a combination of HOBO and Maxim i-button temperature loggers to record temperatures at index reaches. We estimated average temperature from hourly-averaged water temperatures collected over the entire study period (10 November 2009–9 November 2010), summer water temperatures from 1 July 2010 to 31 Aug 2010, and winter temperatures from 10 November 2010 to 30 April 2011. We estimated a covariate describing the number of days with anchor ice cover during the winter (November 2010 and April 2011) as the number of days where the water temperature was <1 °C. We used the ‘gam’ package in R to identify a smoothing function to best explain the longitudinal pattern for each temperature covariate; the function with the best performance according to restricted maximum likelihood was used to predict values at non-index reaches (R Core Development Team 2011).
Finally, we calculated the elevation at each of our reaches using average reach elevations obtained from longitudinal surveys performed using a Topcon Satellite GPS unit. We did not consider elevation to be representative of any particular mechanism; however, given many studies demonstrate a decline in brown trout densities with elevation, we wanted to compare the performance of a model containing only elevation to that of models containing abiotic and propagule pressure covariates.
Estimating propagule pressure
To estimate propagule pressure, we determined the historic abundance of brown trout individuals stocked to each reach in fish/m using methods carried out within ArcMap 10.1 (Esri 2014). Our estimate was based on stocking records for the Logan River drainage obtained from the Utah Department of Wildlife Resources, which existed from 1940 to present. The beginning and ending location of each section, as well as the number stocked at each stocking event, were found in this record. We used the following method to determine the abundance of individuals stocked to each reach: (1) we summed across multiple events to obtain a total number stocked to each section; (2) we converted the polyline for each section into a raster (10 m × 10 m pixel size) describing the total fish stocked/m); (3) we added rasters representing unique, sometimes overlapping sections to obtain the fish stocked/m to each pixel; (4) finally, we used the zonal sum tool in ArcMap to determine the average fish/m stocked to each reach.
We also estimated a covariate describing the distance of the midpoint of each reach from the highly-stocked Third Dam Impoundment (0–36 km). We based this covariate on our finding that Third Dam Impoundment had a historic level of stocking many times higher than any other reach (Fig. 4) indicating that this area may act as a source of brown trout to upstream areas.
Environmental conditions (a–g) and measures of propagule pressure (h–j) exhibited obvious spatial patterns along a longitudinal gradient of the Logan River. We measured temperature covariates (a–d) at nineteen index reaches and interpolated across reaches to estimate values at the remaining reaches. The remaining covariates were measured for each study reach (e–j). Points represent actual measurements whereas lines show interpolated spatial patterns. Distance upstream from the impoundment is represented by a 1:1 relationship
Finally, we estimated a measure of stocking pressure that considered how an abiotic condition influenced resistance to movement upstream. Specifically, we observed anchor ice forming in low-temperature reaches as early as November, covering spawning gravels and appearing to limit access to spawning gravels and movement upstream during spawning (a high-movement time period). We therefore hypothesized that the inability of brown trout to move from the Third Dam Area and invade upstream reaches may be due to the presence of anchor ice. Further, inclusion of an interaction between the number of days with anchor ice and distance from Third Dam would not be adequate to capture the cumulative effect of this resistance. To estimate total resistance to movement from this stocking area due to anchor ice, which we refer to as “anchor ice barrier,” we determined the cumulative number of days with anchor ice between each reach and the impoundment. For each index reach, we estimated the number of days covered in anchor ice (≤1° C) during the spawning season (15 November–31 December 2010). We then used a spline interpolation to obtain a value indicating the number of days with anchor ice for each 200 m section of river in our sampling area. We summed the number of anchor ice days across all reaches located between a given reach and the impoundment. We then rescaled this distance from 0 to 1, with the most downstream reach immediately upstream from the impoundment received a 0, and the farthest upstream reach received a 1.
Statistical analysis
We used a zero-inflated negative binomial generalized linear mixed model with fixed and random effects to explain redd abundance. Our fixed effects included environmental (e.g., temperature and substrate) and propagule pressure predictor variables, while our random effect accounted for spatial structure beyond what was provided by the predictor variables. Ver Hoef and Peterson (2010) and others have shown that accounting for latent spatial structure in stream ecosystems can be essential for providing appropriate inference. Our model approach uses a conditional autoregressive (CAR) formulation for the random spatial effects (Schabenberger and Gotway 2004). Because we initially found high correlation between our predictors and the spatial random effect in our models, which affected coefficient estimates, we used a CAR modeling approach called SPOCK (Spatial Orthogonal Centroid “K”orrection) that addresses the spatial confounding (Assunção et al. 2016). We provide the full Bayesian hierarchical model statement and details about the priors in “Appendix”. We used integrated nested Laplace approximation (INLA; Rue et al. 2009) to fit the statistical model to our data because it is computationally efficient for large spatial datasets such as ours. Our model is similar to other negative binomial models in that a one unit increase in a covariate increases the mean of the response by exp(βj). The benefit of the Bayesian spatial approach is that it allows us to account for the amount of spatial structure that further explains redd abundance but is not accounted for by existing covariates. As such, we can be more sure that coefficients of our non-spatial covariates reflect actual relationships to redd abundance and are not influenced by collinearity with this extra spatial structure.
We tested multiple combinations of covariates based on a priori hypothesis about what conditions were most limiting. We fit models containing covariates that did not exhibit high collinearity (r > 0.40), which resulted in a reduced set of possible models. We used the logarithmic score (LS) to evaluate model performance, which is based on the conditional predictive ordinate. The conditional predictive ordinate (CPO) is essentially performing a Bayesian version of cross-validation (Hooten and Hobbs 2015) which allowed us to rank models based on the posterior predictive distribution in the Bayesian Analysis. It automatically penalizes overly complex models in the same way cross-validation does (because over-parameterized models predict out of the sample data poorly). Smaller values of LS indicate better predictive ability (Borgstrøm and Museth 2005; Schrödle et al. 2010). We chose to use the LS method rather Deviance Information Criteria (DIC), which is often used in Bayesian analysis, because it is better suited for predictive scoring in Bayesian models with random effects (Hooten and Hobbs 2015). In addition to LS, we report model weights and pseudo r-squared. We calculated pseudo model weights as: exp(−LSj)/∑ (exp(−LSj)), which provides insight about the relative importance of the models for prediction. We determined pseudo r-squared by regressing the predicted values of redd abundance against the actual values of redd abundance. We also report spatial precision, which is a parameter indicating the amount of spatial structure with smaller values equating to greater spatial structure (Rue et al. 2009).
We provide unstandardized coefficients for each model we tested. The coefficients (β) can be interpreted as such: holding all other variables constant, a 1 unit increase in the covariate xj increases the mean of the response variable Y by exp(βj). However, to further interpret the strength of coefficients for our best-performing covariates, we also calculated the average increase in redd abundance with percent change in covariate. We determined this by identifying the best model containing each covariate but potentially also containing other covariates. Holding the other covariates at their medians, we calculated the redd abundance at the lowest value of the covariate of interest in the study area and at the highest value of the covariate of interest. We then divided the difference in these values by 100 to obtain the average increase in redd abundance with one percent increase in the covariate.
Finally, we chose several of the best models based on a combination of LS Score and pseudo-r-squared. For these models, we examined both the actual and predicted values across a longitudinal gradient to visualize how well models captured the spatial distribution of redd abundance from downstream to upstream.
Temporal trends in anchor ice prevalence and summer temperatures
Temporal trends in the distribution of spawning season anchor ice and summer temperature could contribute to potential expansion of brown trout to upstream reaches. As a result, we also explored possible trends in these environmental covariates over time. First, we used available temperature data collected at a nearby SNOTEL site [Tony Grove Lake (823)] during 1992–2013 to estimate mean daily air temperature for the spawning and summer time periods (Fig. 6a). We developed a relationship between daily mean temperature estimates at the SNOTEL site and daily mean water temperature collected at a high-elevation sampling reach on the Logan River for the years 2006–2011 (Fig. 1). This relationship was based on an s-shaped function as described in Mohseni et al. (1998). We used this relationship to predict average daily water temperatures at the high-elevation Logan River reach over the time-period of record available for the SNOTEL site (1992–2013). Based on daily temperature data, we estimated the number of anchor ice days during the spawning season (15 November–31 December 2010) as well as mean summer water temperature (1 July 2010–31 Aug 2010) for each year (Fig. 6c, d) of record. We evaluated trends in number of anchor iced days and average summer temperature using linear regression.
Results
We documented notable patterns in many of our covariates with increasing elevation and distance upstream (Fig. 4). Average temperature decreased linearly with increasing distance upstream. Average summer temperature remained nearly constant at 12 °C until near the confluence of Temple Fork, and then exhibited a general decrease with distance upstream. The reaches with the lowest winter temperatures and greatest number of days with anchor ice were immediately above the Temple Fork confluence, where the highest summer temperatures also occurred. Although water depth decreased with increasing distance upstream, it exhibited high variability along the longitudinal gradient. The pattern in the spawning gravel index indicated that localized areas of moderate gravel availability were present along the entire length of the Logan River in the study area, although the general trend was a decrease in gravel availability with distance upstream.
Many of our covariates were strongly correlated (Table 2; correlation coefficient was >0.4). Most notably, we observed strong correlations among our temperature covariates. Winter, annual average, and summer temperatures were strongly positively correlated with each other and with water depth and negatively correlated with the number of days with anchor ice, elevation, distance from the impoundment, and the anchor ice barrier index. In addition to being correlated with temperature covariates, water depth was strongly negatively correlated with distance from the impoundment and the anchor ice barrier index. The anchor ice barrier index was also strongly positively correlated with distance from the impoundment and elevation. Our index of gravel availability was negatively correlated with elevation, positively correlated with temperature, and negatively correlated with the number of ice days with ice and the distance from the impoundment weighted by the presence of anchor ice.
Our model weights indicated that a large proportion of the models we tested had similar performance in explaining brown trout abundance. Models with the highest weights also tended to have high pseudo-r-squared but we did not observe a direct relationship between these two measures. A model could have a higher pseudo-r-squared values but a lower weight than another model if some subset of observations was predicted poorly. Note that there are no well-accepted rules of thumb for setting a model selection cut-off score in complicated Bayesian models with random effects (Table 3).
In our analysis, covariates in models with the lowest logarithmic scores (e.g., best performance) included average, summer, and winter temperature and days below 0 °C, as well as gravel availability, distance to a concentrated stocking area, and distance to a concentrated stocking area weighted by the presence of anchor ice. Models containing water depth and stocking intensity to a reach exhibited low performance when alone; when included in combination with other covariates performance decreased. Although summer temperature was included in several of the top-performing models, the low pseudo-r-squared for the model containing just summer temperature indicated that much less variance was explained compared to many of the other covariates. Because of the strong correlations (>0.40) among covariates, we were not able to include many of them together in the same models, even though they may simultaneously influence brown trout abundance.
The strength of the effect on abundance was similar among many of the best-performing covariates. We found that the average change in redd counts for each percent increase in summer temperature, winter temperature, average temperature, days with anchor ice, anchor ice barrier index, gravel availability, and distance from concentrated stocking area was 0.132, 0.061, 0.124, −0.053, −0.090, 0.139, and −0.127, respectively. This equated to changes in redd counts/reach across the study area of 13 (summer temperature), 6 (winter temperature), 12 (average temperature), −5 (days with anchor ice), −9 (anchor ice barrier index), 13 (gravel availability), and −12 (distance from a concentrated stocking area). Thus, the estimated change in redd abundance with decreasing average winter temperature or increasing days with anchor ice was lower than for many of the other covariates in the best-performing models. The spatial precision parameter was relatively high across models, indicating low spatial structure remaining after accounting for the covariates.
Although many models performed well, our final graphs illustrated the explanatory power of three of our top models (Fig. 5.). These included the anchor ice barrier + gravel availability model, the maximum temperature + gravel availability model, and the average temperature/distance to concentrated stocking area + gravel availability model (the average temperature and distance to concentrated stocking covariates had the same effect on redd abundance due to a direct relationship). All of these models exhibited high performance based on cross-validation as well as a high pseudo-r-squared.
The spatial distribution of actual redd abundance closely matched the distribution of predicted redd abundance, according to the top performing models. We illustrate the three best-performing models, including the a anchor ice barrier + gravel availability index model (b), the summer temperature + gravel availability index model, and c the average temperature/distance upstream from concentrated stocking area + gravel availability index model. These models explained 48, 39, and 51% of the variation in redd abundance, respectively
Temporal trends in anchor ice prevalence and summer temperatures
Spawning season air temperature at the SNOTEL site exhibited positive trends during the time period of the study (Fig. 6a). However, only the trend in spawning season temperature was significant. Given the relationship between air and water temperature that we observed (Fig. 6b), our results provide some evidence of an increasing trend in anchor ice cover at the high-elevation test reach (Fig. 6c; p = 0.031; R2 = 0.172), but no evidence for an increasing trend in summer water temperature (Fig. 6d; p = 0.45; R2 = 0.022).
We observed increases in average (a) spawning (15 November–31 December) and summer (1 July–31 August) air temperatures at the Tony Grove SNOTEL site for the years 1992–2012, although only the spawning season trend was significant (p < 0.05); b we used a Mohseni curve to develop a relationship between mean daily air temperatures at the SNOTEL site and daily water temperatures at a high-elevation reach; c based on the relationship in the Monseni curve, we predicted that the number of days with anchor ice has increased significantly (p < 0.03); and d we predicted that the average daily summer temperature has not changed significantly over this same time period (p = 0.45)
Discussion
While other research has reported significant correlations between environmental conditions and brown trout abundance, these studies focused on one or a few potential conditions. In contrast, we assessed multiple conditions and associated mechanisms potentially influencing the distribution of exotic brown trout abundance on the Logan River, Utah including those describing both environment and propagule pressure. Because we used a spatial-modeling approach, we can be more confident the relationships we observed between covariates and brown trout abundance were not due to unidentified, spatially-varying environmental covariates (e.g., Lichstein 2002). We expected this approach to identify one or several conditions that could best explain the pattern of abundance. Instead, we found that multiple abiotic conditions affecting both early and adult life stages, as well as measures of propagule pressure, were good predictors.
It is difficult to determine what temperature-related mechanism could be driving the distribution of brown trout from just a correlative study alone. In our study, we found that all of the temperature conditions could partly explain the distribution in redd abundance. While average temperature showed one of the strongest relationships to redd abundance, it is integrative of conditions over the entire year while summer and winter temperatures are typically considered drivers of growth and survival. However, we know from experimental research that summer temperature did not limit growth and survival of adult brown trout transplanted to high elevation-reaches during the summer season (McHugh and Budy 2005) and that low winter temperatures did not lead to high mortality of early life-stages of developing brown trout (Wood and Budy 2009). Winter temperature also had a low magnitude of effect on redd abundance in our study compared to many other covariates. While it is possible that summer or winter temperature conditions are affecting different life stages other than what has been tested experimentally (such as potential effects of summer temperatures on age-0, see Borgstrøm and Museth 2005), we also hypothesize that the pattern in redd abundance could be driven by the location of the concentrated stocking area coupled with anchor ice acting as a barrier to movement upstream.
The failure to include propagule pressure in models of species distributions can lead to incorrectly associate invasion success with species traits or other factors when it is actually a result of propagule pressure (Colautti 2005). In our study, the location of the concentrated stocking area at the downstream end of the study area and the positive significant relationship between temperature and distance to this area makes it impossible to eliminate stocking pressure as a driver of the distribution. Had the concentrated stocking area been in the middle of the study area, we may have been able to better assess its influence. However, we know that the impoundment and reach immediately upstream had a history of high stocking pressure that far exceeded that of other areas in this system. Unpublished historical records also indicated that there may have been a hatchery near the impoundment. Therefore, it is possible that the impoundment is structuring the brown trout distribution in this system by providing a source of individuals to upstream sections. In Newfoundland, the presence of brown trout was similarly associated with the distance from heavily stocked areas (Westley and Fleming 2011). We suggest movement studies coupled with genetic testing, used to determine relatedness, could help determine how brown trout movement from stocking areas contributes to their longitudinal pattern on the Logan River and elsewhere (Hudy et al. 2010).
Anchor ice may act as a barrier to movement from this stocking area given that most brown trout movement often occurs during spawning season (Young 1994). In higher elevation reaches of the Logan River, most spawning gravels are located in channel margins where anchor ice typically forms. In 2009 and 2010, we visually observed anchor ice covering these spawning gravels as early as November and appearing to restrict spawning as well as movement into the same reaches that had low redd abundance. Elsewhere, anchor ice has acted as a barrier and forced cutthroat trout into large congregations within areas of less suitable habitat (Brown and Mackay 1995). Juvenile Atlantic salmon have been found to migrate away from stream sections containing anchor ice (Linnansaari et al. 2008). To our knowledge, the role of anchor ice in affecting distributions of trout along an elevational gradient of a river has not been previously investigated.
In our analyses, including gravel availability in models with other covariates increased model performance and explanatory power. Gravel availability explained much of the local variation, rather than the general trend, in abundance along a longitudinal gradient. Abundance was also correlated with gravel availability within a Pennsylvania stream (Beard and Carline 1991) and in rivers across New Zealand (Jowett and Richardson 1996). Given its potential importance to structuring trout populations (Hudy et al. 2010) the role of gravel availability in structuring trout populations in this and other systems also deserves further investigation. The use of redd abundance as a response variable makes it more difficult to interpret the importance of gravel availability. Given the importance of gravel for spawning, it is possible that gravel availability could be affecting redd abundance but not necessarily adult abundance. However, our preliminary analysis (Fig. 2) showed a strong correlation between redd abundance and adult abundance, and other factors that are typically important to the distribution of adults (e.g., summer temperature, anchor ice, and propagule pressure), were also strongly correlated with adult abundance. These considerations provided support for our use of redd abundance as a general measure of the brown trout distribution and the probable role of gravel availability in structuring the brown trout distribution.
It is only from a combination of experimental and statistical analysis that we have gained the most insight into the most likely causal mechanisms driving the distribution of brown trout on the Logan River. Our findings suggest that multiple environmental conditions may interact to influence patterns of exotic brown trout in mountain rivers. In a study of conditions influencing fishes in France (where brown trout are native), brown trout was one of the few species with a probability of occurrence explained by a combination of many climatic and physical factors instead of just a few (Buisson et al. 2008). In the species’ introduced range, is typical to find a combination of a high abundance of spawning gravels, warm summer temperature conditions, and low anchor ice cover within the same sections (Cunjak and Power 1986; Bozek and Hubert 1992; Weigel and Sorensen 2001); the permeability of gravel substrate is what promotes groundwater inflow and low anchor ice formation. Thus, the availability of such ideal conditions may help explain why brown trout is one of the world’s most successful invaders (Lowe et al. 2000; McIntosh et al. 2012).
Nonetheless, in the Logan River and other systems brown trout appear to be more limited by conditions at high elevations than native trout. It has been suggested that brown trout could move into high elevation sections of mountain streams as a changing climate results in warmer summer temperatures (Borgstrøm and Museth 2005). While this could vary by system, our findings support that conditions other than summer temperature (e.g., anchor ice and gravel availability) may limit brown trout at these elevations. Further, it should not be assumed that any negative relationship between elevation and abundance is due to a lack of suitable summer temperature conditions at high elevations. The potential influences of anchor ice to the brown trout distribution on the Logan River is compelling given our findings that the prevalence of anchor ice on the Logan River may be decreasing. Other research has similarly found that of spring floods on early life-stages could limit the expansion of brown trout despite warming temperatures (Wenger et al. 2011), although this has not yet been demonstrated for the Logan River (Meredith 2012). Such findings demonstrate how a changing climate can impact biota in unexpected ways (Stenseth and Mysterud 2002).
The use of approaches similar to ours, to better understand how abiotic factors contribute to a species’ expansion, would provide more insight if conducted over multiple locations including in brown trout’s native and introduced range. For instance, with regard to exotic brown trout, additional research could be conducted in riverine systems with alternate and potentially unique geomorphic arrangements, including those characterized by a range of elevations, climates, stream sizes, and stream gradients (e.g., New Zealand, southern USA, France or Spain, and the Oregon Cascades mountain range, USA) to determine if the same relationships to abiotic conditions are observed. We originally planned to use exiting datasets for such a comparison, however, the different methods and metrics measured between studies are not conducive for this type of analysis. Better knowledge of the history of the locations and intensity of brown trout introductions (e.g., such as in Westley and Fleming 2011), would also improve our understanding of how propagule pressure factors interact with abiotic conditions to influence the distribution of exotic brown trout in river systems. Spatially-explicit modeling approaches such as ours, in combination with findings from experimental and other field studies, can provide insight into mechanisms driving patterns of species invasion.
References
Al-Chokhachy R, Budy P, Schaller H (2005) Understanding the significance of redd counts: a comparison between two methods for estimating the abundance of and monitoring bull trout populations. N Am J Fish Manag 25:1505–1512
Assunção RM, Prates MO, Castilho ER (2016) Where geography lives? A projection approach for spatial confounding. https://arxiv.org/abs/1407.5363
Ayllon D, Almodovar A, Nicola GG, Elvira B (2010) Ontogenetic and spatial variations in brown trout habitat selection. Ecol Freshw Fish 19:420–432
Baltz DM, Moyle PB (1993) Invasion resistance to introduced species by a native assemblage of California stream fishes. Ecol Appl 3:246–255
Baxter JS, McPhail JD (1999) The influence of redd site selection, groundwater upwelling, and over-winter incubation temperature on survival of bull trout (Salvelinus confluentus) from egg to alevin. Can J Zool 77:1233–1239
Beard TD, Carline RF (1991) Influence of spawning and other stream habitat on spatial variability of wild brown trout. Trans Am Fish Soc 120:711–722
Borgstrøm R, Museth J (2005) Accumulated snow and summer temperature—critical factors for recruitment to high mountain populations of brown trout (Salmo trutta L.). Ecol Freshw Fish 14:375–384
Bozek MA, Hubert WA (1992) Segregation of resident trout in streams as predicted by three habitat dimensions. Can J Zool 70:886–890
Brown RS, Mackay WC (1995) Fall and winter movements and habitat use by cutthroat trout in the Ram River, Alberta. Trans Am Fish Soc 124:873–885
Budy P, Thiede GP, McHugh P, Hansen ES, Wood J (2008) Exploring the relative influence of biotic interactions and environmental conditions on the abundance and distribution of exotic brown trout (Salmo trutta) in a high mountain stream. Ecol Freshw Fish 17:554–566
Buisson L, Blanc L, Grenouillet G (2008) Modelling stream fish species distribution in a river network: the relative effects of temperature versus physical factors. Ecol Freshw Fish 2:244–257
Burner CJ (1951) Characteristics of spawning nests of Columbia River salmon. Fish Bull 52:95–110
Colautti RI (2005) Are characteristics of introduced salmonid fishes biased by propagule pressure? Can J Fish Aquat Sci 62:950–959
Cunjak RA (1988) Physiological consequences of overwintering in streams: the cost of acclimization? Can J Fish Aquat Sci 45:443–452
Cunjak RA, Power G (1986) Winter habitat utilization by stream resident brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta). Can J Fish Aquat Sci 43:1970–1981
de la Hoz Franco E, Budy P (2005) Effects of biotic and abiotic factors on the distribution of trout and salmon along a longitudinal stream gradient. Environ Biol Fishes 72:379–391
Elliott JM (1976) The energetics of feeding, metabolism, and growth of brown trout (Salmo trutta L.) in relation to body weight, water temperature and ration size. J Anim Ecol 45:923–948
ESRI (2014) ArcGIS Desktop: Release 10.2. Environmental Systems Research Institute, Redlands
Gesch D, Oimoen M, Greenlee S, Nelson C, Steuck M, Tyler D (2002) The National Elevation Dataset. Photogramm Eng Remote Sens 68:5–11
Golden LA, Springer GS (2006) Channel geometry, median grain size, and stream power in small mountain streams. Geomorphology 78:64–76
Hooten MB, Hobbs NT (2015) A guide to Bayesian model selection for ecologists. Ecol Monogr 85:3–28
Hudy M, Coombs JA, Nislow KH, Letcher BH (2010) Dispersal and within-stream spatial population structure of brook trout revealed by pedigree reconstruction analysis. Trans Am Fish Soc 139:1276–1287
Jowett IG, Richardson J (1996) Distribution and abundance of freshwater fish in New Zealand rivers. N Z J Mar Freshw Res 30:239–255
Kondolf GM (1997) Application of the pebble count: notes on purpose, method, and variants. J Am Water Resour Assoc 33:79–87
Kondolf GM, Wolman GM (1993) The sizes of salmonid spawning gravels. Water Resour Res 29:2275–2285
Lichstein JW (2002) Spatial autocorrelation and autoregressive models in ecology. Ecol Monogr 72:445–463
Linnansaari T, Cunjak RA, Newbury R (2008) Winter behaviour of juvenile Atlantic salmon Salmo salar L. in experimental stream channels: effect of substratum size and full ice cover on spatial distribution and activity pattern. J Fish Biol 72:2518–2533
Lobon-Cervia J, Utrilla C, Rincon P, Amezcua F (1997) Environmentally induced spatio-temporal variations in the fecundity of brown trout Salmo trutta L: trade-offs between egg size and number. Freshw Biol 38:277–288
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Lowe S, Browne M, Boudjelas S, P. Global Invasive Species, and I. S. I. S. S. Group (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Invasive Species Specialist Group, Auckland
McHugh P, Budy P (2005) An experimental evaluation of competitive and thermal effects on brown trout (Salmo trutta) and Bonneville cutthroat trout (Oncorhynchus clarkii utah) performance along an altitudinal gradient. Can J Fish Aquat Sci 62:2784–2795
McIntosh A, McHugh P, Budy P (2012) Chapter 24: Salmo trutta (brown trout). In: A handbook of global freshwater invasive species, New York, NY, pp 285–298
Meredith CS (2012) Factors influencing the distribution of brown trout (Salmo trutta) in a mountain stream: implications for brown trout invasion success. Dissertation, Utah State University
Meyer KA, Griffith JS (1997) First-winter survival of rainbow trout and brook trout in the Henrys Fork of the Snake River, Idaho. Can J Zool 75:59–63
Mohseni O, Stefan HG, Erickson TR (1998) A nonlinear regression model for weekly stream temperatures. Water Resour Res 34:2685–2692
Moir JJ, Gibbins CN, Buffington JM, Webb JH, Soulsby C, Brewer MJ (2009) A new method to identify the fluvial regimes used by spawning salmonids. Can J Fish Aquat Sci 66:1404–1408
Montgomery DR, Beamer EM, Pess GR, Quinn TP (1999) Channel type and salmonid spawning distribution and abundance. Can J Fish Aquat Sci 56:377–387
Needham PR, Jones AC (1959) Flow, temperature, solar radiation, and ice in relation to activities of fishes in Sagehen Creek. California 40:465–474
Peterson EE, Ver Hoef JM (2010) A mixed-model averaging approach to geostatistical modeling in stream networks. Ecology 91:644–651
R Core Development Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahel FJ, Nibbelink NP (1999) Spatial patterns in relations among brown trout (Salmo trutta) distribution, summer air temperature, and stream size in Rocky Mountain streams. Can J Fish Aquat Sci 56:43–51
Rue H, Martino S, Chopin N (2009) Approximate bayesian inference for latent gaussian models by using integrated nested Laplace approximations. J R Stat Soc B 71:319–392
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Schabengerger O, Gotway CA (2004) Statistical methods for spatial data analysis. Chapman and Hall, Boca Raton
Schrödle B, Held L, Riebler A, Danuser J (2010) Using integrated nested Laplace approximations for the evaluation of veterinary surveillance data from Switzerland: a case-study. J R Stat Soc Ser C (Appl Stat) 60:261–279
Sepulveda AJ, Colyer WT, Lowe WH, Vinson MR (2009) Using nitrogen stable isotopes to detect long-distance movement in a threatened cutthroat trout (Oncorhynchus clarkii utah). Can J Fish Aquat Sci 66:672–682
Shirvell CS, Dungey RG (1983) Microhabitats chosen by brown trout for feeding and spawning in rivers. Trans Am Fish Soc 112:355–367
Smith RW, Griffith JS (1994) Survival of rainbow trout during their first winter in the Henrys Fork of the Snake River Idaho. Trans Am Fish Soc 123:747–756
Stenseth NC, Mysterud A (2002) Climate, changing phenology, and other life history traits: nonlinearity and match-mismatch to the environment. Proc Natl Acad Sci USA 99:13379–13381
Stonecypher RJ, Hubert WA, Gern WA (1994) Effect of reduced temperatures on survival of trout embryos. Progress Fish Cult 56:180–184
Thurow RF, Peterson JT, Guzevich JW (2006) Utility and validation of day and night snorkel counts for estimating bull trout abundance in first to third order streams. N Am J Fish Manag 26:217–232
Weigel DE, Sorensen PW (2001) The influence of habitat characteristics on the longitudinal distribution of brook, brown, and rainbow trout in a small Midwestern stream. J Freshw Ecol 16:599–613
Wenger SJ, Isaak DJ, Luce CH, Nelville HM, Fausch KD, Dunham JB, Dauwalter DC, Young MK, Elsner MM, Rieman BE, Hamlet AF, Williams JE (2011) Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. Proc Natl Acad Sci USA 108:14175–14180
Westley PAH, Fleming IA (2011) Landscape factors that shape a slow and persistent aquatic invasion: brown trout in Newfoundland 1883–2010. Divers Distrib 17:566–579
Williamson MH (1996) The origins and the success and failure of invasion. In: Biological invasions. Chapman and Hall, Boca Raton, pp 28–54
Wolman MG (1954) A method of sampling coarse river-bed material. Earth Space News 35:951–956
Wood J, Budy P (2009) The role of environmental factors in determining early survival and invasion success of exotic brown trout. Trans Am Fish Soc 138:756–767
Young MK (1994) Mobility of brown trout in south-central Wyoming streams. Can J Zool 72:2078–2083
Acknowledgements
The Utah Division of Wildlife Resources, the U. S. Geological Survey Utah Cooperative Fish and Wildlife Research Unit (in-kind), the U.S. Forest Service, Utah State University Ecology Center, Utah State University School of Graduate Studies, and a George L. Disborough Trout Unlimited Research grant provided funding and/or materials towards this study. We would like to thank Gary Thiede for providing logistical support for this project as well as numerous field technicians and volunteers who helped in data collection, especially L. Goss, P. Mason, E. Castro, M. Weston, J. Randall, and C. Saunders. Brett Roper, Chris Luecke and Jack Schmidt reviewed previous versions of this manuscript. We also thank the Utah Division of Wildlife Resources, especially Matt McKell, for providing stocking records. In addition, we are grateful to our anonymous reviewers for providing suggestions to improve the manuscript. We performed this research under the auspices of Utah State University IACUC Protocol 2022. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U. S. Government.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Model Statement:
where Eq. 1 describes the negative binomial likelihood, with µ equal to the negative binomial mean, \(\varphi\) represents the overdispersion parameter, and p is the zero inflation probability; Eq. 2 represents the process model, with regression coefficients \(\beta\) and error arising from a multivariate normal distribution; Eq. 3 describes the spatial CAR model, where Σ is the precision parameter and matrix Q describes the spatial structure; and Eqs. 4–6 represent the prior distributions for the remaining model parameters. We used relatively vague priors for the regression coefficients and relied on standard default priors for the remaining parameters.
The LS (logarithmic score), as described in Schrödle et al. (2010), can be estimated as follows:
where CPO is based on the conditional predictive ordinate at each observation.
Rights and permissions
About this article
Cite this article
Meredith, C.S., Budy, P., Hooten, M.B. et al. Assessing conditions influencing the longitudinal distribution of exotic brown trout (Salmo trutta) in a mountain stream: a spatially-explicit modeling approach. Biol Invasions 19, 503–519 (2017). https://doi.org/10.1007/s10530-016-1322-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1322-z