Abstract
In Southampton Water, UK, the recent (c. 150 years ago) interspecific hybridisation between Spartina alterniflora (2n = 6x = 62; A-genome) and S. maritima (2n = 6x = 60; M-genome) gave rise to the homoploid hybrid (S. × townsendii, 2n = 6x = 62), and subsequently to the invasive allododecaploid species S. anglica (2n = 12x = 120–124) that has since spread worldwide. To address the question of dynamics of mixed ploidy populations involving these plants, we analysed several Spartina populations (fifty one individuals) in Southern England, UK, one of which was the presumed place of origin of the homoploid hybrid (Hythe). Using a combination of flow cytometry and ribosomal DNA (rDNA) genotyping we were able to identify the genomic composition and ploidy level of each individual analysed. The data show that the homoploid hybrid still dominates the population at Hythe (82 % of individuals collected in that locality) since its origin in the nineteenth century. We also identified S. × townsendii for the first time on Hayling Island (66 % individuals), indicating dispersal beyond its likely origin. The fertile allododecaploid S. anglica was mainly found in populations outside the initial hybridisation site, on Hayling Island and at Eling Marchwood. Quantification of the rDNA contributions from each parental genome showed that the ratios were mostly balanced in S. × townsendii. However, two (3 %) S. anglica individuals analysed have lost nearly all M-genome homeologs, indicating extensive repeat loss. Such variation indicates that despite the presumed single allopolyploid origin of S. anglica and genetic uniformity at other loci, it has undergone substantial changes at the rDNA loci following genome duplication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reticulate evolution (resulting from interspecific hybridization) and hybrid genome duplication (allopolyploidy) have long been recognized as major processes generating new lineages frequently involved in biological invasions (Stebbins 1985; Ainouche and Schierenbeck 2006; te Beest et al. 2012). The particularly dynamic nature of hybrid and polyploid genomes plays a central role in the adaptive success of the newly formed lineages (Adams and Wendel 2005; Wood et al. 2009; Mable 2013; Chen and Yu 2013).
Interspecific hybridisation between the hexaploid Spartina alterniflora (♀, 2n = 6x = 62, genomic composition AA) from North America and the native British hexaploid S. maritima (♂, 2n = 6x = 60, genomic composition MM) produced the largely infertile homoploid hybrid S. × townsendii (2n = 6x = 62, genomic composition AM), first collected in 1870 at Hythe, Southampton, UK and named in 1880 (Groves and Groves 1880). The derived allopolyploid Spartina anglica C.E. Hubbard dodecaploid (2n = 12x = 120–124, genomic composition AAMM) is thought to have formed by spontaneous duplication of chromosome sets soon after the hybridisation event (Marchant 1963; Hubbard 1968). All populations analysed to date in France (where Spartina anglica was naturally introduced (first recorded in 1906) or Australia [where it was deliberately introduced in 1920 and has since rapidly invaded salt marshes (Boston 1981)], are composed of dodecaploid individuals (Baumel et al. 2001; Ainouche et al. 2009; Strong and Ayres 2013; Ainouche unpublished data). In evolutionary ecology, S. anglica has become recognized as a model system of recent allopolyploid speciation (Ainouche et al. 2004, 2012). Its population dynamics may be complex as local contractions in population size and even die-out have been reported (Goodman and Williams 1961; Gray and Pearson 1984).
While the original populations of S. × townsendii exhibit additivity of multilocus parental molecular markers (Raybould et al. 1991; Ayres and Strong 2001; Baumel et al. 2001), the situation in S. anglica is more complex. Some nuclear markers indicate the presence of several S. anglica genotypes that have spread worldwide (Ayres and Strong 2001) whereas the genetic uniformity at other loci suggest a single origin or multiple origins involving similar genotypes (Raybould et al. 1991; Baumel et al. 2001, 2002). Further, the subgenome integrity of S. anglica seems to have been maintained without bursts of retroelement activity (Baumel et al. 2002; Parisod et al. 2009). Such an observation agrees with several other studies focused on examining TE transposition in newly formed allopolyploids (reviewed in Parisod et al. 2010), and adds support to the view that bursts of TE transposition are not a common response to allopolyploid formation.
Instead, it has been suggested that there has always been a lack of genomic diversity or that most variation was purged following a genetic bottleneck around the time of formation (Ainouche et al. 2004). Rapid evolutionary changes in this system appear to affect mainly epigenetic regulation following hybridization (Salmon et al. 2005; Parisod et al. 2009), and transcriptome evolution (Chelaifa et al. 2010).
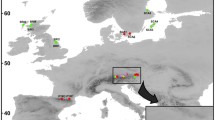
The hybridisation zone in Southampton Water, UK has been intensively studied since S. × townsendii was first discovered in the early 1870s (Groves and Groves 1880). Using classical cytogenetics, Marchant discovered populations of mixed cytotypes (hexaploids, nonaploids and dodecaploids) at Hythe (Marchant 1963, 1968a, b). Charman (1990) proposed that the expansion of S. anglica had been concomitant with the reduction of the homoploid hybrid and parental species across the entire southern coast of Britain. More recently, Renny-Byfield et al. (2010), taking a flow cytometry approach, showed that the Hythe population (Fig. 1) was composed mainly of homoploid hybrids (94 %), co-occurring with a low proportion of S. anglica plants (6 %). In contrast, the nearby population at Eling Marchwood consisted of S. anglica (36.4 %), nonaploids (2n = 90; 27.2 %) and S. alterniflora (36.4 %). S. alterniflora is restricted to one or two localities in Southampton water and is probably derived directly from the original colonisation event. The native species S. maritima, is now relatively rare in both UK (Raybould et al. 1991) and France (Yannic et al. 2004), whereas it was abundant before the arrival of S. alterniflora. Raybould et al. (1991) investigated the UK populations and suggested that its decline in UK is largely due to (1) erosion of suitable habitats due to a rise in sea level and (2) successional change in species composition.
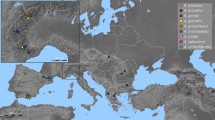
Location of the salt marshes sampled for ploidy and rDNA genotyping. The species and cytotypes identified along transects in 2007 and 2012/2013 are marked by black and green symbols, respectively. Terrain projections are shown in Online resource 2. In Eling Marchwood, the S. anglica clones with (A) and without (B) uniparental rDNA deletion are indicated in the north-west part of the transect. Dark grey meadow or field, dashed shaded areas forest, white areas tidal zone, light grey areas water
Here, we studied mixed populations of this recently formed Spartina homoploid hybrid and allopolyploid and addressed the question of their possible genetic diversification. Population-level studies are often complicated by difficulties in species and cytotype identification due to morphological similarities and phenotypic plasticity (Thompson et al. 1991). To overcome these problems, we applied flow cytometry to assess the variation in ploidy level, together with the analysis of genes encoding 18S-5.8S-26S ribosomal RNA (rDNA, nucleolar organizer region, NOR) on samples collected from three populations (at Hythe, Eling Marchwood and Hayling Island, see Fig. 1 and Online resource 1) in Southern England during 2012 and 2013. The ploidy diversity patterns were compared with those of previous analysis conducted in 2007 (Renny-Byfield et al. 2010).
Materials and methods
Plant material
The sampling sites included the place of S. × townsendii origin (Hythe, Southampton Water), and Eling Marchwood (5 km to the north). The samples were randomly collected along transects. Plants were also sampled at Hayling Island as it is one of the places where remnant S. maritima populations may still occur (Raybould et al. 2000). Particular attention was then paid to sample S. maritima like plants. In general, samples labelled with “A” originated from individuals located landwards; samples “D–E” were from individuals growing nearer to the sea. Sampling was carried out during low tides at variable times of the day. For localities and direction of transects, see (Online resource 1, 2 and Fig. 1). Some samples were also collected from French localities (mostly Brittany). Fresh leaf material (~1 g) was collected and either used directly for flow cytometry or stored in RNAlater ® solution at −20 °C until use. At each locality we collected plant material from 4 to 6 clones.
Southern blot hybridisation
DNA was extracted from the leaf samples stored in RNAlater ® using the Plant DNA extraction kit (Qiagen, Germany). Southern blotting followed the protocol described by Kovarik et al. (2005) using genomic DNA samples digested with BstNI and rDNA probes labelled with [α-32P] dCTP (Izotop, Hungary) in a random-primed reaction (DekaPrime kit, Fermentas, Lithuania). The hybridisation signals were visualized by Phosphor imaging (Typhoon, GE healthcare, USA) and signals were quantified using ImageQuant software. The probe was a 220 bp-long 26S rRNA gene fragment from Nicotiana tabacum (tobacco) (Lim et al. 2000).
Flow cytometry
Flow cytometry was used to screen for ploidy level using methods described in Hanson et al. (2005) and a Partec CyFlow SL flow cytometer fitted with a Cobalt Samba 532 nm 100 mM laser. Parsley (Petroselinum crispum ‘Curled Moss’ (2C = 4.50 pg; Obermayer et al. 2002) was used as a calibration standard for assessing ploidy levels. Nuclei were isolated by chopping the fresh leaf tissue with a razor in the GPB buffer of Loureiro et al. (2007) supplemented with 3 % polyvinylpyrrolidone (PVP-40).
AFLP analysis
The AFLP analysis followed standard procedures (Salmon et al. 2005). Briefly, genomic DNA (100 ng) was digested with MseI and EcoRI restriction enzymes (both New England Biolabs, Beverly, MA), and resulting fragments were ligated to the MseI and EcoRI adaptors. A combination of the primers MseI + C and EcoRI + A (0.3 µM in the reaction mixture) were used in the pre-amplification step in a final volume of 20 µl containing 1× Kapa buffer B, 0.12 mM dNTP and 1U Taq Kapa polymerase (Kapa Biosystems, USA). The PCR conditions were as follows: 1 cycle 72 °C, 5 min; 29 cycles 94 °C, 30 s; 56 °C, 30 s; 72 °C, 2 min; and 1 cycle 60 °C, 10 min. The pre-amplification products were diluted 1:100 (v/v) with distilled H2O and 1.25 μl was used in the selective amplification reaction with 0.625 µM of the primers MseI + CT and EcoRI + ACT labelled with a fluorescent HEX dye in a final volume of 20 μl. The PCR reactions were performed with the following program: 94 °C, 2 min; 12 cycles 94 °C, 30 s, 65 °C (with a–0.7 °C/cycle fall) for 30 s; 72 °C, 2 min; 23 cycles 94 °C, 30 s; 56 °C, 30 s; 72 °C, 2 min and final extension 72 °C, 10 min. The fragments were separated by capillary electrophoresis on the ABI PRISM® 3100 Genetic Analyzer. Only reproducible DNA fragments (there was c. 0.5 % variation between 3 technical replicates), ranging in size from 55 to 500 bp, were scored for presence or absence using Gene-Marker Software version 2.4.0. (SoftGenetics, State College, PA, USA).
Quantitative PCR
The relatively high (12 %) ITS1 divergence (Baumel et al. 2001) allowed the design of homeolog-specific primers. The forward and reverse primers were 5′-TCATCCATGGCATCGGGTGGC-3′ and 5′-CAGCACCGCAACGCATGGCTC-3′, respectively. Selective 3′-terminal nucleotides discriminating between M- and A-genome homeologs are underlined. The primers were confirmed to be highly specific for the M-genome repeat (Online resource 3a). Quantitative PCR was carried out in a 20 µl reaction mixture containing 10 and 0.1 ng of genomic DNA template or a dilution of standard, 0.75 µl of each 10 µM primer, and 10 µl of the 2x Fast Start SYBR Green Master mix (Roche, Germany). The amplification conditions were as follows: 1 cycle 95 °C, 10 min; 40 cycles 95 °C, 30 s; 59 °C, 30 s; 72 °C, 30 s. PCR was carried out in a 96 well plate format on the Applied Biosystems 7300 thermocycler. There was a linear relationship between cycle threshold (Ct) value and amount of standard across a broad range of concentrations (Online resource 3b). Three technical replicates were performed. Differences between the parallel samples were <0.2 cycle. We calculated genome representation of homeologs by comparing Ct values of a sample to those of the standards. We considered 10 kb the repeat unit size and 5330 Mb the size of the S. anglica haploid genome (Bennett and Leitch 2012). The total rDNA content was determined by slot blot hybridisation.
Results
Distribution of ploidy levels
Table 1 and Fig. 2 summarize the ploidy levels identified for the samples collected in 2012 and 2013, together with data for 2007 taken from Renny-Byfield et al. (2010). Combining the data for 2012 and 2013 shows that the population at Hythe is dominated by hexaploids corresponding to the S. × townsendii homoploid hybrid (82 % of individuals analysed). In 2012 one individual was found to be the S. anglica dodecaploid, whereas no dodecaploids were identified among the 2013 collection. These results are broadly similar to those of Renny-Byfield et al. (2010) who also predominantly identified hexaploid S. × townsendii homoploid hybrids (94 %) in their analyses. Three individuals (11 %) were assigned as S. alterniflora.
Variation in the percentage of different Spartina hybrid cytotypes and parental species (S.a: S. alterniflora and S.m: S. maritima) growing at Hythe, Hayling Island and Eling Marchwood. The percentage of individuals of each cytotype is taken from the combined data for 2007, 2012 and 2013 for Hythe and Eling Marchwood and the combined data for 2012 and 2013 for Hayling Island given in Table 1 (N.B. no collections were made from Hayling Island in 2007)
The Hayling Island salt marsh was also visited twice. Both samplings in 2012 and 2013 found only dodecaploids (34 %) and hexaploid (66 %) hybrids (Table 1). Neither S. alterniflora nor S. maritima was found on Hayling Island based on rDNA genotyping (see below), even though some plants phenotypically resembled S. maritima.
At Eling Marchwood, analyses of collections made in 2012 and 2013 resulted in mainly dodecaploid S. anglica (44.5 %) with one nonaploid cytotype (11 %). In contrast to the other two sites, (i) no homoploid 6x hybrids were found and (ii) S. alterniflora was relatively frequent (44.5 %), especially in the southern part of the locality.
Identification of hybrids by Southern blot hybridisation
We used Southern blot hybridisation to genotype the samples. Genomic DNAs were digested with BstNI and hybridised with the 26S rDNA probe. There is one conserved BstNI recognition site in the 26S coding region and a second, more variable site in the intergenic spacer (IGS) region (Fig. 3a). Examples of Southern blot analyses of representative samples are shown in Fig. 3b while additional blots supplemented with quantitative analysis of signals are shown in Online resources 4 and 5. In S. alterniflora (A-genome), a 1.9 kb band was visualized after probe hybridisation (Fig. 3b, right panel). In contrast, digests of S. maritima DNA (M-genome) generated a single 2.3 kb band. In most hybrid and allopolyploid digests, the rDNA probe hybridised to both parental bands. The ratio between the 1.9 kb (A-genome) and 2.3 kb (M-genome) band was approximately balanced in homoploid hybrids (mean A/M ratio = 0.46, Fig. 4). In contrast, most S. anglica samples from the British (Online resource 4) and French (Online resource 5) populations showed weaker hybridisation to the 1.9 kb A-genome band indicating the homeolog ratios were skewed against the A-genome variants (mean A/M ratios = 0.31 and 0.30 for French and British populations respectively). However, some individuals of S. anglica from Eling Marchwood represented notable exceptions to this with high A/M ratios, while two individuals (20A, 36A) lacked the M-genome rDNA band completely (Online resource 4).
Example of Southern blot hybridisation analysis of homeologous rDNAs. a Diagram representing part of the ribosomal DNA (rDNA) unit showing the position of the diagnostic BstNI restriction sites. The region of probe hybridisation is indicated. b Southern blot analysis of selected DNA samples from Hayling Island, Hythe and Eling Marchwood. Parental DNAs originated from the French populations. The hybridisation profiles were mapped to ploidy levels (given below the blots) determined by flow cytometry. For a complete analysis, see Online resource 4. Note two 6x individuals with a S. alterniflora morphology (asterisks) from Hythe lacked M-genome bands, confirming they are S. alterniflora. In contrast, plants 19A-C which also have an S. alterniflora morphology, have rDNA bands from both the M and A genome indicating the plants are of hybrid origin. For plant 36A, which has typical S. anglica morphology, AFLP analysis (see Fig. 5) indicated it was a hybrid while flow cytometry confirmed it was 12x. The absence of the rDNA locus from the M-genome in this plant indicates that the locus has been lost post-polyploidy
Box plots representing homeolog rDNA gene ratios in the homoploid hybrid S. × townsendii and allopolyploid S. anglica. The data were taken from quantification of bands in the Southern blot hybridization analyses (Online resources 4 and 5). There was ~90 % overlap between the ploidy and rDNA analyses among the samples from southern England (from Eling Marchwood, Hayling Island, Hythe and Isle of Wight, Online resource 4). The French populations (from localities at the Atlantic coast, mostly Brittany (Online resource 5) were previously reported to be dodecaploids (Baumel et al. 2001). Differences between S. × townsendii and both S. anglica were significant (Mann-Witney U test, p < 0.01)
AFLP
Phenotypic plasticity of species in Spartina often prevents unambiguous identification based on morphology. The 12x individuals from Eling Marchwood showing monomorphic rDNA Southern profiles (i.e. samples 20A and 36A) could represent S. alterniflora autopolyploids rather than allopolyploids. To exclude this possibility, we genotyped these individuals using AFLP markers. Figure 5 shows the results of AFLP fragmentation analysis of S. alterniflora, S. maritima and S. anglica (36A, 36B) samples. Clearly, both 36A (non-additive rDNA genotype and 36B (additive genotype) individuals have inherited AFLPs from both parents confirming both are allopolyploids containing A- and M-genomes. Sample 20A (other putative rDNA deletion mutant) showed a similar pattern as 36A (data not shown), confirming it too is an allopolyploid rather than autopolyploid. The 20A and 36A individuals could have originated from multiple sampling of the same clone at the north-west edge of the transect (Fig. 1). Only about 50 % of the AFLP bands are shared between the parents (Table 2) indicating their high genomic divergence. Both S. anglica samples 20A and 36A have inherited >98 % of these parental-specific bands providing further support of their hybridogenic origin.
Locus-specific PCR
As noted above, Southern blot analysis failed to detect any M-genome rDNA signal in two S. anglica individuals from Ealing Marchwood. However, since the current methods used for Southern blotting may not be sensitive enough to reveal a few residual M-genome rDNA copies we employed a repeat-specific PCR approach that has previously been used to detect rare variants in the Glycine tomentella polyploid complex (Rauscher et al. 2002). The analysis of four S. anglica individuals from Eling Marchwood, including 20A and 36A, is shown in Table 3. From these results it is evident that the 36A and 20A individuals have lost thousands of M-genome units, retaining only 20–30 copies in the genome.
Discussion
Understanding population dynamics in the presence of mixed populations of parental species, homoploid hybrids and derived polyploids is a considerable challenge to ecological and genetic research and is of particular interest in the context of biological invasion. The Spartina model offers a rare opportunity to explore those dynamics under natural conditions, and in association with the expansion of a highly invasive species.
The usefulness of combined ploidy and rDNA marker analysis for the identification of hybrids
Spartina species exhibit important morphological plasticity. Often, plants first identified as parental species according to morphology have subsequently turned out to be hybrids following genotyping. First genetic analyses, including the exhaustive chromosome counts performed by Marchant (1968a, b), enzyme electrophoresis (Guénégou et al. 1988; Gray et al. 1990; Raybould et al. 1991) and DNA multilocus fingerprinting (Baumel et al. 2001; Ferris et al. 1997) have greatly helped our understanding of hybrid and allopolyploid formation and distribution. In this study, we further show the importance of combining various approaches, as no single method (e.g. rDNA profiling, plant habit, flow cytometry, or AFLP) is sufficient to determine the identity of plants in mixed populations. Flow cytometry analysis of Spartina provides information about the ploidy levels but it cannot distinguish homoploid hybrids from their parents. On the other hand, molecular analysis of rDNA homeologs distinguishes between hybrids and parents while it cannot assess ploidy. Thus these two methods are clearly complementary, and together they enable the efficient determination of plant identity in mixed populations.
Long term persistence of Spartina × townsendii homoploid hybrids at the first recorded site of hybridisation
Interspecific hybridisation is generally thought to generate “genomic stress” arising from incompatibilities between parental genomes and chromosome pairing problems during meiosis. In many cases, allopolyploidy restores fertility by enabling chromosome pairing. Consequently, newly formed allopolyploids may be expected to competitively replace parental hybrids (Mandak et al. 2004). Indeed, Charman (1990) argued that the expansion of S. anglica had been concomitant with the reduction of the homoploid hybrid and parental species across the entire southern coast of Britain. Surprisingly, we found no evidence of homoploid loss. At Hythe, the place where S. × townsendii was first recorded, we found only a few S. anglica plants, the majority (82 %) of individuals analysed in 2012 and 2013 were the homoploid hexaploid hybrids. A similar cytotype frequency was previously recorded at the same place in 2007 (Renny-Byfield et al. 2010) indicating that the homoploid hybrid population is stable and abundant (Table 1).
In contrast to this, the occurrence of nonaploid hybrids is rare at Hythe. We identified just one nonaploid (2n = 90) individual in 2013 and none in 2012 (this study) or 2007 (Renny-Byfield et al. 2010). One nonaploid (2n = 90) and one irregular ploidy individual (2n = 76) were reported in the 1960s near this locality ‘south of Hythe’ by Marchant (1967). Higher frequencies of nonaploids have been reported nearby at Eling Marchwood [22 % of individuals analysed, based on this study and Renny-Byfield et al. (2010)]. In one of these nonaploid individuals the genomic composition was assessed by genomic in situ hybridisation (GISH) and shown to be AAM suggesting that it arose as a backcross between S. anglica and S. alterniflora, as originally proposed by Marchant (1967). The sporadic occurrence of nonaploids suggests that they may be scattered throughout the whole Southampton Water region at low frequency. It will be interesting to cytogenetically characterise further individuals by GISH to see if reciprocal genotypes also occur.
Despite the ploidy homogeneity, phenotypically, the Hythe population was not uniform. Most homoploid hybrids (87 %) took their phenotype from the S. maritima parent (as seen in S. anglica) while four individuals (13 %) resembled S. alterniflora according to morphological features (Marchant 1967). This can be explained by the known morphological plasticity of hybrid genotypes (Raybould et al. 2000). However, we cannot exclude the possibility that the parental species have hybridised more than once in Southampton Water (Ayres and Strong 2001), and that these early clones persist today. It is, perhaps, noteworthy that an independent homoploid hybrid between S. alterniflora and S. maritima (=S. × neyrautii) has been formed in the South of France that is morphologically more similar to S. alterniflora (Jovet 1941), in spite of having the same maternal parent (S. alternifora) as S. × townsendii (Baumel et al. 2003).
Decline of progenitors at the site of species hybridisation
Previous records of S. maritima (Marchant 1967, 1968b; Raybould et al. 1991) suggest this species had disappeared from the initial hybridization site at Hythe. Our data support this as we found no evidence of S. maritima in the area based on our collections in 2007, 2012 and 2013 (Table 1). The previous reports also indicated a few surviving clones in Hayling Island and Isle of Wight (Raybould et al. 2000). While we did not detect S. maritima in Hayling Island, the extant population at the Isle of Wight reported by Raybould et al. (2000) could represent one of the last remnants of this species in Southern England.
The repeated finding of S. alterniflora, the female genome donor of hybrids, at Hythe in 2012 and 2013 deserves some attention since it has not been reported here since 1930 (Gray et al. 1990; Renny-Byfield et al. 2010). One explanation for this could be that S. alterniflora has always been present at Hythe but due to the morphological plasticity we expose here, (which can make species identification difficult) previous studies based on plant morphology alone simply failed to distinguish S. alterniflora from the homoploid hybrid. Data from the literature indicate that this species was recorded in Hythe at the end of the nineteenth century, growing together with S. maritima (which has now disappeared at this location) and S. × townsendii (Groves and Groves 1880; Townsend 1883). Since then, S. alterniflora has undergone a major decrease and in 1963, it was recorded only in Marchwood (Marchant and Goodman 1969; Gray et al. 1991). Our finding then would result from a recent re-introduction of S. alterniflora at Hythe, where these plants were observed since the early 2000s (Baumel, Misset and Ainouche unpublished). It is only through the combination of ploidy level and genotype analysis, employed here, that we can be confident that S. alterniflora is indeed present at Hythe. However, it is also possible that S. alterniflora did disappear and has only re-colonised Hythe from nearby Eling Marchwood relatively recently. Its occurrence in the channels closest to the sea (Fig. 1) is in line with their vegetative introduction through tillers. In China, the reduction of S. anglica populations was attributed to out-competition with S. alterniflora (Zhi et al. 2007). Certainly, this is not happening in Hythe where S. alterniflora comprises only a small proportion of the population (Table 1; Fig. 2) but may be happening at Eling Marchwood.
Introduction of S. × townsendii to distal locations
A mixed ploidy population at Hayling Island located about 40 km east of Hythe is identified for the first time. In this population both hexaploid and dodecaploid individuals were encountered. However, while the hexaploid plants exhibited morphological similarities with S. maritima, the rDNA patterns suggest a hybrid origin with additive S. maritima-type and S. alterniflora-type diagnostic markers, as in S. × townsendii. How can the presence of S. × townsendii in distant localities be best explained? One possibility is that the Hayling Island population may have an independent origin from the original population at Hythe. Although multiple origins seem to be frequent attributes of many successful allopolyploids (Soltis and Soltis 2009), we consider this possibility unlikely since there is no evidence of S. alterniflora being introduced to Hayling Island. Previous reports have indicated that, in England, its occurrence is restricted to Southampton Bay (Charman 1990). In addition, while the second parent, S. maritima, has been reported on Hayling Island in the past (Raybould et al. 2000), the only hexaploid plants encountered during our sampling in 2007, 2012 and 2013 exhibited hybrid nuclear rDNA patterns indicative of the homoploid hybrid. An alternative explanation for the occurrence of S. × townsendii at this additional locality is that they have originated from Hythe. Indeed, vegetative fragments (tillers), which have a high regenerative capability, could have been introduced to these locations by sea currents or human activity. It is noteworthy that S. anglica has spread worldwide due, in part, to its deliberate planting (Gray et al. 1990; Nehring and Adsersen 2006). Hence, a lower occurrence of S. × townsendii (compared to S. anglica) may reflect a biased pattern of sampling of S. anglica in Britain for shipment overseas rather than its overall sterility. One further possibility is that S. × townsendii may actually be partially fertile and produce seeds which can be dispersed. In this context, despite irregular chromosome pairing, rare bivalents have been noted in their pollen mother cells (Marchant 1977).
The origin of S. anglica dodecaploid
While it is clear that the initial hybridisation that subsequently led to the formation of S. anglica occurred in Southampton Water around 1870, the time frame, mechanism and exact geographical location of the subsequent polyploidisation event(s) remain debatable. We can envisage several scenarios:
-
1.
The hybridisation and polyploidisation sites overlapped; there was a single polyploidisation event that gave rise to S. anglica (Ainouche et al. 2004). If so, it is puzzling why the highly invasive, successful and fertile S. anglica dodecaploid does not occur in significant numbers at the place of species hybridisation (i.e. Hythe). One explanation could be that the locality was simply already occupied by homoploids at the time of polyploid formation—according to historical records, there is at least a 30 year interval since the initial hybridisation at Hythe and the appearance of the dodecaploid (Gray et al. 1990). During this interval the size of the homoploid population might have expanded tremendously. In addition, homoploid hybrids could be better adapted to the ecological conditions (substratum, tidal range, salinity) possibly as a result of dramatic epigenetic reprogramming (Salmon et al. 2005; Parisod et al. 2009) that immediately followed hybridisation. It is conceivable that such ecological and physiological factors could have highly skewed the cytotype ratios towards 2n = 6x homoploids. Certainly, the fertility of a newly formed dodecaploid might have facilitated its spread to nearby localities.
-
2.
The hybridisation and polyploidisation sites did not overlap and thus S. anglica may have arisen at a site outside the original hybridisation zone. This possibility would require spreading of F1 hybrids beyond Hythe. Indeed, we have identified a mixed population of 6x and 12x cytotypes in Hayling Island (40 km from Hythe) and another mixed population could have existed in Poole Harbour that is about 15 km from Hythe (Hubbard 1965; Hubbard and Stebbing 1968). Subtle but significant differences have been reported to exist between S. anglica genotypes (Ayres and Strong 2001) and here we show that the rDNA homeolog ratios differ between S. anglica and S. × townsendii as well as between S. anglica individuals. It is also noteworthy that the first reports of dodecaploid individuals were not from Hythe, but from Lymington in 1892 (Hubbard 1957) and Isle of Wight in 1893 (Goodman et al. 1969). These observations support the hypothesis that duplication of S. × townsendii chromosomes occurred outside the initial hybridisation zone, possibly involving a distinct genotype.
-
3.
The formation of S. anglica involved unreduced gametes of the progenitor species and not somatic doubling of F1 hybrids (Marchant 1963; Hubbard 1968). Ayres et al. (2008) speculated that triploid-bridges (Husband 2004) may have played a role in the evolution and recurrent formation of S. anglica. There are several observations which support such a hypothesis. First, it is puzzling why we do not witness recurrent polyploidisation events in Hythe where the extensive population of homoploid hybrids may comprise thousands of individual which may be ~150 years old. Similarly, fertile allopolyploids of the independently arising S. × neyrautii hybrid have never been reported (Jovet 1941; Marchant 1977). Second, attempts to artificially double the S. × townsendii chromosomes have so far been unsuccessful (Gray and Pearson 1984), suggesting that many genotypes (and epigenotypes) might be incompatible with chromosome duplication. Finally, nonaploids (Marchant 1963, 1968a, b; Renny-Byfield et al. 2010; and this work) and individuals with irregular karyotypes (Huskins 1931) reported in Southampton Water could represent remnants of complex hybridisation events involving fertilization with unreduced gametes.
Elimination of paternally inherited NORs in two S. anglica individuals
The Eling Marchwood site probably represents one of the oldest populations of S. anglica. It exhibits the largest known genomic diversity in terms of both ploidy levels (i.e. hexaploids, docecaploids, nonaploids) and variable rDNA ratios. The increased A/M ratio in a nonaploid individual is consistent with the AAM genome composition determined by GISH for another individual from this location (Renny-Byfield et al. 2010). Significantly, we also identified two individuals (3 % of all S. anglica individuals analysed) that have lost nearly all the paternal rRNA genes of S. maritima origin. A sensitive homeolog-specific quantitative PCR revealed few, probably about 30, residual M-genome copies in these plants. This contrasts with the situation in most plants that inherited, on average, 4000 M-genome copies (Table 3). In these individuals genomic AFLP revealed additive patterns of parental fragments indicating that rDNA repeat elimination has probably affected just the nucleolar organizing region (NOR) and not the whole chromosome. Plants with the eliminated rDNA homoelog could comprise the whole clone since the same genotype has been repeatedly recorded at Eling Marchwood. Interestingly, homozygous deletions have also been reported in populations of other recently formed allotetraploid systems (Lowe and Abbott 1996; Zozomova-Lihova et al. 2014; Dobesova et al. 2015) suggesting that these heterochromatic deletions may occur in different systems. There is no evidence for impact of rDNA homeolog loss on plant viability in any of the systems.
Variability in genetic markers among S. anglica, including those from Eling Marchwood, has previously been reported by Ayres and Strong (2001). These authors argued that the genotypes may originate from different genome donors during repeated formation of hybrids. In the case of rRNA genes we can exclude the possibility of progenitor variation since rRNA genes must have been present in the progenitor. Thus, the results are more consistent with rDNA rearrangement after the formation of the allopolyploid. In this context, elimination of rDNA sequences have been reported in synthetic lines of Arabidopsis (Pontes et al. 2004), Tragopogon (Malinska et al. 2010), Brassica (Książczyk et al. 2011), wheat (Guo and Han 2014) and in forage grasses of the Lolium-Festuca complex (Książczyk et al. 2010), indicating that lineages with different rDNA genotypes might be formed in the early generations of allopolyploids. Our results clearly indicate that the previously reported “structural genomic stasis” in recent Spartina hybrids and allopolyploids (Baumel et al. 2002; Ainouche et al. 2004) is not confirmed for the ribosomal gene family which appears more dynamic than previously thought.
Conclusions
We found that the Spartina populations in the hybridisation zone appeared stable. Our survey of Spartina in Southern England found no evidence for the somatic age-related decline of the homoploid hybrid S. × townsendii since the middle of the last century when Christopher Marchant investigated the area and efficiently mapped the occurrence and distribution of Spartina cytotypes using traditional cytogenetic methods. We also show that S. × townsendii has the capacity to spread to distal localities forming mixed populations with S. anglica. Certainly, the long-term survival of homoploid hybrids together with their large effective population sizes means that there is an opportunity for further evolution.
References
Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8:135–141
Ainouche M, Schierenbeck K (2006) The role of evolutionary genetics in studies of plant invasions. In: Cadotte MW, McMahon SM, Fukami T (eds) Conceptual ecology and invasion biology: reciprocal approaches to nature. Springer, Berlin, pp 193–221
Ainouche ML, Baumel A, Salmon A (2004) Spartina anglica C. E. Hubbard, a natural model system for analysing early evolutionary changes that affect allopolyploid genomes. Biol J Linn Soc 82:475–484
Ainouche ML, Fortune PM, Salmon A, Parisod C, Grandbastien MA, Fukunaga K, Ricou M, Misset MT (2009) Hybridization, polyploidy and invasion, lessons from Spartina (Poaceae). Biol Invasions 11:1159–1173
Ainouche M, Chelaifa J, Ferreira S, Bellot A, Ainouche A, Salmon A (2012) Polyploid evolution in Spartina: dealing with highly redundant hybrid genomes. In: Soltis DE, Soltis PS (eds) Polyploidy and genome evolution. Springer, Berlin, pp 225–243
Ayres DR, Strong DR (2001) Origin and genetic diversity of Spartina anglica (Poaceae) using nuclear DNA markers. Am J Bot 88:1863–1867
Ayres DR, Grotkopp E, Zaremba K et al (2008) Hybridization between invasive Spartina densiflora (Poaceae) and native S. foliosa in San Francisco Bay, California, USA. Am J Bot 95:713–719
Baumel A, Ainouche ML, Levasseur JE (2001) Molecular investigations in populations of Spartina anglica C.E. Hubbard (Poaceae) invading coastal Brittany (France). Mol Ecol 10:1689–1701
Baumel A, Ainouche M, Kalendar R, Schulman AH (2002) Retrotransposons and genomic stability in populations of the young allopolyploid species Spartina anglica CE Hubbard (Poaceae). Mol Biol Evol 19:1218–1227
Baumel A, Ainouche ML, Misset MT, Gourret JP, Bayer RJ (2003) Genetic evidence for hybridization between the native Spartina maritima and the introduced Spartina alterniflora (Poaceae) in South-West France, Spartina × neyrautii re-examined. Plant Syst Evol 237:87–97
Bennett MD, Leitch IJ (2012) Angiosperm DNA C-values database. http://data.kew.org/cvalues/. (Release 8.0). Accessed December 2012
Boston KG (1981) The introduction of Spartina townsendii (S.L) to Australia. Melbourne State College Occasional Papers vol 6, pp 1–57
Charman K (1990) The current status of Spartina anglica in Britain. In: Gray AJ, Benham PEM (eds) Spartina anglica, a research review. Institute of Terrestrial Ecology Research Publication, vol 2. HMSO, London, pp 11–14
Chelaifa H, Monnier A, Ainouche M (2010) Transcriptomic changes following recent natural hybridization and allopolyploidy in the salt marsh species Spartina × townsendii and Spartina anglica (Poaceae). New Phytol 186:161–174
Chen ZJ, Yu HH (2013) Genetic and epigenetic mechanisms for polyploidy and hybridity. In: Chen ZJ, Birchler JA (eds) Polyploid and hybrid genomics. Chapter 21. Wiley-Blackwell, New York, pp 335–354
Dobesova E, Malinska H, Matyasek R, Leitch AR, Soltis DE, Soltis PS, Kovarik A (2015) Silenced rRNA gene homeologs are activated and substitute for partially eliminated active homoelogs in the recently formed allotetraploid, Tragopogon mirus (Asteraceae). Heredity 114:356–365
Ferris C, King RA, Gray AJ (1997) Molecular evidence for the maternal parentage in the hybrid origin of Spartina anglica. Mol Ecol 6:185–187
Goodman PJ, Williams WT (1961) Investigations into dieback in Spartina × townsendii agg. 3. Physiological correlates of dieback. J Ecol 49:391–398
Goodman PJ, Braybrooks EM, Marchant CJ, Lambert JM (1969) Spartina × townsendii H. & J. Groves sensu lato. (Biological flora of the British Isles.). J Ecol 57:298–313
Gray AJ, Pearson JM (1984) Spartina marshes in Poole Harbour, Dorset, with particular reference to Holes Bay. In: Doody JP (ed) Spartina anglica in Great Britain (Focus on nature conservation no. 5). Nature Conservancy Council, Attingham, pp 11–14
Gray AJ, Benham PEM, Raybould AF (1990) Spartina anglica—the evolutionary and historical background. In: Gray AJ, Benham PEM (eds) Spartina anglica—a research review. London: Institute of Terrestrial Ecology. Natural Environment Research Council, pp 5–10
Gray AJ, Marshall DF, Raybould AF (1991) A century of evolution in Spartina anglica. Adv Ecol Res 21:1–62
Groves H, Groves J (1880) Reports of the botanical society and exchange club of the British isles. Great Britain 1:37
Guénégou MC, Citharel J, Levasseur JE (1988) The hybrid status of Spartina anglica (Poaceae). Enzymatic analysis of the species and the presumed parents. Can J Bot 66:1830–1833
Guo X, Han F (2014) Asymmetric epigenetic modification and elimination of rDNA sequences by polyploidization in wheat. Plant Cell 26:4311–4327
Hanson L, Boyd A, Johnson MAT, Bennett MD (2005) First nuclear DNA C-values for 18 eudicot families. Ann Bot 96:1315–1320
Hubbard JCE (1957) Report on the British ecological society symposium on Spartina. J Ecol 57:612–616
Hubbard JCE (1965) Spartina marshes in southern England. VI. Pattern of invasion in Poole Harbour. J Ecol 53:799–813
Hubbard JCE (1968) Grasses, 2nd edn. Penguin Books, London
Hubbard JCE, Stebbing RE (1968) Spartina marshes in Southern England. VII. Stratigraphy of Keysworth Marsh Poole Harbour. J Ecol 56:707–722
Husband BC (2004) The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol J Linn Soc 82:537–546
Huskins CL (1931) The origin of Spartina townsendii. Genetica 12:531–538
Jovet P (1941) Notes systematiques et ecologiques sur les Spartines du Sud-Quest. Bulletin de la Société Botanique de France 88:115–123
Kovarik A, Pires JC, Leitch AR, Lim KY, Sherwood AM, Matyasek R, Rocca J, Soltis DE, Soltis PS (2005) Rapid concerted evolution of nuclear ribosomal DNA in two Tragopogon allopolyploids of recent and recurrent origin. Genetics 169:931–944
Książczyk T, Taciak M, Zwierzykowski Z (2010) Variability of ribosomal DNA sites in Festuca pratensis, Lolium perenne, and their intergeneric hybrids, revealed by FISH and GISH. J Appl Genet 51:449–460
Książczyk T, Kovarik A, Eber F, Huteau V, Khaitova L, Tesarikova Z, Coriton O, Chèvre A-M (2011) Immediate unidirectional epigenetic reprogramming of NORs occurs independently of rDNA rearrangements in synthetic and natural form of a polyploid species Brassica napus. Chromosoma 20:557–571
Lim KY, Kovarik A, Matyasek R, Bezdek M, Lichtenstein CP, Leitch AR (2000) Gene conversion of ribosomal DNA in Nicotiana tabacum is associated with undermethylated, active gene units. Chromosoma 109:161–172
Loureiro J, Rodriguez E, Dolezel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888
Lowe AJ, Abbott RJ (1996) Origins of the new allopolyploid species Senecio cambrensis (Asteraceae) and its relationship to the Canary Islands endemic Senecio teneriffae. Am J Bot 83:1365–1372
Mable BK (2013) Polyploids and hybrids in changing environments: winners or losers in the struggle for adaptation? Heredity 110:95–96
Malinska H, Tate JA, Matyasek R, Leitch AR, Soltis DE, Soltis PS, Kovarik A (2010) Similar patterns of rDNA evolution in synthetic and recently formed natural populations of Tragopogon (Asteraceae) allotetraploids. BMC Evol Biol 10:291
Mandak B, Pysek P, Bimova K (2004) History of the invasion and distribution of Reynoutria taxa in the Czech Republic: a hybrid spreading faster than its parents. Preslia 76:15–64
Marchant CJ (1963) Corrected chromosome numbers for Spartina × townsendii and its parent species. Nature 199:929
Marchant CJ (1967) Evolution in Spartina (Gramineae). I. The history and morphology of the genus in Britain. J Linn Soc Bot 60:1–24
Marchant CJ (1968a) Evolution in Spartina (Gramineae). II. Chromosomes, basic relationships and the problem of S. × townsendii agg. J Linn Soc Bot 60:381–409
Marchant CJ (1968b) Evolution in Spartina (Gramineae), III. Species chromosome numbers and their taxonomic significance. J Linn Soc Bot 60:411–417
Marchant CJ (1977) Hybrid characteristics in Spartina × neyrautii four taxon rediscovered in Northern Spain. J Linn Soc Bot 74:289–296
Marchant CJ, Goodman PJ (1969) Spartina alterniflora Loisel. Biological Flora of the British Isles. J Ecol 57:291–295
Nehring S, Adsersen H (2006) NOBANIS—invasive alien species fact sheet—Spartina anglica. From: online database of the European network on invasive alien species—NOBANIS www.nobanis.org. Accessed 01 June 2015
Obermayer R, Leitch IJ, Hanson L, Bennett MD (2002) Nuclear DNA C-values in 30 species double the familial representation in pteridophytes. Ann Bot 90:209–217
Parisod C, Salmon A, Zerjal T, Tenaillon M, Grandbastien MA, Ainouche M (2009) Rapid structural and epigenetic reorganization near transposable elements in hybrid and allopolyploid genomes in Spartina. New Phytol 184:1003–1015
Parisod C, Alix K, Just J, Petit M, Sarilar V, Mhiri C, Ainouche M, Chalhoub B, Grandbastien MA (2010) Impact of transposable elements on the organization and function of allopolyploid genomes. New Phytol 186:37–45
Pontes O, Neves N, Silva M, Lewis MS, Madlung A, Comai L, Viegas W, Pikaard CS (2004) Chromosomal locus rearrangements are a rapid response to formation of the allotetraploid Arabidopsis suecica genome. Proc Natl Acad Sci USA 101:18240–18245
Rauscher JT, Doyle JJ, Brown AHD (2002) Internal transcribed spacer repeat-specific primers and the analysis of hybridization in the Glycine tomentella (Leguminosae) polyploid complex. Mol Ecol 11:2691–2702
Raybould AF, Gray AJ, Lawrence MJ, Marshall DF (1991) The evolution of Spartina anglica Hubbard, C.E. (Gramineae)—genetic-variation and status of the parental species in Britain. Biol J Linn Soc 44:369–380
Raybould AF, Gray AJ, Hornby DD (2000) Evolution and current status of the salt marsh grass, Spartina anglica, in the Solent. In: Collins M, Ansell K (eds) Solent science: a review. Elsevier, Amsterdam, pp 299–302
Renny-Byfield S, Ainouche M, Leitch IJ, Lim KY, Le Comber SC, Leitch AR (2010) Flow cytometry and GISH reveal mixed ploidy populations and Spartina nonaploids with genomes of S. alterniflora and S. maritima origin. Ann Bot 105:527–533
Salmon A, Ainouche ML, Wendel JF (2005) Genetic and epigenetic consequences of recent hybridization and polyploidy in Spartina (Poaceae). Mol Ecol 14:1163–1175
Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Annu Rev Plant Biol 60:561–588
Stebbins GL (1985) Polyploidy, hybridization, and the invasion of new habitats. Ann Mo Bot Gard 72:824–832
Strong DR, Ayres DR (2013) Ecological and evolutionary misadventures of Spartina. In: Futuyma DJ (ed) Annual review of ecology, evolution, and systematics, vol 44. Ann Rev, Palo Alto, pp 389–410
te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubesova M, Pysek P (2012) The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot 109:19–45
Thompson JD, McNeilly T, Gray AJ (1991) Population variation in Spartina anglica Hubbard C.E. 1. Evidence from a common garden experiment. New Phytol 117:115–128
Townsend F (1883) Flora of hampshire. Reeve, London, pp 400–401
Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH (2009) The frequency of polyploid speciation in vascular plants. Proc Natl Acad Sci USA 106:13875–13879
Yannic G, Baumel A, Ainouche M (2004) Uniformity of the nuclear and chloroplast genomes of Spartina maritima (Poaceae), a salt-marsh species in decline along the Western European Coast. Heredity 93:182–188
Zhi YB, Li HL, An SQ, Zhao L, Zhou CF, Deng ZF (2007) Inter-specific competition: Spartina alterniflora is replacing Spartina anglica in coastal China. Estuar Coast Shelf Sci 74:437–448
Zozomova-Lihova J, Mandakova T, Kovarikova A, Mühlhausen A, Mummenhoff K, Lysak MA, Kovarik A (2014) When fathers are instant losers: homogenization of rDNA loci in recently formed Cardamine × schulzii trigenomic allopolyploid. New Phytol 203:1096–1108
Acknowledgments
We thank the New Forest District Council, Associated British Ports, and Northney Marina for granting us permission to collect Spartina plants. We thank Dr Sonia Garcia (University of Barcelona for helpful discussion in the course of manuscript preparation. The work was funded by the Czech Science Foundation (P501/13/10057S), the French CNRS—University of Rennes 1 International Associated Laboratory Ecological Genomics of Polyploidy (ECOGEN), the Partner University Funds, NERC (UK), and the Czech-French research Barrande program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Alan Gray and Malika Ainouche/Invasive Spartina.
Dalibor Huska and Ilia Leitch contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huska, D., Leitch, I.J., de Carvalho, J.F. et al. Persistence, dispersal and genetic evolution of recently formed Spartina homoploid hybrids and allopolyploids in Southern England. Biol Invasions 18, 2137–2151 (2016). https://doi.org/10.1007/s10530-015-0956-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0956-6