Abstract
Ecological impacts of invasive species are mediated by the environmental characteristics of the invaded habitats. The invasive round goby Neogobius melanostomus is a brackish-water adapted invader with high predatory and competitive impacts on native communities. We test the hypothesis that both body mass gain and predation rates of the round goby are reduced in low-dissolved ion waters in which they can establish, using calcium (Ca2+) as a focal ion of great importance to the physiology of aquatic animals. Round gobies were first acclimated for 36 days on a diet of shrimp pellets in either high (35 mg L−1) or low (12 mg L−1) Ca2+ concentration [Ca]. We then assessed the functional response (FR)—the relationship between predation rate and prey supply—of the round goby from high and low [Ca] on amphipod prey in either condition. Round gobies in high [Ca] consumed more pellets during the acclimation period and gained more mass compared to fish in low-[Ca] conditions. Functional response experiments revealed that round gobies at high-[Ca] levels for both acclimation and FR experiments had the highest predatory impact, whereas fish in low-[Ca] FR treatments had similarly low predation rates regardless of acclimation [Ca]. In a second experiment, non-acclimated round gobies had a higher FR on mayfly nymph prey at high [Ca] than fish at low-[Ca] levels. Our results indicate that the round goby has stronger ecological impacts in conditions that are more physiologically optimal for the species. Identifying key physico-chemical factors that mediate impacts of invaders would help prioritize habitats for management attention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Physico-chemical gradients can dramatically alter the distribution, abundance and per capita effect (e.g. individual consumption rate) of invasive species (Hellmann et al. 2008; Rahel and Olden 2008; Walther et al. 2009), thereby mediating their impact on native communities (Parker et al. 1999; Ricciardi et al. 2013). Invaders have been found to have higher predatory and competitive impacts when in environmental conditions for which they are adapted (Dick 1996; MacNeil et al. 2004; Costanzo et al. 2005). For instance, an experimental study of intraguild predation between a brackish-water adapted invasive amphipod and a freshwater-adapted native amphipod found that the invader shifts from being the inferior predator in ion-poor water to being the superior predator in ion-rich water (Kestrup and Ricciardi 2009). Similarly, predation and aggression of the freshwater-adapted invasive mosquitofish Gambusia affinis have been shown to be reduced in high salinity (Alcaraz et al. 2008). The Environmental Matching Hypothesis predicts that the impacts of an invader—as defined by its per capita effect and abundance (see Parker et al. 1999)—decline as habitat conditions move further from its physiological optimum (Ricciardi et al. 2013; Iacarella et al. 2015). In support of this hypothesis, a meta-analysis found that maximal consumptive impacts of invasive fishes and crustaceans declined as habitat temperatures moved further from their thermal growth optima (Iacarella et al. 2015).
Here, we test the Environmental Matching Hypothesis by assessing variation in body mass gain and per capita effect of an invasive species across contrasting water chemistries. The round goby Neogobius melanostomus is a voracious and aggressive Ponto-Caspian invader with impacts across multiple trophic levels (reviewed by Kornis et al. 2012). Ponto-Caspian species are generally euryhaline, owing to their evolution in a region with a geological history of extreme salinity fluctuations (Reid and Orlova 2002). However, round gobies may be unable to establish in freshwater habitats characterized by calcium (Ca2+) concentrations (hereafter [Ca]) <8 mg L−1 and conductivity <100 μS cm−1 (Baldwin et al. 2012). The ecological impacts of the round goby may also be constrained by dissolved ion gradients across its invaded range.
Analysis of functional response (FR), defined as the relationship between predation rate and prey supply (Holling 1959), is an emerging method for explaining and predicting relative per capita effects of invasive species (Dick et al. 2014). Functional response analyses have been used to compare invader impacts in different abiotic and biotic contexts, such as the presence of higher-order predators (Barrios-O’Neill et al. 2014; Paterson et al. 2014), different levels of habitat complexity (Alexander et al. 2012) and temperature gradients (Iacarella et al. 2015). Maximum feeding rate is a physiologically-dependent parameter of FR (indicated by the asymptote of the FR curve) and is the inverse of the time required to handle and digest a prey item (Holling 1959, 1966; Jeschke et al. 2002). Functional response serves as a metric of per capita effect that can be used to identify environmental conditions in which an invader has its highest potential impact (Dick et al. 2014; Iacarella et al. 2015).
We assessed the effect of two dissolved ion levels on body mass gain and per capita predation rates of round gobies. We focused our study on the round goby, as it and other Ponto-Caspian invaders have been shown to be physiologically influenced by [Ca] gradients; we expect that freshwater-adapted invaders will not be similarly affected (see “Discussion”). As such, we encourage further tests of the Environmental Matching Hypothesis on species with different evolutionary histories. In addition, we use [Ca] as our metric of dissolved ionic strength, given that it is a key element that can limit growth of freshwater animals (Hunn 1985; Wheatly 1999) and is well-correlated with conductivity (Kestrup and Ricciardi 2010). We first acclimated fish in high and low [Ca] and then measured their FR in both [Ca] conditions. In a second set of experiments, we measured the FR of non-acclimated round gobies to (1) verify responses to high and low [Ca] using size-matched fish without any potential effects of acclimation conditions, and (2) determine the generality of the response to [Ca] on a different prey item. We predicted a relative match between greater increases in body mass and higher predation rates of round gobies (the Environmental Matching Hypothesis; Ricciardi et al. 2013; Iacarella et al. 2015), and that both these variables would be highest in ion-rich waters for which the species is adapted (Reid and Orlova 2002; Brandner et al. 2013).
Methods
Acclimation and holding period: data collection
Round gobies were collected from Melocheville (45.3192°N, 73.9278°W) and Chateauguay (45.3356°N, 73.8183°W) along the St. Lawrence River in June 2014, and river water was collected throughout the summer from Chateauguay. Water conditions in this area represent high-[Ca] conditions, with [Ca] ranging from 32 to 35 mg L−1, conductivity from 276 to 294 μS cm−1 and pH from 8.5 to 8.9 (Kestrup and Ricciardi 2009). Upon collection, we measured the mass of the fish, but not length, because mass is more directly related to consumption potential, and we wished to minimize handling of the fish. Previous length-weight relationships of round gobies collected from Chateauguay (unpublished data, J.C.I.) yielded estimates of initial total lengths of 43–58 mm. At this size, round gobies feed primarily on macroinvertebrates, including amphipods and mayfly nymphs (French and Jude 2001; Campbell et al. 2009). The invasion of round gobies in the St. Lawrence River is correlated with reduced diversity and biomass of macroinvertebrates, attributed to goby predation (Kipp and Ricciardi 2012).
Round gobies were acclimated at high- and low-[Ca] levels for 36 days, and were maintained for an additional 20 days during and after their use in FR experiments. During all acclimation and holding periods, fish were kept in a single row of 70 L opaque, plastic mesocosms outdoors and were shaded with high-density polyethylene agricultural shade cloth. Fish were first held in a 50:50 mix of river and de-chlorinated tap water for 2 days and were then tagged for identification with green or orange visible implant elastomer (VIE; Northwest Marine Technology) in the caudal peduncle. Fish were held for an additional 2 days to reduce stress, prior to placement in acclimation conditions. VIE does not affect fish growth (Malone et al. 1999; Olsen and Vollestad 2001; Astorga et al. 2005), and no fish died or appeared injured after tagging.
Acclimation mesocosms were prepared with the same 50:50 mix of river and tap water, and each stocked with five rocks, three PVC tubes (9.5 cm × 5 cm) and two bunches of eelgrass, replenished biweekly, for habitat structure and shelter. Mesocosms were randomly assigned to high- or low-[Ca] levels, with 23 of each, and on the first day of the acclimation period, 28 L of water from low-[Ca] mesocosms were replaced with deionized water to establish low-[Ca] conditions. The submerged mass of each fish was then measured (1.6 ± 0.1 g, ±1 SE) and individuals were randomly assigned to each mesocosm, with 1 each of orange- and green-tagged fish per mesocosm.
A 30–50 % water change and cleaning of the mesocosms were conducted weekly, with newly collected river water added biweekly. The same amount of river water was added to each mesocosm with a measured amount of de-chlorinated tap water and deionized water to maintain high- and low-[Ca] conditions, respectively. Calcium concentration was measured at least once a week using a combination ion selective electrode (Cole Parmer, #RK-27504-06). Dissolved [Ca] supplement (Aquavitro) was added to high-[Ca] mesocosms a total of three times owing to reduced [Ca] from heavy rainfall. Calcium concentrations throughout the holding period were 35.1 mg L−1 (±0.6 mg L−1; conductivity: 275.2 ± 5.0 μS cm−1) in high-[Ca] mesocosms and 12.0 mg L−1 (±0.1 mg L−1; conductivity: 109.4 ± 1.3 μS cm−1) in low-[Ca] mesocosms.
Other soluble water components, particularly ions, differed between high and low [Ca] treatments owing to our dilution design. However, [Ca] is a key mediator of fish growth and function, and is especially limiting for brackish-water adapted Ponto-Caspian invaders (see “Discussion”). Another method to assess performance across ionic concentrations is to use water obtained from different locations (e.g. Kestrup and Ricciardi 2010; Baldwin et al. 2012). We chose to use a dilution design to keep the proportion of all soluble water components the same.
Water temperatures of all mesocosms were recorded on 9 days throughout the holding period; a preliminary test confirmed that round gobies in high- and low-[Ca] mesocosms were not differentially influenced by temperature (linear mixed-effects model with random effect of mesocosm compared to random effect only model, analysis of variance (ANOVA) Chi square test, χ 2 = 0.03, p > 0.10). A temperature logger (Hobo Water Temp Pro V2) was also attached at mid-water level in a middle mesocosm to record daily fluctuations in water temperature; temperatures ranged from 16.2 to 28.7 °C, with an average of 21.3 °C.
The feeding regime was modified throughout the acclimation period based on the amount eaten by the fish and waste levels in the mesocosms. Round gobies were fed sinking shrimp pellets (Nutrafin) daily, with the exception of 3 days distributed over the acclimation period when they were not fed. For the first 10 days, fish were given only one pellet each to prevent ammonia build-up. Feeding was then increased to 2–4 pellets/fish (the same amount always provided in each mesocosm) for the next 19 days. During this time, the amount eaten in each mesocosm was recorded by counting the pellets that were removed before each subsequent feeding and on mesocosm maintenance days; partially eaten pellets were visually estimated to the nearest half pellet. Fish were each provided with one pellet daily for the remainder of the time spent in mesocosms. Fish were reweighed before FR experiments began (after 36 days of acclimation) and again 10 days after completion of all FR trials, with 56 days of holding at high- and low-[Ca] levels. Two fish died after 20 days of acclimation in low-[Ca] mesocosms; the remaining fish in these two mesocosms were not used for any analysis.
Functional response experiments I: acclimated round gobies
The FRs of round gobies in high- and low-[Ca] acclimation treatments were measured using live native amphipods (Gammarus fasciatus) as prey in high- and low-[Ca] conditions. Water was premixed in 98.4 L bins with river water poured over a 63 μm Nitex mesh filter held between two 1 mm sieves to exclude debris. Deionized water was then added to obtain low [Ca] (10.4 ± 0.2 mg L−1); no water was added for high [Ca] (31.8 ± 1.5 mg L−1). Functional response experiments were conducted in a climate-controlled room (16.8 °C) in 18.9 L glass aquaria with 800 cm3 of gravel, one PVC tube and 6 L of aerated water. Fish were randomly assigned to the [Ca] at which they would be tested and to a prey density of 2, 3, 5, 10, 20 and 30; amphipods (6.8 ± 0.2 mm) were collected from Parc René-Lévesque, Lake Saint-Louis, St. Lawrence River (45.4281°N, 73.6811°W). Fish were placed individually in experimental aquaria and starved 24 h prior to the start of the experiment. Prey were introduced into aquaria at 17:00 and subsequently removed and counted at 09:00 the following day. All prey densities were replicated three times. Controls without fish present were run simultaneously at the highest prey density in either high- or low-[Ca] conditions, with two replicates of each, to ensure prey survival rates of at least 85 %.
Functional response experiment II: non-acclimated round gobies
A second set of FR experiments were run on non-acclimated round gobies to assess their response to high- and low-[Ca] levels without any potential influence of acclimation conditions, and to make a comparison between size-matched individuals. In addition, we tested the generality of this predatory response by using a different prey item of mayfly nymphs (Stenonema spp.). Both round gobies (1.4 ± 0.0 g) and mayfly nymphs (7.5 ± 0.3 mm) were collected from Chateauguay. Round gobies were collected 6 days prior to experiments and held in aquaria (12 L river water, 58 L dechlorinated tap water) with one shrimp pellet/fish provided daily. In the FR experiments, fish were provided with prey densities of 2, 3, 5, 10, 15 and 20, whereas all other protocol remained the same. During the experiments, some mayfly nymphs emerged from the water as sub-imago forms; these were counted as prey that were not eaten. One control each of high- and low-[Ca] conditions were run simultaneously, with a minimum of 90 % of the mayfly nymphs surviving and remaining in nymph form.
Data analysis
We assessed the amount of shrimp pellets eaten during acclimation, as well as change in body mass of the round gobies over the duration of the holding period. The total amount of pellets eaten out of the amount provided was compared between high- and low-[Ca] levels from acclimation days 10–28 using a generalized linear model (GLM) with a binomial distribution. Change in submerged mass of round gobies (final–initial mass) was compared between fish that had only been placed in high-[Ca] conditions (i.e. high-[Ca] acclimation and FR treatments) and fish only from low-[Ca] conditions using analysis of variance (ANOVA). Data were normally distributed (Shapiro–Wilk test, W = 0.98, N = 36, p > 0.58).
For the first series of FR experiments, we used a GLM with a binomial distribution to determine the effect of acclimation [Ca], FR [Ca] and initial prey density on the amount of amphipod prey eaten by round gobies. Tukey’s honest significant difference tests were used for post hoc analysis to compare the amount of prey eaten between each acclimation and FR [Ca] combination. We analyzed the second set of FR experiments using a GLM to assess the effect of FR [Ca] level and initial prey density on the amount of mayfly nymph prey eaten by non-acclimated round gobies. Interaction terms for both models were reduced using ANOVA Chi square tests to compare models.
We determined whether the FR was of Type II or Type III, following the methods of Alexander et al. (2012) and Dick et al. (2013). We derived FR types using logistic regressions of the proportion of prey consumed as a function of prey density. Functional response curves were then modeled using maximum likelihood estimation (bbmle R package; Bolker 2010) with Rogers’ random predator equation (Rogers 1972) for Type II curves with non-replacement of prey (Juliano 2001). This provided estimates of handling times ‘h’, which were converted to maximum feeding rates (1 h −1). Our FR experiments allowed for prey depletion to minimize disturbance during the experiments. Both prey depletion and replacement designs provide similar estimates of maximum feeding rates (Alexander et al. 2012).
Results
During the acclimation period, round gobies in high-[Ca] mesocosms consumed significantly more shrimp pellets than they did in low-[Ca] mesocosms (GLM, z 43 = −7.30, p < 0.001) (Fig. 1a). Prior to beginning the FR experiments, round gobies from high-[Ca] acclimation tended to have gained more mass than fish from low [Ca] (ANOVA, F 1,42 = 3.34, p = 0.08). Upon completion of the holding period, round gobies in high [Ca] for both the acclimation and FR experiments increased in weight more than those in low [Ca] (F 1,34 = 5.32 = , p = 0.03) (Fig. 1b).
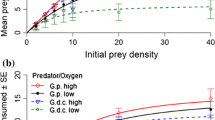
In the first series of FR experiments, high-[Ca] acclimated fish (GLM, z 71 = −2.47, p = 0.01) and fish in high-[Ca] FR treatments (z 71 = −3.50, p < 0.001) consumed more amphipod prey than at low-[Ca] levels, with a tendency for an interaction between acclimation and FR [Ca] (z 71 = 1.87, p = 0.06) (Fig. 2). High-[Ca] acclimated round gobies in high-[Ca] FR treatments tended to have a higher predatory impact compared to low-[Ca] acclimated fish (Tukey’s tests, z = −2.34, p = 0.09) and to fish from both acclimation conditions in low-[Ca] FR treatments (p < 0.01). Fish had similarly low predation rates when in low-[Ca] FR treatments, regardless of acclimation (z = 0.20, p > 0.10). Round gobies also consumed more at higher prey densities (GLM, z 71 = −8.79, p < 0.001).
Non-acclimated, size-matched, round gobies in the second set of FR experiments also had a higher predatory impact on mayfly nymphs when in high-[Ca] FR treatments than fish in low [Ca] (GLM, z 35 = −2.48, p < 0.01). Prey consumption increased with the amount of mayfly nymphs provided (z 35 = −4.13, p < 0.001).
Logistic regressions returned significantly negative linear coefficients (GLM, p < 0.05), indicating that all FRs were of Type II (Table 1; Fig. 3). Both high-[Ca] acclimated and non-acclimated round gobies had higher maximum feeding rates in high-[Ca] FR treatments, whereas low-[Ca] acclimated fish had a higher maximum feeding rate in low [Ca] (Table 1).
Type II functional response (FR) curves of round gobies on (a–d) amphipod and (e, f) mayfly nymph prey. Round gobies were acclimated in (a, b) high-[Ca] and (c, d) low-[Ca] conditions and their FR was measured in (a, c) high-[Ca] and (b, d) low-[Ca] conditions. In separate experiments, the FR of non-acclimated round gobies was measured in e high-[Ca] and f low-[Ca] conditions
Discussion
Our results demonstrate that round goby body mass gain and predation rates are reduced at low-dissolved ion levels in which they can establish, in support of the Environmental Matching Hypothesis. The effect of [Ca] and conductivity on the survival of round gobies has been used to predict their spread and establishment (Baldwin et al. 2012), whereas our study explains, in part, spatial variation in impact levels of round gobies across invaded habitats of differing water chemistry. Other invasive species have been found to have higher impacts in physico-chemical conditions for which they are adapted (i.e. MacNeil et al. 2004; Costanzo et al. 2005), and temperature has been shown to mediate inland water fish and crustacean impacts (Iacarella et al. 2015). By continuing to identify key abiotic conditions that affect performance and impacts of invasive species, environmental matching tools can be extended from spread and establishment (i.e. niche-based modeling; Peterson 2003; Bomford et al. 2010; Britton et al. 2010) to impact risk assessments.
Importance of calcium ions for aquatic animal physiology and ecology
Calcium is a key element influencing fish osmoregulation, growth and other physiological processes (Hunn 1985). Low-[Ca] levels increase gill permeability to water, reducing the osmotic regulatory functioning of the gill (Hunn 1985). Round gobies held at lethal levels of [Ca] and conductivity (<2 mg L−1, <42 μS cm−1, respectively) increase ventilation rates, and ventilate with larger volumes of water; their survival increases along a dissolved ion gradient, up to an asymptote near 20 mg L−1 [Ca] (Baldwin et al. 2012). We believe that our body mass gain and predation measurements at high [Ca] represent a threshold response, as has been demonstrated for round goby survival (Baldwin et al. 2012) and other Ponto-Caspian invaders mediated by [Ca] (Jones and Ricciardi 2005; Jokela and Ricciardi 2008).
For some fish species, growth is aided more by absorption of dissolved calcium ions than by calcium taken up through feeding (Berg 1970; Hunn 1985; Flik et al. 1986). Food provided during both acclimation and FR experiments contained calcium from crustaceans. However, round gobies that were acclimated in low [Ca] appeared to have a weakened condition, as they ate less and gained less mass throughout the acclimation period, and they had a reduced response to high- versus low-[Ca] FR treatments. Though low-[Ca] acclimated fish tended to eat more overall in high-[Ca] FR treatments, they had a higher maximum feeding rate in low [Ca] (indicated by the asymptote at high prey densities); however, both FR curves showed a substantive degree of overlap.
Despite the finding that high-[Ca] acclimated fish tended to be larger at the start of the FR experiments, predation rates were similarly reduced in low-[Ca] FR experiments regardless of the acclimation condition. It appears that low-[Ca] has a relatively immediate influence on predation rates, but may incur longer lasting health effects; this is corroborated by the response of the non-acclimated, size-matched, round gobies. Both high-[Ca] acclimated and non-acclimated round gobies ate more overall and had greater maximum feeding rates at high-[Ca] than low-[Ca] levels. The similar response of acclimated and non-acclimated fish to [Ca] also indicates that the difference in predatory impact is an effect of water chemistry and not differences that may have occurred over time in the mesocosms (i.e. nutrient availability). Our results show that the predatory impact of round gobies is reduced at low-dissolved ion levels, in part owing to the effect of dissolved calcium ions on osmoregulation.
We also found that three high-[Ca] acclimated fish contained eggs at the end of the experiments, whereas none from low-[Ca] had eggs. Females with eggs may have higher consumption rates, though male round gobies grow more quickly and thus also have high energetic demands (Charlebois et al. 1997). We did not attempt to determine the gender of the fish as this is unreliable for juveniles less than 50 mm in total length (Brandner et al. 2013). However, we do not believe that our results were biased by gender, given the random assignment of fish to treatments and the expectation that both females with eggs and males may have higher predation rates.
Dissolved calcium ions also limit the spread and establishment of aquatic crustacean and molluscan invaders, and may mediate their impact across invaded ranges. As with fish, aquatic crustaceans meet the majority of their calcium requirements through uptake at the gills (Wheatly 1999). The freshwater invader Bythotrephes longimanus has very low-[Ca] requirements for growth; in lakes that are declining in [Ca], it may have synergistic negative impacts on native daphniids with high-[Ca] requirements (Kim et al. 2012). Invasive crayfish display more vigilant and escape behaviours exposed to predation risk in [Ca] conditions at the lower end of their tolerance range (Edwards et al. 2013). The native amphipod prey used in our FR experiments have similar growth and survival rates in waters matching our high- and low-[Ca] treatments (Kestrup and Ricciardi 2010); the mayfly nymphs used in the second set of FR experiments are also freshwater-adapted and unlikely to have been affected by our low-[Ca] treatments. Conversely, in low-conductivity waters of invaded habitats, the Ponto-Caspian amphipod Echinogammarus ischnus has been found to have slower growth rates (Kestrup and Ricciardi 2010) and is more vulnerable to intraguild predation by adult native amphipods (Kestrup and Ricciardi 2009). The distribution and abundance of another group of invasive Ponto-Caspian animals, the zebra mussel (Dreissena polymorpha) and the quagga mussel (D. bugensis), are limited by low-[Ca] (Jones and Ricciardi 2005). Zebra and quagga mussels are macofouling species that overgrow any available hard substrate, including the shells of other molluscs, and can thus severely damage native mussel (Unionidae) populations (Jokela and Ricciardi 2008). However, they have substantively greater [Ca] requirements than native unionid mussels (McMahon and Bogan 2001), and exert reduced fouling intensities on native mussels at lower [Ca] levels (Jokela and Ricciardi 2008); therefore, low-[Ca] habitats offer a refuge for native unionid mussels against the impacts of dreissenid mussel invasion. Filtration rates of dreissenid mussels could also be affected by varying [Ca] levels, although this has not been reported to our knowledge. These examples suggest that calcium concentration is a key mediator of impacts of Ponto-Caspian species in their invaded habitats.
Environmental matching to predict impacts of invasive species
Environmental matching between habitat conditions and physiological optima can be used to predict per capita effect and abundance, both components of impact. Per capita effects of invasive species are often difficult to assess (Parker et al. 1999), and standardized methods are needed for comparing them in different environmental contexts (Dick et al. 2014). Functional response analysis provides relative comparisons of predatory efficiency that can be used to predict impact potential (Dick et al. 2013; Alexander et al. 2014); furthermore, maximum feeding rates identify the highest predatory response within the physiological limitations of the invader (Jeschke et al. 2002). Ecological impacts of invasive species also increase with abundance (Thomsen et al. 2011), though the relationship between per capita effect and abundance is not generally known (Parker et al. 1999) and likely varies across taxa (Yokomizo et al. 2009). Performance metrics such as growth rates across physico-chemical gradients may partially explain differences in field abundances (Pörtner and Knust 2007). Habitats characterized by physiologically optimal conditions for an invader may promote both high per capita effects and abundance levels of the invader.
Per capita effects of invasive species are not limited to predatory impacts and, for fishes in particular, include competition for food and habitat. For instance, the round goby has displaced many native fishes through resource competition (Kornis et al. 2012). Water chemistry conditions have also been found to mediate aggressive behaviours of invasive fishes (Alcaraz et al. 2008). We expect that round goby competitive ability will be reduced in low-[Ca] conditions, given their decline in body mass gain and consumption in such conditions. The Environmental Matching Hypothesis generally predicts that all direct per capita effects of an invader are highest in physiologically optimal conditions; however, responses of different per capita effect measures across physico-chemical gradients have not yet been assessed.
Measurements of performance of an invasive species along key environmental gradients can be assembled to develop a multidimensional niche-space that delineates the impact potential of the species. This would enable predictions of relative impact across an invaded range, or across habitats at-risk for invasion, based on a suite of environmental characteristics. Biotic factors that influence impacts, such as the presence of functionally-similar natives (Ricciardi and Atkinson 2004; Ricciardi and Ward 2006), could also be incorporated into risk assessment. More precise predictions may account for potential interacting effects of abiotic conditions on invader responses (e.g. Epifanio et al. 1998). Developing such impact risk assessments would better enable managers to prioritize invasion threats.
References
Alcaraz C, Bisazza A, Garcia-Berthou E (2008) Salinity mediates the competitive interactions between invasive mosquitofish and an endangered fish. Oecologia 155:205–213
Alexander ME, Dick JTA, O’Connor NE, Haddaway NR, Farnsworth KD (2012) Functional responses of the intertidal amphipod Echinogammarus marinus: effects of prey supply, model selection and habitat complexity. Mar Ecol Prog Ser 468:191–202
Alexander ME, Dick JTA, Weyl OLF, Robinson TB, Richardson DM (2014) Existing and emerging high impact invasive species are characterized by higher functional responses than natives. Biol Lett 10:20130946
Astorga N, Afonso JM, Zamorano MJ, Montero D, Oliva V, Fernandez H, Izquierdo MS (2005) Evaluation of visible implant elastomer tags for tagging juvenile gilthead seabream (Sparus auratus L.); effects on growth, mortality, handling time and tag loss. Aquac Res 36:733–738
Baldwin BS, Carpenter M, Rury K, Woodward E (2012) Low dissolved ions may limit secondary invasion of inland waters by exotic round gobies and dreissenid mussels in North America. Biol Invasions 14:1157–1175
Barrios-O’Neill D, Dick JTA, Emmerson MC, Ricciardi A, MacIsaac HJ, Alexander ME, Bovy HC (2014) Fortune favours the bold: a higher predator reduces the impact of a native but not an invasive intermediate predator. J Anim Ecol 83:693–701
Berg A (1970) Studies on the metabolism of calcium and strontium in freshwater fish. II. Relative contribution of direct and intestinal absorption in growth conditions. Memorie dell’Istituto Italiano di Idrobiologia 26:241–255
Bolker BM (2010) bbmle: Tools for general maximum likelihood estimation. R package version 1.0.15. http://CRAN.R-project.org/package=bbmle
Bomford M, Barry SC, Lawrence E (2010) Predicting establishment success for introduced freshwater fishes: a role for climate matching. Biol Invasions 12:2559–2571
Brandner J, Cerwenka AF, Schliewen UK, Geist J (2013) Bigger is better: characteristics of round gobies forming an invasion front in the Danube River. PLoS ONE 8:e73036
Britton JR, Cucherousset J, Davies GD, Godard MJ, Copp GH (2010) Non-native fishes and climate change: predicting species responses to warming temperatures in a temperate region. Freshw Biol 55:1130–1141
Campbell LM, Thacker R, Barton D, Muir DCG, Greenwood D, Hecky RE (2009) Re-engineering the eastern Lake Erie littoral food web: the trophic function of non-indigenous Ponto-Caspian species. J Great Lakes Res 35:224–231
Charlebois PM, Marsden JE, Goettel RG, Wolfe RK, Jude DJ, Rudnicka S (1997) The round goby, Neogobius melanostomus (Pallus), a review of European and North American literature. INHS Special Publication No. 20. Illinois–Indiana Sea Grant Program, Urbana, Illinois, USA and Illinois Natural History Survey, Champaign, Illinois, USA
Costanzo KS, Kesavaraju B, Juliano SA (2005) Condition-specific competition in container mosquitoes: the role of noncompeting life-history stages. Ecology 86:3289–3295
Dick JTA (1996) Post-invasion amphipod communities of Lough Neagh, Northern Ireland: influences of habitat selection and mutual predation. J Anim Ecol 65:756–767
Dick JTA, Gallagher K, Avlijas S, Clarke H, Lewis S, Leung S, Minchin D, Caffrey J, Alexander M, Maguire C, Harrod C, Reid N, Haddaway N, Farnsworth K, Penk M, Ricciardi A (2013) Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol Invasions 15:837–846
Dick JTA, Alexander ME, Jeschke JM, Ricciardi A, MacIsaac HJ, Robinson TB, Kumschick S, Weyl OLF, Dunn AM, Hatcher MJ, Paterson RA, Farnsworth KD, Richardson DM (2014) Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol Invasions 16:735–753
Edwards BA, Lewis VRE, Rodd FH, Jackson DA (2013) Interactive effects of calcium decline and predation risk on the potential for a continuing northward range expansion of the rusty crayfish (Orconectes rusticus). Can J Zool 91:328–337
Epifanio CE, Dittel AI, Park S, Schwalm S, Fouts A (1998) Early life history of Hemigrapsus sanguineus, a non-indigenous crab in the Middle Atlantic Bight (USA). Mar Ecol Prog Ser 170:231–238
Flik G, Fenwick JC, Kolar Z, Mayergostan N, Bonga SEW (1986) Effects of low ambient calcium levels on whole-body Ca2+ flux rates and internal calcium pools in the freshwater cichlid teleost, Oreochromis mossambicus. J Exp Biol 120:249–264
French JRP, Jude DJ (2001) Diets and diet overlap of nonindigenous gobies and small benthic native fishes co-inhabiting the St. Clair River, Michigan. J Great Lakes Res 27:300–311
Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS (2008) Five potential consequences of climate change for invasive species. Conserv Biol 22:534–543
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 92:385–398
Holling CS (1966) The functional response of invertebrate predators to prey density. Mem Entomol Soc Can 48:1–86
Hunn JB (1985) Role of calcium in gill function in freshwater fishes. Comp Biochem Physiol 82A:543–547
Iacarella JC, Dick JTA, Alexander ME, Ricciardi A (2015) Ecological impacts of invasive alien species along temperature gradients: testing the role of environmental matching. Ecol Appl 25:706–716
Jeschke JM, Kopp M, Tollrian R (2002) Predator functional responses: discriminating between handling and digesting prey. Ecol Monogr 72:95–112
Jokela A, Ricciardi A (2008) Predicting zebra mussel fouling on native mussels from physicochemical variables. Freshw Biol 53:1845–1856
Jones LA, Ricciardi A (2005) Influence of physicochemical factors on the distribution and biomass of invasive mussels in the St. Lawrence River. Can J Fish Aquat Sci 62:1953–1962
Juliano S (2001) Nonlinear curve fitting. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 179–196
Kestrup AM, Ricciardi A (2009) Environmental heterogeneity limits the local dominance of an invasive freshwater crustacean. Biol Invasions 11:2095–2105
Kestrup A, Ricciardi A (2010) Influence of conductivity on life history traits of exotic and native amphipods in the St. Lawrence River. Fund Appl Limnol 176:249–262
Kim N, Walseng B, Yan ND (2012) Will environmental calcium declines hinder Bythotrephes establishment success in Canadian Shield lakes? Can J Fish Aquat Sci 69:810–820
Kipp R, Ricciardi A (2012) Impacts of the Eurasian round goby (Neogobius melanostomus) on benthic communities in the upper St. Lawrence River. Can J Fish Aquat Sci 69:469–486
Kornis MS, Mercado-Silva N, Vander Zanden MJ (2012) Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. J Fish Biol 80:235–285
MacNeil C, Prenter J, Briffa M, Fielding NJ, Dick JTA, Riddell GE, Hatcher MJ, Dunn AM (2004) The replacement of a native freshwater amphipod by an invader: roles for environmental degradation and intraguild predation. Can J Fish Aquat Sci 61:1627–1635
Malone JC, Forrester GE, Steele MA (1999) Effects of subcutaneous microtags on the growth, survival, and vulnerability to predation of small reef fishes. J Exp Marine Biol Ecol 237:243–253
McMahon RF, Bogan AE (2001) Mollusca: Bivalvia. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego, pp 331–429
Olsen EM, Vollestad LA (2001) An evaluation of visible implant elastomer for marking age-0 brown trout. N Am J Fish Manage 21:967–970
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Paterson RA, Dick JTA, Pritchard DW, Ennis M, Hatcher MJ, Dunn AM (2014) Predicting invasive species impacts: a community module functional response approach reveals context dependencies. J Anim Ecol 84:453–463
Peterson AT (2003) Predicting the geography of species’ invasions via ecological niche modeling. Q Rev Biol 78:419–433
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Rahel FJ, Olden JD (2008) Assessing the effects of climate change on aquatic invasive species. Conserv Biol 22:521–533
Reid DF, Orlova MI (2002) Geological and evolutionary underpinnings for the success of Ponto-Caspian species invasions in the Baltic Sea and North American Great Lakes. Can J Fish Aquat Sci 59:1144–1158
Ricciardi A, Atkinson SK (2004) Distinctiveness magnifies the impact of biological invaders in aquatic ecosystems. Ecol Lett 7:781–784
Ricciardi A, Ward JM (2006) Comment on ‘‘Opposing effects of native and exotic herbivores on plant invasions’’. Science 313:298a
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of non-native species. Ecol Monogr 83:263–282
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:369–383
Thomsen MS, Olden JD, Wernberg T, Griffin JN, Silliman BR (2011) A broad framework to organize and compare ecological invasion impacts. Environ Res 111:899–908
Walther GR, Roques A, Hulme PE, Sykes MT, Pysek P, Kuhn I, Zobel M, Bacher S, Botta-Dukat Z, Bugmann H, Czucz B, Dauber J, Hickler T, Jarosik V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vila M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Wheatly MG (1999) Calcium homeostasis in crustacea: the evolving role of branchial, renal, digestive and hypodermal epithelia. J Exp Zool 283:620–640
Yokomizo H, Possingham HP, Thomas MB, Buckley YM (2009) Managing the impact of invasive species: the value of knowing the density-impact curve. Ecol Appl 19:376–386
Acknowledgments
We thank Rachael Ryan, Sandrine Vigneron and Jean-Michel Matte for assistance with the experiments. This research was funded by the Canadian Aquatic Invasive Species Network and by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to A.R. Additional support was provided by the Group for Interuniversity Research in Limnology and Aquatic Environment (to J.C.I.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iacarella, J.C., Ricciardi, A. Dissolved ions mediate body mass gain and predatory response of an invasive fish. Biol Invasions 17, 3237–3246 (2015). https://doi.org/10.1007/s10530-015-0949-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0949-5