Abstract
Objective
To examine the influence of widely used protein affinity tags and the tobacco PR1a signal peptide (SP) on detection, purification and bioactivity analyses of the small oomycete apoplastic effector SCR96 in planta.
Results
Through agroinfiltration, the phytotoxic effector SCR96 of Phytophthora cactorum was expressed in Nicotiana benthamiana leaf apoplast as a fusion protein carrying single affinity tag (His, HA or FLAG) at either C- or N-terminus. Leaf necrosis caused by different affinity-tagged SCR96 varied among tags and replicates. All of tagged proteins can be detected by antibodies against SCR96. All of SCR96 fusions except N-terminally fused 6His-tagged protein were detected using tag antibodies, indicating that 6His tag may be degraded when fused at N-terminus. Interestingly, C-terminal His- and FLAG-tagged SCR96 maintained the biological activity after purification. In the substitution assay of SCR96 SP, we observed that PR1a SP can lead chimeric SCR96 expression in N. benthamiana, but the replacement totally disrupted its bioactivity.
Conclusion
C-terminal His or FLAG tag, along with its original SP, is efficient enough to enable detection and purification of functional SCR96 from N. benthamiana leaf apoplast, which would facilitate plant–pathogen interaction studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Phytophthora within the class Oomycetes under the kingdom Chromista contains over 100 plant pathogens that severely devastate agricultural production and natural ecosystems, including potato and tomato late blight agent Phytophthora infestans, and the soybean root and stem rot agent Phytophthora sojae (Erwin and Ribeiro 1996). Unlike these species with narrow host ranges, Phytophthora cactorum is a soil borne pathogen that has a very broad host range, spanning 54 plant families worldwide. It can cause root, collar, and crown rot, as well as foliar and fruit infections and is among the most destructive oomycete pathogens for many economically important plants, such as strawberry, apple, pear, tomato and rhododendron (Erwin and Ribeiro 1996).
To establish colonization and accomplish infection, the oomycete pathogens are believed to secret an array of proteins, namely effectors (i.e. apoplastic and cytoplasmic effectors), into plants to manipulate the physiological and defense network of host cells (Kamoun 2006). The initial interaction between plants and oomycete pathogens takes place in host apoplast, which often determines the battle outcomes (Kamoun 2006). This implies that the apoplastic effectors need to survive in the harsh acidic and protease-rich environment of apoplast to execute their functions. Of the apoplastic effectors, there is a subfamily called small cysteine-rich (SCR) effectors that shows features similar to fungal avirulence proteins (Avr), such as being secreted during infection, relatively small (3–15 kDa) and harboring abundant cysteine (Cys) residues (Stergiopoulos and de Wit 2009), which enable them to be a research focus. Notably, Cys residues are involved in formation of disulfide bridges, which helps to stabilize the effector structures in the host apoplast. To date, the SCR effectors have been found in several genome-sequenced oomycete species including P. infestans, P. sojae, Phytophthora ramorum and Hyaloperonospora arabidopsidis, based on genomic analysis (Tyler et al. 2006; Haas et al. 2009; Baxter et al. 2010), and are often named by the abbreviation ‘SCR’ appended with their residue number (Chen et al. 2016). However, only three members, namely P. cactorum PcF and SCR96, and Phytophthora capsici SCR82 were functionally characterized (Orsomando et al. 2011; Chen et al. 2016; Zhang et al. 2021). They functioned as selective phytotoxic proteins that lead to plant cell death (PCD)/leaf necrosis of strawberry, tomato and/or Nicotiana benthamiana, suggesting a cell surface pattern recognition receptor (PRR)-based mode of action (Orsomando et al. 2011; Chen et al. 2016; Zhang et al. 2021). SCR effectors also invoked expression of the key genes or pathways related to plant defense, such as phenylalanine ammonia-lyase, pathogenesis-related (PR) genes and pattern-triggered immunity (PTI) in tomato (Orsomando et al. 2011; Zhang et al. 2021). Intriguingly, silencing or disruption of P. cactorum scr96 and P. capsici scr82 significantly attenuated the virulence of the pathogens and reduced their tolerances to oxidative stresses, indicating that they may function as both plant defense elicitors and virulence factors (Chen et al. 2016; Zhang et al. 2021).
Due to its complex nature, the SCR protein family has been less studied than other effector families. All of the SCR effectors are small (≤ 300 amino acids), hydrophobic and show high Cys contents, which make them difficult to be studied at biochemical level. For instance, these effector proteins are often prone to aggregation due to the high contents of Cys residues when expressed in Escherichia coli, yeast and mammalian cells (Orsomando et al. 2011; Zhang et al. 2019, 2021; Huang et al. 2020). Additionally, it is unclear whether widely used protein affinity tags and heterologous signal peptides (SPs) could facilitate the study of the SCR effectors or not. Generation of fusion proteins by adding tags or heterologous SPs has been widely used to analyze protein functions or interactions in biological studies. Fusion tags exist for a variety of applications, including affinity purification, solubility enhancement, and protein detection (Terpe 2003). While affinity tags were previously used for Phytophthora SCR effector studies (Song 2007), some tags adversely affected the bioactivity of the recombinant proteins, such as the SUMO tag used for P. capsici SCR82 expression in E. coli (Zhang et al. 2019). The SUMO-tagged SCR82 totally lost its ability to induce PCD in N. benthamiana. Previous studies also revealed that affinity tags were prone to be removed from the effectors of Cladosporium fulvum and P. infestans when they were expressed in the tomato leaf apoplast, leading to failures of protein detection (van Esse et al. 2006; Song 2007). In order to facilitate production and secretion of a soluble form of foreign proteins in the Solanaceae plants, modifications are often engineered into the gene cDNA sequences, including substitution of their native SP encoding sequences with the one of tobacco (Nicotiana tabacum) PR1a (Agarwal et al. 2008; Kanagarajan et al. 2012). However, whether such a plant derived SP could improve the ectopic expression of the oomycete SCR effectors in Nicotiana plants has not been revealed.
To study the biochemical functions of the SCR effectors, the host partners that they interact with, and their posttranslational modifications, it is necessary to get them expressed, detected and even purified from the in vivo situation with the assistance of epitope-tags. Hence, appropriate protein affinity-tags should be selected to minimize their influence on the tertiary structures and biological activities of the effector proteins. In this study, we employed a well-characterized SCR effector, the P. cactorum phytotoxic SCR96 (Chen et al. 2016; Huang et al. 2020), as a model to test the influence of the commonly used small protein tags, such as the FLAG, Hemagglutinin (HA) and Histidine (His) tags with different replicates on the expression and phytotoxicity of the SCR effectors in N. benthamiana. Based on the apoplastic localization and expression profile of the SCR effectors (Chen et al. 2016), SCR96 was extracted from the apoplast at 48 h post-infiltration (hpi) and subsequently purified utilizing the FLAG and His tags, respectively. Additionally, we evaluated the impact of the N. tabacum PR1a (NtPR1a) SP on the expression and secretion of SCR96 in N. benthamiana.
Materials and methods
Strains, plants and reagents
P. cactorum isolate 10,300 was routinely cultured as previously described (Huang et al. 2020). E. coli DH5α was utilized for plasmid propagation. N. benthamiana was grown in a greenhouse under a 16-h/8-h light/dark rhythm (light intensity 130 mE m−2 s−1) at 22 ± 3 °C. The vector pBINPLUS was used for the ectopic expression of the scr96 gene in N. benthamiana leaves (Zhang et al. 2021). All the chemicals and kits were purchased from commercial sources.
The peptide fragment encoded by scr96 (GenBank accession No. KT215393) without its SP sequence was synthesized using solid phase peptide synthesis technology and provided at 91.82% HPLC purity by DGpeptides (Hangzhou, China). Polyclonal antibodies purified from the antiserum (ELISA titer > 1:128, 000) of New Zealand white rabbits against the synthesized peptide were ordered from DGpeptides.
Construction of plasmids expressing affinity tag or SP fused SCR96
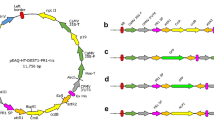
To examine the performance of affinity tags, a DNA fragment coding for SCR96 was synthesized de novo (GenScript, Nanjing, China), appended with a N-terminal or C-terminal fusion tag. Tested tags include FLAG (peptide sequence DYKDDDDK), HA (YPYDVPDYA) and His (HHHHHH) tags with different replicates (Fig. 1a).
To test if plant-derived SP could facilitate the expression and secretion of the SCR effectors, the native 23-amino acid (aa) SP of SCR96 was designed to be replaced with the 30-aa SP of NtPR1a (GenBank accession No. X06930) (Fig. 1b). All the genes were assembled as shown in Fig. 1, synthesized by GenScript and cloned into pBINPLUS using appropriate restriction sites (Supplementary File 1).
Agroinfiltration of N. benthamiana
The plasmid pBINPLUS harboring different version of the scr96 expression cassettes (Fig. 1) was individually introduced into Agrobacterium tumefaciens strain GV3101 using the standard electroporation method. Agrobacteria suspension (OD600 = 0.5) and leaf infiltration were made as previously described (Chen et al. 2016) with minor modifications. Briefly, each SCR96 fusion plasmid was co-agroinfiltrated with the plasmid pJL3:p19 that contains the viral gene-silencing suppressor p19 originated from Tomato bushy stunt virus, using a 1:1 ratio. Twenty leaves for each plasmid were infiltrated unless specifically stated. Infiltration buffer (10 mM MES, 0.1 mM acetosyringone, 10 mM MgCl2) and the plasmid carrying the P. infestans elicitin inf1 gene (GenBank accession No. AY830094) served as the negative and positive controls, respectively. Cell death symptom was monitored visually at a 2–15 days interval after infiltration. Biologically repeated infiltrations with different sets of plants were conducted three times, producing similar results.
Extraction of total proteins
Three N. benthamiana leaves were excised from plants at 48 hpi, cleaned and ground into fine powder in a mortar using a pestle and liquid nitrogen. About 0.5 g powder was immediately transferred into a 2-mL tube containing 495 μL of NP-40 Lysis Buffer (Beyotime Biotech, Shanghai, China) and 5 μL of 100 mM phenylmethylsulfonyl fluoride. The samples were vibrated for 1 min, and centrifuged at 4 °C, 12, 000 rpm for 15 min. The supernatant was saved for SDS-PAGE analysis.
Isolation of apoplastic fluid (AF)
AF was obtained from N. benthamiana leaves at 48 hpi according to a previously described method (O’Leary et al. 2014) with minor modifications. Briefly, ten agroinfiltrated leaves were detached, rinsed with sterile water, and placed in a 1000-mL vacuum suction flask filled with distilled water. Vacuum (about 60 mbar) was applied for 15–30 s repeatedly until the leaves appeared as dark translucent. The vacuumed leaves were taken out, dried with clean tissue, and sandwiched in parafilm sheets (10 cm × 10 cm). Next, the wrapped leaves were rolled and placed into a 20-mL syringe, and centrifuged in a 50-mL conical tube for 10 min at 1000 g, 4 °C. AF extract was collected from the 50-mL conical tube and further centrifuged at 15,000×g for 5 min. The supernatant was transferred into a fresh tube on ice and utilized on the same day or stored at − 75 °C for later use.
Protein purification from AF extracts
AF extracts containing the soluble SCR96 fused with FLAG tag at N- or C-terminus were purified using anti-FLAG magnetic beads (Abmart, Shanghai, China). The 1.5-mL tube containing 40 μL beads was placed in an appropriate magnetic separator to collect the beads. The storage buffer was aspirated and discarded. The beads were washed twice by 400 μL of TBS buffer (50 mM Tris/HCl, 150 mM NaCl, pH 7.4) and then incubated with 500 μL apoplastic fluid on a roller shaker for 2 h at room temperature. After washing the beads three times with 800 μL of TBS-washing buffer (50 mM Tris/HCl, 150 mM NaCl, 250 μM EDTA, 0.25% Triton X-100, 0.25% Na deoxycholate, 0.025% SDS, pH 7.4), the FLAG fusion protein was eluted by the elution buffer (0.1 M Glycine/HCl, pH 3.5) at room temperature and saved for further analysis. The recombinant protein SCR96 fused with C-terminal 6His tag was purified using Ni–NTA affinity purification as previously described (Huang et al. 2020). Protein concentration was quantified using an enhanced BCA protein assay kit (Beyotime Biotech).
SDS-PAGE and Western blotting
Ten μL of protein solution was mixed with an equal volume of the loading buffer (4% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.04% bromophenol blue, and 125 mM Tris/HCl, pH 6.8), boiled for 10 min, and then kept on ice for 5 to 10 min. After centrifugation at 12,000×g for 10 min at 4 °C, the supernatant was taken to SDS-PAGE gel electrophoresis. After electrophoresis, the proteins in gels were electrotransferred onto 0.22-μm nitrocellulose filter membranes (Pall Corporation). Proteins on the membranes were stained with Ponceau S (Aladdin, Shanghai, China), then destained by distilled water washing, and further detected by tag antibody (Abmart) or custom anti-SCR96 polyclonal antibodies (DGpeptides) as previously described (Huang et al. 2020).
Results
Different epitope tagged SCR96 induced differentiated PCD in N. benthamiana leaves
In our previous studies, we observed that some epitope tagged SCR effectors lost their ability to induce PCD, such as the SUMO tagged SCR82 (Zhang et al. 2019). To study the influence of epitope tags on expression of the P. cactorum phytotoxic effector SCR96 (Chen et al. 2016; Huang et al. 2020), we fused SCR96 with the commonly used small epitope tags including HA, His and FLAG either at the N or C terminus and transiently expressed them in N. benthamiana leaves. We found that these tagged SCR96s showed differentiated PCD-inducing abilities, compared with the untagged SCR96 (Fig. 2). Notably, the 6His fused SCR96 at either N or C-terminus (6His-SCR96 and SCR96-6His) caused obvious PCD as both the untagged SCR96 and the positive control INF1 did, while the SCR96 C-terminally fused with 3HA (SCR96-3HA) barely induced PCD. In comparison, other tagged SCR96 such as HA-tagged SCR96 and FLAG-tagged SCR96 displayed relatively mild PCD (Fig. 2a). We also noticed that the PCD/necrosis caused by tagged SCR96 varied among replicates in each tag case. To demonstrate the PCD/necrosis phenotype better, we categorized the necrosis severity into six levels, namely from Level 1 to Level 6, representing no symptom to most severe necrosis/PCD (Fig. 2b). In general, N-terminal or C-terminal 6His tagged SCR96 (6His-SCR96 or SCR96-6His) induced the strongest PCD (Level 5 and 6, ratio > 86%), which was quite similar to the untagged SCR96. FLAG-SCR96 caused a bit milder necrosis (mostly at Level 5). The remainder versions of tagged SCR96 seemed to gradually lose the PCD-causing activity. Taken together, the results indicated that the tested protein affinity tags exhibited different effects on the PCD-inducing activity of SCR96. His tag and the N-terminally fused FLAG may be the most appropriate ones that could be used in the studies of the SCR effectors.
Expression of His-, HA- and FLAG-tagged SCR96 in N. benthamiana. a PCD phenotypes produced by different tagged SCR96. The untagged SCR96 and INF1 served as the positive controls. Photos were taken at 6 days post-infiltration. The numbers below the panel indicated the ratios of leaves showing PCD to the total leaves that were infiltrated. b Analysis of leaves showing different PCD/necrosis severity. Different necrosis degree was represented by different color blocks. c Detection of tagged SCR96 using antibodies specific to SCR96 or selected affinity tags. The experiment was repeated three times, producing similar results
Epitope tagged SCR96 can be highly expressed in N. benthamiana
To address the issue of tag stability in planta, we checked the expression of each epitope tagged SCR96 described above by Western blot using antibodies against either tags or the SCR96 protein (Fig. 2c). We identified that all versions of tagged SCR96 can be detected by the customized antibody against SCR96, while no signal was detected from N. benthamiana leaves infiltrated with A. tumefaciens containing an empty binary vector (EV). This indicated that all versions of tagged SCR96 were successfully expressed in plants. Next, the same samples were subjected to Western blotting employing antibodies specific to each tag. Distinct bands were detected in samples from leaves expressing all versions of tagged SCR96 except N-terminal 6His-tagged SCR96 (6His-SCR96) (Fig. 2c). However, an expected band was detected in leaf sample expressing C-terminally 6His-tagged SCR96 (SCR96-6His) using the same anti-His antibody. In contrast, we detected SCR96 tagged by other epitopes regardless of the tag positions. These findings suggest that the N-terminal 6His tag was possibly unstable, or could be structurally buried in the protein so that detection of the protein failed. This is in agreement with the observation that when equal amount of the SCR96 proteins were loaded, the 6His-tagged SCR96 (both 6His-SCR96 and SCR96-6His) detected by the SCR96-specific antibody showed weaker signals comparing to the other samples tagged by different epitopes (Fig. 2c).
SCR96 fused with C-terminal His or FLAG tag is stable during affinity purification
It is widely recognized that SCR effectors function in plant apoplast (Kamoun 2006). Based on the above results, we used anti-FLAG magnetic beads or Ni–NTA resin to purify SCR96 tagged with FLAG or 6His from N. benthamiana leaf apoplast, respectively. The samples were subsequently subjected to SDS-PAGE followed by Western blot using tag antibodies. As shown in Fig. 3a, both tags remained stable during the purification process and tagged SCR96 was detected in the final eluted protein fractions. It was noted that unlike SCR96-FLAG, FLAG-SCR96 was not detected in the original AF, but detected in the final purified sample, indicating the original low quantity of FLAG-SCR96 and later successful enrichment (Fig. 3a). We quantified the eluted SCR96 using a BCA method (Zhang et al. 2021), which displayed 10–20 μg purified SCR96 from 1 mL of total N. benthamiana leaf apoplastic fluid. These results indicate that both FLAG and C-terminal His tags are stable and can be used to effectively label and purify heterologous proteins expressed in N. benthamiana leaves.
Purification and re-infiltration of His- and FLAG-tagged SCR96. a Western blot analysis of SCR96. Letters above the films indicate samples collected from different fractions during protein purification process: AF apoplastic fluid, F column flow-through, W washing-away, E eluted sample from the column. Lane M, a protein marker. The asterisks indicate the target bands of ~ 9 kDa. b Phenotype of re-infiltrated SCR96. Twenty leaves were re-infiltrated with the purified SCR96 protein, and showed the same symptoms. Photos were taken at 4 days post re-infiltration. The experiment was repeated three times, producing similar results
As described above, while assaying the different tags, we noticed that ectopic expression of the tagged SCR96 induced various PCD in N. benthamiana leaves (Fig. 2). To determine whether the purification process affected the bioactivity of SCR96, the purified proteins of SCR96-6His, SCR96-FLAG and FLAG-SCR96 were re-infiltrated into N. benthamiana leaves. Intriguingly, the purified SCR96 recombinant proteins also caused PCD, indicating that affinity purified SCR96 was functional (Fig. 3b). Noticeably, both N- and C-terminally FLAG-tagged SCR96 produced mild PCD, which was like water-soaked. In comparison, the purified SCR96-6His protein induced the strongest PCD, which was similar to the result observed in the agroinfiltration assay (Fig. 3b). These findings suggest that C-terminal 6His may be the best tag choice for the analyses of SCR96.
The NtPR1a SP does not affect the expression of SCR96, but impairs its phytotoxic activity in N. benthamiana
The NtPR1a SP has been extensively utilized to drive the high expression of exogenous genes in the Solanaceae plants (Agarwal et al. 2008; Kanagarajan et al. 2012). To examine if higher expression of SCR96 could be obtained in N. benthamiana, we replaced its original SP with the NtPR1a SP. The chimeric SCR96 protein demonstrated comparable expression and accumulation of SCR96 in N. benthamiana as the protein expressed with its native SP did (Figs. 2, 4). Surprisingly, we found that despite the proteins labeled with different tags were properly expressed, none of the NtPR1a SP fused proteins induced visible PCD in N. benthamiana (Fig. 4). Collectively, these results illustrated that the expression of SCR96 driven by the NtPR1a SP was comparable to that driven by its original SP; however, such SP substitution disrupted the PCD-inducing activity of SCR96 in N. benthamiana.
Expression of SCR96 driven by the signal peptide (SP) derived from the N. tabacum PR1a protein. a Phenotypes of tagged SCR96 driven by the NtPR1a SP. SCR96 driven by its own SP and tagged with C-terminal 6His was used as a positive control while empty vector (EV) as a negative control. The numbers shown on the top left corner of each leaf indicate that twenty leaves were infiltrated for each plasmid and demonstrated the same results (SCR96-6His totally infiltrated into 40 leaves). Photos were taken at 6 days post-infiltration. b Western blot analysis of the NtPR1a SP fused SCR96 protein using the SCR96-specific antibodies. The experiment was repeated three times, producing similar results
Discussion
Epitope fusion tags are important for detection, purification and functional analysis of heterologous proteins expressed in plants, especially expressed in plant apoplast. Expression of epitope tagged proteins in plant apoplast is reputed to be problematic (van Esse et al. 2006; Song 2007). In a previous study, several epitope tagged proteins were shown to be unstable in the tomato apoplast due to protein degradation or removal of affinity-tags preventing detection and affinity purification (van Esse et al. 2006). In this study, we selected three of small size and commonly used affinity tags (His, HA and FLAG), and examined their influence on protein expression of the apoplastic effector SCR96 in N. benthamiana. Our results indicate that His-, HA- and FLAG-tagged proteins enable expression and detection assays in N. benthamiana apoplast. We found that when SCR96 was labeled by His tag at the C-terminus, or FLAG tag at either the N- or C-terminus, the protein fusions were stable during expression and purification. We also found that the protein fusions were functional before and after affinity purification. This study represents a powerful tool for studying plant or microbial secreted proteins in planta, especially for those small apoplastic proteins such as PcF/SCR effectors.
Previous studies reported that some fusion tags were unstable in the apoplast of Solanaceous plants (van Esse et al. 2006; Song 2007). In this study, we found that His tag fused at the N terminus cannot be detected by Western blot, while the C-terminal fusion can (Fig. 2c). This may be due to cleavage of the N-terminal His tag in the apoplast as reported by van Esse et al. (2006). Alternatively, the instability of the N-terminal His-tagging may be due to the post-translational modification of the N-terminal His tag that interfered with the protein crystallization, like the case of chicken src tyrosine kinase (Kim et al. 2001), which disrupted the tag detection. The other two tags, namely FLAG and HA, were also successfully used for SCR96 detection, regardless of the position where the tag was attached. The results indicated that these three commonly used fusion tags could be used for SCR effector expression and detection with caution of appendage protein end choice.
Our previous work demonstrated that SCR96 is an important virulence factor of P. cactorum (Chen et al. 2016). To elucidate its action mechanism during infection, it is important to identify its interacting partners in plants. To identify the targets of SCR96, the recombinant protein was expressed in planta as a fusion with an affinity tag. Many protein tags are available for such studies, each with their own characteristics. The Phytophthora SCR effectors are very small, so small affinity tags (His, FLAG and HA) were chosen to minimize the effects on tertiary structures and biological activities of the effectors. Choosing of small affinity tags is a major concern as in some cases it has indeed been reported that an affinity tag can interfere with a biologically relevant target binding site (Goel et al. 2000). In our study, we noticed that compared with other tested tags, His tag displayed minimal effect on the PCD-inducing bioactivity of SCR96 (Fig. 2). This is probably because of the smaller size of 6His tag (840.86 Da, versus FLAG, 1.01 kDa, and HA, 1.10 kDa). Indeed, larger tags, such as 3HA, almost eliminated the bioactivity of SCR96 (Fig. 2). This is in agreement with our previous report that the SUMO tag (~ 24 kDa) adversely affected the bioactivity of the recombinant SCR82 protein in plants (Zhang et al. 2019). Furthermore, we found that the positions of tags exhibited an obvious difference on detection of the effector even if the same tag was used, for instance 6His-SCR96 versus SCR96-6His. The results of our study demonstrate that the tag characteristics, and its position (N- versus C-terminus) should be carefully considered when applied to the studies on SCR effectors and other small apoplastic effectors.
In the targeted proteomics approach, affinity-tagged SCR96 was designed to work as a bait to fish for its targets. By doing so, affinity purification of the tagged effector protein should be performed. It was previously shown that of eight elutable affinity tags, FLAG produced the highest purity protein for all crude extracts (Lichty et al. 2005). We presume that FLAG tag may also work for purification of the fused SCR96. His tag may be qualified for the protein purification as well, since it has been commonly used for affinity purification of target proteins including apoplastic effectors (van Esse et al. 2006; Song 2007; Huang et al. 2020). We found that the apoplastic SCR effectors can be expressed and purified from N. benthamiana apoplast using either FLAG or C-terminal His tags. The tags remained intact during expression in apoplast and survived the affinity purification procedures (Fig. 3). However, the N-terminally fused FLAG tag affected production of the recombinant protein, leading to low protein production on average (Figs. 2 and 3). This may indicate the cleavage of the N-terminal FLAG tag. In summary, the C-terminal 6His tag is the best choice for the expression, purification and bioactivity analysis of SCR96.
The apoplast expression system that we described in this study shows several advantages for the apoplastic effector studies. Firstly, relatively high yield of target proteins can be obtained from N. benthamiana apoplast. In a typical trial, we were able to get ~ 8 mL of crude apoplastic extract from twenty N. benthamiana plants with an average of four leaves per plant. From these leaves, we obtained ~ 800 μg purified proteins. This method can be easily scaled up to obtain milligrams of purified proteins. Secondly, the expressed proteins maintain their tag stability and biological properties for functional analysis. For example, the C-terminal 6His tag remained integrity during expression in apoplast and through the affinity purification procedures. We identified that re-infiltration of the purified proteins into plants did not alter bioactivity of the protein (Fig. 3), which makes the C-terminal 6His tag the ideal tag for SCR effector studies.
In practice, we found that there are some limitations of using N. benthamiana apoplast for heterologous expression of Phytophthora SCR effectors. Firstly, the tags may interfere with the functions of the effectors. We identified that the PCD phenotype caused by SCR96 varied among tag versions and tag replicates (Fig. 2). Similar results were observed in other cases (Kim et al. 2001; Zhang et al. 2019). A possible way to resolve this problem is to use other smaller affinity tags on either N- or C-terminus of the proteins, depending on the stability and functional integrity of the proteins. Secondly, this system is probably not suitable for expression of cytoplasmic proteins, because these proteins can be easily degraded in harsh acidic and protease-rich apoplast.
Comparing to harness the pathogen itself to study individual effectors, the advantage of the in planta expression method is that it does not interrupt other P. cactorum effectors. We utilized the Agrobacterium-mediated transient expression system in N. benthamiana, which is simple and enables expression of the recombinant affinity-tagged proteins in the infiltrated leaves easily. To seek for a better production of the recombinant proteins, the NtPR1a SP is often employed to drive protein expression in Nicotiana plants (Agarwal et al. 2008; Kanagarajan et al. 2012). In this study, the native SP sequence of P. cactorum SCR96 was replaced by the counterpart of NtPR1a. However, we did not observe the NtPR1a SP helped to increase the production of the recombinant proteins, rather such SP substitution severely interfered with the biological activity of the effector (Fig. 4). The result suggests that the NtPR1a SP may be not suitable to drive the expression of the Phytophthora SCR effectors in N. benthamiana plants.
In summary, our data demonstrate that the C-terminal 6His is the best one for studying Phytophthora SCR effectors. It provides acceptable performance of detection and purification with good yields at a mild cost of effector bioactivity. In addition, we conclude that the commonly used NtPR1a SP is not appropriate to drive the SCR effector expression in planta. In brief, the in planta apoplastic protein expression system described in this study provides us a simple tool for expression, detection and purification of Phytophthora apoplastic effectors to facilitate plant-pathogen interaction studies.
References
Agarwal S, Singh R, Sanyal I, Amla DV (2008) Expression of modified gene encoding functional human alpha-1-antitrypsin protein in transgenic tomato plants. Transgenic Res 17:881–896
Baxter L, Tripathy S, Ishaque N, Boot N et al (2010) Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330:1549–1551
Chen XR, Li YP, Li QY, Xing YP, Liu BB, Tong YH, Xu JY (2016) SCR96, a small cysteine-rich secretory protein of Phytophthora cactorum, can trigger cell death in the Solanaceae and is important for pathogenicity and oxidative stress tolerance. Mol Plant Pathol 17:577–587
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide. American Phytopathological Society, St. Paul
Goel A, Colcher D, Koo JS, Booth BJ, Pavlinkova G, Batra SK (2000) Relative position of the hexahistidine tag effects binding properties of a tumor-associated single-chain Fv construct. Biochim Biophys Acta 1523:13–20
Haas BJ, Kamoun S, Zody MC, Jiang RH et al (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461:393–398
Huang SX, Zhang ZH, Liu W, Tao H, Zhang Y, Shi NX, Zhu F, Ji ZL, Chen XR (2020) Expression of the small cysteine-rich protein SCR96 from Phytophthora cactorum in mammalian cells: phytotoxicity and exploitation of its polyclonal antibody. Biotechnol Lett 42:125–133
Kamoun S (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol 44:41–60
Kanagarajan S, Tolf C, Lundgren A, Waldenström J, Brodelius PE (2012) Transient expression of hemagglutinin antigen from low pathogenic avian influenza A (H7N7) in Nicotiana benthamiana. PLoS ONE 7:e33010
Kim KM, Yi EC, Baker D, Zhang KYJ (2001) Post-translational modification of the N-terminal His tag interferes with the crystallization of the wild-type and mutant SH3 domains from chicken src tyrosine kinase. Acta Crystallogr D Biol Crystallogr 57:759–762
Lichty JJ, Malecki JL, Agnew HD, Michelson-Horowitz DJ, Tan S (2005) Comparison of affinity tags for protein purification. Protein Expr Purif 41:98–105
O’Leary BM, Rico A, McCraw S, Fones HN, Preston GM (2014) The infiltration-centrifugation technique for extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an example. J vis Exp 94:e52113
Orsomando G, Brunetti L, Pucci K, Ruggeri B, Ruggieri S (2011) Comparative structural and functional characterization of putative protein effectors belonging to the PcF toxin family from Phytophthora spp. Protein Sci 20:2047–2059
Song J (2007) Functional characterization of extracellular protease inhibitors of Phytophthora spp. and their targets tomato proteases. Dissertation, Ohio State University
Stergiopoulos I, de Wit PJGM (2009) Fungal effector proteins. Annu Rev Phytopathol 47:233–263
Terpe K (2003) Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60:523–533
Tyler BM, Tripathy S, Zhang X, Dehal P et al (2006) Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313:1261–1266
van Esse HP, Thomma BP, van’t Klooster JW, de Wit PJ (2006) Affinity-tags are removed from Cladosporium fulvum effector proteins expressed in the tomato leaf apoplast. J Exp Bot 57:599–608
Zhang ZH, Huang SX, Tao H, Zhang Y, Zhao Y, Ji ZL, Chen XR (2019) Gene transcriptional pattern, prokaryotic expression and functional analysis of an apoplastic, hydrophobic and small effector SCR82 from Phytophthora capsici. Acta Microbiol Sinica 59:1586–1599
Zhang ZH, Jin JH, Sheng GL, Xing YP, Liu W, Zhou X, Liu YQ, Chen XR (2021) A Small cysteine-rich phytotoxic protein of Phytophthora capsici functions as both plant defense elicitor and virulence factor. Mol Plant Microbe Interact 34:891–903
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (Grant Nos. 32272477, 31871907, 31671971, 32102154), Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) (Grant No. CX(20)3125), Guangdong Provincial Key Laboratory of High Technology for Plant Protection (Grant No. Zhizhong2021-04), the Open Project of Joint International Research Laboratory of Agriculture and Agri-Product Safety of Ministry of Education of China (Grant No. JRK20180012), College students’ innovation and entrepreneurship training program of Yangzhou University, the Yangzhou University 2016 Project for Excellent Young Key Teachers, and High-Level Talent Support Program of Yangzhou University.
Supporting information
File 1 List of synthesized gene fragments used in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, JH., Zhou, X., Liu, W. et al. Influence of affinity tags and tobacco PR1a signal peptide on detection, purification and bioactivity analyses of the small oomycete apoplastic effectors. Biotechnol Lett 45, 115–124 (2023). https://doi.org/10.1007/s10529-022-03324-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03324-0