Abstract
Finger millet [Eleusine coracana (L.) Gaertn.] is an important cereal because of its mineral-nutrition value. With the increasing demand, there is a pressing need to conserve it through biotechnological approaches. High-frequency somatic embryogenesis from seed-derived callus of E. coracana was developed on Murashige–Skoog (MS) medium supplemented with a combination of auxins [Indole-3-acetic acid (IAA), 2,4-Dichlorophenoxy acetic acid (2,4-D)] and cytokinins [6-Benzylaminopurine (BAP), kinetin (KN)] in different concentrations, ranging from 0.1 to 5.0 mg L−1. Seeds cultured on this medium produced three different types of primary callus. Type I callus was very compact and dark brown, type II callus was light brownish and type III callus appeared whitish and light brown. All three types of calli had differential proliferation responses. Type II compact brown calli were obtained on the MS medium supplemented with 1.0 and 1.5 mg 2,4-Dichlorophenoxy acetic acid L−1 and 0.5 mg kinetin L−1. Friable yellowish embryogenic calli with a large number of somatic embryos were developed within 60 days after being transferred to auxins and cytokinin (1.0 and 1.5 mg 2,4-Dichlorophenoxy acetic acid L−1 and 0.5 mg Kinetin L−1) along with 200 mg casein hydrolysate L−1. Germination of somatic embryos on a half-strength MS medium supplemented with 0.1% Kinetin led to the development of healthy plantlets within 30 days. Genetic fingerprinting using random amplified polymorphic DNA (RAPD) revealed high levels of genetic fidelity. The study provides methods and hormonal concentrations required to develop somatic embryos in E. coracana for its genetic improvement and conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Finger millet [Eleusine coracana (L.) Gaertn.] is the world’s sixth most important crop among cereals and is considered one of the future smart crops to address zero hunger (Goldman et al. 2003; FAO 2018). According to Food and Agriculture Organization (FAO), the gap between production and nutrition should be compensated by the underutilized crops, such as finger millet. However, the cultivation of finger millet has been declining over years due to pests and diseases, high weed infestation (Oduori 2008), inadequate knowledge about finger millet seeding rate (Kidoido et al. 2002), awareness of nutritional values, limited usage, weeds, and moisture stress (Oduori 2005). Finger millet is placed in an important position in the evolution of C4 plants since the transition of the C3–C4 plant occurred in the sub-family Chloridoideae (Pilatti et al. 2018). The water use efficiency is high due to the proportionate allocation of its shoots in a moist environment and roots in a dry environment (Sage and Zhu 2011).
The finger millet grains have added value to human health, as it has antitumorigenic, antiulcer, anti-atherosclerogenic, anti-diabetic, anti-inflammatory, and antioxidant properties (Chandra et al. 2016). However, finger millet is still considered an under-utilized crop (Vijayalakshmi et al. 2012).
Genetic improvement of finger millet by conventional approaches is a tedious and time-consuming process (Miah et al. 2013). The biotechnological intervention for the conservation of finger millet genetic resources has been recommended (Joshi 2017). Rangan (1976) first reported the development of plant regeneration from mesocotyl explants of finger millet. Somatic embryogenesis from the callus of immature inflorescence and apical meristem (Vasil 1987) and whole seedling explant (Thiru and Mohan 1990) has been reported. Regeneration of plantlets through shoot bud induction and high-frequency shoot proliferation has also been reported (Wakizukia and Yamaguchi 1987). The influence of genotypes and plant growth regulators on the morphogenic potential of finger millet has been well studied (Ngetich et al. 2018).
Therefore, our objective was to optimize the conditions and concentrations of growth hormones for developing an efficient regeneration method. Different combinations of growth regulators involving IAA, 2,4-D, KN, and BAP were tested for efficient callus induction from seed-derived explants. The beneficial role of casein hydrolysate in combination with other growth hormones in improving the regeneration of plantlets through somatic embryogenesis is explored.
Materials and methods
Explant preparation and callus induction
Mature seeds of finger millet of GPU 26 (Germplasm Unit 26), a derivative of Indo-African germplasm released by Gandhi Krishi Vignan Kendra (GKVK), Bangalore, India were used. Seeds were dehusked and rinsed with water 4–5 times and surface sterilized with 0.1% mercuric chloride (w/v) for 5 min. Seeds were repeatedly washed with sterile distilled water under aseptic conditions. Excess water was removed by blotting the seeds on sterile filter paper and inoculated on semi-solid MS (Murashige and Skoog 1962) medium fortified with 28 different combinations of auxins Indole-3-acetic acid (IAA) and 2,4-Dichlorophenoxy acetic acid (2,4-D) and cytokinin 6-Benzylaminopurine (BAP) and Kinetin (KN) (Table 1). The pH of the medium was adjusted to 5.8 before adding 0.7% agar (Himedia, Mumbai, India). The medium was autoclaved for 20 min at 121 °C. The dried seeds were placed on medium for two weeks and kept in dark to develop a callus. Primary cultures established on the above media were subcultured onto their respective media after three weeks and maintained for another 45 days. All the cultures were maintained at 25 ± 2 °C and grown under a 16 h photoperiod provided by cool white fluorescent tubes (Philips, India) with a light intensity of 40 μmol m−2 s−1. Callus developed during primary culture on a medium containing a combination of 2,4-Dichlorophenoxy acetic acid (0.2–2.5 mg L−1) and 0.5 mg Kinetin L−1 was transferred to the same media with an addition of 200 mg casein hydrolysate L−1 and maintained for 45 days under dark. These cultures were observed for the development of embryogenic calli and somatic embryogenesis.
Germination of somatic embryos
Mature somatic embryos were obtained on two combinations of medium containing 1.0 mg 2,4-Dichlorophenoxy acetic acid L−1 and 0.5 mg Kinetin L−1 and 1.5 mg 2,4-Dichlorophenoxy acetic acid L−1 and 0.5 mg Kinetin L−1 with 200 mg Casein Hydrolysate (CH) L−1 in both the combinations were transferred to Murashige and Skoog basal medium and cultured for 3–4 weeks. About 80% of the somatic embryos were developed into complete plantlets with healthy shoots and roots.
Assessment of genetic fidelity
Extraction of genomic DNA
Genomic DNA was isolated from finger millet seeds using the CTAB method by following the protocol of Nandhini et al. (2013) with few modifications. Finger millet seeds (100 mg) were ground into a fine powder using liquid N2 in a sterile/chilled mortar and pestle. The homogenized samples were added to one ml of extraction buffer (100 mM Tris, pH 8.0, 1.4 M NaCl, 20 mM EDTA, pH 8.0, 2% CTAB, 0.3% β Mercaptoethanol, 5% SDS, 1% PVP and 50 µg Proteinase K of Sigma Aldrich, Mumbai, India) in a centrifuge tube and incubated for 60 min at 60 °C in a heating block (Amkette Industries, Mumbai, India). The samples were kept for 5 min at room temperature to cool down and equal volumes of (24:1) chloroform and isoamyl alcohol were added and gently mixed. Centrifuged the samples at 16,099×g for 10 min (Kubota, Japan). After centrifugation at 16,099×g for 10 min, the supernatant was transferred into a fresh tube and treated once again with chloroform and isoamyl alcohol. DNA precipitation was performed with 1/4 of NaOAc 3 M and 2/3 of chilled isopropanol and then samples were stored at − 80 °C (Cryo Scientific Systems Private Limited, Chennai, India) for 60 min. Following centrifugation, the DNA pellet was washed in chilled ethanol 70% and resuspended in 50 μl of 0.1X TE buffer, pH 8.0 (10 mM Tris, 1 mM EDTA), and stored at − 20 °C. The quality of DNA was checked by running in 0.8% agarose gel (Sigma Aldrich, Mumbai, India).
Screening of RAPD primers
To screen for suitable primers, the genomic DNA of all the traditional as well as hybrids were bulked and used as template DNA. The mixture of template DNA was amplified with 16 randomly selected RAPD primers (Table 3). The PCR condition followed for the amplification was denaturation for 5 min at 95 °C, followed by 45 cycles of 1 min at 94 °C, 1 min at 37 °C, 2 min at 72 °C, and final stage of 4 min at 72 °C in a Thermal Cycler (Prima-96, Hi-media Laboratories Pvt. Ltd, India). PCR products were resolved in 1.5% agarose gel in 1 × TAE buffer with EtBr (5 µg/ml) and were visualized under Gel doc (Bio Rad Laboratories Inc., 1000, USA). The number of bands with a varying range of molecular weights was counted and data were generated to select the most suitable primer for assessing the genetic fidelity of micro propagated plants of finger millet (Figs. 1, 2).
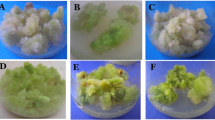
Effect of auxin and cytokinin on callus induction and somatic embryogenesis of Eleusine coracana- a Initiation of primary callus from the seeds of Eleusine coracana; b Simultaneous induction and proliferation of callus from the seeds; c Non-embryogenic calli showing active proliferation of brown callus-Type I; d Non-embryogenic calli with pale and brown colored base-Type III; eYellowish proliferating callus transforming into embryogenic calli-Type II; f Embryogenic calli produces minute globular somatic embryos; g–j Maturation of somatic embryos into plantlets; k Healthy plantlets regenerated from somatic embryos of Eleusine coracana. Scale bar = a 4 mm; b 10 mm; c 12 mm; d 8 mm; e 15 mm; f 18 mm; g 25 mm; h 13 mm; i 26 mm; j 28 mm; k 110 mm
Assessment of genetic fidelity of micropropagated plants
Out of 25 primers, one primer (OPL 12) which showed the maximum number of bands from the previous experiment was selected for determining the genetic fidelity of micropropagated plants of finger millet. Genomic DNA was isolated from the randomly selected micropropagated plants and amplified with OPL12 primer by adopting the PCR protocol as described in the previous section. PCR products from all the micropropagated plants were resolved in a 0.8% agarose gel and observed for genetic uniformity.
Results and discussion
Variations in callus induction and somatic embryogenesis
Seeds of finger millet cultured on MS basal medium without any hormones developed into plantlets in two weeks. However, seeds cultured on varying combinations of auxin (IAA and 2,4-D) and cytokinin (KN and BAP) produced soft whitish transparent callus mostly from the part of root stem transition during the initial two weeks (Fig. 1a, b). Of the 29 combinations, explants that responded for embryogenesis on the two combinations of media (1.0 and 1.5 mg 2,4-D L−1 and 0.5 mg Kinetin L−1) had produced more amount of callus than others. The primary callus had developed into three different types of secondary callus (Type I, II, and III). Type I calli are compact, dark brown (Fig. 1c), and Type II calli are light brownish with compact callus (Fig. 1d, f), and Type III calli are a combination of whitish and lighter brown (Fig. 1e). Type I and Type III callus were non-embryogenic, while type II callus was embryogenic. At the end of 60 days, numerous, translucent globular-like growth was observed from type II callus (Fig. 1e) and turned into greenish-yellow upon their maturity (Fig. 1h; Tables 2, 3). Characteristics of color change in all three types of calli could be possibly due to differences in hormone concentration and their combinations.
Soft friable callus mostly embryogenic in nature was observed in 65% of the callus in a combination of 1.0 mg 2,4-Dichlorophenoxy acetic acid L−1 and 2.5 mg kinetin L−1 and 70% of the callus in a combination of 1.5 mg 2,4-Dichlorophenoxy acetic acid L−1 and 0.5 mg kinetin L−1. It was observed that both 2,4-Dichlorophenoxy acetic acid and kinetin induced embryogenesis irrespective of their concentrations.
Effect of casein hydrolysate on somatic embryogenesis
Embryogenic callus and subsequent development of somatic embryos were observed only in presence of Casein Hydrolysate (CH) along with 2,4-D and KN while other combinations in absence of Casein Hydrolysate (CH) did not induce embryogenesis irrespective of the types of callus. The presence of 0. 5 mg Kinetin L−1 and 200 mg Casein hydrolysate L−1 along with lower concentration of 1.0 mg 2,4-Dichlorophenoxy acetic acid L−1 and higher concentrations of 1.5 mg 2,4-Dichlorophenoxy acetic acid L−1 of 2,4-D induced 65% and 70% embryogenesis respectively, while other combinations containing Glutamine (Glu) did not induce any embryogenesis, indicating the clear role of Casein hydrolysate (CH) on somatic embryogenesis as evidenced in a few species such as Abelmoschus esculentus L. monech (Daniel et al. 2018), Setaria italica L. (Sood and Prasad 2020), Eleusine coracana [L.] Gaertn. (Sathish et al. 2015). About 90% of the somatic embryo were germinated into plantlets with tiny leaves and numerous adventitious roots on the medium containing full strength MS basal medium within 60 days of culture (Figs. 1f–k, 3).
Micropropagated plantlets developed through somatic embryogenesis show improved genetic fidelity
Of the 16 RAPD primers, only seven primers (OPAL-12, OPN-02, OPL-12, OPU-06, OPI-07, OPAJ-14, OPO-14) had generated 5–8 scorable bands while the use of other primers resulted in a lesser and inconsistent banding pattern. The size range of RAPD bands in all the tested primers was from 300 to 2500 bp. However, only one primer (OPL-12) was randomly selected for the determination of genetic fidelity of micropropagated plantlets of finger millet due to reproducibility with seven consistent bands of which four bands are prominent and three bands are faint but scorable. Genomic DNA from randomly selected plantlets showed a consistent and uniform banding pattern with 500–1500 bp when amplified with OPL 12, indicating the high degree of genetic uniformity of micropropagated plantlets of finger millet regenerated through somatic embryogenesis.
Regeneration of plantlets through somatic embryogenesis is a very critical and important step for crop improvement through genetic transformation (Dosad and Chawla 2015). However, the consistency of somatic embryogenesis is one of the major problems in many crop species (Garcia et al. 2019). A few numbers of research on invitro regeneration of finger millet were reported using casein hydrolysate and glutamine (Dosad and Chawla 2015). However, these reports did not reveal the consistency and reproducibility of somatic embryogenesis. The present work reveals the optimal and reproducible protocol for the regeneration of plantlets. The significance of our finding includes the critical role of Casein hydrolysate (CH) on callus induction. Although, the beneficial role of CH on callus induction and somatic embryogenesis was reported in a few crops such as rice (Khaleda and Al-Forkan 2006), date palm (Ageel and Elmeer 2011), and sorghum (Indra Arulselvi and Krishnaveni 2009). Our study confirms its beneficial role and was found to induce morphogenetic potential of callus very consistently in combination with casein hydrolysate. Therefore, our protocol is expected to be very useful in genetic improvement through embryogenesis by employing Agrobacterium or biolistic-mediated genetic transformation. Since finger millet is a self-pollinated crop with a narrow genetic base (Sood et al. 2016), the present methods optimized for somatic embryogenesis are expected to be useful for developing somaclonal variation to broaden the genetic base for utilization in the breeding of finger millet.
Genetic uniformity is more emphasized in micropropagated plants as this process often induces genetic variation due to different culture conditions (Rani and Raina 2000). As RAPD markers are co-dominant, this has been applied to ensure the genetic uniformity of several cereal crops such as Setaria italica (Shingane et al. 2018), Panicum miliaceum (M'ribu and Hilu 1994), and Pennisetum glaucum (Mohammed and Hamza 2018). RAPD method has been used widely even to detect somaclonal variation (Mukhopadhyay et al. 2016). In our study, all the randomly selected micropropagated plants of finger millet showed genetic uniformity as revealed by RAPD fingerprints and this result also indicated that the various culture conditions employed for induction of somatic embryogenesis were safer for large-scale multiplication of finger millet germplasm without any genetic variation.
Data availability
All the data generated and analyzed during this study are included in this article.
Abbreviations
- RAPD:
-
Random amplified polymorphic DNA
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- NAA:
-
1-Naphthalene acetic acid
- IAA:
-
Indole-3-acetic acid
- BAP:
-
6-Benzylaminopurine
- KN:
-
Kinetin
- CH:
-
Casein hydrolysate
- Glu:
-
Glutamine
- TDZ:
-
Thidiazuron
- EtBr:
-
Ethidium bromide
- NaoAc:
-
Sodium acetate
- EDTA:
-
Ethylenediaminetetraacetic acid
- CTAB:
-
Cetyl trimethyl ammonium bromide
- TAE:
-
Tris–acetate-EDTA
- FAO:
-
Food and Agriculture Organization
- SDS:
-
Sodium dodecyl sulfate
- MS media:
-
Murashige and Skoog media
References
Ageel S, Elmeer K (2011) Effects of casein hydrolysates and glutamine on callus and somatic embryogenesis of date palm (Phoenix dactylifera L.). N Y Sci J 4(7):121–125
Arulselvi I, Krishnaveni S (2009) Effect of hormones, explants, and genotypes in vitro culturing of sorghum. J Biochem Technol 1(4):96–103
Chandra D, Chandra S, Sharma AK (2016) Review of Finger millet (Eleusine coracana (L) Gaertn.): a powerhouse of health benefiting nutrients. Food Sci Hum Wellness 5(3):149–155. https://doi.org/10.1016/j.fshw.2016.05.004
Daniel DRHA, Caesar SA, Ramakrishnan M, Duraipandiyan V, Ignacimuthu S, Al-Dhabi NA (2018) Effect of l-glutamine and casein hydrolysate in the development of somatic embryos from cotyledonary leaf explants in okra (Abelmoschus esculentus L. monech)”. S Afr J Bot 114:223–231. https://doi.org/10.1016/j.sajb.2017.11.014
Dosad S, Chawla HS (2015) In vitro plant regeneration from mature seeds of finger millet (Eleusine coracana) through somatic embryogenesis. Indian J Plant Physiol 20(4):360–367. https://doi.org/10.1007/s40502-015-0191-2
FAO (2018) Future smart food Rediscovering hidden treasures of neglected and underutilized species for Zero Hunger in Asia Executive summary Bangkok, p 36. http://www.fao.org/3/I8907EN/i8907en.pdf
Garcia C, De Almeida AAF, Costa M, Britto D, Valle R, Royaert S, Marelli JP (2019) Abnormalities in somatic embryogenesis caused by 2, 4-D: an overview. Plant Cell Tissue Organ Cult (PCTOC) 137(2):193–212. https://doi.org/10.1007/s11240-019-01569-8
Goldman JJ, Hanna WW, Fleming G, Ozais-akins P (2003) Fertile transgenic pearl millet (Pennisetum glaucum (L.) R.Br.) plants recovered through microprojectile bombardment and phosphinothricin selection of apical meristem-, inflorescence-, and immature embryo-derived embryogenic tissues. Plant Cell Rep 21:999–1009. https://doi.org/10.1007/s00299-003-0615-8
Joshi BK (2017) Biotechnology for conservation and utilization of agricultural plant genetic resources in Nepal. J Nepal Agric Res Counc 3:49–59. https://doi.org/10.3126/jnarc.v3i1.17276
Khaleda L, Al-Forkan M (2006) Stimulatory effects of casein hydrolysate and proline in vitro callus induction and plant regeneration from five deepwater rice (Oryza sativa L.). Biotechnology 5(3):379–384. https://doi.org/10.3923/biotech.2006.379.384
Kidoido MM, Kasenge V, Mbowa S, Tenywa JS, Nyende P (2002) Socioeconomic factors associated with finger millet production in eastern Uganda. Afr Crop Sci J 10(1):111
Miah G, Rafii MY, Ismail MR, Puteh AB, Rahim HA, Asfaliza R, Latif MA (2013) Blast resistance in rice: a review of conventional breeding to molecular approaches. Mol Biol Rep 40(3):2369–2388. https://doi.org/10.1007/s11033-012-2318-0
Mohammed HI, Hamza NB (2018) Genetic diversity analysis of forty pearl millet (Pennisetum glaucum (L.)R.Br) accessions from Sudan using agronomical descriptors and DNA molecular markers. Adv Biosci Biotechnol 9(07):322
M’ribu HK, Hilu KW (1994) Detection of interspecific and intraspecific variation in Panicum millets through random amplified polymorphic DNA. Theor Appl Genet 88(3–4):412–416. https://doi.org/10.1007/BF00223653
Mukhopadhyay M, Bantawa P, Mondal TK, Nandi SK (2016) Biological and phylogenetic advancements of Gaultheria fragrantissima: economically important oil-bearing medicinal plant. Ind Crops Prod 81:91–99. https://doi.org/10.1016/j.indcrop.2015.11.042
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nandhini RB, Rahul RN, Thilaga S, Rao NSP, Ganesh D (2013) Molecular distinction of C × R hybrid (Coffea congensis× Coffea canephora) from morphologically resembling male parent using rbcL and matK gene sequences. S Afr J Bot 88:334–340. https://doi.org/10.1016/j.sajb.2013.08.011
Ngetich A, Mweu C, Ngugi M, Murakami A, Ojuolong H, Mbinda W (2018) Efficient plant regeneration protocol for finger millet [Eleusine coracana (L.) Gaertn] via somatic embryogenesis. Afr J Biotechnol 17(21):660–667. https://doi.org/10.5897/AJB2018.16452
Oduori COA (2005) The importance and research status of finger millet in Africa. In: Paper presented at the Mcknight Foundation Collaborative Research Program Workshop on tef and finger millet: comparative genomics of the chloridoid cereals at the Biosciences for east and Central Africa (BECA) ILRI, Nairobi, Kenya, pp 28–30
Oduori COA (2008) Breeding investigations of finger millet characteristics including blast disease and striga resistance in Western Kenya. Dissertation
Pilatti V, Muchut SE, Uberti-Manassero NG, Vegetti AC, Reinheimer R (2018) Diversity, systematics, and evolution of Cynodonteae inflorescences (Chloridoideae–Poaceae). Syst Biodivers 16(3):245–259. https://doi.org/10.1080/14772000.2017.1392371
Rangan TS (1976) Growth and plant regeneration in plant tissue culture of some Indian millets; Paspalum scorbiculatum (L); Eleusine coracana (L.) Gaertn. and Pennisetum typhoideum. Perspflawzen Physiol 78:208–216. https://doi.org/10.1016/S0044-328X(73)80003-0
Rani V, Raina SN (2000) Genetic fidelity of organized meristem-derived micro propagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant 36(5):3199–3330. https://doi.org/10.1007/s11627-000-0059-6
Sage RF, Zhu XG (2011) Exploiting the engine of C4 photosynthesis. J Exp Bot 62(9):2989–3000. https://doi.org/10.1093/jxb/err179
Satish L, Ceasar SA, Shilpha J, Rency AS, Rathinapriya P, Ramesh M (2015) Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev Biol Plant 51:192–200. https://doi.org/10.1007/s11627-015-9672-2
Shingane S, Patil JV, Gomashe S, Chand D (2018) Assessing genetic diversity among Foxtail millet (Setaria italica (L.) P. Beauv.) accessions using RAPD and ISSR markers. Int J Bio-Res Stress Manag 9(1):1–6. https://doi.org/10.23910/IJBSM/2018.9.1.3C0574
Sood RKS, Prasad M (2020) An efficient Agrobacterium-mediated genetic transformation method for foxtail millet (Setaria italica L.). Plant Cell Rep 39:511–525. https://doi.org/10.1007/s00299-019-02507-w
Sood AK, Babu BK, Gaur VS, Pandey D, Kant L, Pattnayak A (2016) Gene discovery and advances in finger millet [Eleusine coracana (L.) Gaertn.] genomics: an important nutri-cereal of future. Front Plant Sci 7:1634. https://doi.org/10.3389/fpls.2016.01634
Thiru AN, Mohan R (1990) Tissue culture studies in ragi (Eleusine coracana(L.)Gaertn. Plant Cell Rep 18:93–96
Vasil IK (1987) Developing cell and tissue culture systems for the improvement of cereal and grass crops. J Plant Physiol 128:193–218. https://doi.org/10.1016/S0176-1617(87)80234-1
Vijayalakshmi D, Gowda KN, Jamuna KV, Ray BRM, Sajjan JT (2012) Empowerment of self-help group women through value addition of finger millet. J Dairy Foods Home Sci 31:223–226
Wakizukia T, Yamaguchi T (1987) The induction of enlarged apical domes invitro and multiple shoot formation from finger millet Eleusine coracana (L.). Ann Bot 60:331–336. https://doi.org/10.1093/oxfordjournals.aob.a087452
Acknowledgements
The authors thank Gandhi Krishi Vignan Kendra, Bangalore for providing finger millet seed samples of various genotypes. We also acknowledge Jawaharlal Nehru Memorial Fund, New Delhi and Department of Science & Technology, Promotion of University Research and Scientific Excellence (DST-PURSE), New Delhi, and Rashtriya Uchchattar Shiksha Abhiyan (RUSA) Ministry of Human Resource Development, Government of India for their financial support. We thank Dr. Ramu S Vemanna, Regional Centre for Biotechnology for the revision of the research paper.
Funding
This work was supported by Jawaharlal Nehru Memorial Fund, New Delhi (SU-1/124/2018-19/92) and Department of Science & Technology, Promotion of University Research and Scientific Excellence (DST-PURSE), New Delhi for their financial support.
Author information
Authors and Affiliations
Contributions
JV set up the experiment, carried out the entire experimental work, and analyzed the data. VR assisted the experiment. TS helped in preparing manuscript draft and interpretation of data. CS and GD (Research Guide) guided in setting up the experiment, data analysis, and manuscript preparation. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Venkatesan, J., Ramu, V., Sethuraman, T. et al. Assessing the genetic fidelity of somatic embryo-derived plantlets of finger millet by random amplified polymorphic DNA analysis. Biotechnol Lett 44, 1379–1387 (2022). https://doi.org/10.1007/s10529-022-03305-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03305-3