Abstract
The digestion efficiency of liquid industrial wastes increases when using bioreactors colonized by microbial biofilms. High concentrations of proteins derived from the fish processing industry lead to the production of ammonia, which inhibits methane production. Two bioreactors were constructed to compare methanogenic activity: one enriched with mMPA (methylaminotrofic methane production archaea) consortia (control bioreactor), and the second with NH3 tolerant consortia (treatment bioreactor). Ammonia tolerant activity was assessed by applying an ammonia shock (755 mg NH3/L). Methane production, consumption of total organic carbon (TOC) and the taxonomic composition of bacteria and archaea was evaluated using 16S rDNA in the acclimatization, ammonia shock, and recovery phases.The ammonia shock significantly affected both methane production and the consumption of TOC in the control reactor (p < 0.05) and taxonomical composition of the microbial consortia (OTU). These values remained constant in the treatment reactor. The analysis of biofilm composition showed a predominance of Methanosarcinaceae (Methanomethylovorans sp., and probably two different species of Methanosarcina sp.) in bioreactors. These results demonstrate that using acclimated biofilms enriched with ammonia tolerant methanogens control the inhibitory effect of ammonia on methanogenesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A common waste management practice that controls odour and organic matter pollution is anaerobic digestion (Vidal et al. 2000). It is defined as the biological conversion of organic material into a variety of by-products, during which microorganisms perform cooperative and syntrophic processes to convert complex substrate mixtures into biogas that contains mainly methane (65–70%) and carbon dioxide (Kato and Watanabe 2010). This process allows energy to be recovered in the form of methane, which can replace fossil fuels for in-situ heating and electricity, reducing negative environmental effects, including global warming and acid rain (Gupta et al. 2012). Another advantage is that it is a relatively inexpensive process, making it a cost-effective way to produce energy (Angelidaki et al. 2011). Anaerobic biofilm reactors, including the anaerobic fluidized bed reactors (AFBR), anaerobic composite biofilm reactors (ACBRs) and anaerobic moving bed biofilm reactors (AMBBRs) applied in this study are among the oldest and most efficient methods for wastewater treatment (Zhong et al. 2021). The high levels of active biomass retention within these systems allow the application of high organic loading rates with low hydraulic retention times, qualities that lead to a tremendous practical applicability of these biofilm-based systems for wastewater treatment (McHugh et al. 2003, 2005).
Treatment efficiency depends on the taxonomical composition of the microbial consortia. Methanogenesis is the limiting step in anaerobic processes, affected by physicochemical parameters such as high levels of H2S and NH3 in wastewater derived from the fishing industry (Sossa et al. 2004). The treatment of liquid industrial wastes (LIW) from the fishing industry faces periods of high organic and nutrient loads, as it is a seasonal activity. During this time, high concentrations of ammonia and sulphate cause the inhibition of methane production and poor bioreactor functionality. These bioreactors are containers with specific parameters for growing microbial communities that decompose organic material input. SRM (sulphate reduction microorganisms) exhibit a higher affinity for common substrates when compared to MPA (methane production archaea), therefore, reactions usually favour sulphate reduction (Mohan et al. 2005).
Moreover, protein hydrolysis produces α-acetoacids which are reabsorbed as nourishment and cause ammonia discharge, which increases alkalinity. It has been reported that free ammonia (NH3) has higher inhibitory effects on methanogenesis than NH4+ (Sossa et al. 2004). NH3 concentration depends largely on the pH of the medium and, to a lesser degree, temperature. For example, the NH3 concentration at pH 7 is close to 1% but increases to 10.2% at pH 8 (Kayhanian 1999). High ammonia concentration and susceptibility of methanogenic archaea to ammonia have been demonstrated in several studies (Procházka et al. 2012). Decreases in methanogenic activity have been reported due to restricted availability of acetate as a carbon source for MPA (Sung and Liu 2003). However, microorganisms acclimated to high ammonia concentrations can sustain their growth and digestive activity in protein-rich effluents (Calli et al. 2005). Omil et al. (1995) recommend that substrates with high initial total ammonia nitrogen (TAN) and protein content (e.g., seafood processing industry) should be treated anaerobically using selected sludge containing up to 3000 mg/L of NH3 (corresponding to 150 mg of free ammonia nitrogen (FAN)/L at pH 7.6). Strategies have been described in order to solve the problems associated with high H2S production by enriching the microbial community with mMPA (methylaminotrofic methane production archaea). Methylated amines are abundant in fishery waste and cannot be used by SRM as a substrate, but it has been reported that NH3 effects can be minimized by using mMPA tolerant to high ammonia concentrations (Sossa et al. 2004).
Given this background, the main objective of this study was to evaluate the use of biofilms enriched with a consortium of ammonia tolerant mMPA in reactors used for treatment of industrial fishery waste, in order to improve ammonia shock tolerance during the purification process of liquid discharge effluents rich in proteins and sulphates, and to characterize the main taxonomic groups composing the biofilm before, during and after ammonia shock in a bioreactor, this improvement of the biofilm within the concept of bioaugmentation of microorganisms.
Materials and methods
Isolation and growth kinetics of ammonia tolerant mMPA consortia biofilm
Ammonia tolerant mMPA consortia (hereinafter (NH3)-mMPA: methylaminotrofic methane production archaea) were isolated from a fishing industry methanogenic reactor exposed to increasing ammonia concentrations, following the physicochemical conditions and protocols described by Sossa et al. (2004). The inoculum was obtained directly from industrial fishing effluent (Camanchaca fishery factory). A final concentration of 1 × 106 cells/mL was inoculated into each vial to isolate consortia. Triplicate samples were taken every two days from the vials to determine methane concentrations in the biogas by using gas chromatography (Pham et al. 2013). Total mMPA concentration was assessed using the Most Probable Number technique, as recommended by the American Public Health Association (APHA 1992).
Scanning electron microscopy
To evaluate the attachment of (NH3)-mMPA consortia on ceramic, the support was washed in a 35 kHz ultrasound bath and observed with a scanning electron microscope (SEM) (ETEC Autoscan U-1), as described by Anderson et al. (1951).
Design of an anaerobic biofilm enriched with (NH 3 )-mMPA under NH 3 -N shock
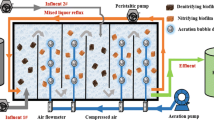
Two anaerobic upflow fixed bed reactors were used: control (CR) and treatment (TR). CR was enriched with mMPA, while TR was enriched with (NH3)-mMPA. Fixed bed reactors with retained biomass systems improve when the biofilms formed are fixed to ceramic rings, avoiding large dangerous pH fluctuations and liberating micronutrients to microbial communities (Urrutia et al. 1999). Total and bed volume for both CR and TR were 2 L. The bioreactors were semi-continuous fed with fishing industry model effluent medium (2.45 g/L Total Organic Carbon, 48.8 mg NH3-N/L, 82.3 mg/L sulphate, 181 mg/L sulphide and a salinity of 13.7 g/L, at pH 7.5, Sossa et al. 2004) at organic loading rate (OLR) of 0.98 g DOC added/L-reactor/d and hydraulic retention time (HRT) of 5 d. Methylamine was used as the main carbon source. Ammonia concentration was 320–430 mg/L, in all cases, ammonium chloride was added to obtain the unionized ammonia concentration (mg NH3-N/L). TR was inoculated with (NH3)-mMPA at a final concentration of 106 cells/mL.
In the enrichment phase (phase A), bacterial activity was inhibited with piperacillin (0.128 g/L), SRM activity with 2-mercaptoethane sodium sulfonate (mesna, 0.16 mg/L) and yeast activity with cycloheximide (0.4 g/L) in both reactors. The bioreactors were kept under these conditions for 3 months, allowing biofilm settlement (phase A) and acclimatization (phase B) before carrying out further analysis. Both bioreactors were supplemented with the fishing industry model effluent without inhibitors and maintained at a concentration of 37.7–128 mg NH3-N/L in the medium at pH 7.5 for a hydraulic retention time of 5 days and incubated at 30° C for 3 months to allow anaerobic biofilm formation. The bioreactor temperature was maintained at 30° C because the inoculum originated in a natural marine environment dominated by the cold Humboldt marine current After acclimatization (phase B), both bioreactors were exposed to an ammonia shock treatment (755 mg NH3-N/L) (phase C). The methanogenic activity at different ammonia concentrations was monitored.
Chemical analysis of bioreactor performed to evaluate performance
Total organic carbon (TOC), alkalinity, sulphate concentration, and volatile suspended solids were analysed according to standard methods (APHA 1992). Methane determination by gas chromatography consisted of the injection of 1 mL of biogas in a 1000 AGC Hach Carle Series chromatograph. Ammonia concentration was estimated (expressed as mg NH3-N/L) according to the corrected formula (Clegg and Whitfield 1995).
Total microbial count
Biofilm analysis was carried out according to the protocol described by Hobbie et al. (1977). Samples were taken every 48 h from both CR and TR. Total bacterial count was performed using the direct epifluorescence technique with a LEICA Confocal Laser Microscope, mod. TCS NT.
Amplification, cloning and sequencing of (NH3)-mMPA consortia 16S rDNA
Genomic DNA of (NH3)-mMPA consortia was extracted using a mini Beadbeater (Biospec Products, Bartlesville, Okla) and the FastDNA® SPIN Kit for Soil (MP Biomedicals LLC). Genomic DNA cloning was carried out by amplifying (NH3)-mMPA 16S rDNA, using 21F (Abdallah et al. 2016) and 9R primers (Jurgens et al. 1997). The PCR amplification mix used was: DNA template, 1X PCR buffer (with 1.5 mM MgCl2, Roche), 2 mM dNTPs, 2 pM of each primer and 0.04 U/µL pfu polymerase (Roche). The PCR program amplification cycling protocol for amplification was DNA melting at 95 °C for 2 min, followed by 30 cycles of 30 s at 95° C, annealing at 40° C for 30 s, extension at 73° C for 3 min, and a final extension at 73° C for 5 min. PCR products were loaded in an agarose gel (1.5%), isolated and purified using the MiniElute gel purification kit (Qiagen), concentrated and quantified. Amplified fragments of purified rDNA were cloned using the Zero Blunt Cloning kit (Invitrogen). Amplified fragments obtained in the PCR reaction with M13F/M13R primers were cleaned using PCR clean-up kit plates and sequenced using ABI PRISM Big Dye Terminator v3.1 Cycle Sequencing kit, using an AB3100 Genetic Analyser from Applied Biosystems (Hitachi).
Taxonomic diversity determination by 16S rRNA analysis
To extract total rRNA, the 38 mL of bacteria resuspended from the biofilm were centrifuged at 9300×g for 5 min. The cells were resuspended in a protoplastic buffer (0.45 M sucrose) with a final concentration of 0.1% (v/v) of tween 20, and cell aggregates were disaggregated in a sublethal ultrasound bath (Brasonic Ultrasonic Cleaner-80 W) for 15 min at 4° C. The rRNA was then centrifuged and extracted by the simple extraction method, based on bacterial lysis in the presence of diethylpyrocarbonate (DEPC), described by Reddy and Gilman (1993). The integrity of the total rRNA extracted was visualized by gel electrophoresis in 1% agarose-formaldehyde (70 V for 1.5 h) and stained with Ethidium Bromide, as described by Rosen and Villa-Komaroff (1990). The final concentration of extracted rRNA was determined by spectrophotometric analysis at 260 nm (Ausbel et al. 1998).
Approximately five µg of total rRNA and known concentrations of Escherichia coli 16S rRNA (from 20 µg to 0.002 µg, used as standard) were resuspended in 50 µL of DEPC-treated deionized water and serial dilutions (up to 1:100) of each sample, all were denaturated with 150 µL of denaturant solution (Formamide: Formaldehyde: MOPS [5:1.62:1]). The denatured samples were transferred to the membrane (Hybond-N+ ; Amersham) with a dot-blotting apparatus (Bio-Rad) and, subsequently, immobilized by baking at 80° C for 2.5 h according to the protocol described by Raskin et al. (1994a and b).
Hybridization was performed using specific probes for 16S rRNA, oligonucleotides labeled with dioxygen at the 5′ end and according to the protocol recommended by Raskin et al. (1994a, b). Hybridization was carried out at 46° C for 18 h, and the Tm of each probe was adjusted using formamide. The probes used were EUB338 (Amann et al. 1990) for Bacteria, ARCH915 (Stahl and Amann 1991) for Archaea, SRB385 (Amann et al. 1992) for sulfate-reducing bacteria, and the probes described by Raskin et al. (1994a, b) for methanogens, MS821 for Methanosarcina, MX825 for Methanosaeta, MB310 for Methanobacteriaceae, MSMX860 for Methanoplanaceae, MC1109 for Methanococcaceae and MS1414 for Methanosarcina, Methanococcoides, Methanolobus, Methanohalophilus.
All the probes were detected by a chemiluminescent reaction between alkaline phosphatase and its substrate CSPD (3- (4-methoxispiro {1,2-dioxetane-3,2- (5-chloro) tricyclo [3.3.1.13,7] decane} -4- (Disodium phenyl phosphate), as recommended by Roche Diagnostics Corp. The film that detected the hybridization signal (SH) was digitized in grayscale and processed for image analysis through the Quantity One software package (Bio-Rad, version 4.2). This program automatically transforms the gray average measurement of each SH into RNA concentration, using a calibration curve constructed with serial dilutions of known concentrations of Escherichia coli 16S rRNA (MRE600), which were hybridized with the EUB338 probe.
PCR-DGGE community comparison (gel electrophoresis with denaturing gradient)
Biofilms obtained from the bioreactor were suspended and concentrated by centrifugation at 9.300×g × 5 min for DNA isolation using the FastDNA® SPIN Kit for Soil (MP Biomedicals LLC). 16S rRNA gene was amplified using the primers described in the following section. A nested PCR was performed to obtain 16S rRNA gene fragments (Vissers et al. 2009). The primers used for archaea were 21F/915R (PCR 1) and 344F-GC/915R (Abdallah et al. 2016) (PCR 2). A PCR program for archaeal DNA amplification was DNA melting at 95° C for 2 min, followed by 10 cycles of 30 s at 95° C, annealing at 71° C for 45 s (decreasing 1° C/cycle), extension at 72° C for 1 min, followed by 20 cycles of 30 s at 95° C, annealing at 61° C for 45 s, extension at 72° C for 1 min, and finally the extension at 72° C for 10 min. The primers used for bacteria were 341F/907R (Beck et al. 2013) (PCR 1) and 341F-GC/534R (PCR 2) (Dar et al. 2005). The PCR reaction mix contained DNA template 1× PCR buffer (with 1.5 mM MgCl2, Promega), 0.2 mM dNTPs, 0.5 µM of each primer and 0.025 U/µL Gotaq polymerase. A PCR program for bacterial DNA amplification was DNA melting at 95° C for 2 min, followed by 20 cycles of 30 s at 95° C, annealing at 65° C for 45 s (decreasing 0.5° C/cycle), extension at 72° C for 1 min, followed by 10 cycles of 30 s at 95° C, annealing at 55° C for 45 s, extension at 72° C for 1 min, and a final extension at 72° C for 2 min. PCR amplicon were kept at 4° C. The DGGE primers used for Archaea are shown in Table 1.
DGGE primers used for most bacteria and archaea
1492R (5′-GGY TAC CTT GTT ACG ACT T-3′) (Leadbetter and Breznak 1996).
DGGE was performed using a Dcode Universal Mutation Detection System (Bio Rad Laboratories, Hercules, CA, USA), following system recommendations (Muyzer et al. 1993). Electrophoresis was performed at a constant voltage for 4 h (archaea) and 8 h (bacteria). Gels were stained with silver nitrate (Sanguinetti et al. 1994), while ethidium bromide (0.5 µg/mL) was used to obtain bands for subsequent sequencing. Excised gel bands were re-amplified (Valdebenito-Rolack et al. 2011), quantified and sequenced (Macrogen, Korea). Images (densitograms) were analysed by Quantity One software, BioRad version 4.2.3 using WPGAMA for cluster analysis (Chen et al. 2017; Putra et al. 2020).
Estimation of (NH3)-mMPA consortia growth kinetics in biofilm
The growth kinetics of (NH3)-mMPA in biofilms were estimated using the Gompertz model as described by Zwietering et al. (1994). Data were adjusted to the model by nonlinear regression and the most useful model was selected (Sokal and Rohlf 1968). To obtain the maximum specific growth rate (µ), lag phase duration (λ) and maximum count (A), results free R software for Windows was used (R core Team 2018). To compare the control reactor with the experimental reactor, t-tests were performed for independent groups, with a statistical significance of 0.005 using the SYSTAT Software package. Normal distribution was assumed, given that the measurements were performed in triplicate.
Nucleotide accession numbers
The archaeal sequences obtained and described in this study of mMPA rRNA 16S gene sequence data were submitted to the NCBI GenBank database under accession numbers: EU544305, EU544306 and EU544307.
Phylogenetic analysis
Sequences for selected clones in this study have been submitted to NCBI/GenBank. These were aligned with the NCBI nucleotide database using BLAST (Altschul et al. 1997), as those sequences were at least 90% identical and above. To compare the diversity of OTU in CR and TR in different phases of the bioreactors, a multiple alignment was performed using ClustalW (Mount 2004). Phylogenetic trees were calculated with MEGA4 software (Tamura et al. 2007), using the Kimura two-parameter correction and the Neighbour-Joining method.
Results and discussion
Isolation and growth kinetics of ammonia tolerant consortia (NH3)-mMPA biofilm
Ammonia tolerant consortia, (NH3)-mMPA, were isolated from the reactor enriched with mMPA communities. Consortia showed a Methanosarcinaceae MPA family prevalence. Consortia were able to grow in a planktonic phase at 848.8 mg NH3/L with methane representing a mean of approximately 70% of the produced biogas. (NH3)-mMPA consortia were inoculated into vials with ceramic spheres as support for 20 days, allowing biofilm formation. Growth kinetics determined by the Gompertz model showed a λ of 3.7 d (days) and μm of 0.67 d−1, with r2 = 0.95 (Fig. 1). In addition, a correlation was observed between the growth of the methanogenic community and methane production, which was monitored during biofilm formation until methane peaked at 80% of total biogas after 20 days of incubation. A time lag of 5—6 days was observed in methane production. By performing SEM analysis, (NH3)-mMPA consortia were detected on the support surfaces after 4 days of incubation, consistent with the kinetic growth data that predicted micro-colony formation after 20 days of incubation with morphotypes similar to Methanomethylovorans sp. (Methanosarcinaceae, Methanosarcinales).
Design of an anaerobic biofilm enriched with (NH3)-mMPA under NH3 shock
After a full year of operation, the control bioreactor (CR) showed a decrease of approximately 80% in TOC (Fig. 2A), with 85% methane content in the produced biogas. The methane production curve obtained for the treatment bioreactor (TR) gradually increased, approaching 70% of the biogas produced in the first 50 days (Fig. 2B). The TOC consumption varied between 80 ± 20% in CR and 62 ± 17% in TR. Methane content in the biogas ranged from 86 ± 8% in CR, and 81 ± 18% in TR (Fig. 2A, B). The alkalinity ratio remained below 0.3 in both reactors. The alkalinity ratio is defined as the alkalinity that sets the buffer strength of the reactor, which decreases due to the increase of fatty acids produced during the instability stages of the system. The intermediate alkalinity (IA) was calculated by determining the difference between partial alkalinity (PA, measured by titration with 0.062 N H2SO4 at pH 5.75) and total alkalinity (TA, measured at pH 3.7). The IA corresponds to the contribution of salts of volatile fatty acids (pKa between 4.6 and 4.9). The IA/TA ratio (alpha factor) was determined and the results were expressed as bicarbonate concentration (mg/L or ppm). It has been empirically proven that in general, an IA/TA ratio (factor a) lower than 0.3 provides adequate operational stability (Huang et al. 2015).
Total Organic Carbon consumption, methanization and ammonia concentration in an anaerobic filter type reactor enriched with mMPA from control (CR) and treatment reactors (TR). 2A. CR. 2B. TR. Phase A: CR anaerobic biofilm development with ammonia operating range of 27.45–75 mg NH3-N/L; Phase B. Reactor acclimatization- Initial phase bioreactor functioning. Acclimatization at 37.7–128 mg NH3-N/L. Phase C: Ammonia shock at 755 mg NH3-N/L; Phase D: Final phase bioreactor functioning, reactor recovery from 37.1 to 41.2 mg NH3-N/L. ■- % Total Organic Carbon (TOC) consumption; - □ -% methane; --- ○ --- NH3 input concentration; --●-- NH3 output concentration
Morphological and genotyping characterization of (NH3)-mMPA consortia and ammonia Tolerant biofilm
To determine the microbial morphologies present in (NH3)-mMPA consortia, concentrated planktonic cultures and ceramic support fragments were observed by SEM. Sarcina morphotypes characteristic of Methanosarcinaceae were found. Analysis of the 16S rDNA from (NH3)-mMPA consortia revealed that gene sequences were grouped with Methanosarcinaceae members (Methanosarcinaceae, Methanosarcinales). Phylogenetic relationships of archaeal 16S rDNA partial sequences (EU544306; EU544307), archaeal mMPA ammonium tolerant strain 16S rDNA partial sequence (EU544305) and methanogenic species sequences from the Gene bank show that mMPA sequence EU544305 (868 bp), was grouped in the Methanomethylovorans genus. This is because it showed higher similarities with uncultured archaeon (99%) and Methanomethylovorans hollandica (Lomans 1999) (98%), and two different species of Methanosarcina genera which were predominant components of the consortia, and similar to cloned sequences. EU544306 (547 bp), uncultured Methanosarcina sp. clone HUB A5, and EU544307 (521 bp), were grouped into the Methanosarcina genus, showing high similarity with Methanosarcina sp. (95%) and Methanosarcina baltica (von Klein 2002) (95%). When a BLAST analysis compared the sequences with stored species Types of uncultured Methanosarcina sp. clone HUB A5, this is located near Methanosarcina baltica in a phylogenetic tree at a Neighbor Joining distance of 0.05 (not shown). M. baltica is an archaeon isolated from anoxic layers of sediment in the Baltic Sea that grows fast in marine culture, in methylamines, methanol, and acetate (von Klein et al. 2002). Uncultured Methanosarcina sp. clone HUB A6 sequence EU544307 was also grouped in the Methanosarcina genus, showing high similarity to Methanosarcina acetivorans (Sowers et al. 1984) (93%) and uncultured Methanosarcina sp. (92%). Uncultured Methanosarcina sp. clone HUB A6 is located near M. acetivorans in a phylogenetic tree at a Neighbor Joining distance of 0.2 (not shown), also described in anoxic marine environments and found as a single cell in marine cultures (Maeder et al. 2006).
Microbiological and molecular description of biofilm in bioreactors
The microbial biofilm composition of anaerobic communities in both reactors was determined through 16S rRNA analysis (by membrane hybridization, Dot Blott procedure). In the control reactor, the amount initial of bacteria and archaea was found to be 78.3% and 21.7%, respectively. However, in the enriched reactor, the proportion initial was 38.4% bacteria and 61.6% archaea. As expected, in the ammonia-tolerant APMm reactor a higher proportion of Archaea was found, especially representatives of the Methanococcaceae family (41%), a group to which the community of ammonia-tolerant APMm belongs, with which the biofilm of this reactor was enriched. The proportion of Methanococcaceae in the control reactor was 19.9%. Sulfate-reducing bacteria (SRB) represented 19.5% in the control reactor and 29.9% in the reactor with ammonia-tolerant APMm, given the symbiotic relationship of methanogenic Archaea with SRB (Shi et al. 2020).
Ammonia concentration in both bioreactor influents ranged between 37.7 and 128 mg NH3-N/L until day 152, when both bioreactors were subjected to ammonia shock, increasing ammonia concentrations to 755 mg NH3-N/L. Between day 180 and 208, ammonia concentrations returned to average levels (37.2–41.1 mg NH3-N/L). Significant differences in ammonia concentration were observed in phases C and D in both TR and CR effluent outputs (Fig. 2A, B). The experimental bioreactor showed a lower concentration of ammonia residual (137–184 mg NH3/L) when compared with the control bioreactor (327–128 mg NH3/L). Shock NH3-N effects in CR and TR are shown in Fig. 2A and B, respectively. Methanation in CR began at 90%, dropping to 70% during ammonia shock (Fig. 2A). A different response was observed in TR, where no significant variations were observed during the ammonia shock, reaching methanation levels above 90% throughout the experiment (t-test, p 0.0005–0.01) (Fig. 2B). In both cases (CR and TR), correlations between the percentage of TOC consumed and the concentration of methane produced were significant (r2: 0.72 p ≤ 0.05 for CR, and r2 = 0.77 p ≤ 0.05 for TR) (Fig. 2). Production of methane specific was determined based on biomass as volatile suspended solids (VSS) were registered in both reactors. In CR, a production of 0.32 ± 0.04 mL CH4/g (VSS * d) was registered, while TR showed a production of 0.65 ± 0.08 mL CH4/g (VSS * d), this activity was re-controlled post ammonia shock where it was found a production of 0.30 ± 0.05 mL CH4/g (VSS * d) in CR and 0.38 ± 0.04 mL CH4/g (VSS * d) in TR. Increased methanogenic activity per biomass unit from day 152 to 166 was 20.5-fold higher in TR when compared to CR, which indicates that methanogenic archaea maintained their activity under ammonia shock conditions.
When comparing the first 100 days of both reactors, the control bioreactor consumed significantly more TOC than the experimental bioreactor (t-test, p < 0.0001–0.007). Methane production measure by gas chromatography was also significantly higher in the CR (89.48–95%) when compared to the TR (29–75%) (p < 0.0001–0.029). These differences diminished from day 54 to 82 (Fig. 2A, B). During and after the ammonia shock, (phase C—day 152 to 208), a higher consumption of TOC was observed in both bioreactors, although it was significantly higher in the TR until day 180, after which it decreased significantly to 65.78% on day 208. Methane production in the TR remained above 90%, significantly higher than in the CR (p < 0.0005–0.0417), which showed a methane production that fluctuated between 69 and 86% (Fig. 2A, B). Significant differences (t-test, α ≤ 0.05) were observed for both methane production and TOC concentrations when comparing the CR and TR during the ammonia shock step. Figure 2A and B show how the ammonia shock caused a significant decline (t-test, α = 0.05) in methane production and TOC consumption in the CR. These results clearly indicate that the TR was resistant to the deleterious effect of ammonia, as the mMPA biofilm community was able to maintain its methanogen activity even under high ammonia concentrations.
The analysis of 16 s RNA through gel electrophoresis showed that the abundance of archaea increased between phase B, the recovery phase, and phase D, the final phase. Besides, lower archaea biodiversity was observed in the CR throughout the profile when compared with TR biofilm (Fig. 3A). Moreover, it was observed that (NH3)-mMPA enriched biofilm produced fewer bands in the bacteria domain in the CR than biofilm enriched with mMPA tolerant to ammonia. However, 16S rDNA bacterial profile similarities were higher in the CR than in the TR. Similarities were lower for archaea than bacteria, as shown in Fig. 3A and B.
Clustering of the DGGE profiles by similarity analysis (WPGMA) obtained from PCR-amplified 16S rRNA gene segments of Archaea (A) and Bacteria (B). Samples were recovered from clays collected from control (CR) and treatment reactors (TR). Conditions: B. initial acclimatization at 37.7–128 mg NH3-N/L; C. ammonia shock at 755 mg NH3-N/L; D. final reactor recovery from 37.1 to 41.2 mg NH3-N/L. NH3-mMPA is isolated methanogenic ammonia tolerant Archaea
This study demonstrates that using anaerobic biofilms formed in fixed bed reactors containing (NH3)-mMPA (ammonia tolerant archaea biofilms) can achieve efficient wastewater treatment and stable methane production under high ammonia concentrations. Zheng et al. (2015) demonstrated that selecting a suitable support is crucial for fixed-bed reactors designed for the anaerobic digestion of ammonium-rich livestock wastes. The authors found that when zeolites were used as the support, methane production increased by over 80% compared to other types of support, which is comparable to the results of this study.
The ammonia tolerant acclimated microbial consortium, composed of archaea and bacteria, gives rise to long-term tolerance to ammonia stress in the anaerobic digestion process. Methane producing archaea (MPA) were represented by the Methanomethylovorans genus (EU544305 sequence), and the Methanosarcina genus (EU544306 and EU544307 sequences), which is considered an obligate methylotrophic methanogen (Supplementary Material SI 1 to SI 3).
Members of Methanosarcinaceae have been found in a wide variety of anaerobic environments where methane is produced, can grow on a diversity of substrates, and represent the most versatile group of Archaea (Oren 2014). The predominant archaea throughout the experiment were Methanosarcina and Methanomethylovorans (He et al. 2017; Cadavid-Rodríguez et al. 2019). Some researchers consider these species to be keystone species. For instance, M. acetivorans (Galagan et al. 2002) is a unique species with a wide range of metabolisms that support the reactor’s operation under ammonia shock.
In a study by Wu and Song (2021), adding inoculum during anaerobic co-digestion of waste activated sludge (WAS) and fish waste (FW) produced high methane production rates with mixtures of 1.5% WAS and 3% FW. However, methanogenesis was inhibited with an addition of 6% or higher of FW, due to high ammonia and fatty acid accumulation. In a similar trial, Cadavid-Rodríguez et al. (2019) found that methanogenesis is inhibited when more than 1.5% of FW is used in anaerobic digestion. Thus, the alternative of enriching the inoculum with ammonia-tolerant mMPA is an attractive and feasible alternative to avoid the inhibition of methanogenesis in the treatment of protein-rich fishery waste.
Furthermore, the microbial community analyses carried out by Wu and Song (2021) showed that hydrogenotrophic and methylotrophic methanogenic archaea dominated methane production. This result was expected since fishery residues are methylated amine enriched substrates, which favors the growth and maintenance of the ammonia tolerant mMPA consortia.
Physiological studies of this strain have reported that it tolerates NaCl concentrations of 100 mM. In this study, the NH3 tolerant consortia grew and maintained constant methanogenic activity at 400 mM NaCl, a characteristic that favors their use for fishery waste treatment at high salt concentrations (Aloui et al. 2009; Chen et al. 2018; He et al. 2017) because salinity has been reported to diminish removal performance, activated sludge characteristics, and change the microbial community in anaerobic reactors (Chen et al. 2017; He et al. 2017).
We identified a strong relationship between reactor performance and the abundance of Methanosarcina cells. Until day 398 (750 FAN mg/L; 6000 TAN mg/L), highly active Methanosarcina cells were also found in large multicellular structures (clusters). These results agree with the findings of Calli et al. (2005), which found an abundance of Methanosarcina species at high ammonia concentrations (160–747 mg).
The experimental bioreactor showed consistently low ammonia values in the output effluent after an ammonia shock compared to the control bioreactor. Similar results have been previously reported (Dai et al. 2016). The 16S rDNA profile of archaea and bacteria obtained by PCR-DGGE shifted at OTU (Operational Taxonomic Units) indicated that acetoclastic methanogenesis dominated acetate utilization in the bioreactor. Similarly, Galagan et al. (2002) described the metabolic and physiological diversity of M. acetivorans, suggesting that the formation of multicellular structures (cell envelope and extracellular matrix) is an adaptation to stress and likely plays an essential role in the ability of Methanosarcina to colonize diverse environments. This species metabolizes a broad spectrum of carbon compounds into methane, a process controlled by different gene repertoires. Individual copies of the duplicated genes may display differential regulation and kinetic properties, allowing the species to change between substrates, consistent with the reported plasticity that Methanosarcina species show in nature (Angelidaki et al. 2011). This critical characteristic allows the community to tolerate stress. High bacterial biodiversity was observed in the communities under study, but we did not find significant differences between these communities. This information has been noted in previous studies about anaerobic bioreactor stability (Fernandez et al. 2000), where bacteria was found in the biofilm, even under stress. Nevertheless, according to our results, when the communities were exposed to changing conditions, the bacterial activity was modified and archaea did not exhibit significant differences in abundance.
The Chilean coastal zone is characterized by high primary production, upwelling-favorable winds, and the marine oceanographic current of the southeastern Pacific Ocean. This oceanographic system produces constant upwelling and has registered significant fish strandings for a long time (Hernandez-Miranda et al. 2010, 2012, 2017). Therefore, it is probable that an unknown, endemic, and rich microbial ammonia methanogenic community (bacteria and archaea) potentially has evolved and is associated with both the water column and sediments because of the high protein content product of the strandings. This potential microbial community sampled from industrial fishery effluent was used in the anaerobic treatment bioreactor after being bioaugmented in a bioreactor, with the objective of characterizing archaea components.
Data availability
The data used for this article is available to be requested by the reviewers if they deem it appropriate.
Code availability
Not aplicable.
References
Abdallah MB, Karray F, Mhiri N, Mei N, Quéméneur M, Cayol JL, Erauso G, Tholozan JL, Alazard D, Sayadi S (2016) Prokaryotic diversity in a Tunisian hypersaline lake, Chott El Jerid. Extremophiles 20:125–138. https://doi.org/10.1007/s00792-015-0805-7
Aloui F, Khoufi S, Loukil S, Sayadi S (2009) Performances of an activated sludge process for the treatment of fish processing saline wastewater. Desalination 246(1–3):389–396. https://doi.org/10.1016/j.desal.0000.00.000
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402. https://doi.org/10.1093/nar/25.17.3389
Amann R, Krumholz I, Stahl D (1990) Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 172:762–770. https://doi.org/10.1128/jb.172.2.762-770.1990
Amann R, Stromley J, Devereux R, Key R, Stahl D (1992) Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol 58:614–623
Anderson TF (1951) Techniques for the preservation of three-dimensional structure in preparing specimens for the electron microscope. Trans NY Acad Sci 13(4 Series II):130–134. https://doi.org/10.1111/j.2164-0947.1951.tb01007.x
Angelidaki I, Karakashev D, Batstone DJ, Plugge CM, Stams AJ (2011) Biomethanation and its potential. Methods Enzymol 494:327–351. https://doi.org/10.1016/B978-0-12-385112-3.00016-0
APHA (1992) Standard methods for the examination of waste and wastewater. American Public Health Association, New York
Ausubel M, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K (1998) Quantitation of DNA and RNA with absorption and fluorescence spectroscopy. Current protocols in molecular biology, vol 1–3. Wiley, New York (appendix 3D)
Beck BR, Holzapfel W, Hwang CW, Do HK (2013) Bacterial community structure shift driven by salinity: analysis of DGGE band patterns from freshwater to seawater of Hyeongsan River, Korea. J Life Sci 23(3):406–414. https://doi.org/10.5352/JLS.2013.23.3.406
Cadavid-Rodríguez LS, Vargas-Muñoz MA, Plácido J (2019) Biomethane from fish waste as a source of renewable energy for artisanal fishing communities. Sustain Energy Technol Assess 34:110–115. https://doi.org/10.1016/j.seta.2019.05.006
Calli B, Mertoglu B, Inanc B, Yenigun O (2005) Methanogenic diversity in anaerobic bioreactors under extremely high ammonia levels. Enzyme Microb Technol 37:448–455. https://doi.org/10.1016/j.enzmictec.2005.03.013
Chen WY, Kraková L, Wu JH, Pangallo D, Jeszeová L, Liu B, Yasui H (2017) Community and proteomic analysis of anaerobic consortia converting tetramethylammonium to methane. Archaea 3:1–14. https://doi.org/10.1155/2017/2170535
Chen Y, He H, Liu H, Li H, Zeng G, Xia X, Yang C (2018) Effect of salinity on removal performance and activated sludge characteristics in sequencing batch reactors. Bioresour Technol 249:890–899. https://doi.org/10.1016/j.biortech.2017.10.092
Clegg SL, Whitfield M (1995) A chemical model of seawater including dissolved ammonia and the stoichiometric dissociation constant of ammonia in estuarine water and seawater from −2 to 40°C. Geochim Cosmochim Acta 59(12):2403–2421. https://doi.org/10.1016/0016-7037(95)00135-2
Dai X, Yan H, Li N, He J, Ding Y, Dai L, Dong B (2016) Metabolic adaptation of microbial communities to ammonium stress in a high solid anaerobic digester with dewatered sludge. Sci Rep 6:28193. https://doi.org/10.1038/srep28193
Dar SA, Kuenen JG, Muyzer G (2005) Nested PCR-denaturing gradient gel electrophoresis approach to determine the diversity of sulfate-reducing bacteria in complex microbial communities. Appl Environ Microbiol 71(5):2325–2330. https://doi.org/10.1128/AEM.71.5.2325-2330.2005
Fernandez AS, Hashsham SA, Dollhopf SL, Raskin L, Glagoleva O, Dazzo FB, Hickey RF, Criddle CS, Tiedje JM (2000) Flexible community structure correlates with stable community function in methanogenic bioreactor communities perturbed by glucose. Appl Environ Microbiol 66(9):4058–4067
Galagan JE, Nusbaum C, Roy A, Endrizzi MG, Macdonald P, FitzHugh W, Brown A (2002) The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res 12(4):532–542. https://doi.org/10.1101/gr.223902
Gupta P, Shekhar R, Sachan A, Vidyarthi AS, Gupta A (2012) A re-appraisal on intensification of biogas production. Renew Sustain Energy Rev 16:4908–4916. https://doi.org/10.1016/j.rser.2012.05.005
He H, Chen Y, Li X, Cheng Y, Yang C, Zeng G (2017) Influence of salinity on microorganisms in activated sludge processes: a review. Int Biodeterior Biodegrad 119:520–527. https://doi.org/10.1016/j.ibiod.2016.10.007
Hernández-Miranda E, Quiñones RA, Aedo G, Valenzuela A, Mermoud N, Román C, Yañez F (2010) A major fish stranding caused by a natural hypoxic event in a shallow bay of the eastern South Pacific Ocean. J Fish Biol 76(7):1543–1564. https://doi.org/10.1111/j.1095-8649.2010.02580.x
Hernández-Miranda E, Veas R, Labra FA, Salamanca M, Quiñones RA (2012) Response of the epibenthic macrofaunal community to a strong upwelling-driven hypoxic event in a shallow bay of the southern Humboldt current system. Mar Environ Res 79:16–28. https://doi.org/10.1016/j.marenvres.2012.04.004
Hernández-Miranda E, Veas R, Anabalón V, Quiñones RA (2017) Short-term alteration of biotic and abiotic components of the pelagic system in a shallow bay produced by a strong natural hypoxia event. PLoS ONE 12(7):e0179023
Hershberger KL, Barns SM, Reysenbach AL, Dawson SC, Pace NR (1996) Wide diversity of Crenarchaeota. Nature 384(6608):420. https://doi.org/10.1038/384420a0
Hobbie JE, Daley RJ, Jasper S (1977) Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33(5):1225–1228
Huang LY, Lee DJ, Lai JY (2015) Forward osmosis membrane bioreactor for wastewater treatment with phosphorus recovery. Bioresour Technol 198:418–423
Jurgens G, Lindström K, Saano A (1997) Novel group within the kingdom Crenarchaeota from boreal forest soil. Appl Environ Microbiol 63(2):803–805
Kato S, Watanabe K (2010) Ecological and evolutionary interactions in syntrophic methanogenic consortia. Microbes Environ 25(3):145–151. https://doi.org/10.1264/jsme2.ME10122
Kayhanian M (1999) Ammonia inhibition in high-solids biogasification: an overview and practical solutions. Environ Technol 20(4):355–365. https://doi.org/10.1080/09593332008616828
Leadbetter JR, Breznak JA (1996) Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol 62(10):3620–3631
Lomans B, Maas R, Luderer R, Op den Camp H, Pol A, van der Drift C, Vogels G (1999) Isolated and characterization of Methanomethylovorans hollandica gen. nov., sp. nov., isolated from freshwater sediment, a methylotrophic methanogen able to grow on dimethyl sulfide and methanethiol. Appl Environ Microbiol 65:3641–3650
Maeder DL, Anderson I, Brettin TS, Bruce DC, Gilna P, Han CS, Lapidus A, Metcalf W, Saunders E, Tapia R, Sowers KR (2006) The Methanosarcina barkeri genome: comparative analysis with Methanosarcina acetivorans and Methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J Bacteriol 188(22):7922–7931. https://doi.org/10.1128/JB.01858-06
McHugh S, O’Reilly C, Mahony T et al (2003) Anaerobic granular sludge bioreactor technology. Rev Environ Sci Biotechnol 2:225–245. https://doi.org/10.1023/B:RESB.0000040465.45300.97
McHugh S, Collins G, Mahony T, O’Flaherty V (2005) Biofilm reactor technology for low temperature anaerobic waste treatment: microbiology and process characteristics. Water Sci Technol 52:107–113. https://doi.org/10.2166/wst.2005.0188
Mohan SV, Rao NC, Prasad KK, Sarma PN (2005) Bioaugmentation of an anaerobic sequencing batch biofilm reactor (AnSBBR) with immobilized sulphate reducing bacteria (SRB) for the treatment of sulphate bearing chemical wastewater. Process Biochem 40:2849–2857. https://doi.org/10.1016/j.procbio.2004.12.027
Mount D (2004) Bioinformatics: sequence and genome analysis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Muyzer G, de Waal EC, Uittierlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59(3):695–700
Omil F, Mendez R, Lema JM (1995) Anaerobic treatment of saline wastewaters under high sulphide and ammonia content. Bioresour Technol 54:269–278. https://doi.org/10.1016/0960-8524(95)00143-3
Oren A (2014) The family methanosarcinaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes. Springer, Berlin, Heidelberg
Pham CH, Triolo JM, Cu TTT, Pedersen L, Sommer SG (2013) Validation and recommendation of methods to measure biogas production potential of animal manure. Asian Australas J Anim Sci 26(6):864–873. https://doi.org/10.5713/ajas.2012.12623
Procházka J, Dolejš P, Máca J, Dohányos M (2012) Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl Microbiol Biotechnol 93(1):439–447. https://doi.org/10.1007/s00253-011-3625-4
Putra AA, Watari T, Maki S, Hatamoto M, Yamaguchi T (2020) Anaerobic baffled reactor to treat fishmeal wastewater with high organic content. Environ Technol Innov 17:100586. https://doi.org/10.1016/j.eti.2019.100586
Raskin L, Stromley JM, Rittmann BE, Stahl DA (1994a) Group-specific16S rRNA hybridization probes to describe natural communities of methanogens. Appl Environ Microbiol 60(4):1232–1240
Raskin L, Poulsen L, Noguera D, Rittmann B, Stahl D (1994b) Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probes hybridization. Appl Environ Microbiol 60:1241–1248
Reddy KJ, Gilman M (1993) Preparation of bacterial RNA. In: Ausbel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K (eds) Current protocols in molecular biology. Suplement 21. Wiley, Massachusetts, p 4.4.1-4.4.7
Rosen K, Villa-Komaroff L (1990) An alternative method for the visualization of RNA in formaldehyde agarose gels. Focus 12:23–24
Sanguinetti CJ, Dias Neto E, Simpson AJ (1994) Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 17(5):914–921
Shi X, Gao G, Tian J, Wang XC, Jin X, Jin P (2020) Symbiosis of sulfate-reducing bacteria and methanogenic archaea in sewer systems. Environ Int 143:105923
Sokal R, Rohlf F (1968) Biometría, Principios y Métodos estadísticos para la investigación biológica. Trad de Biometrics por ML Leon, Malaga, España
Sossa K, Alarcón M, Aspé E, Urrutia H (2004) Effect of ammonia on the methanogenic activity of methylaminotrophic methane producing archaea enriched biofilm. Anaerobe 10(1):13–18. https://doi.org/10.1016/j.anaerobe.2003.10.004
Sowers KR, Baron SF, Ferry JG (1984) Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl Environ Microbiol 47(5):971–978
Stahl D, Amann R (1991) Development and application of nucleic acid probes. In: Stakebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 205–248
Sung S, Liu T (2003) Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 53(1):43–52. https://doi.org/10.1016/S0045-6535(03)00434-X
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596–1599
Urrutia H, Vidal R, Baeza M, Aspé E (1999) Effect of fishing industries effluents pH and organic load on the methanogenic bacteria biofilm developed over support in fixed biomass reactor. Anaerobe 5(3):325–327. https://doi.org/10.1006/anae.1999.0301
Valdebenito-Rolack EH, Araya TC, Abarzua LE, Ruiz-Tagle NM, Sossa KE, Aroca GE, Urrutia HE (2011) Thiosulphate oxidation by Thiobacillus thioparus and Halothiobacillus neapolitanus strains isolated from the petrochemical industry. Electron J Biotechnol 14(1):7–8. https://doi.org/10.2225/vol14-issue1-fulltext-10
Vidal G, Carvalho A, Mendez R, Lema JM (2000) Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour Technol 74(3):231–239. https://doi.org/10.1016/S0960-8524(00)00015-8
Vissers EW, Bodelier PL, Muyzer G, Laanbroek HJ (2009) A nested PCR approach for improved recovery of archaeal 16S rRNA gene fragments from freshwater samples. FEMS Microbiol Lett 298(2):193–198
von Klein D, Arab H, Völker H, Thomm M (2002) Methanosarcina baltica, sp. Nov., a novel methanogen isolated from the Gotland Deep of the Baltic Sea. Extremophiles 6(2):103–110. https://doi.org/10.1007/s007920100234
Wu Y, Song K (2021) Anaerobic co-digestion of waste activated sludge and fish waste: methane production performance and mechanism analysis. J Clean Prod 279:123678. https://doi.org/10.1016/j.jclepro.2020.123678
Zheng H, Li D, Stanislaus MS, Zhang N, Zhu Q, Hu X, Yang Y (2015) Development of a bio-zeolite fixed-bed bioreactor for mitigating ammonia inhibition of anaerobic digestion with extremely high ammonium concentration livestock waste. Chem Eng J 280:106–114. https://doi.org/10.1016/j.cej.2015.06.024
Zhong D, Zhu K, Ma W et al (2021) Optimization of a completely mixed anaerobic biofilm reactor (CMABR) based on brewery wastewater treatment. Water 13:606. https://doi.org/10.3390/w13050606
Zwietering M, De Wit JC, Cuppers AM, Riet VTK (1994) Microbiology modeling of bacterial growth with shifts in temperature. Appl Environ Microbiol 60:204–213
Acknowledgements
DIC UDEC 203.031.095-1.0. Ammonia effects on taxonomic diversity of methanogenic Archaea in anaerobic biofilms used to purify industrial discharges high in protein. DIUC-Ordinario, 10-2003, 10-2005. DAAD Grant.
Funding
The research was supported by internal funds from the Universidad de Concepción obtained by Fidelina Gonzalez. DIC UDEC 203.031.095–1.0. Ammonia effects on taxonomic diversity of methanogenic Archaea in anaerobic biofilms used to purify industrial discharges high in protein. DIUC-Ordinario, 10-2003, 10-2005. DAAD Grant.
Author information
Authors and Affiliations
Contributions
MA-V: data curation, formal analysis, investigation, methodology, writing—original draft. Writing—review and editing. NR-T: conceptualization, formal analysis, investigation, writing—original draft. FG: conceptualization, data curation, formal analysis, writing—original draft. PJ-C: conceptualization, investigation, methodology. EA: conceptualization, formal analysis, investigation. HUB: conceptualization, formal analysis, supervision, visualization. KSF: conceptualization, funding acquisition, project administration, supervision, visualization.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
All authors subscribe to the Singapore Statement of Ethics for Life Science Work.
Informed consent
All authors give their consent to participate in this article and in general in the research presented.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alarcón-Vivero, M., Moena, N.RT., Gonzalez, F. et al. Anaerobic biofilm enriched with an ammonia tolerant methanogenic consortium to improve wastewater treatment in the fishing industry. Biotechnol Lett 44, 239–251 (2022). https://doi.org/10.1007/s10529-021-03213-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03213-y




 Experimental values; ---Values predicted by Gompertz model;
Experimental values; ---Values predicted by Gompertz model;  methane in biogas (%). Bars represent standard deviation of average methane %.
methane in biogas (%). Bars represent standard deviation of average methane %.
