Abstract
Objectives
To assess the extracellular synthesis of silver nanoparticles using marine derived fungi Aspergillus brunneoviolaceus with their antibacterial and antioxidant activities.
Results
The biosynthesis of silver nanoparticles was estimated by the change in color from light yellow to dark brown within 36 h as the reaction progressed. UV-Visible spectroscopy exhibited its stability at 411 nm; ATR-FTIR spectroscopy depicted the functional group responsible for its production; X-Ray Diffraction denoted its crystalline FCC structure resembling the peaks in XRD pattern, corresponding to [111], [200], [220], [311] and [222] planes; TEM imaging revealed its spherical morphology with the particle size ranging from 0.72 to 15.21 nm and Tauc’s plot analysis that disclosed its band gap energy as 2.44 eV that manifested the potential of AgNPs to be semiconductors. The characterization data henceforth, confirmed the efficient production of silver nanoparticles. The biosynthesized AgNPs expressed strong antibacterial activity against two Gram-positive and three Gram-negative bacteria. They also proved to possess higher antioxidative potentials by showing their potent radical scavenging activity against DPPH (2, 2-diphenyl-1-picrylhydrazyl).
Conclusions
The study unfolds the prospect for further utilization of this mycogenically synthesized AgNPs as antibacterial, antioxidative and anticancer agents.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthesis of nanoparticles is often achieved by physical, chemical, and biological methods. The chemical method is most frequently used for the synthesis of silver nanoparticles (AgNPs) employing reagents that reduce silver ions and stabilize the nanoparticles. These reagents are toxic and may present risks to human health as well as the environment (Guilger-Casagrande and Lima 2019), which has drawn increasingly higher attention towards the green synthesis of AgNPs asanother alternative. Such methods enable the synthesis of nano-sized particles that show better physicochemical characteristics, eco-friendliness, cost-effectiveness and higher stability than chemical methods (Iravani et al. 2014). Biological methods involve the use of living organisms including plants, bacteria and fungi, which can reduce metal ions and facilitate the formation of nanoparticles that possess the desired size and morphology (Durán et al. 2011). Fungi are the most suitable microorganism for the biogenic synthesis of AgNPs, because they present a high tolerance to metal, easy to handle and also secrete many extracellular proteins that contribute in providing stability to the AgNPs (Netala et al. 2016). Marine procured fungi have been found to produce important enzymes including NADPH dependent and nitrate dependent reductases which are main reducing agents for silver ions to form nanoparticles (Manivasagan et al. 2016). Main advantages of the fungal system over bacterial cultures are that they offer attractive biomass production and do not require any robust process to extract the filtrate (Gade et al. 2008). In contrast to synthesis using plants, the fungal mycelial mass is highly resistant to the pressure and agitation process of the fermentor. So, the use of the fungus instead of the plant is more suitable for large-scale synthesis of AgNPs (Velusamy et al. 2016).
Silver is popular for its antimicrobial activity for many centuries.Amidst various types of metal nanoparticles, AgNPs have attained greater attention because of its distinctive properties as well as their broad-spectrum antimicrobial and antioxidative potential (Loo et al. 2018). The potential applications of AgNPs in various fields have been stated in several studies. Moreover, usage of AgNPs in the control of pathogenic microorganisms in different areas such as healthcare and agriculture has been extensively accepted and reported (Nayak et al. 2018). It has been reported that many fungal species like Fusarium oxysporum (Birla et al. 2013), Aspergillus niger (Zomorodian et al. 2016), Cladosporium cladosporioides (Hulikere and Joshi 2019) and Trichoderma spp. (Ramos et al. 2020) showed extracellular and stable synthesis of AgNPs.
This study is aimed at using cell free filtrate (CFF) of marine fungi Aspergillus brunneoviolaceusfor synthesis of AgNPs. The synthesized nanoparticles were characterized for morphological and structural analysis by using Uv-Visible spectroscopy, TEM imaging, FTIR and XRD. Antibacterial activity of the synthesized AgNPs were tested against human pathogenic bacterial strains along with AgNO3 and standard ampicillin as a reference drug molecule. The antioxidant activity of AgNPs was also performed by using the DPPH method furthermore, considering it as a potent anticancer agent in the future.
Experimental
Biosynthesis of nanoparticles
Aspergillus brunneoviolaceus (GenBank accession number: MT645337)was grown in potato dextrose broth in shaking condition (120 rpm) for 96 h at 28 °C. Biomass (6 g) was filtered through Whatman® filter paper no. 42 followed by washing with sterilized double-distilled water and resuspended in 100 mL of sterilized double distilled water for 24 h at 60 °C. Biomass was then again filtered through Whatman® filter paper no. 42 and CFF was used for biosynthesis of silver nanoparticles (AgNPs).
For the biosynthesis of silver nanoparticles (AgNPs), 10 mL of CFF (pH-9.0) was reacted with 90 mL of 10 mM silver nitrate solution (AgNO3, M.W. 169.87, HiMedia) at 60 °C in the water bath. The reaction was carried out in dark condition to avoid any photochemical reaction.
Characterization of mycosynthesized silver nanoparticles
The absorption spectra of synthesized AgNPs were measured in the range of 300 nm to 700 nm by a UV–Vis spectrophotometer. The shape and size of the synthesized AgNPs were determined by transmission electron microscopy (TEM). The surface chemistry of the sample was studied using attenuated total reflection- Fourier transform Infrared Spectroscopy (ATR-FTIR). XRD pattern of silver nanoparticles was performed on an X-ray diffractometer. Spectrum was recorded by CuKα radiation with a wavelength of 1.5406 nm in the 2θ range from 0º to 90º and operated at 30 kV and 100 mA. The optical property of the silver nanoparticles was also performed by calculating the optical band gap Eg by using Tauc’s plot.
Antibacterial activity of silver nanoparticles (AgNPs)
Antibacterial activity of synthesized AgNPs was carried out via a well diffusion method reported by Hulikere and Joshi(2019) with some modifications. All the test bacterial strains were grown in nutrient broth at 37 °C overnight and adjusted to 0.5 as per McFarland standards. Under sterile conditions, 100 µL of two Gram-positive (B. subtilis and S. aureus) and three Gram-negative strains (P. aeruginosa, E. coli and S. typhi)were spread on each nutrient agar plate. A diameter well of 12 mm was punched on the agar plate using a cork borer and the synthesized AgNPs and AgNO3were inoculated in each well. Similarly, 100 µL of ampicillin (1 mg/mL) served as a positive control. Plates were incubated at 37 °C for 24 h and the antibacterial activity was evaluated by measuring the diameter of the inhibition zone using zone scale (HiMedia).
Antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) method
Antioxidant capacity of synthesized AgNPs was performed according to Keshari et al. (2020)with slight modification. The radical scavenging activity of AgNPs and vitamin C was determined using the DPPH. Various concentrations (10, 20, 30, 40, 50, 75 and 100 µg/mL) of 1 mL AgNPs were mixed with 1 mL of 1 mM freshly prepared DPPH solution followed by vortex. Then after, the solution was kept for 30 min in dark at room temperature. The absorbance was recorded at 517 nm. DPPH with all reagents except sample was used as a control and methanol was used as a blank. The free radical scavenging activity was represented as the percentage of inhibition which was calculated by using the following formula,
where Pc is the absorbance of control and Ps is the absorption of AgNPs/vitamin C.
Statistical analysis
Statistical analysis was carried out using IBM SPSS Statistics 23. All the experimental values were expressed as a mean ± SE. Significance level also indicated after performing 1 way ANOVA analysis.
Result and discussion
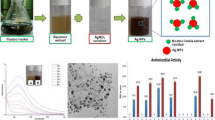
Aspergillus brunneoviolaceus was isolated from water collected from the sea coast of Diu, India (20° 42′ 17.3″ N 70° 54′ 55.8″ E) subjected to the biosynthesis of AgNPs (Fig. 1). The addition of the 10 mM of AgNO3 to the CFF of Aspergillus brunneoviolaceus resulted in an immediate change in the color of the solution from colorless to light yellow after which the solution color changed to light brown at 12 h and to a dark brown solution at 36 h as the reaction progressed as shown in Fig. 2.
UV–visible spectroscopic analysis
The continuous formation of the AgNPs was monitored using UV–Vis spectrophotometer. The synthesized CFF reduced AgNPs that had shown a unique absorption peak attributing to their surface plasmon resonance (SPR). The intensity of the absorption peak at 411 nm was increased with incubation time till 48 h and no additional change in the location of the absorption peak was observed even after 72 h of incubation (Table 1), which exhibited the consistent particle shape of AgNPs(Wu et al. 2018). Furthermore, the synthesis of highly dense nanoparticles is shown to be accountable for raising the intensity of absorptionpeaks (Anandan et al. 2019).Results obtained has been found consistent with the observations of many researchers reported globally (Li et al. 2009; Singh et al. 2017). Figure 3 shows no changein SPR of synthesized AgNPs even after 6 months (stored at room temperature).
Band gap energy by Tauc’s plot
Band gap energy of synthesized silver nanoparticles was calculated from Tauc’s plot by extrapolating the linear portion of the UV-Visible curve. Figure 4 show that the synthesized AgNPs have a value of band gap energy 2.44 eV. These particles with the large value of band gap energy can be further used in advance optoelectronic devices, batteries and sensors as a semi-conductive material. The value of band gap is much similar to earlier reported literature and this value could be due to quantum confinement effect (Das et al. 2016).
Fourier-transform infrared spectroscopy (FTIR) analysis
The FTIR spectrum of synthesized AgNPs is shown in Fig. 5, which manifests absorption peaks located between the region about 4000 cm−1 and 500 cm− 1. FTIR spectra showed absorption bands at 3290 cm−1, 2113 cm−1, 2047 cm−1, 1635 cm−1, 1372 cm−1 and 1212 cm−1 indicating the presence of capping and stabilizing biomolecules with the nanoparticles. Spectra of ATR-FTIR was analyzed using software SpectraGryph (version 1.2) developed by Dr. Friedrich Menges. The peaks and their corresponding functional groups are shown in Table 2, which are in consent with the observations reported by several researchers globally.
The incorporation of silver salt to CFF solution at optimum conditions results in immediate binding of silver ions with the protein and other molecules present in CFF solution with functional groups such as –OH and C=C get captured leading towards conformational changes in proteins which represent its hydrophobic residues to aqueous phase resulting in infiltration of reducing agents from CFF solution and hence, provides capping to silver ions with the formation of stable silver nanoparticles (Rheder et al. 2018). Considering the CFF of marine fungi Aspergillus brunneoviolaceus used in this study, it can be suggested that alkyne, sulfur compounds, alcohol and phenolic compounds, proteins and other water-soluble biomolecules served as a reducing and stabilizing agent (Gopinath et al. 2013).
X-ray diffraction analysis
The unique XRD patterns of the mycosynthesized AgNPs is shown in Fig. 6. XRD pattern exhibited diffraction peaks corresponding to [111], [200], [220], [311] and [222] appearing at 2θ representing the value of 37.96°, 46.08°, 64.4°, 76.83° and 81.1° respectively. These peaks represent crystallographic planes of the face centered cubic (fcc) Ag in accordance with COD ID no. 1100136. The mean particle size of AgNPs was calculated using the Debye-Scherrer formula given as D = 0.9λ/β cos θ, where D is the crystalline size (nm), λ is the wavelength of X-ray (0.1541 nm), β represent the angular line full width at half maximum (FWHM) of the peak (in radians) and θ is the Braggs angle (in radians) (Cullity and Stock 2013). The mean size of the Ag nanoparticles was estimated 4.3 ± 0.49 nm as in the range between 2.8 and 5.39 nm as represented in Table 3. The extensive pattern of the XRD diffraction peaks are accredited to the nanocrystalline nature of the mycosynthesized NPs.
Transmission electron microscopy (TEM) analysis
TEM observation proved the synthesis of nanocrystalline silver particles, as illustrated in Fig. 7. The AgNPs had predominantly taken up a spherical morphology, uniform size with average particle size of 1.4 ± 0.8 nm. In exceptional cases, particles with larger sizes were also observed in the sample, but their numbers were quite less. TEM image of synthesized AgNPs showed the existence of lattice fringes indicating the crystalline nature of the AgNPs with the‘d’ spacing values interrelated to the XRD observations. Moreover, the selected area electron diffraction (SAED) pattern revealed the ring patterns accompanied by the single spots in a ring (Fig. 7) which are in accordance with the XRD patterns. The size distribution curve obtained from the TEM analysis is presented in Fig. 8. The size of the particles ranges between 0.72 and 15.21 nm. Larger size range of AgNPs synthesized using different fungi as well as plant extract was also reported till the date (Jemilugba et al. 2019).
Antibacterial activity of AgNPs
The antibacterial potential of AgNPs was assessed by measuring the inhibition zone diameter and the observations are hown in Table 4. Maximum zone of inhibition was observed for Gram-positive compared to Gram-negative bacteria. This result evidently recognized the possibility of using these AgNPs as broad-spectrum antimicrobial agents. The present study is in concurrence with the view of Ma et al. (2016) that the probable antimicrobial potential of AgNPs is because of their higher surface-to-volume ratio and their crystalline structure (Kharat et al. 2016). The AgNPs are shown to disrupt the cell membrane resulting in the release of reactive oxygen species (ROS) which damage the DNA and protein followed by the killing of the microorganism (Ismail et al. 2018). Many preceding reports have shown the toxicity of AgNPs against Gram-negative bacteria than Gram-positive bacteria, as they possess less amount of peptidoglycan layer through which AgNPs get admitted inside them leading towards the destruction of their proteins and DNA ultimately, resulting in microbial death. Conversely, due to the thicker peptidoglycan layer in gram positive bacteria, a higher concentration of AgNPs is required to act against it (Saravanan et al. 2018) Hence, AgNPs are not much useful against Gram-positive pathogens. In this study interestingly, AgNPs derived from CFF of marine fungi Aspergillus brunneoviolaceus were found to be similarly effective against Gram-positive and Gram-negative bacteria. This property of synthesized AgNPs will aid in the expansion of a broad-spectrum antimicrobial activity.
Antioxidant capacity of CFF derived AgNPs
DPPH is a steady compound which can be reduced by accepting the hydrogen or electrons and has been widely applied to determine the antioxidant activity. AgNPs showed effective antioxidant potential as their radical scavenging ability was increasing with the increment in their concentration. Figure 9 shows the antioxidant activity of the AgNPs was about 63.97% and vitamin C was about 28.13%. Results confirmed that the AgNPs have more antioxidant activity than vitamin C.
Antioxidant property of the silver nanoparticles is due to the adsorption of fungal constituents from CFF on the silver nanoparticle (Keshari et al. 2020). Marine fungi are proficient producers of metal nanoparticles that have a wide range of biological properties such as antioxidant, antimicrobial and antimalarial. Recently, marine endophytic fungi Cladosporium cladosporioides isolated from seaweed have been found to synthesize AgNPs with high antioxidant activity (Hulikere and Joshi 2019). The result strongly suggests the potent application of AgNPs as natural antioxidants for health protection against many oxidative stresses allied with degenerative diseases (Sulaiman et al. 2015). In fact, this antioxidant evaluation is necessary for synthesized AgNPs before its application for experimental models and also for humans.
Conclusions
Nanoparticles synthesized using biological method is an easy alternative over physical and chemical methods. In this study, the prospective wide-ranging antimicrobial and antioxidant silver nanoparticles have been synthesized using the aqueous extract of the marine fungus Aspergillus brunneoviolaceus. The synthesized AgNPs were characterized by different analytical methods including UV–Vis, FTIR, TEM and XRD analysis. This study shows that the biogenic synthesis of AgNPs using fungi proposes several advantages and these materials have promising potential for a range of applications in the areas of health and agriculture. The synthesized nanoparticles possess capping components secreted from the fungi, which confer stability. Based on the fungi used, this capping may also show biological activity, acting synergistically with the effect of the nanoparticles. Further studies at the molecular level may help in exploiting these nanoparticles in the development of broad-spectrum antibiotic and anticancer agents.
References
Anandan M, Poorani G, Boomi P et al (2019) Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaeaviscosa with their antibacterial and anticancer activities. Process Biochem 80:80–88. doi:https://doi.org/10.1016/j.procbio.2019.02.014
Birla SS, Gaikwad SC, Gade AK, Rai MK (2013) Rapid Synthesis of Silver Nanoparticles from Fusarium oxysporum by Optimizing Physico cultural Conditions. Sci World J 2013:1–12. doi:https://doi.org/10.1155/2013/796018
Cullity BD, Stock SR (2013) Elements of x-ray diffraction. Pearson Education Press, Harlow
Das AJ, Kumar R, Goutam SP (2016) Sunlight irradiation induced synthesis of silver nanoparticles using glycolipid bio-surfactant and exploring the antibacterial activity. J Bioeng Biomed Sci. https://doi.org/10.4172/2155-9538.1000208
Durán N, Marcato PD, Durán M et al (2011) Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl Microbiol Biotechnol 90:1609–1624. doi:https://doi.org/10.1007/s00253-011-3249-8
Gade AK, Bonde P, Ingle AP et al (2008) Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy 2:243–247. https://doi.org/10.1166/jbmb.2008.401
Gopinath V, Priyadarshini S, Priyadharsshini NM et al (2013) Biogenic synthesis of antibacterial silver chloride nanoparticles using leaf extracts of Cissusquadrangularis Linn. Mater Lett 91:224–227. doi:https://doi.org/10.1016/j.matlet.2012.09.102
Guilger-Casagrande M, Lima RD (2019) Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotech. https://doi.org/10.3389/fbioe.2019.00287
Hulikere MM, Joshi CG (2019) Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus- Cladosporium cladosporioides. Process Biochem 82:199–204. doi:https://doi.org/10.1016/j.procbio.2019.04.011
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385–406
Ismail RA, Sulaiman GM, Mohsin MH, Saadoon AH (2018) Preparation of silver iodide nanoparticles using laser ablation in liquid for antibacterial applications. IET Nanobiotechnol 12:781–786. doi:https://doi.org/10.1049/iet-nbt.2017.0231
Jemilugba OT, Sakho EHM, Parani S et al (2019) Green synthesis of silver nanoparticles using Combretum erythrophyllum leaves and its antibacterial activities. Colloid Interface Sci 31:100191. https://doi.org/10.1016/j.colcom.2019.100191
Jyoti K, Baunthiyal M, Singh A (2016) Characterization of silver nanoparticles synthesized using Urticadioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci 9:217–227. https://doi.org/10.1016/j.jrras.2015.10.002
Keshari AK, Srivastava R, Singh P et al (2020) Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integr Med 11:37–44. doi:https://doi.org/10.1016/j.jaim.2017.11.003
Kharat SN, Mendhulkar VD (2016) Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopusscaber leaf extract. Mater Sci Eng C 62:719–724. https://doi.org/10.1016/j.msec.2016.02.024
Koyyati R, Nagati V, Nalvothula R et al (2014) Antibacterial activity of silver nanoparticles synthesized using Amaranthus viridis twig extract. Int J Res Pharm Sci 5:32–39
Kumar DA, Palanichamy V, Roopan SM (2014) Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A 127:168–171. doi:https://doi.org/10.1016/j.saa.2014.02.058
Li W-R, Xie X-B, Shi Q-S et al (2009) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85:1115–1122. doi:https://doi.org/10.1007/s00253-009-2159-5
Loo YY, Rukayadi Y, Nor-Khaizura M-A-R et al (2018) In vitro antimicrobial activity of green synthesized silver nanoparticles against selected Gram-negative foodborne pathogens. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01555
Ma J, Fu K, Shi J et al (2016) Ultraviolet-assisted synthesis of polyacrylamide-grafted chitosan nanoparticles and flocculation performance. Carbohydr Polym 151:565–575. https://doi.org/10.1016/j.carbpol.2016.06.002
Manivasagan P, Venkatesan J, Sivakumar K, Kim S-K (2016) Actinobacteria mediated synthesis of nanoparticles and their biological properties: a review. Critical Rev Microbiol 42(2):209–221
Nayak B, Nanda A, Prabhakar V (2018) Biogenic synthesis of silver nanoparticle from wasp nest soil fungus, Penicillium italicum and its analysis against multi drug resistance pathogens. Biocatal Agri Biotechnol 16:412–418. https://doi.org/10.1016/j.bcab.2018.09.014
Netala VR, Kotakadi VS, Bobbu P, Gaddam SA, Tartte V (2016) Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti microbial studies. 3 Biotech 6(2):132
Ramos MM, Morais EDS, Sena IDS et al (2020) Silver nanoparticle from whole cells of the fungi Trichoderma spp. isolated from Brazilian Amazon. Biotechnol Lett 42:833–843. https://doi.org/10.1007/s10529-020-02819-y
Rheder DT, Guilger M, Bilesky-José N et al (2018) Synthesis of biogenic silver nanoparticles using Althaea officinalis as reducing agent: evaluation of toxicity and ecotoxicity. Sci Rep. https://doi.org/10.1038/s41598-018-30317-9
Saravanan M, Arokiyaraj S, Lakshmi T, Pugazhendhi A (2018) Synthesis of silver nanoparticles from Phenerochaetechrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. MicrobPathog 117:68–72. doi:https://doi.org/10.1016/j.micpath.2018.02.008
Singh T, Jyoti K, Patnaik A et al (2017) Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J Genet Eng Biotechnol 15:31–39. https://doi.org/10.1016/j.jgeb.2017.04.005
Sulaiman GM, Hussien HT, Saleem MMNM (2015) Biosynthesis of silver nanoparticles synthesized by Aspergillus flavus and their antioxidant, antimicrobial and cytotoxicity properties. Bull Mater Sci 38:639–644. https://doi.org/10.1007/s12034-015-0905-0
Thirunavoukkarasu M, Balaji U, Behera S et al (2013) Biosynthesis of silver nanoparticle from leaf extract of Desmodiumgangeticum (L.) DC. and its biomedical potential. Spectrochim Acta A 116:424–427. https://doi.org/10.1016/j.saa.2013.07.033
Velusamy P, Kumar GV, Jeyanthi V et al (2016) Bio-inspired green nanoparticles: synthesis, mechanism, and antibacterial application. Toxicol Res 32:95–102. https://doi.org/10.5487/tr.2016.32.2.095
Wu T, Lu F, Wen Q et al (2018) Novel strategy for obtaining uniformly dispersed silver nanoparticles on soluble cotton wound dressing through carboxymethylation and in-situ reduction: antimicrobial activity and histological assessment in animal model. Cellulose 25:5361–5376. doi:https://doi.org/10.1007/s10570-018-1907-z
Zomorodian K, Pourshahid S, Sadatsharifi A et al (2016) Biosynthesis and characterization of silver nanoparticles by Aspergillus species. BioMed Res Int 2016:1–6. https://doi.org/10.1155/2016/543539
Acknowledgements
The Authors highly acknowledge the Department of Biotechnology and Department of Life Sciences for providing the research facilities.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that the content of this manuscript has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mistry, H., Thakor, R., Patil, C. et al. Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnol Lett 43, 307–316 (2021). https://doi.org/10.1007/s10529-020-03008-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-03008-7