Abstract
Fixed-bed bioreactors packed with macrocarriers show great potential to be used for vaccine process development and large-scale production due to distinguishing features of low shear force, high cell adhering surface area, and easy replacement of culture media in situ. As an initial step of utilizing this type of bioreactors for Pseudorabies virus production (PRV) by African green monkey kidney (Vero) cells, we developed a tube-fixed-bed bioreactor in the previous study, which represents a scale-down model for further process optimization. By using this scale-down model, here we evaluated impacts of two strategies (use of serum-free medium and low cell inoculum density) on PRV production, which have benefits of simplifying downstream process and reducing risk of contamination. We first compared Vero cell cultures with different media, bioreactors and inoculum densities, and conclude that cell growth with serum-free medium is comparable to that with serum-containing medium in tube-fixed-bed bioreactor, and low inoculum density supports cell growth only in this bioreactor. Next, we applied serum-free medium and low inoculum cell density for PRV production. By optimization of time of infection (TOI), multiplicity of infection (MOI) and the harvesting strategy, we obtained total amount of virus particles ~ 9 log10 TCID50 at 5 days post-infection (dpi) in the tube-fixed-bed bioreactor. This process was then scaled up by 25-fold to a Xcell 1-L fixed-bed bioreactor, which yields totally virus particles of 10.5 log10 TCID50, corresponding to ~ 3 × 105 doses of vaccine. The process studied in this work holds promise to be developed as a generic platform for the production of vaccines for animal and human health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudorabies virus (PRV) is a member of the family Herpesviridae and the causative agent of Aujeszky’s disease (Dong et al. 2014; Nauwynck et al. 2007), which is consider to be the most economically devastating viral disease for swine industry. Swine is the natural host of PRV, but other mammals, including cattle, sheep and goats, can also be infected. Nowadays, attenuated live or inactivated vaccines have been developed and applied to prevent swine from this fatal disease in industry (Slivac et al. 2006; Wittmann 1991), among which the strain PRV Bartha-K61 is the most common one used worldwide (Green 1986; McFerran and Dow 1975).
Chick embryo fibroblasts (CEF), BHK-21 fibroblast cell line and Swine testicular (ST) cell line were used for PRV production (Onyekaba et al. 1987; Srcek et al. 2004), while Vero cells from the kidney of an African green monkey are mammalian cell lines regularly used for human vaccine production (Barrett et al. 2017; Organization 1987). Considering high safety and productivity of Vero cells, along with the broad market of PRV, we intend to develop an optimized industry-scale process of using such cell lines for PRV production.
Regarding to large-scale production of adherent Vero cell-based vaccines, stirred tank bioreactors (STR bioreactors) with microcarriers and fixed-bed bioreactors with macrocarriers are the two main systems used for process development (Wen and Yang 2011). Shear force is typically generated by agitation and collisions between microcarriers in STR bioreactors, which causes cell damage (Cherry and Papoutsakis 1986; Wen and Yang 2011). In contrast, fixed-bed bioreactors contain statically packed macrocarriers where cells adhere and grow, and the design results in physical sequestration of cells from the agitation system, which greatly reduces shear force and hence becomes more preferred for vaccine production (Meuwly et al. 2007; Wen and Yang 2011). In our previous study, we successfully developed and characterized the tube-bed-fixed bioreactor, a scale-down model of fixed-bed bioreactors, which was used for PRV production by Vero cells in a serum-containing medium with initial cell inoculum density of 2 × 105 cells/mL (Nie et al. 2019).
Serum in culture media and cell inoculum density are two key factors impacting the whole process of vaccine production. Serum is typically supplied in a mammalian cell culture medium, which provides growth factors and attachment factors supporting cell growth. However, addition of serum also increases potential risk of virus contamination, as well as costs of down-stream process (Toriniwa and Komiya 2007). As an alternative, serum-free media for adherent mammalian cells have been developed and successfully used for vaccine production in STR bioreactors and fixed-bed bioreactors (Mattos et al. 2015; Toriniwa and Komiya 2007), in which recombinant growth factors and attachment factors are additionally supplied (Gallo-Ramirez et al. 2015; Varani et al. 1993). Cells become more sensitive in a serum-free medium than in a serum-containing medium, since quite a few components of serum increase viscosity and hence protect cells from shear force (Ozturk and Palsson 1991). This issue can be solved through addition of the shear protectant Pluronic F-68 (Wu 1999; Zhang et al. 1992).
Large-scale production of vaccine by mammalian cells requires considerable seed inoculum, and hence use of great number of seed fermenters (such as roller bottles or Cell Factory systems). Not only this rises cost, but more importantly, manual operations in a large-scale process increase risk of contamination during inoculation. Meanwhile, gene expression and phenotype can vary between low passage and high passage cell lines, which is related to the so-called passage number effects and should be seriously considered (Abdul-Hamid et al. 2019; Krell 1996). Reduced inoculum density is thus desired. Since cells are able to adhere, migrate and then propagate on macrocarriers (Gallo-Ramirez et al. 2015), fixed-bed bioreactors may provide a solution for effective vaccine production with low inoculum density.
In this study, we validated PRV production by Vero cells in fixed-bed bioreactors using serum-free medium with low inoculum density. We first verified that the tube-fixed-bed bioreactor developed in previous study (Nie et al. 2019) is suitable for Vero cell culture in serum-free medium with low inoculum density. We then optimized TOI, MOI and the harvesting strategy for PRV production. Finally, this process was scaled-up by 25-fold to a 1 L Xcell fixed-bed bioreactor, which totally yields virus particles of 10.5 log10 TCID50, corresponding to ~ 3 × 105 doses of vaccine. Such process could be developed as a generic platform for production of other vaccines for animal and human health.
Materials and methods
Culture media, cell line and virus strain
The ready-to-use Vero-A serum-free medium was purchased from TBD (TBD Science, Cat. No. JNVAS001, China). To protect cells from shear force, Pluronic F-68 (Gibco, Cat. No. 24040032, USA) was supplemented into this medium with final concentration of 0.75 mL/L in all experiments. The DMEM medium (HyClone, Cat. No. SH30243.01, USA) supplemented with 10% fetal bovine serum (Biological Industries, Cat. No. 04-001-1ACS, Israel) was used as the serum-containing medium.
Adherent Vero cells were obtained from ATCC (CCL-81) and pre-cultured in a T-flask (Thermo Scientific Nunc, Cat. No. 156367, USA) with serum-containing medium at 37 °C and 5% CO2 atmosphere. Before inoculation, sub-culture was prepared by passing seed culture three times with serum-free medium using direct adaptation strategy according to TrypLE Express Enzyme (GIBCO, Cat. No. 12605028, USA) user manual.
PRV strain Bartha-K61 was obtained from Zhejiang Ceva Ebvac Biotech Co., Ltd. (Hangzhou, China). Bartha-K61 continuously passed three times through Vero cells in the serum-free medium to produce working seed virus. Virus stocks were stored in 1-mL aliquots (7.5 log10 TCID50/mL) at − 70 °C.
Cell cultures in the tube-fixed-bed bioreactor and the spinner flask
As for culture in tube-fixed-bed bioreactor, Vero cells were inoculated into 20 mL working media. Culture media were replaced with fresh media daily post inoculation. Cells were grown at 37 °C, 50 rpm and 5% CO2 atmosphere. As for culture in 250 mL spinner flasks, Vero cells were inoculated into 50 mL culture media with 4 g/L Cytodex-1 microcarriers (GE Healthcare, Cat. No.17-0448-04, Sweden). Half culture media were replaced daily with fresh media when glucose concentration was lower than 1.5 g/L. Normal inoculum density and low inoculum density are 2 × 105 cells/mL and 5 × 104 cells/mL, respectively. Samples were taken daily to measure cell density and metabolites (glucose and lactate) in supernatants. Experiments were run in triplicate.
Optimization of PRV production in the tube-fixed-bed bioreactor
Vero cells grown in serum-free medium were infected by PRV with different doses (MOI = 0.0001, 0.001, 0.01, 0.05, 0.1) and at different time points (TOI = day 3, 5 and 7). Three harvesting strategies were compared, including “one harvest” (no medium replaced), “half-medium harvest” (50% medium replaced with fresh medium daily) and “full-medium harvest” (100% medium replaced with fresh medium daily). Samples were collected daily to measure virus titer by a TCID50 assay described in Sect. “Analytical methods” below, and experiments were carried out in duplicate.
PRV production in a Xcell 1-L fixed-bed bioreactor
PRV production was scaled up to a Xcell 1-L fixed-bed bioreactor, using the serum-free medium. Two inoculum densities (2 × 105 cells/mL and 5 × 104 cells/mL) were compared. MOI = 0.001, TOI = day 3 (for normal inoculum density) and TOI = day 6 (for low inoculum density), and full-harvest process were optimized parameters used. Temperature was set to 37 °C, pH was maintained at 7.2 by injection of CO2 or addition of 7.5% (w/v) NaHCO3, and pO2 was controlled at 50% air-saturation by injecting air or pure oxygen. Experiments were carried out in duplicate.
Analytical methods
Cell density was measured using crystal violet staining method (Ho et al. 2004; Nie et al. 2019). Macrocarriers in a tube-fixed-bed bioreactor or microcarriers from a spinner flask, attached with adherent Vero cells, were transferred into a centrifuge tube, mixed with crystal violet reagent (0.1% crystal violet in 0.1 M citric acid solution), incubated in water bath for 2 h at 37 °C and shaked with interval to detach cells from carriers. Cells were counted using blood cell counting chamber and hence cell density was calculated.
Concentrations of glucose and lactate in culture media were determined using commercially available enzymatic kits (Nanjing Jiancheng Bioengineering Institute, Cat. No. F006-1-1 & Cat. No. A019-2-1, China).
PRV titer was quantified by the median endpoint of a tissue culture’s infectious dose (TCID50) described by (Liu et al. 2011). Cytopathic effects (CPE) in infected Vero cells were counted and the TCID50 value was calculated by the Reed-Muench method (MUENCH 1937). Number of virus particles was calculated by multiplying virus titer (TCID50/mL) with volume of the medium harvested (mL). Cumulative virus particles were summation of virus particles harvested each day. All assays were performed in duplicate.
Results
Culture of Vero cells in the serum-free medium
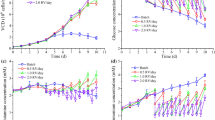
PRV production in fixed-bed bioreactors with serum-containing medium was described in our previous work (Nie et al. 2019). Due to obvious advantages of serum-free medium in vaccine production, we first compared growth of Vero cells in these two media with normal inoculum density (2 × 105 cells/mL). As shown in Fig. 1a, during seven days of culture, serum-containing medium supported better cell growth than serum-free medium in either tube-fixed-bed bioreactor or spinner flask. Serum-free medium was refreshed daily in tube-fixed-bed bioreactor, while only half of the used medium in spinner flask was refreshed daily, due to lack of a filter module to separate microcarriers from the medium completely. This may help to explain the observed more glucose (Fig. 1c) and less lactate (Fig. 1d) detected in tube-fixed-bed bioreactor, and hence better cell growth (Fig. 1a). Next, we briefly evaluated morphological characteristics of adherent Vero cells grown on the microcarriers in serum-free medium with serum-containing medium on day 3 and day 7, which indicates Vero cells are able to propagate well on both media (Fig. 1b). Based on results above, we conclude that with normal inoculum density, Vero cells are able to grow in either serum-containing medium or serum-free medium, although better in the former. However, difference is almost negligible in the tube-fixed-bed bioreactor (Fig. 1a).
Comparison of Vero cell growth in tube-fixed-bed bioreactor and in spinner flask, with inoculum density 2 × 105 cells/mL. a Cell growth with serum-free medium and serum-containing medium. b Glucose and c lactate concentrations measured from culture media. Tube-fixed-bed bioreactor with serum-containing medium (open circle); tube-fixed-bed bioreactor with serum-free medium (open square); spinner flask with serum-containing medium (open inverted triangle); spinner flask with serum-containing medium (open triangle). d Microscopic images of Vero cells on Cytodex 1 microcarriers in serum-free medium and serum-containing medium at day 3 and day 7. Scale bars represent 200 μm. Standard deviations are for triplicate flasks/bioreactors
Next, we examined growth of Vero cells with reduced inoculum densities (Fig. 2). We set inoculum densities 5 × 104 cells/mL for tube-fixed-bed bioreactor and 1.8 × 104 cells/mL for spinner flask, respectively, which results in equal cell density per surface area of the carriers (1 × 103 cells/cm2). As shown in Fig. 2a, after 14 days of culture in tube-fixed-bed bioreactor, Vero cells were grown to 1.1 × 107 cells/mL in serum-containing medium and 7.7 × 106 cells/mL in serum-free medium, respectively, which are 220-fold and 154-fold over the initial density. In comparison, after 14 days of culture in spinner flask, Vero cells reached 3.2 × 106 cells/mL in serum-containing medium and only 2 × 105 cells/mL in serum-free medium, respectively, which represent 178-fold and only 11-fold over the initial density. Actually, cells were grown very poorly with serum-free medium in spinner flask (Fig. 2a). We also measured glucose and lactate during these culture processes. Besides the case of poor cell growth in spinner flask with serum-free medium (no fresh media replaced due to low nutrients consumption), all other three culturing processes follow the trend that faster growing cells consume more glucose and accumulate more lactate (Fig. 2b, c). With low inoculum density, Vero cells grown in serum-free medium reached 70% final density of that in serum-containing medium in tube-fixed-bed bioreactor.
Cell growth in tube-fixed-bed bioreactor and spinner flask with low cell inoculation densities. Vero cells were inoculated into tube-fixed-bed bioreactor and spinner flask at 5 × 104 cells/mL (1042 cells/cm2) and 1.8 × 104 cells/mL (1042 cells/cm2), respectively. a Growth curves. b Glucose and c lactate concentration. Tube-fixed-bed bioreactor with serum-containing medium (open circle); tube-fixed-bed bioreactor with serum-free medium (open square); spinner flask with serum-containing medium (open inverted triangle); spinner flask with serum-containing medium (open triangle). Standard deviations are for triplicate flasks/bioreactors
To summarize, Vero cells show close (slightly inferior) growth in serum-free medium to serum-containing medium. But considering its potential benefits of avoiding virus contamination and simplifying down-stream process, we still used it for following PRV production in fix-bed bioreactors.
PRV production and process optimization in tube-fixed-bed bioreactor
We then infected Vero cells with PRV and optimized its production process in serum-free medium in tube-fixed-bed bioreactor by evaluating different TOIs, MOIs and harvesting strategies, similar to our previous study in serum-containing medium (Nie et al. 2019).
We first tested effect of TOI on PRV production with normal inoculum density of Vero cells (2 × 105 cells/mL). As shown in Fig. 3a, the highest virus titer reached 7.7 log10 TCID50/mL at 4 days post infection (dpi) with TOI = day 3. In general, virus titer reached the plateau at 2 dpi, and TOI = day 3 and day 5 resulted in similar PRV titer, higher than TOI = day 7. Therefore, TOI = day 3 was chosen for the following experiments. We next evaluated effect of MOI on PRV production (Fig. 3a). PRV titer also reached the plateau at 2 dpi, and obviously higher MOI resulted in lower virus production. The highest virus titer reached 7.6 log10 TCID50/mL at 3 dpi with MOI of 0.001, which was applied in the following experiments. We finally tested different virus harvesting strategies on PRV production (Fig. 3c). Virus titers varied among different strategies, but there’s a trend that virus titer decreases with increased daily refreshed medium at 5 dpi, which is probably due to detachment of infected cells from macrocarriers and subsequently removal from bioreactors during change of the medium. However, full-medium harvesting strategy indeed increased total PRV yield. We obtained cumulative PRV virus particles of 9.3 log10 TCID50 at 5 dpi in a total of 100 mL virus-containing medium by this strategy, as compared to 8.9 log10 TCID50 by one harvesting and 9.1 log10 TCID50 by half-medium harvesting, respectively (Fig. 3c).
PRV production process optimization in tube-fixed-bed bioreactor. Effects of a TOIs (day 3, day 5 and day 7), b MOI (0.0001, 0.001, 0.01, 0.05, and 0.1) and c harvest strategies (one harvest, half-medium harvest and full-medium harvest) on PRV yield were tested. In c, virus titers and cumulative total virus particles are shown in columns and lines, respectively. One harvest ( , open square); half-medium harvest (
, open square); half-medium harvest ( , open triangle); full-medium harvest (
, open triangle); full-medium harvest ( , open inverted triangle). Standard deviations are for duplicate bioreactors
, open inverted triangle). Standard deviations are for duplicate bioreactors
We next examined effect of reduced Vero cell inoculum density (5 × 104 cells/mL) on PRV production. This time we applied TOI = day 6 for infection with virus (MOI = 0.001) in tube-fixed-bed bioreactor, when cell density reached the similar value (~ 1.2 × 106 cells/mL) at TOI = day 3 at normal inoculum density. In this case, the highest daily virus titer and the highest cumulative PRV particles reached 7.3 log10 TCID50/mL at 2 dpi and 8.9 log10 TCID50 at 5 dpi, respectively (Fig. 4), which are close to normal inoculum density.
PRV production in tube-fixed-bed bioreactor (low cell inoculum density of 5 × 104 cells/mL ( , open inverted triangle)) and Xcell 1 L fixed-bed bioreactor (normal cell inoculum density of 2 × 105 cells/mL (
, open inverted triangle)) and Xcell 1 L fixed-bed bioreactor (normal cell inoculum density of 2 × 105 cells/mL ( , filled square), low cell inoculum density of 5 × 104 cells/mL (
, filled square), low cell inoculum density of 5 × 104 cells/mL ( ,open square)). Virus titers and cumulative virus particles are shown in columns and lines, respectively. Error bars represent standard deviations from two independent determinations
,open square)). Virus titers and cumulative virus particles are shown in columns and lines, respectively. Error bars represent standard deviations from two independent determinations
PRV production in a Xcell 1-L fixed-bed bioreactor
Finally, we scaled up PRV production from the tube-fixed-bed bioreactor to the Xcell 1-L fixed-bed bioreactor with normal (2 × 105 cells/mL) and low (5 × 104 cells/mL) densities, using process parameters optimized in previous section: MOI = 0.001, TOI = day 3 (for normal inoculum density) and TOI = day 6 (for low inoculum density), and full-medium harvesting strategy. As shown in Fig. 4 and compared to Fig. 3, two bioreactors show similar virus titers at each dpi, and in this 1-L bioreactor two inoculum densities resulted in comparable total virus particles. With low inoculum density, the highest virus titer reached 7.5 log10 TCID50/mL at 2 dpi and the highest cumulative virus particles reached 10.5 log10 TCID50 from a total of 2.5 L PRV-containing medium at 5 dpi, which corresponds to 3.1 × 105 doses of vaccine [one vaccine dose requires 5.0 log10 TCID50/mL (Srcek et al. 2004)].
Discussion
The attenuated modified live Bartha-K61 virus is nowadays used worldwide to control pseudorabies, providing sound protection against PRV challenge in growing pigs (Zhou et al. 2017). Development of PRV vaccine production process include two steps: (1) Optimization of production by continuous cell lines (BHK-21 cells, swine testicular cells and PK-15 cells) in scale-down models (T-flasks, roller bottles or lab-scale bioreactors) (Slivac et al. 2006). Key process parameters, such as MOI, TOI (Hu et al. 2008; Silva et al. 2008; Yuk et al. 2006) and harvesting strategy (Srcek et al. 2004; Tapia et al. 2014), are optimized to improve virus yield in this step. Especially by using tube-fixed-bed bioreactors, optimization can be done in a high through-put manner (Nie et al. 2019); (2) Large-scale production by STR bioreactors with microcarriers or fixed-bed bioreactors with macrocarriers (Gallo-Ramirez et al. 2015). STR bioreactors with microcarriers (surface-to-mass ratio 4400 cm2/g, 2–12 g/L (Bock et al. 2011) in culture) are commonly used for virus production, due to several merits including quasi-suspension and bead-to-bead transfer process (Gallo-Ramirez et al. 2015). A sparger is introduced into the bottom of a STR bioreactor, which helps to improve oxygen transfer efficiency. However, shear force (caused by the stirred impeller or break of bubbles) and collision (between microcarriers or between impeller and microcarriers) often result in severe cell damage and hence sub-optimal PRV production (Cherry and Papoutsakis 1986; Wen and Yang 2011).
As an alternative, fixed-bed bioreactors with macrocarriers overcome limitations of STR bioreactors, and have gained growing attention in culture of mammalian cells in past a few years (Portner et al. 2007). This type of bioreactors uses a unique mixing strategy which allows separation of cells from foams and hence protects them from shear force caused by break of bubbles (Wang et al. 1995). Meanwhile, the impeller of a fixed-bed bioreactor is typically designed far away from static macrocarriers, where cells adhere, which further avoids the negative impact of shear force on cells (Wen and Yang 2011). This type of bioreactor also allows easy full-medium exchange without loss of cells during the culturing process, which enables replenishment of nutrients (e.g. glucose) and removal of accumulated metabolites that impair cell growth (e.g. lactate). In contrast, spinner flask or STR bioreactor only allows replacement of up to 70% media. Therefore, fixed-bed bioreactor has been widely applied for production of antibodies and virus vaccines (Gallo-Ramirez et al. 2015; Meuwly et al. 2007). We also developed a tube-fixed bed bioreactor in the previous study, which represents a scale-down model of a large-scale fixed-bed bioreactors (Nie et al. 2019). The only demerit of fixed-bed bioreactors is that cells adhere to macrocarriers, so that cell density can only be estimated based on concentrations of metabolites, such as glutamine or glucose (Kaufman et al. 2000; Tapia et al. 2014).
In order to achieve optimized large-scale vaccine production in a fixed-bed bioreactor, two factors, serum in the medium and cell inoculum density, also need to be seriously considered. As for an important component for mammalian cell culture, animal serum provides nutrients, hormones and growth factors for cell growth, which is widely used for virus production (Butler 2015). However, addition of serum also results in complication of the downstream purification process and increases risk of virus contamination, especially for production of biopharmaceuticals (Butler 2015). In some special cases, serum in media even needs to be removed in the upstream process. For example, the hemagglutinin glycoprotein HA needs be cleaved into HA1 and HA2 by trypsin to achieve cell infection for influenza virus production (Le Ru et al. 2010). Presence of serum leads to loss of trypsin activity and thus it must be removed before virus infection (Le Ru et al. 2010). Therefore, use of serum-free medium began to draw attention in recent years in pharmaceutical industry, which overcomes drawbacks of using serum-containing media (Butler 2015; Genzel and Reichl 2009; Huang et al. 2015). Cells grown in serum-free media show increased sensitivity to shear force due to impaired adhesive ability on macrocarriers without serum (Ozturk and Palsson 1991), but this issue typically can be solved through addition of shear protectant Pluronic F-68 (Wu 1999). In this study, we comprehensively investigated growth of Vero cells in a serum-free medium with different culture conditions. We found use of serum-free medium resulted ~ 30% less cell density than serum-containing medium (2.5 × 106 cells/mL vs. 3.7 × 106 cells/mL) after seven days in spinner flask. When culture was transferred into tube-fixed bed bioreactor, cell densities in both serum-free and serum-containing media reached similar level (> 5 × 106 cells/mL), which may be due to significantly reduced shear stress. These values are all at close range from other reports of bioreactors with different process parameters (1.5–3.6 × 106 cells/mL in batch mode and 1 × 107 cells/mL in recirculation mode in serum-containing media of STR with microcarriers (Silva et al. 2008; Trabelsi et al. 2006), and 1.8–2.5 × 106 cells/mL in in serum-free media VP-SFM (Liu et al. 2011; Rourou et al. 2007).
Traditional expansion in roller bottles or Cell Factory systems is still the most common method to prepare inoculum for virus production in fixed-bed bioreactor. Thus substantial numbers of vessels are needed for large-scale culture process, and hence risk of contamination increases. Since cells are able to migrant and propagate on macrocarriers in fixed-bed bioreactor, we hypothesized inoculation of Vero cells with low inoculum density is possible. In this study, we tested cell growth with inoculum density of 5 × 104 cells/mL (equal to 1042 cells/cm2), fourfold lower than the normal inoculum density. In this case, cells are able to grow up either in serum-containing medium or serum-free medium. During the first eight days of culture, cell densities are comparable for two media. At the end of culture on 14th day, cell density in serum-free medium still shows 70% of that in serum-containing medium (Fig. 2a). In contrast, low inoculum density resulted in poor growth in spinner flask, which is probably due to high shear force in this type of bioreactor (Fig. 2a).
We then applied PRV production in tube-fixed-bed bioreactor using serum-free medium. TOI = day 3, MOI = 0.001 and full-harvesting strategy turned out to be the optimal parameters, which resulted in cumulative virus particles of ~ 9 log10 TCID50 at 5 dpi for either normal inoculum density or low inoculum density. Such process was then finally scale-up to a Xcell 1-L fixed-bed bioreactor, which gives cumulative virus particles of 10.5 log10 TCID50 in totally 2.5 L PRV containing medium, which corresponds to 3.1 × 105 doses of vaccine.
In summary, we investigated PRV production process with serum-free medium and low inoculum density in fixed-bed bioreactors, which proves its comparable performance to the strategy with serum-containing medium and normal inoculum density in our previous study (Nie et al. 2019). Such strategy has benefits of simplifying downstream process and reducing risk of contamination, which holds potential as a generic platform for producing other vaccines for animal and human health.
References
Abdul-Hamid NA, Abas F, Maulidiani M, Ismail IS, Tham CL, Swarup S, Umashankar S (2019) NMR metabolomics for evaluating passage number and harvesting effects on mammalian cell metabolome. Anal Biochem 576:20–32. https://doi.org/10.1016/j.ab.2019.04.001
Barrett PN, Terpening SJ, Snow D, Cobb RR, Kistner O (2017) Vero cell technology for rapid development of inactivated whole virus vaccines for emerging viral diseases. Expert Rev Vaccines 16:883–894. https://doi.org/10.1080/14760584.2017.1357471
Bock A, Schulze-Horsel J, Schwarzer J, Rapp E, Genzel Y, Reichl U (2011) High-density microcarrier cell cultures for influenza virus production. Biotechnol Prog 27:241–250. https://doi.org/10.1002/btpr.539
Butler M (2015) Serum and protein free media. In: Butler M (ed) Animal cell culture. Cell engineering. Springer, Cham, pp 223–236
Cherry RS, Papoutsakis ET (1986) Hydrodynamic effects on cells in agitated tissue culture reactors. Bioprocess Eng 1:29–41. https://doi.org/10.1007/bf00369462
Dong B, Zarlenga DS, Ren X (2014) An overview of live attenuated recombinant pseudorabies viruses for use as novel vaccines. J Immunol Res 2014:824630. https://doi.org/10.1155/2014/824630
Gallo-Ramirez LE, Nikolay A, Genzel Y, Reichl U (2015) Bioreactor concepts for cell culture-based viral vaccine production. Expert Rev Vaccines 14:1181–1195. https://doi.org/10.1586/14760584.2015.1067144
Genzel Y, Reichl U (2009) Continuous cell lines as a production system for influenza vaccines. Expert Rev Vaccines 8:1681–1692. https://doi.org/10.1586/Erv.09.128
Green JA (1986) Unusual monocyte-lymphocyte interactions determine the specificity of the immune-specific interferon response induced by newcastle disease and herpes simplex viruses. J Med Virol 20:381–389. https://doi.org/10.1002/jmv.1890200411
Ho L, Greene CL, Schmidt AW, Huang LH (2004) Cultivation of HEK 293 cell line and production of a member of the superfamily of G-protein coupled receptors for drug discovery applications using a highly efficient novel bioreactor. Cytotechnology 45:117–123. https://doi.org/10.1007/s10616-004-6402-8
Hu AY et al (2008) Microcarrier-based MDCK cell culture system for the production of influenza H5N1 vaccines. Vaccine 26:5736–5740. https://doi.org/10.1016/j.vaccine.2008.08.015
Huang D et al (2015) Serum-free suspension culture of MDCK cells for production of influenza H1N1 vaccines. PLoS ONE 10:e0141686. https://doi.org/10.1371/journal.pone.0141686
Kaufman JB, Wang G, Zhang W, Valle MA, Shiloach J (2000) Continuous production and recovery of recombinant Ca2+ binding receptor from HEK 293 cells using perfusion through a packed bed bioreactor. Cytotechnology 33:3–11. https://doi.org/10.1023/A:1008143132056
Krell PJ (1996) Passage effect of virus infection in insect cells. Cytotechnology 20:125–137. https://doi.org/10.1007/BF00350393
Le Ru A, Jacob D, Transfiguracion J, Ansorge S, Henry O, Kamen AA (2010) Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing. Vaccine 28:3661–3671. https://doi.org/10.1016/j.vaccine.2010.03.029
Liu CC et al (2011) Purification and characterization of enterovirus 71 viral particles produced from vero cells grown in a serum-free microcarrier bioreactor system. PLoS ONE 6:e20005. https://doi.org/10.1371/journal.pone.0020005
Mattos DA, Silva MV, Gaspar LP, Castilho LR (2015) Increasing Vero viable cell densities for yellow fever virus production in stirred-tank bioreactors using serum-free medium. Vaccine 33:4288–4291. https://doi.org/10.1016/j.vaccine.2015.04.050
McFerran JB, Dow C (1975) Studies on immunisation of pigs with the Bartha strain of Aujeszky’s disease virus. Res Vet Sci 19:17–22. https://doi.org/10.1016/s0034-5288(18)33548-3
Meuwly F, Ruffieux PA, Kadouri A, von Stockar U (2007) Packed-bed bioreactors for mammalian cell culture: bioprocess and biomedical applications. Biotechnol Adv 25:45–56. https://doi.org/10.1016/j.biotechadv.2006.08.004
Muench LJRH (1937) A simple method of estimating fifty per cent endpoints. Am J Epidemiol 27:493–497
Nauwynck H, Glorieux S, Favoreel H, Pensaert M (2007) Cell biological and molecular characteristics of pseudorabies virus infections in cell cultures and in pigs with emphasis on the respiratory tract. Vet Res 38:229–241. https://doi.org/10.1051/vetres:200661
Nie J et al (2019) Production process development of pseudorabies virus vaccine by using novel scale-down model of fixed-bed bioreactor. J Pharm Sci. https://doi.org/10.1016/j.xphs.2019.10.002
Onyekaba C, Bueon L, King P, Fahrmann J, Goyal SM (1987) Susceptibility of various ceil culture systems to pseudorabies virus. Comp Immunol Microbiol Infect Dis 10:163–166. https://doi.org/10.1016/0147-9571(87)90027-0
Organization WH (1987) Requirements for continuous cell lines used for biological substances. Tech Rep Ser 745(1):99–115
Ozturk SS, Palsson BO (1991) Examination of serum and bovine serum albumin as shear protective agents in agitated cultures of hybridoma cells. J Biotechnol 18:13–28. https://doi.org/10.1016/0168-1656(91)90232-k
Portner R, Platas OB, Fassnacht D, Nehring D, Czermak P, Markl H (2007) Fixed bed reactors for the cultivation of mammalian cells: design, performance and scale-up. Open Biotechnol J 1:41–46. https://doi.org/10.2174/1874070700701010041
Rourou S, van der Ark A, van der Velden T, Kallel H (2007) A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine 25:3879–3889. https://doi.org/10.1016/j.vaccine.2007.01.086
Silva AC, Delgado I, Sousa MF, Carrondo MJ, Alves PM (2008) Scalable culture systems using different cell lines for the production of Peste des Petits ruminants vaccine. Vaccine 26:3305–3311. https://doi.org/10.1016/j.vaccine.2008.03.077
Slivac I, Srček VG, RadoŠević K, Kmetič I, Kniewald Z (2006) Aujeszky’s disease virus production in disposable bioreactor. J Biosci 31:363–368. https://doi.org/10.1007/bf02704109
Srcek VG, Cajavec S, Sladic D, Kniewald Z (2004) BHK 21 C13 cells for Aujeszky's disease virus production using the multiple harvest process. Cytotechnology 45:101–106. https://doi.org/10.1007/s10616-004-2551-z
Tapia F, Vogel T, Genzel Y, Behrendt I, Hirschel M, Gangemi JD, Reichl U (2014) Production of high-titer human influenza A virus with adherent and suspension MDCK cells cultured in a single-use hollow fiber bioreactor. Vaccine 32:1003–1011. https://doi.org/10.1016/j.vaccine.2013.11.044
Toriniwa H, Komiya T (2007) Japanese encephalitis virus production in Vero cells with serum-free medium using a novel oscillating bioreactor. Biologicals 35:221–226. https://doi.org/10.1016/j.biologicals.2007.02.002
Trabelsi K, Rourou S, Loukil H, Majoul S, Kallel H (2006) Optimization of virus yield as a strategy to improve rabies vaccine production by Vero cells in a bioreactor. J Biotechnol 121:261–271. https://doi.org/10.1016/j.jbiotec.2005.07.018
Varani J, Inman DR, Fligiel SE, Hillegas WJ (1993) Use of recombinant and synthetic peptides as attachment factors for cells on microcarriers. Cytotechnology 13:89–98. https://doi.org/10.1007/bf00749935
Wang S-J, Zhong J-J, Chen Y-L, Yu J-T (1995) Characterization and modeling of oxygen transfer in a 20-l modified cell-lift bioreactor with a double-screen cage. J Ferment Bioeng 80:71–77. https://doi.org/10.1016/0922-338x(95)98179-o
Wen Y, Yang ST (2011) Microfibrous carriers for cell culture: a comparative study. Biotechnol Prog 27:1126–1136. https://doi.org/10.1002/btpr.625
Wittmann G (1991) Spread and control of aujeszky's disease (AD). Comp Immunol Microbiol Infect Dis 14:165–173. https://doi.org/10.1016/0147-9571(91)90129-2
Wu S-C (1999) Influence of hydrodynamic shear stress on microcarrier-attached cell growth: cell line dependency and surfactant protection. Bioprocess Eng 21:201. https://doi.org/10.1007/s004490050663
Yuk IH et al (2006) A serum-free Vero production platform for a chimeric virus vaccine candidate. Cytotechnology 51:183–192. https://doi.org/10.1007/s10616-006-9030-7
Zhang S, Handa-Corrigan A, Spier RE (1992) Foaming and media surfactant effects on the cultivation of animal cells in stirred and sparged bioreactors. J Biotechnol 25:289–306. https://doi.org/10.1016/0168-1656(92)90162-3
Zhou J, Li S, Wang X, Zou M, Gao S (2017) Bartha-k61 vaccine protects growing pigs against challenge with an emerging variant pseudorabies virus. Vaccine 35:1161–1166. https://doi.org/10.1016/j.vaccine.2017.01.003
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No.2018YFA0900804); the National Natural Science Foundation of China (No. 21878124 and No. 31570034), the Collaborative Innovation Center of Jiangsu Modern Industrial Fermentation, the 111 Project (No. 111-2-06), and TaiShan Industrial Experts Programme (tscy20160307).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nie, J., Sun, Y., Peng, F. et al. Pseudorabies virus production using a serum-free medium in fixed-bed bioreactors with low cell inoculum density. Biotechnol Lett 42, 2551–2560 (2020). https://doi.org/10.1007/s10529-020-02987-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02987-x