Abstract
Objective
Earlier, we have found that the enteropathogenic Yersinia enterocolitica have evolved the survival mechanisms that regulate the expression of laccase-encoding genes in the gut. The present study aims to characterize the purified recombinant laccase from Y. enterocolitica strain 8081 biovar 1B and understand its effect on the midgut of cotton bollworm, Helicoverpa armigera (Hübner) larvae.
Results
The recombinant laccase protein showed high purity fold and low molecular mass (~ 43 kDa). H. armigera larvae fed with laccase protein showed a significant decrease in body weight and damage in the midgut. Further, transmission electron microscopy (TEM) studies revealed the negative effect of laccase protein on trachea, malpighian tubules, and villi of the insect. The proteome comparison between control and laccase-fed larvae of cotton bollworm showed significant expression of proteolytic enzymes, oxidoreductases, cytoskeletal proteins, ribosomal proteins; and proteins for citrate (TCA cycle) cycle, glycolysis, stress response, cell redox homeostasis, xenobiotic degradation, and insect defence. Moreover, it also resulted in the reduction of antioxidants, increased melanization (insect innate immune response), and enhanced free radical generation.
Conclusions
All these data collectively suggest that H. armigera (Hübner) larvae can be used to study the effect of microbes and their metabolites on the host physiology, anatomy, and survival.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yersinia enterocolitica is a potential human pathogen with extensive serotype diversity, which is further differentiated into six biovars based on their biochemical and physiological characteristics (Howard et al. 2006; Virdi et al. 2012). Y. enterocolitica biovar 1B is a food- and water-borne pathogen, but high mortality has also been reported due to the blood-transfusion associated septicemia (Leclercq et al. 2005). The enteropathogenic Y. enterocolitica penetrates the intestinal epithelium and proliferates in the lymphatic system (Fallman et al. 2001). The virulence factor type-III secretion system (T3SS) of the pathogen inhibits its phagocytosis in the host (Barison et al. 2013). Y. enterocolitica biovar 1A strains were also found to survive phagocytosis (Dhar and Virdi 2013) in the absence of virulent genes and plasmid (Burnens et al. 1996); further, causing nosocomial infection (Greenwood and Hooper 1990). One of the factors which facilitate the pathogen to survive phagocytosis in the host is reported to be the free radical scavenging characteristic of multicopper oxidases (MCOs) (Singh et al. 2016). Laccases (EC 1.10.3.2), the member of the MCOs family, catalyzes the oxidation of various aromatic compounds (Sharma and Kuhad 2009). Interestingly, the antioxidative property of laccase is well known in the virulence of a basidiomycetes yeast Cryptococcus neoformans (Zhu and Williamson 2004; Sharma et al. 2018). Laccases are also prevalent in several pathogenic bacteria, e.g., Salmonella enterica, and are reported to be involved in their pathogenicity (Achard et al. 2010). Y. enterocolitica, which is considered as a mammalian pathogen, also confers toxicity to insects (Heermann and Fuchs 2008). It is orally toxic to Manduca sexta (Bresolin et al. 2006) and colonizes the intestine of Caenorhabditis elegans, expanding its intestinal lumen, thus, killing the nematode (Spanier et al. 2010).
Helicoverpa armigera or cotton bollworm classified in family Noctuidae and order Lepidoptera, is a polyphagous insect with broad host spectra. Its polyphagy feeding habit is due to the highly complex and diverse environment of the insect gut (Shinde et al. 2019). The proteins carry out almost all the functions of a cell collectively forming the proteome. It is a highly diverse ‘-omics’ approach, concerned with protein identification and quantification from high-throughput data. In general, proteomics is a large-scale analysis of proteins used to reveal the biological functions in the quantitative and qualitative terms (Tsigaridas et al. 2017). The proteome is studied mostly with mass spectrometry (MS)-based approaches. It involves the biological interpretation of their subcellular localizations, concentration changes, post-translational modifications (PTMs), and interactions (Chen et al. 2017; Sinitcyn et al. 2018; Jain et al. 2019).
Previous reports suggested greater wax moth (Galleria mellonella) as a rapid, cost-effective model to assess the virulence for a range of microorganisms and for the rapid evaluation of antimicrobial drug effectiveness (Tsai et al. 2016). In an earlier work, silkworm (Bombyx mori) was used as a model animal to test the toxicity of neonicotinic pesticides and its biodegradation metabolites (Phugare et al. 2013). Therefore, the present study was undertaken to characterize the recombinant laccase from Y. enterocolitica strain 8081 biovar 1B and to study its effect on the midgut of H. armigera (Hübner) larvae. Finally, the gut proteome profile was also studied.
Materials and methods
Cloning, expression, and purification of laccase
The laccase (yacK) gene amplified from the genomic DNA of Y. enterocolitica strain 8081 (Accession No. YP_001005057.1) (Singh et al. 2014) using forward primer 5′ ATGGATCCGAATTCATGCCATGCATCGCCGTGATTTTAT 3′ with restriction sites BamHI, EcoRI and reverse primer 5′ AAGCGGCCGCCTCGAGCTAAGCACTGACAGTAAGCC 3′ with restriction site NotI, XhoI was cloned in pTZ57R/T (TA cloning vector). Further, the amplified yacK gene was digested with EcoRI/NotI, cloned in expression vector, pET28a (+), and transformed in host cells, Escherichia coli BL21 (DE3).
The 1% (v/v) 12 h old transformed culture was used to inoculate LB medium containing kanamycin (50 µg/mL) and were grown at 37 °C, 200 rpm until attains an optical density (OD) of 0.5–0.6 at wavelength 600 nm. Further, isopropyl-β-d-1-thiogalactopyranoside (IPTG; 1 mM) was added to induce the laccase expression under different incubation conditions of temperature (16, 25, 30, or 37 °C), aeration (100, 150, or 200 rpm), incubation temperature after induction (4, 16, 25, or 30 °C), incubation time after induction (2, 3, 4, 5, or 16 h), and copper (CuCl2) concentration (0.25, 0.5, 1.0, 1.5, or 2.0 mM) (Supplementary Fig. 1). The laccase from inclusion bodies (IBs) was solubilized by the modified method of Sambrook and Russell (2001). The cell pellet obtained by harvesting cells at 5000×g, 15 min, 4 ºC was washed and suspended in lysis buffer [Tris–HCl (50 mM, pH 8.0); EDTA (1 mM, pH 8.0); NaCl (100 mM)] followed by addition of protease inhibitor (phenylmethylsulfonyl fluoride), lysozyme, and deoxycholic acid. The laccase was solubilized using urea (8 M) and β-mercaptoethanol (β-ME; 0.4 mM) in potassium-phosphate buffer (50 mM, pH 8.0) and refolded using refolding buffer [potassium-phosphate buffer (50 mM, pH 8.0); glutathione reduced (5 mM); glutathione oxidized (1 mM); CuCl2 (1 mM)] by pulsatile dilution method (Kamen and Woody 2002). Thereafter, the protein was purified via nickel–nitrilotriacetic acid (Ni–NTA) affinity column chromatography (GE Health care), pre-equilibrated with 10 bed volume of suspension buffer for affinity purification, washed with 10 bed volume of wash buffer [NaCl (150 mM), Tris–HCl (50 mM), β-ME (5 mM)] to remove nonspecific binding, and eluted by elution buffer [NaCl (150 mM), imidazole (250 mM), Tris–HCl (50 mM), β-ME (5 mM)]. The purity of recombinant protein was analysed on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). The positive fractions were concentrated using 10 kDa cutoff Centricon (Millipore) and loaded on a pre-equilibrated [Tris–HCl (50 mM, pH 8.0); NaCl (50 mM); β-ME (5 mM)] Superdex 200 HiLoad 16/60 column (GE Healthcare, New Jersey, USA). The active protein was confirmed as laccase using zymogram with [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] ABTS as substrate followed by biochemical and electrochemical characterization. As reported earlier, the cloning, expression, and purification of laccase from Bacillus pumilus strain DSKK1 (Ahlawat et al. 2019) was done similarly.
Biochemical characterization of laccase
Guaiacol was used for the determination of optimal catalysis conditions. The enzyme was incubated with guaiacol (25 mM) in Tris–HCl buffer (0.2 M, pH 9.0) at 70 ºC for 30 min. One unit laccase was defined as the absorbance change of 0.01/mL/min at wavelength 470 nm (Singh et al. 2016). Various buffers such as citrate–phosphate buffer (pH 3.0–7.0), potassium-phosphate buffer (pH 8.0), Tris–HCl buffer (pH 9.0), and glycine–NaOH buffer (pH 10.0) were used to determine the effect of pH (3.0–10.0) on purified laccase. While, the effect of temperature on enzyme activity was studied in the range from 20 to 90 °C with a difference of 10 °C. Guaiacol (0.5 mM to 4.0 mM) was used to determine the Michaelis–Menten constant (Km) and the maximum rate of reaction (Vmax) from the Eadie–Hofstee plot. The effect of various metal ions (0.00625–0.5 mM), surfactants (0.025–1.0 mM), and inhibitors (0.025–1.0 mM) on laccase activity was determined by measuring the residual activity under standard assay conditions.
Electrochemical characterization of laccase
Yersinia enterocolitica strain 8081 recombinant laccase was electrochemically characterized by cyclic voltammetry. Cyclic voltammetric (CV) experiments were carried out with a computer-controlled potentiostat (Autolab Potentiostat/Galvanostat PGSTAT204, Metrohm, USA) using software Nova version 1.6. Cyclic voltammograms were performed on screen printed carbon electrode (SPCE; Dropsens) having surface diameter of 4.0 mm; where working, counter, and reference electrodes were made of carbon, carbon, and silver (Ag), respectively.
Before experiment, the electrode was washed with milli Q water and dried at RT. Thereafter, the reactive ester groups were generated on electrode after incubation for 2–3 h with 6 µl mixture of 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide (EDC; 10 mM) and N-hydroxysuccinimide (NHS; 10 mM) (1:1) followed by washing and drying. Now, the modified electrode was incubated overnight at RT with 6 µL of Y. enterocolitica strain 8081 or B. pumilus strain DSKK1 recombinant laccase, followed by repeated washing. Cyclic voltammetry was done at three pH’s i.e., 5.0 (citrate–phosphate buffer), 7.0 (Tris–HCl buffer), and 10.0 (carbonate-bicarbonate buffer) with and without 1 mM ABTS at a scan rate of 20 mV/s between − 0.9 and + 1.2 V v/s Ag|AgCl|KCl.
Effect of laccase on insect gut
The effect of purified laccase was studied on the growth of H. armigera larvae at different concentrations (0.5, 5.0, and 10.0 μg/g insect diet). The laccase was added to the insect diet (Supplementary Table 1) followed by their growth till third larval stage. The experiments were done in five replicates with initial weight, final weight, and mortality in insect observed and recorded. The data of insect weights was calculated as mean values [± standard deviation (SD)]. Statistical analysis was done by one-way analysis of variance (ANOVA) using Microcal Origin Version 6.0. The mean values obtained from each sample were compared using an unpaired t test. Probability values ≤ 0.05 were considered as statistical significant. Thereafter, the midguts of the insects were excised aseptically to study the biochemical changes. Catalase (CAT) assay was performed as per the procedure described by Beers and Sizer (1952). Lipid peroxidation (LPO) was assayed by the modified method of Wills (1966). Glutathione (GSH) was estimated as described by Kumar et al. (2008). Further, transmission electron microscopy (TEM) was done commercially at AIRF, Jawaharlal Nehru University (JNU), New Delhi, India. For TEM, the aseptically excised gut tissues were fixed in 2% paraformaldehyde and 2% glutaraldehyde in potassium-phosphate buffer (0.1 M, pH 8.0). Ultra-thin sections were sliced using Leica EM UC 6 ultramicrotome and examined using JEOL-2100F TEM at 120 kV. The samples were studied at various magnifications of × 1000–6000.
Proteomic analysis of laccase-fed insect gut
The excised gut were lysed in extraction buffer containing 125 mM NaCl, 0.5 M Tris, and 1 M EDTA with protease inhibitors for 4 min and, then centrifuged for 20 min at 10,000 rpm, 4 °C. The extracted proteins were precipitated using TCA: acetone (1:8) at − 20 °C for overnight and, then centrifuged at 15,000 rpm, 4 °C for 40 min. The obtained pellets were purified by repeatedly washing with 5 mM dithiothreitol (DTT)-acetone. Thereafter, the total protein was solubilized in guanidine hydrochloride (GdmHCl; 6 M) at 90 °C for 30 min, reduced using 10 mM DTT for 45 min at 56 °C, and alkylated with 55 mM indole acetic acid at RT for 30 min in dark. Then, the samples were diluted up to 1 M of GdmHCl using 25 mM ABC followed by digestion with trypsin (MS grade) in ratio 1:50 at 37 °C. After that, 2% formic acid (FA) was added to stop the digestion and desalting was performed by the manufacturer’s protocol (Thermo Fisher Scientific). Finally, the samples were dried by speed vacuum, reconstituted in 0.1% FA, and loaded onto a C18 reverse phase column. Digested peptides were analyzed using nano-LC–MS/MS. Peptides were loaded onto a PepMap RSLC C18 2 µmx50 cm pre-column (Thermo Scientific Easy-nLC 1200 column). The column was eluted using gradient of solvent A (95% water, 5% acetonitrile, and 0.1% FA) and solvent B (90% acetonitrile, 10% water, and 0.1% FA) at a flow-rate of 300 nL/min and total run time of 123 min. For precursor mass tolerance (MS1) and fragment mass tolerance (MS2) maximum ion transfer time was kept 60 ms and 120 ms, respectively. Full-scan MS spectra with mass scan range of m/z 350–2000 were acquired on a Q Exactive Orbitrap (Thermo Scientific) in a positive ion mode with 27% normalized collision energy (NCE).
Raw MS data files were analyzed in proteome discoverer software (ver. 2.2, Thermo Scientific) against the constructed database of Helicoverpa available in UniProt (https://www.uniprot.org/). Trypsin was set as the digestion enzyme with two missed cleavage. The peptides and proteins were inferred using peptide spectrum matches (PSMs) and were validated at false discovery rate (FDR) ≤ 1% estimated using the decoy hit distribution. The results were filtered by Xcorr (< 1.5), ΔCn (< 0.01) for peptides and q-value (≤ 0.05), PSMs (≥ 1) for proteins with high confidence. Protein quantification was conducted using the total spectrum count of identified proteins as described earlier (Kamal et al. 2018). Function assignment and annotation by InterPro terms, gene ontology (GO) terms, enzyme classification (EC) codes, and metabolic pathways (KEGG, Kyoto Encyclopaedia of Gene and Genomes) were determined using Blast2GO (https://www.blast2go.com/) and OmicsBox (https://www.biobam.com/omicsbox/) software suite. Heatmap cluster was generated by MeV software (ver. 4.9; https://www.tm4.org/) and protein–protein interactions were studied by String software (ver. 11.0; https://string-db.org/).
Results
Cloning, expression, and purification of laccase
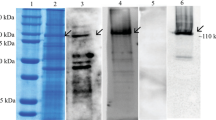
The size of amplified laccase (yacK) gene was 1.6 Kb (Fig. 1a) which was similar to previously studied laccase genes from Y. enterocolitica (Singh et al. 2014, 2016). Yersinia laccase gene (yacK) was cloned in pET28a ( +) (Fig. 1b) and over-expressed in E. coli BL21 (DE3) for the hyperproduction of recombinant protein under varied conditions (Supplementary Fig. 1). The maximum expression was observed after incubation at 30 °C, 200 rpm for 4 h (Supplementary Fig. 2I); however, most of the protein was in the form of IBs. Therefore, the incubation temperature was reduced to 25 °C and 16 °C, and the agitation was changed to 100 rpm. The maximum protein was obtained at 25 °C and 100 rpm, but in the form of IBs. Further, the formation of IBs was avoided by lowering the post-induction temperature, such that the incubation time was increased to 16 h at 30 °C (Supplementary Fig. 2II) and 4 °C under static conditions after incubation at different temperatures (30, 25, or 16 °C) for 4 h at 100 rpm (Papaneophytou and Kontopidis 2014). Then the amount of expressed protein in IBs increased with increasing incubation time, but the yield of refolded protein was reduced drastically. The optimum conditions chosen for induction was incubation for 4 h at 25 °C, 100 rpm, thereafter, under static condition for 16 h at 4 °C (Supplementary Fig. 1, Supplementary Fig. 2III).
a Agarose gel electrophoresis showing band of [a] laccase gene (yacK) of Y. enterocolitica strain 8081 [M] 1 Kb Marker b [a] TA cloning vector (pTZ57R/T) with yacK and [M] Lambda DNA HindIII digest marker c Laccase purification profile using gel filtration chromatography. Inset: Lane M: molecular marker; Lane A: crude lysate; Lane B and C: Ni–NTA purified protein; Lane D: gel filtration purified protein (Molecular mass ~ 43 kDa)
Solubilization of the IBs in urea and β-ME showed protein recovery in supernatant on SDS-PAGE (Supplementary Fig. 3A). The protein was refolded using oxidized and reduced glutathione in sodium-phosphate buffer (pH 8.0) having chloride salt of copper; with activity of 36.71 U/mL and specific activity of 48.94 U/mg. The protein was purified using Ni–NTA affinity chromatography column (43.11 µg/mg) (Supplementary Fig. 3B) with an activity and specific activity of 355.13 µg/mL and 8230 U/mg, respectively. The concentrated protein was loaded on Superdex 200 HiLoad 16/60 column for further purification. The eluted fraction of laccase peak was validated by securing a single band on SDS-PAGE. Finally, the purified laccase was of 82 kDa dimer in a single peak (Fig. 1c) and was further confirmed by zymogram study with [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] (ABTS) as a chromogenic substrate (Supplementary Fig. 3C).
Biochemical characterization of laccase
The maximum activity of the purified laccase was observed at pH 8.0 and 70 °C (Fig. 2a) with residual activity of 91% and 47% after 2 h and 12 h, respectively. At 80 °C, the residual activity of laccase was 73% for 10 min of incubation, but after 30 min drastic loss in enzyme activity (41%) was observed (Fig. 2b). The Km and Vmax for guaiacol were found to be 0.68 mM and 60.5 mM/min, respectively. Lower is the Km value; higher is the affinity towards the substrate (Robinson 2015). Metal ions can stabilize or destabilize the protein conformation by binding to the enzyme and thus, can change its activity. The recombinant laccase was highly stable in the presence of all the metal ions studied (Fig. 2d). The inhibitory effect of sodium azide (NaN3) was also negligible on the activity of laccase. The ability of calcium, copper, magnesium, and manganese to induce the laccase from different bacteria had been reported earlier (Kim et al. 2001; Martins et al. 2002). The non-ionic surfactants Triton X-100 and Tween 80 showed high residual activity. Anionic surfactant (SDS) and the inhibitors of fungal laccase (Kumar et al. 2015), β-ME, DTT, and EDTA showed no significant inhibitory effect on laccase activity (Fig. 2c). However, a higher concentration (1%) of EDTA was inhibitory.
a Effect of different pH and temperature on the activity of purified laccase b Thermostability of purified laccase c Effect of different surfactants and inhibitors on laccase activity at different concentrations (mM) d Effect of different metal ions on laccase activity at different concentrations (mM)
Electrochemical characterization of laccase
Cyclic voltammograms obtained for the reaction of ABTS and Y. enterocolitica strain 8081 recombinant laccase at pH 5.0 and pH 7.0 exhibited a pair of cathodic and anodic peaks Ia/Ic having Eo′ of 0.309 V (at pH 5.0) and 0.381 V (at pH 7.0) (vs Ag|AgCl) with an additional anodic peak (IIa) at 0.790 V (at pH 5.0) (Supplementary Fig. 4A) and 0.845 V (at pH 7.0) (Supplementary Fig. 4B). Where, Ia, IIa, and Ic corresponds to ABTS/ABTS+ oxidation, ABTS+/ABTS+2 oxidation, and ABTS+/ABTS reduction, respectively (Ley et al. 2013). Reduction peak (Ic) was found to be increased strongly in size when compared to the corresponding oxidation peaks which can be explained by the comproportionation reaction between ABTS and its dication ABTS+2, yielding two molecules of ABTS+. It causes the disappearance of ABTS+2/ABTS+ reduction peak (Fernández-Sánchez et al. 2002). No prominent cathodic or anodic peaks were obtained in cyclic voltammograms obtained for the reaction of ABTS and Y. enterocolitica strain 8081 recombinant laccase at pH 10.0 (Supplementary Fig. 4C), suggesting its maximum activity at pH 7.0, followed by pH 5.0 but not at pH 10.0. On contrast, cyclic voltammograms obtained for the reaction of ABTS and non-pathogenic B. pumilus strain DSKK1 recombinant laccase at all three pH’s, exhibited a pair of cathodic and anodic peaks Ia/Ic with Eo′ of 0.348 V (at pH 5.0), 0.349 V (at pH 7.0) and 0.354 V (at pH 10.0) (vs Ag|AgCl) with an additional anodic peak (IIa) at 0.828 V (at pH 5.0), 0.803 V (at pH 7.0), and 0.808 V (at pH 10.0) (Supplementary Fig. 4D–F).
The comparison of the cyclic voltammograms between the recombinant laccases revealed three important differences. First, Y. enterocolitica strain 8081 recombinant laccase was active at pH 5.0 and pH 7.0 but not at pH 10.0, whereas B. pumilus strain DSKK1 recombinant laccase was active at all three pH’s (i.e. 5.0, 7.0, and 10.0) (Ahlawat et al. 2019). Second, the Eo of Y. enterocolitica strain 8081 recombinant laccase varied at pH 5.0 (0.309 V) and pH 7.0 (0.381 V), whereas B. pumilus strain DSKK1 recombinant laccase had almost same Eo′ at all three pH’s 5.0 (0.348 V), 7.0 (0.349 V), and 10.0 (0.354 V). Finally, at pH 7.0, Eo′ for recombinant laccase from pathogenic Y. enterocolitica strain 8081 (0.381 V) was higher than non-pathogenic B. pumilus strain DSSK1 (0.349 V). Further, Eo′ for bacterial laccases (both pathogenic and non-pathogenic) was lower than previously studied fungal laccases (Table 1).
Effect of laccase on insect gut
It was observed that the insect body weight decreased significantly with gradual increase in the concentration of Yersinia laccase in the artificial diet of an insect (Fig. 3). However, no mortality was reported. The gut of third instar larvae was dissected and homogenized for further analysis such that 2.71 fold decreased CAT activity and 1.57 fold decreased GSH was observed in laccase-treated gut (Table 2). The 7.19 fold higher malondialdehyde (MDA) levels in laccase-treated gut showed increased LPO as compared to untreated gut. GSH detoxifies the potentially deleterious substances like oxygen free radicals and modulates the redox state of the cells. The higher MDA levels usually results in the loss of membrane integrity (Ayobola 2012). The increased lipid peroxidation and altered antioxidant enzymes leads to oxidative stress in cells and thus, contributes to the deleterious effects on the growth of H. armigera (Akbar et al. 2012).
I Effect of laccase on H. armigera larvae: A. Control B–D. Treated with different concentrations of laccase from Y. enterocolitica strain 8081; II Reduction in weight of H. armigera larvae after treatment with different concentration of laccases from Y. enterocolitica strain 8081 and Bacillus pumilus DSKK1 (BPL) [control]. *Represent significant differences between the control (artificial diet) and laccase treated larvae (unpaired Student’s t test p < 0.05)
To understand the effect of laccase on the midgut of H. armigera, TEM was done, which showed distorted trachea (Tr), damaged malpighian tubules (MT), and reduced villi (Mv) in laccase-treated insects (Fig. 4).
Proteomic analysis of laccase-fed insect gut
A total of 1105 (387 exclusively in control insect gut, 66 exclusively in laccase-fed insect gut, and 652 shared between both groups) unique protein accessions (with ≥ 1 PSM) were identified and quantified with high confidence (Fig. 5a). Proteins were classified depending on biological process (BP), cellular component (CC), and molecular function (MF) categories in gene ontology (GO) annotations by score distribution. GO analysis of control insect gut proteins revealed 37.6% BP, 19.97% CC, and 42.42% MF (Supplementary Table 2, Fig. 6). Upon strict filtering of the dataset using criterion of ≥ 5 PSMs, 453 proteins were stringently determined. Out of 453 proteins, 221 were commonly shared between both groups and 232 (225 in control, 7 in laccase-fed insect gut) were exclusively identified in single group (Fig. 5b). The differentially expressed proteins were displayed in a heatmap format using normalized PSMs (Fig. 5d, e). The heatmap data clearly demonstrate diverse intensities (81% upregulated, 19% downregulated) of proteins between the control and laccase-fed insect gut (Fig. 5f). Further, the identified proteins (453 proteins, ≥ 5 PSMs) were classified depending on BP, CC, and MF categories in GO annotations by score distribution. GO analysis of control insect gut proteins revealed 29.3% BP, 22.46% CC, and 48.23% MF proteins (Supplementary Table 2, Fig. 7). According to the metabolic pathway analysis, 106 assigned EC codes were identified for the provided protein accessions (Fig. 5c). Further, distribution of differentially regulated proteins (with PSMs ≥ 5) into various enzyme classes showed increased oxidoreductases and reduced hydrolases in response to laccase-feeding (Supplementary Table 3). Among the reported enzymes, majority of them belongs to the families: type-B carboxylesterase/lipase family, peptidase (C1/M1/S1/T1A/S9B) family, thiolase family, thiolase-like superfamily, and glycosyl hydrolase 31 family. Around 72 KEGG pathways were represented by 453 protein accessions (with PSMs ≥ 5) including purine metabolism (KO00230: 11 and 2 EC codes in control and laccase-fed gut, respectively), citrate (TCA cycle) cycle (KO00020: 10 and 3 EC codes in control and laccase-fed gut, respectively), pyruvate metabolism (KO00240: 9 and 1 EC codes in control and laccase-fed gut, respectively), carbon fixation pathways in prokaryotes (KO00720: 8 and 3 EC codes in control and laccase-fed gut, respectively), and amino sugar and nucleotide sugar metabolism (KO00520: 7 and 1 EC codes in control and laccase-fed gut, respectively) pathways.
a Distribution of the identified proteins (PSMs ≥ 1), b Distribution of identified proteins (PSMs ≥ 5), c Distribution of enzymes (PSMs ≥ 5), d Heatmap depicting the changes in protein expression in H. armigera larvae with and without laccase-feeding using proteins common to both conditions, e Heatmap depicting the changes in protein expression in H. armigera larvae with and without laccase-feeding using proteins present in either group, f Distribution of the identified proteins (PSMs ≥ 5) based on expression levels
The proteome comparison between control and laccase-fed larvae of cotton bollworm showed significant expression of enzymes, cytoskeletal proteins, ribosomal proteins, citrate (TCA cycle) cycle & glycolysis proteins, and cell redox homeostasis & stress response proteins. All the identified proteins can be differentiated under three broad categories: (i) proteins for xenobiotic degradation, immune response, and insect defence; (ii) reactive oxygen species (ROS) and antioxidants; and (iii) proteins involved in cellular metabolism. The expression of antioxidant proteins like catalase (CAT; EC 1.11.1.6; Uniprot ID-H9BEW3) and thioredoxin reductase (TrxR; EC 1.8.1.9; Uniprot ID-A0A0A7RB97) along with prophenoloxidase (PPO; EC 1.14.18.1; Uniprot ID-Q2VIY6), NADPH–cytochrome P450 reductase (CPR; EC 1.6.2.4; Uniprot ID-E0A3A7), serpin (Uniprot ID-F5B4G8), and serpin-9 (Uniprot ID-A0A290U612) were found to be significantly reduced in laccase-fed larvae. Further, proteins related to cytoskeleton like Uniprot ID-A0A2W1BVZ9 for motor activity (GO: 0003774) was downregulated and Uniprot ID-A0A2W1BIB1 for actin filament depolymerisation (GO: 0030042) was upregulated. Malic enzyme (Uniprot ID-A0A2W1BVZ1), aminopeptidase (Uniprot ID-Q6R3M5), aminopeptidase N1 (Uniprot ID-A0A1L5JK78), carboxyl/choline esterase (Uniprot ID-D5G3E6), aspartate aminotransferase (Uniprot ID-A0A2W1B2Y8), UDP-glucuronosyltransferase (Uniprot ID-A0A2W1BAL9), and peptidyl-prolyl cis–trans isomerase (Uniprot ID-A0A2W1BDP4) were identified as the significantly downregulated proteins in response to laccase treatment (Table 3). However, significantly upregulated proteins identified were: NADH dehydrogenase (ubiquinone) activity (EC 1.6.99.3; Uniprot ID-A0A2W1BUD9), succinate dehydrogenase (ubiquinone) flavoprotein subunit, mitochondrial (EC 1.3.5.1; Uniprot ID-A0A2W1BZK9), carboxylic ester hydrolases [EC 3.1.1.- (Uniprot ID-D5KXB9; Uniprot ID-D5G3G4; Uniprot ID-D5KX87; Uniprot ID-H9ZVH4; Uniprot ID-S4WMC9)], aminopeptidase (Uniprot ID-A0A2W1BLC5), chemosensory protein (CSP; Uniprot ID-A0A0A0VG76), tubulin beta chain (Uniprot ID-A0A2W1BMR7), actin (Uniprot ID-E2IV62), and glutamine synthetase [GS; EC 6.3.1.2; Uniprot ID-A0A2W1BMY2) (Table 3).
To establish the known and predicted protein interactions, 221 differentially expressed proteins (≥ 5 PSMs) and 30 selected proteins (related to stress and gut damage) were investigated via STRING database (ver. 11.0), using Lepidoptera as a reference organism. Bombyx mori, Danaus plexippus, and Heliconius melpomene match our reference organism Lepidoptera. Maximum match of input proteins was with proteins from B. mori; thus, it was selected for further analysis. On analyzing the interactome of 221 proteins; 186 nodes, 999 edges were found with average node degree of 10.7, average local clustering coefficient of 0.419, and enrichment p-value of < 1.0e−16 PPI (Fig. 8). The resulting network contains 44 proteins with no associations to other proteins. Analysis of the interactome data generated for 30 selected proteins revealed 24 nodes, 11 edges with average node degree of 0.917, average local clustering coefficient of 0.389, and PPI enrichment p-value of < 2e−06 (Fig. 8). Interestingly, the analysis revealed interaction of prophenoloxidase subunit 2 (PPO2) with serpin (serpin 1). Further, actin (A1) was found to interact with proteins for actin filament depolymerisation (100101180; Uniprot ID-A0A2W1BIB1), motor activity (BGIBMGA000613-TA; Uniprot ID: A0A2W1BVZ9), and tubulin beta chain (Tub1). Major cluster involves: succinate dehydrogenase (ubiquinone) flavoprotein subunit, mitochondrial (BGIBMGA009000-TA), aspartate aminotransferase (BGIBMGA003319-TA), NADH dehydrogenase (ubiquinone) activity (BGIBMGA014483-TA; Uniprot ID-A0A2W1BUD9), aminopeptidase (Apn1), methylenetetrahydrofolate dehydrogenase (NADP+) activity (BGIBMGA004950-TA; Uniprot ID-A0A2W1BQ31), thioredoxin reductase (BGIBMGA002218-TA), and catalase (Cat).
Protein interaction network of identified differentially expressed (of interest) proteins generated with STRING (ver. 11.0). Nodes represent proteins and edges represent protein–protein interactions. Empty nodes represent proteins of unknown 3-D structure and filled nodes represents some 3-D structure is known or predicted. Known interactions:  from curated database,
from curated database,  experimentally determined. Predicted interactions:
experimentally determined. Predicted interactions:  gene neighborhood,
gene neighborhood,  gene fusions,
gene fusions,  gene co-occurrence. Others:
gene co-occurrence. Others:  textmining,
textmining,  co-expression and
co-expression and  protein homology. Inset: Protein interaction network of all the identified 221 differentially expressed proteins generated with STRING (ver. 11.0)
protein homology. Inset: Protein interaction network of all the identified 221 differentially expressed proteins generated with STRING (ver. 11.0)
Discussion and conclusion
In the present work, H. armigera was used as a model organism to study the effect of microbial metabolite, i.e., Yersinia laccase (polyphenol oxidase) on host survival, anatomy, and physiology. The yacK gene from Y. enterocolitica strain 8081 was amplified, cloned, and expressed to overproduce the laccase protein. The IBs were formed as a result of aggregation of the overexpressed protein due to the metabolic burden on the host (Hunke and Betton 2003; Sørensen and Mortensen 2005) and can be partially controlled by slowing down the rate of protein synthesis by reducing incubation temperature and agitation (Vera et al. 2007). Previous studies suggests that the IBs formed at low temperatures solubilize faster, improving the efficiency of the refolding strategies (de Groot and Ventura 2006). The IBs were further solubilized in urea and β-ME in corroboration with previous studies which also showed the highest solubilization of IBs using urea and β-ME (Mollania et al. 2013). Besides, refolding was done using glutathione with CuCl2. As reported previously, the addition of prosthetic groups or cofactors essential for proper folding or stability into culture medium prevents IBs formation (Papaneophytou and Kontopidis 2014). The recombinant metallo-oxidase from Aquifex aeolicus was also found to require copper for its maximum catalytic efficiency (Fernandes et al. 2007). After solubilization, the recombinant laccase protein was purified sequentially with the help of Ni-affinity and gel filtration chromatography, which showed high purity fold of 184 and low molecular mass (~ 43 kDa) with activity and specific activity of 355.13 µg/mL and 8230 U/mg, respectively. Recent studies found that 71.5 kDa acid-tolerant recombinant laccase from Setosphaeria turnica had a maximum activity of 127.78 U/mg (Ma et al. 2018). Similarly, recombinant laccases from Y. enterocolitica strain 7 and B. pumilus strain DSKK1 showed the enzyme activity (µg/mL) of 338.48 and 355.15, specific activity (U/mg) of 4630 and 6450, with purity fold of 108 and 148, respectively (Ahlawat et al. 2019).
On characterizing biochemically, the maximum activity was observed at pH 8.0 and 70 °C. This data supports the previous studies, where the optimum pH for bacterial laccases with ABTS was reported to be in the range of 3.0–7.5 (Singh et al. 2016). The results of kinetic constants (Km and Vmax) also corroborate with the earlier studies, which showed lower Km values for laccases from pathogenic bacteria (Hall et al. 2008) as compared to the laccases from non-pathogenic bacteria (Martins et al. 2002). Recombinant laccase was found to be stable in the presence of metal ions and inhibitors. Vertebrates limit pathogens by reducing essential metal availability while at the same time it enriches the infection site with other metals (Palmer and Skaar 2016). Transition metals are essential in trace amount; however, in excess are toxic as they disrupt the normal metabolic processes (Becker and Skaar 2014). The stability of recombinant laccase toward all metal ions studied suggests that the laccase might be helping Y. enterocolitica strain 8081 against the metal toxicity. On characterizing electrochemically, it was evident that at pH 7.0, redox potential (Eo′) for recombinant laccase from pathogenic Y. enterocolitica strain 8081 was higher than non-pathogenic B. pumilus strain DSSK1 but lower than previously studied fungal laccases. Interestingly, immune activation reduces the bacterial load and metabolic activity, which eventually enhances the gut redox potential, under which Enterobacteriaceae and disease-causing species survive (Reese et al. 2018). Redox enzyme laccase contributes to the growth retardation of H. armigera larvae and supports the previous study, showing the toxicity of Y. enterocolitica strain W22703 towards C. elegans by colonizing the nematode gut (Spanier et al. 2010). Also, there is an isolated report on the role of class 1 laccase (MLAC1) from insect pathogenic fungus Metarhizium anisopliae in the virulence to caterpillars at the last instar stage of Galleria mellonella larvae (Fang et al. 2010).
Biochemical assays (LPO, CAT, and GSH) revealed a higher concentration of MDA and reduced level of antioxidant enzymes in laccase-fed larval gut. Earlier, boric acid was reported to increase LPO in the midgut and fat body of G. mellonella (Büyükgüzel et al. 2013). Similarly, thermal stress also increases LPO in Bactrocera dorsalis (Jia et al. 2011). Another study reported decreased CAT activity in larvae of Bombyx mori in response to tryptophan (Priya Bhaskaran et al. 2015). The declined CAT activity causes cell damage due to the accumulation of hydrogen peroxide (H2O2). Catalase (CAT; EC 1.11.1.6) is a first-line defence antioxidant which catalyses the breakdown of H2O2 to less toxic products (Ighodaro and Akinloye 2018). In corroboration to our catalase assay data, the proteomic analysis further suggests significant reduction in catalase (CAT; EC 1.11.1.6; Uniprot ID-H9BEW3) protein in laccase-fed larvae of H. armigera. Similarly, an antioxidant, thioredoxin reductase (TrxR; EC 1.8.1.9; Uniprot ID-A0A0A7RB97) was downregulated in laccase-fed larval gut. It is already known that one-electron reduction of molecular oxygen produces free radicals or reactive species, which in excess are destructive to living organisms. In contrast, antioxidants (enzymatic and non-enzymatic) are the molecules that together act against the free radical attack. Various potential sites for reactive oxygen (H2O2 and O2·−) production have been identified in Kreb’s cycle and electron transport chain (ETC) of mitochondria. In an earlier report, complex II of ETC was suggested as the site for O2·− or H2O2 generation at rates approaching or exceeding the maximum rates achieved by complex I (NADH dehydrogenase; EC 1.6.99.3) or complex III (coenzyme Q: cytochrome c-oxidoreductase; EC 1.10.2.2). Respiratory complex II (succinate dehydrogenase; SDH; EC 1.3.5.1) reduces ubiquinone in ETC and oxidizes succinate to fumarate as a part of Kreb’s cycle (Quinlan et al. 2012). H2O2 inactivates several enzymes, oxidizes keto-acids, degrades haem protein, damages DNA via oxo-copper complexes (Gutteridge 1986), and causes direct irreversible damage to epithelial cells due to its ability to induce Fenton reaction. Further, thioredoxin reductases (TrxRs) belonging to the family of selenium-containing pyridine nucleotide-disulphide oxidoreductases contains a conserved -Cys-Val-Asn-Val-Gly-Cys- redox catalytic site along with another redox-active site, i.e., a C-terminal -Cys-SeCys- (SeCys: selenocysteine). TrxRs protect against oxidant injury and play an essential role in the recycling of ascorbate from its oxidized form, cell growth, and transformation (Mustacich and Powis 2000).
Moreover, downregulated prophenoloxidase subunit 2 (PPO; EC 1.14.18.1; Uniprot ID-Q2VIY6), as reported previously, is detrimental for the defence against bacteria (Cerenius and Söderhäll 2012). Insect PPO, belonging to type-3 copper protein group is an essential innate immunity protein. Its activation occurs via a cascade of pattern recognition proteins, serine proteases, and serine protease inhibitors or serpins after initial pathogen detection. Activated phenoloxidase (PO) oxidizes phenolic molecules to produce melanin around invading pathogens and wounds. During the process of melanization, various toxic molecules like reactive oxygen and cytotoxic quinones are produced. As a result, uncontrolled melanization is lethal to insects; therefore, insects have evolved the mechanisms to regulate melanization. Further, serpins limit the activity of corresponding proteinases; thereby, avoiding excessive melanization (Lu et al. 2014). The present study revealed downregulated serpin (Uniprot ID-F5B4G8) and serpin-9 (Uniprot ID-A0A290U612) proteins in laccase-fed larvae of cotton bollworm; hence, it suggests extensive melanization and reactive oxygen species generation. Whereas, Uniprot ID-A0A2W1BVE6 protein with serine-type endopeptidase activity (GO: 0008236) was upregulated in laccase-fed larvae. Earlier, serine-type endopeptidase has been reported to play a vital role in coagulation, melanization, and antimicrobial peptides generation via protease cascades (Hu et al. 2012).
Moreover, malic enzyme (EC 1.1.1.38; Uniprot ID-A0A2W1BVZ1) which oxidizes malate to pyruvate was underexpressed in the lumen of laccase-fed larvae. According to a previous report, overexpressed malic enzyme extends the lifespan of Drosophila melanogaster by enhancing the free radical production as well as the expression of ROS-scavenging enzymes (Wang et al. 2018). Another protein (Uniprot ID-A0A2W1BQ31) with methylenetetrahydrofolate dehydrogenase (NADP+) activity (GO: 0004488) was underexpressed in the laccase-treated lumen. 5,10‐methylenetetrahydrofolate dehydrogenase (MTHFD; EC 1.5.1.5) is essential for the production of specific nucleic acids (purine) and amino acids (alanine, glycine, methionine, and serine) (Haque et al. 2019). Further, downregulated aminopeptidase (Uniprot ID-Q6R3M5), aminopeptidase N (Uniprot ID-A0A1L5JK78), and metallopeptidase (Uniprot ID-A0A2W1BR38) were found in laccase-fed larval gut. Aminopeptidase N (APN) is a well-known protein-digesting enzyme, abundantly found in brush border membrane of the intestine (Hu et al. 2012). In a previous study of the midgut lumen, various digestive enzymes involved in the digestion of substances including carbohydrates, proteins, and lipids were revealed. Further, many non-digestive enzymes including multidomain lipocalin, arginine kinase, and others with unknown functions were also identified (Pauchet et al. 2008).
Insects lack an adaptive immune response, such that they solely depend on innate immune system to fight infections (Li et al. 2019). They have evolved a detoxification system that includes carboxylesterases (CarEs), cytochrome P450 monooxygenases (P450s), UDP-glucuronosyltransferases (UGTs), and glutathione S-transferases (GSTs) (Sun et al. 2019). In laccase-fed larvae, carboxylic ester hydrolases [EC 3.1.1.- (Uniprot ID-D5KXB9; Uniprot ID-D5G3G4; Uniprot ID-D5KX87; Uniprot ID-H9ZVH4; Uniprot ID-S4WMC9)] were upregulated. Carboxylesterase, a multifunctional superfamily found in all living organisms, plays crucial roles in the process of xenobiotic detoxification, neurogenesis, pheromone degradation, and developmental regulation (Hu et al. 2012). Furthermore, chemosensory protein (CSP; Uniprot ID-A0A0A0VG76) with a role in xenobiotic degradation and insect defence was upregulated in laccase-fed larval gut. In an earlier report, CSPs genes in silkworm moth were significantly upregulated in response to an insecticide. Also, 2 CSPs genes were upregulated during bacterial infection in Drosophila. Besides, CSPs has a role in both innate as well as adaptive immunity against a specific infectious agent such as highly toxic host plant chemical molecule (Liu et al. 2016). Overexpressed glutamine synthetase (GS; EC 6.3.1.2; Uniprot ID-A0A2W1BMY2) and underexpressed NADPH–cytochrome P450 reductase (CPR; EC 1.6.2.4; Uniprot ID-E0A3A7) and carboxyl/choline esterase (CCE; Uniprot ID-D5G3E6) were reported in the lumen of cotton bollworm larvae in response to the feeding of recombinant laccase. Interestingly, P450s along with CCE is involved in conferring resistance against insecticides (Demaeght 2015). Where, GS is an essential detoxification enzyme in stress and immune responses (Wei et al. 2019). While, CPR transfers electrons from NADPH + H+ to ferrous cytochrome P450 monooxygenase (P450), enabling the P450 redox reaction to metabolize insecticides; thus, it leads to detoxification and resistance in insects (Suwanchaichinda et al. 2014). In the present study, UDP-glucuronosyltransferase (Uniprot ID-A0A2W1BAL9), a multi-functional detoxification enzyme with a role in the biotransformation of compounds was found to be significantly downregulated in response to laccase feeding. Other proteins, aspartate aminotransferase (AST; EC 2.6.1.1; Uniprot ID-A0A2W1B2Y8) and peptidyl-prolyl cis–trans isomerase (EC 5.2.1.8; Uniprot ID-A0A2W1BDP4) were found to be significantly suppressed in response to laccase treatment. The previous reports on cotton leafworm suggest the inhibitory effect of studied insecticides on AST (Assar et al. 2016). Another study on destruxin A (DA): a major secondary metabolite and mycotoxin secreted by an entomopathogenic fungus Metarhizium anisopliae reported the interaction between DA and peptidyl-prolyl cis–trans isomerase (BmPPI). DA has various bioactivities including antifeedant, insecticidal, and growth-retarding effects with inhibition of immunity to insects (Wang et al. 2019).
Finally, protein–protein interaction was studied using the STRING database. Three interaction clusters were generated: one between PPO and serpin; another between cytoskeleton proteins; and third among antioxidants (Cat, Trx), digestive enzyme (aminopeptidase), other enzymes (AST, MTHFD), and Kreb’s cycle proteins (succinate dehydrogenase, NADH dehydrogenase). In an earlier report on molecular mechanism of resistance in Drosophila fruit fly, the protein interaction network for a total of 528 proteins constructed by STRING and Cytoscape identified 13,514 protein–protein interactions, in which endopeptidase (Prosα5, Prosα6, Prosβ4, Prosβ6), and ribosomal protein (RPL40) were the central nodes, which if removed, will crash the network (Zhang and Zhang 2018).
In conclusion, increased free radical generation and reduced antioxidants level in the lumen of laccase-fed larvae creates redox imbalance due to altered redox signalling, leading to oxidative stress that deteriorates the biomolecules in the living system. The TEM results reveal the damage in the malpighian tubules, which excretes nitrogenous waste products from the insect body. Also, tracheae are vital for respiration in H. armigera and villi helps in the absorption of digested food. Thus, damaged malpighian tubules and tracheae can result in increased toxicity, and reduced villi leads to decreased absorption, thereby, inhibiting the insect growth. Further, the oxidatively damaged intestinal cells affect functions such as food utilization and nutrients uptake, thus, limiting the insect growth and survival. Hence, we conclude that the cotton bollworm can be used as a model organism to study the effect of microbes and the microbial metabolites.
References
Achard ME, Tree JJ, Holden JA, Simpfendorfer KR, Wijburg OL, Strugnell RA, Schembri MA, Sweet MJ, Jennings MP, McEwan AG (2010) The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun 78:2312–2319. https://doi.org/10.1128/IAI.01208-09
Ahlawat S, Singh D, Virdi JS, Sharma KK (2019) Molecular modeling and MD-simulation studies: fast and reliable tool to study the role of low-redox bacterial laccases in the decolorization of various commercial dyes. Environ Pollut 253:1056–1065. https://doi.org/10.1016/j.envpol.2019.07.083
Akbar SM, Sharma HC, Jayalakshmi SK, Sreeramulu K (2012) Effect of pyrethroids, permethrin and fenvalarate, on the oxidative stress of Helicoverpa armigera. World J Sci Technol 2:1–5
Assar AA, El-Mahasen MA, Dahi HF, Amin HS (2016) Biochemical effects of some insect growth regulators and bioinsecticides against cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). J Biosci Appl Res 8:582–589
Ayobola A (2012) Assessment of lipid peroxidation and activities of antioxidant enzymes in phosphide-powder residue exposed rats. J Drug Metab Toxicol 3(5):132. https://doi.org/10.4172/2157-7609.1000132
Barison N, Gupta R, Kolbe M (2013) A sophisticated multi-step secretion mechanism: how the type 3 secretion system is regulated. Cell Microbiol 15(11):1809–1817. https://doi.org/10.1111/cmi.12178
Becker KW, Skaar EP (2014) Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev 38:1235–1249. https://doi.org/10.1111/1574-6976.12087
Beers R, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133
Bresolin G, Morgan JA, Ilgen D, Scherer S, Fuchs TM (2006) Low temperature-induced insecticidal activity of Yersinia enterocolitica. Mol Microbiol 59(2):503–512. https://doi.org/10.1111/j.1365-2958.2005.04916.x
Burnens AP, Frey A, Nicolet J (1996) Association between clinical presentation, biogroups and virulence attributes of Yersinia enterocolitica strains in human diarrhoeal disease. Epidemiol Infect 116:27–34
Büyükgüzel E, Büyükgüzel K, Snela M, Erdem M, Radtke K, Ziemnicki K, Adamski Z (2013) Effect of boric acid on antioxidant enzyme activity, lipid peroxidation, and ultrastructure of midgut and fat body of Galleria mellonella. Cell Biol Toxicol 29(2):117–129. https://doi.org/10.1007/s10565-013-9240-7
Cerenius L, Söderhäll K (2012) Crustacean immune responses and their implications for disease control. Infectious disease in aquaculture. Woodhead Publishing, Sawston, pp 69–87
Chen B, Zhang D, Wang X, Ma W, Deng S, Zhang P et al (2017) Proteomics progresses in microbial physiology and clinical antimicrobial therapy. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-016-2816-4
de Groot NS, Ventura S (2006) Effect of temperature on protein quality in bacterial inclusion bodies. FEBS Lett 580(27):6471–6476. https://doi.org/10.1016/j.febslet.2006.10.071
Demaeght PA (2015) A genomic approach to investigate resistance mechanisms in the two-spotted spider mite Tetranychus urticae. 9789491407161.
Dhar MS, Virdi JS (2013) Interaction of Yersinia enterocolitica biovar 1A with cultured cells in vitro does not reflect the two previously identified clonal groups. J Med Microbiol 62:1807–1814. https://doi.org/10.1099/jmm.0.056077-0
Fallman M, Deleuil F, McGee K (2001) Resistance to phagocytosis by Yersinia. Int J Med Microbiol 291(6–7):501–509. https://doi.org/10.1078/1438-4221-00159
Fang W, Fernandes ÉK, Roberts DW, Bidochka MJ, Leger RJS (2010) A laccase exclusively expressed by Metarhizium anisopliae during isotropic growth is involved in pigmentation, tolerance to abiotic stresses and virulence. Fungal Genet Biol 47(7):602–607. https://doi.org/10.1016/j.fgb.2010.03.011
Fernandes AT, Soares CM, Pereira MM, Huber R, Grass G, Martins LO (2007) A robust metallo-oxidase from the hyperthermophilic bacterium Aquifex aeolicus. FEBS J 274(11):2683–2694. https://doi.org/10.1111/j.1742-4658.2007.05803.x
Fernández-Sánchez C, Tzanov T, Gübitz GM, Cavaco-Paulo A (2002) Voltammetric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry 58:149–156. https://doi.org/10.1016/S1567-5394(02)00119-6
Greenwood MH, Hooper WL (1990) Excretion of Yersinia spp. associated with consumption of pasteurized milk. Epidemiol Infect 104:345–350. https://doi.org/10.1017/S0950268800047361
Gutteridge JM (1986) Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett 201(2):291–295
Hall SJ, Hitchcock A, Butler CS, Kelly DJ (2008) A multicopper oxidase (Cj1516) and a CopA homologue (Cj1161) are major components of the copper homeostasis system of Campylobacter jejuni. J Bacteriol 190:8075–8085. https://doi.org/10.1128/JB.00821-08
Haque MR, Higashiura A, Nakagawa A, Hirowatari A, Furuya S, Yamamoto K (2019) Molecular structure of a 5, 10-methylenetetrahydrofolate dehydrogenase from the silkworm Bombyx mori. FEBS Open Bio 9(4):618–628. https://doi.org/10.1002/2211-5463.12595
Heermann R, Fuchs TM (2008) Comparative analysis of the Photorhabdus luminescens and the Yersinia enterocolitica genomes: uncovering candidate genes involved in insect pathogenicity. BMC Genomics 9(1):40. https://doi.org/10.1186/1471-2164-9-40
Howard SL, Gaunt MW, Hinds J, Witney AA, Stabler R, Wren BW (2006) Application of comparative phylogenomics to study the evolution of Yersinia enterocolitica and to identify genetic differences relating to pathogenicity. J Bacteriol 188:3645–3653. https://doi.org/10.1128/JB.188.10.3645-3653.2006
Hu X, Chen L, Xiang X, Yang R, Yu S, Wu X (2012) Proteomic analysis of peritrophic membrane (PM) from the midgut of fifth-instar larvae, Bombyx mori. Mol Biol Rep 39(4):3427–3434. https://doi.org/10.1007/s11033-011-1114-6
Hunke S, Betton JM (2003) Temperature effect on inclusion body formation and stress response in the periplasm of Escherichia coli. Mol Microbiol 50(5):1579–1589. https://doi.org/10.1046/j.1365-2958.2003.03785.x
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex Med J 54(4):287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Jain KK, Kumar A, Shankar A, Pandey D, Chaudhary B, Sharma KK (2019) De novo transcriptome assembly and protein profiling of copper-induced lignocellulolytic fungus Ganoderma lucidum MDU-7 reveals genes involved in lignocellulose degradation and terpenoid biosynthetic pathways. Genomics. https://doi.org/10.1016/j.ygeno.2019.01.012
Jia FX, Dou W, Hu F, Wang JJ (2011) Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla Entomol 94(4):956–964
Kamal AHM, Fessler MB, Chowdhury SM (2018) Comparative and network-based proteomic analysis of low dose ethanol- and lipopolysaccharide-induced macrophages. PLoS ONE 13(2):e0193104. https://doi.org/10.1371/journal.pone.0193104
Kamen DE, Woody RW (2002) Folding kinetics of the protein pectate lyase C reveal fast forming intermediate and slow proline isomerisation. Biochemistry 41:4713–4723. https://doi.org/10.1021/bi0115129
Kim C, Lorenz WW, Hoopes JT, Dean JF (2001) Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK gene. J Bacteriol 183:4866–4875. https://doi.org/10.1128/JB.183.16.4866-4875.2001
Kumar A, Sharma KK, Kumar P, Ramchiary N (2015) Laccase isozymes from Ganoderma lucidum MDU-7: isolation, characterization, catalytic properties and differential role during oxidative stress. J Mol Catal B 113:68–75. https://doi.org/10.1016/j.molcatb.2015.01.010
Kumar V, Bal A, Gill KD (2008) Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Res 232:94–103. https://doi.org/10.1016/j.brainres.2008.07.028
Leclercq A, Martin L, Vergnes ML, Ounnoughene N, Laran JF, Giraud P, Carniel E (2005) Fatal Yersinia enterocolitica biotype 4 serovar O: 3 sepsis after red blood cell transfusion. Transfusion 45:814–818. https://doi.org/10.1111/j.1537-2995.2005.04363.x
Ley C, Çekiҫ SZ, Kochius S, Mangoldb KM, Schwaneberg U, Schrader J, Holtmann D (2013) An electrochemical microtiter plate for parallel spectroelectrochemical measurements. Electrochim Acta 89:98–105. https://doi.org/10.1016/j.electacta.2012.10.151
Li S, Yu X, Feng Q (2019) Fat body biology in the last decade. Annu Rev Entomol 64:315–333
Liu G, Ma H, Xie H, Xuan N, Guo X, Fan Z et al (2016) Biotype characterization, developmental profiling, insecticide response and binding property of Bemisia tabaci chemosensory proteins: role of CSP in insect defense. PLoS ONE 11(5):e0154706. https://doi.org/10.1371/journal.pone.0154706
Lu A, Zhang Q, Zhang J, Yang B, Wu K, Xie W et al (2014) Insect prophenoloxidase: the view beyond immunity. Front Physiol 5:252. https://doi.org/10.3389/fphys.2014.00252
Ma S, Liu N, Jia H, Dai D, Zang J, Cao Z, Dong J (2018) Expression, purification, and characterization of a novel laccase from Setosphaeria turcica in Eschericha coli. J Basic Microbiol 58(1):68–75. https://doi.org/10.1002/jobm.201700212
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859. https://doi.org/10.1074/jbc.M200827200
Mollania N, Khajeh K, Ranjbar B, Rashno F, Akbari N, Fathi-Roudsari M (2013) An efficient in vitro refolding of recombinant bacterial laccase in Escherichia coli. Enzym Microb Technol 52(6–7):325–330. https://doi.org/10.1016/j.enzmictec.2013.03.006
Mustacich D, Powis G (2000) Thioredoxin reductase. Biochem J 346(1):1–8
Palmer LD, Skaar EP (2016) Transition metals and virulence in bacteria. Annu Rev Genet 50:67–91. https://doi.org/10.1146/annurev-genet-120215-035146
Papaneophytou CP, Kontopidis G (2014) Statistical approaches to maximize recombinant protein expression in Escherichia coli: a general review. Protein Expr Purif 94:22–32. https://doi.org/10.1016/j.pep.2013.10.016
Pauchet Y, Muck A, Svatoš A, Heckel DG, Preiss S (2008) Mapping the larval midgut lumen proteome of Helicoverpa armigera, a generalist herbivorous insect. J Proteome Res 7(4):1629–1639. https://doi.org/10.1021/pr7006208
Phugare SS, Kalyani DC, Gaikwad YB, Jadhav JP (2013) Microbial degradation of imidacloprid and toxicological analysis of its biodegradation metabolites in silkworm (Bombyx mori). Chem Eng J 230:27–35
Priya Bhaskaran KP, Bindu PU, Akhilesh VP, Rukhsana K, Jisha Krishnan EK, Sebastian CD (2015) Profiling of catalase and hydrogen peroxide activity in tryptophan administered final instar larvae of Bombyx mori L. Int J Pure App Biosci 3(3):201–207
Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD (2012) Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem 287(32):27255–27264. https://doi.org/10.1074/jbc.M112.374629
Reese AT, Cho EH, Klitzman B, Nichols SP, Wisniewski NA, Villa MM, Durand HK, Jiang S, Midani FS, Nimmagadda SN, O'Connell TM, Wright JP, Deshusses MA, David LA (2018) Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. ELIFE 7:e35987. https://doi.org/10.7554/eLife.35987
Robinson PK (2015) Enzymes: principles and biotechnological applications. Essays Biochem 59:1–41. https://doi.org/10.1042/bse0590001
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Sharma KK, Kuhad RC (2009) An evidence of laccases in archaea. Ind J Microbiol 49:142–150. https://doi.org/10.1007/s12088-009-0039-4
Sharma KK, Singh D, Rawat S (2018) Molecular dynamics simulation studies suggests unconventional roles of non-secretary laccases from enteropathogenic gut bacteria and Cryptococcus neoformans serotype D. Comput Biol Chem 73:41–48. https://doi.org/10.1016/j.compbiolchem.2018.01.010
Shinde AA, Shaikh FK, Gadge PP, Padul MV, Govindwar SP, Kachole MS (2019) Conserved nature of Helicoverpa armigera gut bacterial flora on different host plants and in vitro interactions with PI proteins advocates role in host digestive physiology. J Saudi Soc Agric Sci 18(2):141–149
Singh D, Rawat S, Waseem M, Gupta S, Lynn A, Nitin M, Ramchiary N, Sharma KK (2016) Molecular modeling and simulation studies of recombinant laccase from Yersinia enterocolitica suggests significant role in the biotransformation of non-steroidal anti-inflammatory drugs. Biochem Biophys Res Commun 469(2):306–312. https://doi.org/10.1016/j.bbrc.2015.11.096
Singh D, Sharma KK, Dhar MS, Virdi JS (2014) Molecular modeling and docking of novel laccase from multiple serotype of Yersinia enterocolitica suggests differential and multiple substrate binding. Biochem Biophys Res Commun 449:157–162. https://doi.org/10.1016/j.bbrc.2014.05.003
Sinitcyn P, Rudolph JD, Cox J (2018) Computational methods for understanding mass spectrometry–based shotgun proteomics data. Annu Rev Biomed Data Sci 1:207–234. https://doi.org/10.1146/annurev-biodatasci-080917-013516
Solís-Oba M, Ugalde-Saldívar VM, González I, Viniegra-González V (2005) An electrochemical–spectrophotometrical study of the oxidized forms of the mediator 2, 2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) produced by immobilized laccase. J Electroanal Chem 579:59–66. https://doi.org/10.1016/j.jelechem.2005.01.025
Sørensen HP, Mortensen KK (2005) Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Fact 4(1):1. https://doi.org/10.1186/1475-2859-4-1
Spanier B, Starke M, Higel F, Scherer S, Fuchs TM (2010) Yersinia enterocolitica infection and tcaA-dependent killing of Caenorhabditis elegans. Appl Environ Microbiol 76(18):6277–6285. https://doi.org/10.1128/AEM.01274-10
Sun Z, Xu C, Chen S, Shi Q, Wang H, Wang R et al (2019) Exposure to herbicides prime P450-mediated detoxification of Helicoverpa armigera against insecticide and fungal toxin. Insects 10(1):28
Suwanchaichinda C, Sun D, Brattsten LB (2014) Identification and analysis of NADPH-cytochrome p450 reductase in aedes sollicitans (Diptera: Culicidae). J Med Entomol 51(5):958–963. https://doi.org/10.1603/ME13072
Tsai CJY, Loh JMS, Proft T (2016) Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 7(3):214–229
Tsigaridas A, Papanikolaou IS, Vaiopoulou A, Anagnostopoulos AK, Viazis N, Karamanolis G et al (2017) Proteomics and irritable bowel syndrome. Expert Rev Proteomics 14(5):461–468. https://doi.org/10.1080/14789450.2017.1317600
Vera A, González-Montalbán N, Arís A, Villaverde A (2007) The conformational quality of insoluble recombinant proteins is enhanced at low growth temperatures. Biotechnol Bioeng 96(6):1101–1106. https://doi.org/10.1002/bit.21218
Virdi JS, Kumar P, Malik S, Bhagat N, Gulati P (2012) Insights into the genetic relationships between environmental and clinical strains of Yersinia enterocolitica Biovar 1A. In: Satyanarayana T, et al. (eds) Microorganisms in environmental management: microbes and environment. Springer Science, Berlin, pp 61–80. https://doi.org/10.1007/978-94-007-2229-3_3
Wang J, Weng Q, Hu Q (2019) Effects of destruxin A on silkworm’s immunophilins. Toxins 11(6):349. https://doi.org/10.3390/toxins11060349
Wang T, Geng SL, Guan YM, Xu WH (2018) Deacetylation of metabolic enzymes by Sirt2 modulates pyruvate homeostasis to extend insect lifespan. Aging (Albany NY) 10(5):1053. https://doi.org/10.18632/aging.101447
Wei D, Zhang MY, Zhang YX, Zhang SY, Smagghe G, Wang JJ (2019) Reduced glutamine synthetase activity alters the fecundity of female Bactrocera dorsalis (Hendel). Insects 10(7):186. https://doi.org/10.3390/insects10070186
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676. https://doi.org/10.1042/bj0990667
Zhang G, Zhang W (2018) Protein-protein interaction network analysis of insecticide resistance molecular mechanism in Drosophila melanogaster. Arch Insect Biochem Physiol. https://doi.org/10.1002/arch.21523
Zhu X, Williamson PR (2004) Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. https://doi.org/10.1016/j.femsyr.2004.04.004
Acknowledgements
The authors thank Prof. Deepak Pental, CGMCP, University of Delhi South Campus, New Delhi, India for the insect trial facility.
Supplementary information
Supplementary Figure 1—Optimization of recombinant laccase production from Yersinia enterocolitica strain 8081.
Supplementary Figure 2—I Coomassie-stained SDS-PAGE gel of induced E. coli BL21 cells harboring expression plasmid vector pET28a with laccase gene from Y. enterocolitica strain 8081. Induction was done at 200 rpm with 1 mM IPTG. Lanes: a Control (without induction) b Induction for 2 h at 25 °C; c Induction for 3 h at 25 °C; d Induction for 4 h at 25 °C; e Induction for 2 h at 30 °C; f Induction for 3 h at 30 °C; g Induction for 4 h at 30 °C; h Induction for 2 h at 37 °C; i Induction for 3 h at 37 °C; j Induction for 4 h at 37 °C. II Expression at 100 rpm for 4 h and then kept at static for 16 h at 30 °C a–d: from a Induction at 30 °C; b Induction at 25 °C; c Induction at 16 °C and d uninduced III Expression at 100 rpm for 4 h and then kept at static for 16 h at 4 °C. a Induction at 25 °C; b. Induction at 16 °C.
Supplementary Figure 3—A Coomassie-stained SDS-PAGE gel of solubilized inclusion bodies: I Supernatant II Pellet B & C. Purification of laccase protein using B. Ni–NTA affinity chromatography: I Wash (I) II Wash (II) and III Eluted laccase C Zymogram of purified laccase using ABTS as substrate
Supplementary Figure 4—Cyclic voltammograms obtained for Yersinia enterocolitica strain 8081 (A, B, C) and Bacillus pumilis strain DSKK1 (D, E, F) recombinant laccases with 1mM ABTS (in red) and without ABTS (in blue) A, D at pH 5 using citrate-phosphate buffer; B, E at pH 7 using Tris buffer, and C, F at pH 10 using carbonate-bicarbonate buffer.
Supplementary Table 1—Composition of artificial diet
Supplementary Table 2—Gene ontology (GO) analysis of identified proteins (≥ 1 PSMs in black and ≥ 5 PSMs in blue) from H. armigera larvae with and without recombinant laccase feeding
Supplementary Table 3—Distribution of differentially regulated proteins (with PSMs ≥ 5) from normal diet-fed and recombinant laccase-fed H. armigera larvae gut into various enzyme classes
Funding
This work is supported by grants from the UGC-New Delhi [Grant No. 39-204/1010(SR)]. The FIST-DST grant to the Department of Microbiology is sincerely acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research involving animal and/or human rights
This article does not contain any studies with animals performed by any of the authors. Helicoverpa armigera has not been notified under any act or laws and rules thereof of the Government of India as an endangered or threatened species restricting or regulating its collection and observation. Therefore, no permits were required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahlawat, S., Singh, D., Yadav, A. et al. Proteomic analysis reveals the damaging role of low redox laccase from Yersinia enterocolitica strain 8081 in the midgut of Helicoverpa armigera. Biotechnol Lett 42, 2189–2210 (2020). https://doi.org/10.1007/s10529-020-02925-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02925-x