Abstract
The ability of five fungal species belonging to two genera of Aspergillus and Fusarium has been examined in the microbial transformation of androst-4-ene-3, 17-dione (AD). Furthermore, the biotransformation of nandrolone decanoate (2) by F. fujikuroi has been studied. AD (1) was converted by cultures of Aspergillus sp. PTCC 5266 to form 11α-hydroxy-AD (3) as the only product, with a yield of 86% in 3 days. Moreover, two hydroxylated metabolites 11α-hydroxy-AD (3, 65%) and 7β-hydroxy-AD (4; 18%) were isolated in biotransformation of AD by A. nidulans. On the other hand, it was metabolized by F. oxysporum to produce 14α-hydroxy-AD (5; 38%) and testosterone (6; 12%). Microbial transformation of AD by F. solani led to the production of 11α-hydroxy-AD (3; 54%) and testosterone (6; 14%). AD was reduced at the 17-position by F. fujikuroi to produce testosterone in the yield of 42%. Finally, nandrolone decanoate was transformed by F. fujikuroi via hydrolysis and oxidation at the 17-position to produce two metabolites namely 17β-hydroxyestr-4-en-3-one (7, 25.4%) and estr-4-en-3,17-dione (8, 33%), respectively. The all metabolites were purified and subsequently identified based on their spectra data analysis and comparing them to the literature data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroids, many of which are hormones, are a highly valuable class of organic compounds because of their important pharmacological attributes and their key role in the pharmaceutical industry as the primary precursors (Kolet et al. 2013). It has been established that they possess many interesting medicinal, pharmaceutical and agrochemical activities and many of them are widely used in the treatment of chronic inflammatory diseases (Sultana 2018). Among steroidal compounds, androst-4-en-3,17-dione (AD) is a useful steroid intermediate and used as an important starting material to prepare the several invaluable pharmaceutical compounds. It has been for decades a key precursor to synthesis of testosterone and other androgens and anabolic steroids (Malaviya and Gomes 2008). In addition, nandrolone is a natural anabolic steroid which is applied for the treatment of autoimmune hemolytic anemia and memory impairment (Iqbal Choudhary et al. 2008). Decanoate ester of nandrolone is also used to treat breast cancer (Bibby et al. 1981). The valuable pharmaceutical medicinal properties of these compounds have encouraged many researchers to modify their structures with the aim of producing more effective derivatives using various chemical and microbial transformations (Baydoun et al. 2014; Yazdi et al. 2006; Wilds and Nelson 1953; Kolet et al. 2013; Li et al. 2019).
The production of steroids and their derivatives through traditional synthetic procedures involves several steps and they are usually expensive and uneconomical processes. Furthermore, considering the complex structure of the steroids, the production and modification of them by these methods often requires the protection and de-protection of labile functional groups to achieve adequate product selectivity (Kolet et al. 2013; Ye and Guo 2005). The other drawbacks related to the conventional synthetic routes are using nonspecific chemical catalysts and toxic organic solvents (Holland 1999; Koshimura et al. 2010). On the other hand, biocatalytic transformation of steroids is a powerful tool to overcome these limitations and is used to produce bioactive compounds and introduce functional groups to them (Nassiri-Koopaei and Faramarzi 2015; Wang et al. 2019; Janeczko et al. 2009). Among microorganisms that used in the biotransformation of the steroids, fungal species have shown a remarkable ability to perform a diverse range of chemical reactions such as reduction, hydrolysis, hydroxylation, and double bond formation on these compounds (Fernandes et al. 2003; Abourashed et al. 1999; Basso et al. 2016). Especially, fungal species related to the Aspergillus and Fusarium genera are widely distributed and have been successfully applied in the production of steroid derivatives with therapeutic use and commercial value in pharmaceutical industry (Parshikov and Sutherland 2015; Donova and Egorova. 2012; Ghasemi et al. 2014a, 2014b; Al-Aboudi et al. 2017).
As an extension of our previous work, which described the microbial transformaton of AD by three fungal species (Heidary and Habibi 2016), herein, its biotransformation by two fungal species of the genus Aspergillus including A. nidulans and Aspergillus sp. PTCC 5266 as well as three fungi species of the genus Fusarium including F. solani, F. oxysporum, and F. fujikuroi has been investigated for first time. Furthermore, the ability of F. fujikuroi to convert nandrolone decanoate (2) has been studied.
Materials and methods
Instrumental methods
Proton and carbon-13 spectra were recorded using a Bruker Avance-300 and the chemical shifts (δ) were reported in ppm relative to tetramethylsilane (TMS) as an internal reference. The measurement of optical rotation was performed on a Perkin-Elmer 341 polarimeter at the D line of sodium (589 nm). Preparative thin layer chromatography (TLC) plates were based on silica gel 60 mesh GF254 plates (20 × 20 cm) and were viewed under UV light (at 254 nm). Melting points were recorded using an Electrothermal 9100 apparatus and were uncorrected.
Materials, microorganisms and conditions of cultivation
AD (1) and nandrolone decanoate (2) were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and Iran Hormone Company (Tehran, Iran), respectively. The culture media ingredients were supplied by Scharlau (Barcelona, Spain). All solvents and inorganic salts used for in this study were obtained from Merck (Darmstadt, Germany). Five fungal species namely Aspergillus sp. PTCC 5266, A. nidulans PTCC 5014, F. fujikuroi PTCC 5144, F. oxysporum PTCC 5115, and F. solani complex PTCC 5285 were employed in the present study. They were obtained in the Persian Type Culture Collection (PTCC), Iranian research organization for science and technology. The potato-dextrose agar plates (15.0 g agar/L, 300 g diced potatoes, 20 g glucose) were used for cultivation of them. The experiments were carried out in ten 250 mL Erlenmeyer flasks for each fungal species. Then, the autoclaved and pre-cooled broth medium was distributed among them (100 mL in each). In the next step, they were inoculated with freshly obtained spores from well grown agar slope cultures. After cultivation at 25 °C under constant shaking on an orbital shaker at 120 rpm, 100 mg of the steroidal substrate dissolved in DMSO was added to each of the cultures. Furthermore, control samples consisting of non-inoculated sterile medium and the substrate were prepared.
Isolation and purification of products
The obtained products were extracted three times with chloroform (3 times) and dried over anhydrous sodium sulfate. Then, ethyl acetate/n-hexane (1:3 (v/v)) was used as the solvent system to purify of each extracts obtained from the biotransformation of AD by repeated preparative thin layer chromatography. A chloroform/acetone (8:2 (v/v)) solvent system was used for separation of the metabolites 7 and 8 from the unreacted starting material. Identification of the purified metabolites was performed by analysis of their spectral data.
Time course study
In order to investigate time course study of the metabolites, sampling was carried out every 12 h. For this purpose, in each sampling, 20 mL of the broth was taken and extracted with chloroform (3 × 20 mL), then the combined organic phases were dried over Na2SO4 and the solvent was evaporated under reduced pressure. Then, the best timing has been determined by TLC with detection by UV at 254.
Spectral data
Nandrolone decanoate (2), White powder; 1H NMR (300.13 MHz, CDCl3) δ 5.87 (s, 4-H), 4.66 (t, J = 7.9 Hz, 17 α-H), 0.99 (brs, 28-H); 0.86 (s, 18-H); The 13C NMR spectral data (75.47 MHz) are shown in Table 1.
11α-Hydroxy-AD (3), Crystallized from chloroform as colorless crystals; mp: 238–240 °C (lit (Choudhary et al. 2004) 240–241 °C), [α]D20 + 139° (c = 8.0, CHCl3) (lit (Choudhary et al. 2004) [α]D20 + 145° (c = 0.8, CHCl3); 1H NMR (300.13 MHz, CDCl3) δ 5.73 (s, 4-H), 4.01 − 4.11 (m, 11β-H), 1.35 (s, 19-H), 0.96 (3H, s, H-18); The 13C NMR spectral data (75.47 MHz) are shown in Table 1.
7β-Hydroxy-AD (4), White powder: mp 212–216 °C (lit (Kolet et al. 2013) 215–217 °C), [α]D20 + 181° (c = 1.0, CHCl3) (lit (Ghasemi et al. 2014b) [α]D20 + 178° (c = 1.0, CHCl3);1H NMR (300.13 MHz, CDCl3) δ 5.80 (1H, s, H-4), 3.51 (brs, 7 α-H), 1.26 (s, 19-H), 0.94 (s, 18-H); The 13C NMR spectral data (75.47 MHz) are shown in Table 1.
14α-Hydroxy-AD (5), Crystallized from chloroform as colorless crystals; mp 256–259 °C (lit (Kalbasi et al. 2009) 257–262 °C), [α]D20 + 158° (c = 1.0, CHCl3) (lit (Kalbasi et al. 2009) [α]D20 + 160° (c = 1.0, CHCl3)); 1H NMR (300.13 MHz, CDCl3) δ 5.76 (s, 4-H), 1.23 (s, 19-H), 1.06 (s, 18-H); The 13C NMR spectral data (75.47 MHz) are shown in Table 1.
Testosterone (6), Colorless crystals; mp 152–155 °C (lit (Swizdor et al. 2017) 151–153 °C), [α]D20 + 104° (c = 1.0, MeOH) (lit (Kolet et al. 2013) [α]D20 + 106° (c = 1.0, MeOH)); 1H NMR (300.13 MHz, CDCl3) δ 5.74 (1H, s, H-4), 3.66 (t, J = 8.5 Hz, 17α-H), 1.20 (s, 19-H), 0.81 (s, 18-H); The 13C NMR spectral data (75.47 MHz) are shown in Table 1.
17β-Hydroxyestr-4-en-3-one (7), White powder: mp 116–118 °C (lit (Yazdi et al. 2005) 112–115 °C), [α]D20 + 53° (c = 1.0, CHCl3) (lit (Yazdi et al. 2005) [α]D20 + 55° (CHCl3); 1H NMR (300.13 MHz, CDCl3) δ 5.83 (s, 4-H), 3.67 (t, J = 8.4 Hz, 17 α-H), 0.82 (s, 18-H); The 13C NMR spectral data (75.47 MHz) are shown in Table 1.
Estr-4-en-3,17-dion (8), White powder: mp 166–169 °C (lit (Yazdi et al. 2005) 168–170 °C), [α]D20 + 139.5° (c = 1.0, CHCl3) (lit (Yazdi et al. 2005) [α]D20 + 138° (CHCl3); 1H NMR (300.13 MHz, CDCl3) δ 5.87 (s, 4-H), 0.93 (s, 18-H); The 13C NMR spectral data (75.47 MHz) are shown in Table 1.
Results
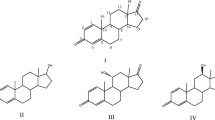
Incubation of AD (1) by Aspergillus sp. PTCC 5266 for 3 days resulted in 11α-hydroxy-AD (3) with the excellent yield of 86% (91 mg) along with recovery of about 14% of the starting material. Moreover, it was hydroxylated with fungus A. nidulans at the 11 and 7-positions to produce 11α-hydroxy-AD (3) as the major product (68 mg, 65%) and 7β-hydroxy-AD (4) in low yield 18% (19 mg) (see Fig. 1). In addition to the metabolites 3 and 4, a number of other metabolites were also produced which could not be identified by NMR spectroscopy because of their very low concentration. Under these conditions, no unreacted precursor remained.
For the biotransformation of AD by three species of the genus Fusarium, time of 5 days was considered as the best time because the maximum amounts of the products were obtained during this time. A mixture of 14α-hydroxy-AD (5; 40 mg, 38%) and testosterone (6; 12 mg, 12%) were obtained from the bioconversion by F. oxysporum. In this case, a significant portion of AD (about 47%) remained unreacted at the end of the biotransformation. Furthermore, it was transformed by F. solani into two metabolites 3 with a yield of 54% (57 mg) and testosterone (6; 14 mg, 14%). The yield for this biotransformation was not 100%; a high concentration of unreacted starting material (29%) and an additional unknown product (3%) were also detected in the final products. It is worth noting that testosterone (6) as the only product was isolated in 42% yield from transformation of AD by F. fujikuroi. Almost 50% of starting material was recovered at the end of the reaction (Fig. 2).
The biotransformation of nandrolone decanoate (2) by F. fujikuroi led to the production derivatives of 17β-hydroxyestr-4-en-3-one (7, 25.4%)) and estr-4-en-3,17-dione (8) with a yield of 33% in 8 days (Fig. 3), Approximately 40% of unreacted nandrolone decanoate was observed at the end of the reaction time.
It seems that the first step of this biotransformation occurred via the hydrolysis of the ester bond to 17β-hydroxyestr-4-en-3-one (7), followed by oxidation at C-17 to yield estr-4-en-3,17-dione (8). In biotransformation of nandrolone decanoate by other fungal species some metabolites were produced, but attempts to identify their structures by NMR spectroscopy were unsuccessful due to the low concentration of them.
The structure of the obtained metabolites was determined based on spectroscopic analysis and comparison with literature data.
In the 1H NMR spectrum of the metabolite 3, a downfield chemical shift for a methine proton at δ 4.06 ppm (ddd, J11α,12β = 14.7 Hz, J11α,9α = 10.4 Hz, J11α,12α = 4.8 Hz) was appeared (Supplementary Fig. 3). Furthermore, the 13C NMR spectrum (Supplementary Fig. 4), revealed a carbon resonance at δ 68.4 ppm. These data indicated that in 3, comparing with AD (1), one of the methylene carbons was hydroxylated. The investigation of splitting pattern and coupling constants enable us to assign the stereochemistry of H-11 as α. In addition, the comparison of the 1H NMR, 13C NMR data, melting point, and optical rotation, with those described in the literature confirmed the metabolite 3 to be 11α-Hydroxy-AD (Choudhary et al. 2004).
The introduction of a hydroxyl group to the parent structure and production of metabolite 4 by the strain of A. nidulans was confirmed by the presence of a new downfield proton signal at δ 3.51 ppm as a broad singlet in the 1H NMR spectrum (Supplementary Fig. 5). Its 13C NMR spectrum (Supplementary Fig. 6) also supported the presence of the hydroxyl group by the downfield shift of C-7 (δ75.1 ppm). Melting point, optical rotation, chemical shifts, and assignments for 4 were in totally agreement with previously published data for 7β-hydroxy-AD (Kolet et al. 2013).
The chemical shift at 80.7 ppm in the 13C NMR spectrum of 14α-Hydroxy-AD (5) suggested the presence of a new hydroxyl group. The 1H NMR spectrum of this metabolite showed no peak in the range of 3.5–4.5 ppm, therefore the existence of the new hydroxyl group with tertiary nature was proposed. In the 13C NMR spectrum of 14α-Hydroxy-AD, the resonance of Me-18 appeared at lower field than the corresponding one in AD (4.5 ppm comparing with AD). These data suggested that the new hydroxyl group should be connected to a tertiary carbon near the Me-18. Based on these findings and comparing of 1H and 13C NMR data (see Supplementary Figs. 7 and 8) with that reported in the literature, metabolite 5 was identified as 14α-Hydroxy-AD (Kalbasi et al. 2009; Faramarzi et al. 2008).
The reduction of 17-carbonyl group was confirmed by the lack of the characteristic signal at δ 220 ppm in 13C NMR spectrum of the metabolite 6 and the appearance of a new signal at 81.6 ppm. The structure of testosterone (6) was further corroborated by the presence of a characteristic signal for proton H-17 as a triplet (3.66 ppm, J = 8.5 Hz). The NMR data (see Supplementary Figs. 9 and 10) of the obtained metabolite were consistent with those reported for testosterone in the literature (Kolet et al. 2013; Swizdor et al. 2017).
The enzymatic ester hydrolysis of nandrolone decanoate into nandrolone was approved by the lack of the characteristic signal of the ester carbonyl group (C-19) in 13C NMR spectrum (Supplementary Fig. 13). Furthermore, the 1H NMR spectrum of 7 (Supplementary Fig. 12) showed the H-17 signal at 3.67 ppm that it was about 0.8 ppm more shielded than the corresponding one (δ 4.5 ppm) of nandrolone decanoate (2), indicating the hydrolysis of the ester group. The spectral data, melting point, and optical rotation of the structure of 7 were in accordance with literature data (Yazdi et al. 2005).
No peak was observed in the range of 3.5–4.5 ppm according to H-17 in 1H NMR spectrum of the metabolite 8 (Supplementary Fig. 14), which indicated the oxidation of C-17. Further evidence for oxidation of C-17 was provided by the appearance of a new signal at 220.4 ppm in 13C NMR spectrum of estr-4-en-3,17-dion (8) (Supplementary Fig. 1).
A summary of the current biotransformation of AD and nandrolonedecanoate by genera of Aspergillus and Fusarium as well as the obtained metabolites and their respective yields have been given in Table 2.
Discussion
The ability of different microorganisms to convert of steroid compounds into their modified derivatives has been subjects of research for many years (Sultana 2018; Zoghi et al. 2019). AD and nandrolone decanoate are two steroid compounds that many attempts have been made to produce their derivatives by some microorganisms due to their pharmaceutical properties (Koshimura et al. 2010; Fernandes et al. 2003; Choudhary et al. 2004; Faramarzi et al. 2008; Kollerov et al. 2020). Most metabolites that result from the microbial biotransformations of these compounds, especially the hydroxylated derivatives, exhibit a wide range of biological activities (Kolet et al. 2013). For example, 14α-hydroxy-AD derivatives that significantly inhibit aromatase activity have been widely used as starting materials to prepare of some steroidal drugs such as proligestone (Andryushina et al. 2013; Hu et al. 1995). Furthermore, 7-hydroxy derivatives of these compounds have been used to prepare of diuretic compounds (Faramarzi et al. 2008; Heidary and Habibi 2016). Despite the great progresses in this area, in many cases, metabolites are produced in considerable number and in very low yields. For instance, Curvularia lunata was able to convert AD into several hydroxylated metabolites and one reductive compound (Choudhary et al. 2004). Biotransformation of AD by Mucor 881 afforded four hydroxylated metabolites: 11α -hydroxy-4-androstene-3,17-dione, 6β -hydroxy-4-androstene-3,17-dione, 6β,11α-dihydroxy-4-androstene-3,17-dione and 7β-hydroxy-4-androstene-3,17-dione (Kolet et al. 2013). The conversion of AD by Neurospora crassa and Mucor racemosus was also investigated, in which the hydroxylation of various positions resulted in the production of different metabolites (Faramarzi et al. 2008).
In most previous studies on AD biotransformation, a large number of metabolites with low yield was obtained; however, in the current study, due to the biotransformation of AD by two species of the genus Aspergillus 11α-hydroxy-AD (3) was produced with a yield of over 60% and in a stereoselective manner. Especially Aspergillus sp. PTCC 5266 which resulted in the production of metabolite 3 as the only product with the desirable yield of 86% could be considered for the commercial preparation of 11α-Hydroxy-AD. Furthermore, F.fujokouri has stereoselectively catalyzed the production of testosterone from AD. Although, various microorganisms have been applied to reduce AD to testosterone, most of them produced the oxidated products beside the reduction of carbonyl group (Choudhary et al. 2004; Xiong et al. 2006; Hu et al. 1995). The selective reduction of AD to testosterone has been previously reported by Faramarzi et al., that in order to improve the bioconversion yield, microalga Nostoc muscorum cells were immobilized in various matrices (Arabi et al. 2010).
Similar efforts have also been made toward structure modification of nandrolone and nandrolone decanoate with the aim of preparing more effective derivatives by microbial transformations. Nandrolone, 19-norandrost-4-en-3,17-dione, and their hydroxylated derivatives at various positions (6 α and β, 9α, 10 β,12 β, 15α, 16α and β) were frequently obtained as a result of these biotransformation reactions (Yazdi et al. 2006, 2005; Baydoun et al. 2014).
Conclusion
Biotransformation of AD which is the key intermediate for synthesis of steroid drugs and nandrolone decanoate (2) catalyzed by two Aspergillus and three Fusarium species was investigated in this study. Due to the biotransformation of AD four products (3–6) were isolated and precisely identified by spectral methods. The applied Aspergillus species preferred to catalyze hydroxylation reaction at the C-11α position of AD, especially for Aspergillus sp. PTCC 5266 with a high yield of 86%. Moreover, it was transformed regio-, and stereoselectively by the mentioned Fusarium species. Particularly, testosterone was produced as the only product due to the reduction of AD by F. fujikuroi with 42% yield. In biotransformation of nandrolone decanoate (2) by F. fujikuroi two metabolites 17β-hydroxyestr-4-en-3-one (7) and estr-4-en-3,17-dione (8) was produced. This is the first study on the microbial transformation of these compounds by these fungal strains which could be applied as the efficient and environmentally friendly biocatalysts for production of pharmaceutical steroid compounds.
References
Abourashed E, Clark A, Hufford C (1999) Microbial models of mammalian metabolism of xenobiotics: an updated review. Curr Med Chem 6:359–374
Al-Aboudi A, Kana'an BM, Zarga MA, Bano S, Atiatul W, Javed K, Choudhary MI (2017) Fungal biotransformation of diuretic and antihypertensive drug spironolactone with Gibberella fujikuroi, Curvularia lunata, Fusarium lini, and Aspergillus alliaceus. Steroids 128:15–22
Andryushina VA, Voishvillo NE, Druzhinina AV, Stytsenko TS, Yaderets VV, Petrosyan MA, Zeinalov OA (2013) 14α -Hydroxylation of steroids by mycelium of the mold fungus Curvularia lunata (VKPM F-981) to produce precursors for synthesizing new steroidal drugs. Pharm Chem J 47:103–108
Arabi H, Tabatabaei Yazdi M, Faramarzi MA (2010) Influence of whole microalgal cell immobilization and organic solvent on the bioconversion of androst-4-en-3,17-dione to testosterone by Nostoc muscorum. J Mol Catal B: Enzym 62:213–217
Basso AV, Nicotra VE, Parra AS, MartÃnez A, Fernandez-Vivas A (2016) Biotransformation of salpichrolides A, C, and G by three filamentous fungi. J Nat Prod 79:1658–1667
Baydoun E, Karam M, Wahab A, Khan MS, Ahmad MS, Samreen SC, Abdel-Massih R, Choudhary MI (2014) Microbial transformation of nandrolone with Cunninghamella echinulata and Cunninghamella blakesleeana and evaluation of leishmaniacidal activity of transformed products. Steroids 88:95–100
Bibby M, Double J, Mughal M (1981) Effects of nandrolone decanoate on the toxicity and anti-tumour action of CCNU and FU in murine tumours. Br J cancer 44:572–577
Choudhary MI, Sultan S, Khan MTH, Yasin A, Shaheen F, Rahman AU (2004) Biotransformation of (+)-androst-4-ene-3,17-dione. Nat Prod Res 18:529–535
Donova MV, Egorova OV (2012) Microbial steroid transformations: current state and prospects. Appl Microbiol Biot 94:1423–1447
Faramarzi MA, Badiee M, Tabatabaei Yazdi M, Amini M, Torshabi M (2008) Formation of hydroxysteroid derivatives from androst-4-en-3,17-dione by the filamentous fungus Mucor racemosus. J Mol Catal B: Enzym 50:7–12
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS (2003) Microbial conversion of steroid compounds: recent developments. Enzym Microb Tech 32:688–705
Ghasemi S, Mohajeri M, Habibi Z (2014a) Biotransformation of testosterone and testosterone heptanoate by four filamentous fungi. Steroids 92:7–12
Ghasemi S, Kheyrabadi R, Habibi Z (2014b) Microbial transformation of hydrocortisone by two fungal species Fusarium fujikuroi PTCC 5144 and Rhizomucor pusillus PTCC 5134. Biocatal Biotransform 32:168–172
Heidary M, Habibi Z (2016) Microbial transformation of androst-4-ene-3,17-dione by three fungal species Absidia griseolla var. igachii, Circinella muscae and Trichoderma virens. J Mol Catal B Enzym 126:32–36
Holland HL (1999) Recent advances in applied and mechanistic aspects of the enzymatic hydroxylation of steroids by whole-cell biocatalyst. Steroids 64:178–186
Hu S, Genain G, Azerad R (1995) Microbial transformation of steroids: contribution to 14α-hydroxylations. Steroids 60:337–352
Iqbal Choudhary M, Adnan S, Shah A, Atta Ur R (2008) Microbial oxidation of anabolic steroids. Nat Prod Res 22:1289–1296
Janeczko T, Dmochowska-Gladysz J, Kostrzewa-Suslow E, Bialonska A, Ciunik Z (2009) Biotransformations of steroid compounds by Chaetomium sp. KCH 6651. Steroids 74:657–661
Kalbasi A, Faramarzi MA, Hejazi MS, Jahandar H, Amini M, Jalali SM (2009) 14α-hydroxylation of androst-4-en-3, 17-dione by the whole cells of cyanobacterium Nostoc piscinale. Biotechnol 8:370–374
Kolet SP, Niloferjahan S, Haldar S, Gonnade R, Thulasiram HV (2013) Biocatalyst mediated production of 6b,11a-dihydroxy derivatives of 4-ene-3-one steroids. Steroids 78:1152–1158
Kollerov V, Shutov A, Kazantsev A, Donova M (2020) Biotransformation of androstenedione and androstadienedione by selected Ascomycota and Zygomycota fungal strains. Phytochemistry 169:112160–112168
Koshimura M, Utsukihara T, Hara A, Mizobuchi S, Horiuchi CA, Kuniyoshi M (2010) Hydroxylation of steroid compounds by Gelasinospora retispora. J Mol Catal B: Enzym 62:72–77
Li J, Tang W, Ren D, Xu J, Yang Z (2019) Iridium-catalysed highly selective reduction-elimination of steroidal 4-en-3-ones to 3, 5-dienes in water. Green Chem 21:2088–2094
Malaviya A, Gomes J (2008) Androstenedione production by biotransformation of phytosterols. Bioresour Technol 67:25–6737
Nassiri-Koopaei N, Faramarzi MA (2015) Recent developments in the fungal transformation of steroids. Biocatal Biotransform 33:1–28
Parshikov IA, Sutherland JB (2015) Biotransformation of steroids and flavonoids by cultures of Aspergillus niger. Appl Biochem Biotechnol 176:903–923
Sultana N (2018) Microbial biotransformation of bioactive and clinically useful steroids and some salient features of steroids and biotransformation. Steroids 136:76–92
Swizdor A, Panek A, Milecka-Tronina N (2017) Hydroxylative activity of Aspergillus niger towards androst-4-ene and androst-5-ene steroids. Steroids 126:101–106
Wang Y, Xiang L, Huang Y, Yi X, He X (2019) Microbial transformation of laxogenin by the fungus Syncephalastrum racemosum. Tetrahedron 75:1440–1449
Wilds AL, Nelson NA (1953) The facile synthesis of 19-nortestosterone and 19-norandrostenedione from estrone. J Chem Soc Chem 75:5366–5369
Xiong Z, Wei Q, Chen H, Chen S, Xu W, Qiua G, Liang S, Hu X (2006) Microbial transformation of androst-4-ene-3,17-dione by Beauveria bassiana. Steroids 71:979–983
Yazdi MT, Amani A, Faramarzi MA, Amini M, Shafiee A, Fathabad EG (2005) Nandrolone decanoate transformation by Neurospora crassa. Pharm Biol 43:630–635
Yazdi MT, Zanjanian SM, Faramarzi MA, Amini M, Amani A, Abdi K (2006) Microbial transformation of nandrolone decanoate by Acremonium strictum. Arch Pharm Chem Life Sci 339:473–476
Ye M, Guo D (2005) Substrate specificity for the 12 beta-hydroxylation of bufadienolides by Alternaria alternate. J Biotech 117:253–262
Zoghi M, Gandomkar S, Habibi Z (2019) Biotransformation of progesterone and testosterone enanthate by Circinella muscae. Steroids 151:108446
Acknowledgements
This work was supported by a Research Council of Shahid Beheshti University, Tehran, Iran and Islamic Azad University of Ilam, Iran for which the authors are thankful.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heidary, M., Ghasemi, S., Habibi, Z. et al. Biotransformation of androst-4-ene-3,17-dione and nandrolone decanoate by genera of Aspergillus and Fusarium. Biotechnol Lett 42, 1767–1775 (2020). https://doi.org/10.1007/s10529-020-02902-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02902-4