Abstract
Objective
l-methionine is an important sulfur-containing amino acid essential for humans and animals. Its biosynthesis pathway is complex and highly regulated. This study aims to explore the bottleneck limiting the improvement of l-methionine productivity and apply efficient strategies to increase l-methionine production in engineered E. coli.
Results
The enzyme O-succinylhomoserine sulfhydrylase involved in thiolation of OSH to form homocysteine was overexpressed in the engineered strain E. coli W3110 IJAHFEBC/PAm, resulting in l-methionine production increased from 2.8 to 3.22 g/L in shake flask cultivation. By exogenous addition of l-glycine as the precursor of one carbon unit, the titer of l-methionine was increased to 3.68 g/L. The glycine cleavage system was further strengthened for the efficient one carbon unit supply and a l-methionine titer of 3.96 g/L was obtained, which was increased by 42% compared with that of the original strain.
Conclusions
Insufficient supply of one carbon unit was found to be the issue limiting the improvement of l-methionine productivity and its up-regulation significantly promoted the l-methionine production in the engineered E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-methionine is an important and unique sulfur-containing essential amino acid playing an integral role in a variety of cellular processes including protein synthesis, regulation of catalytic functions, and post-translational modification of proteins (Zhou et al. 2019). It is widely used in food, feed additive and pharmaceutical fields with a huge market of million tons annually (Jankowski et al. 2014). As a result, the green and efficient production of l-methionine has attracted a great deal of attention.
Based on the advantages including ease of cultivation, high accessibility of genetic information and availability of genetic manipulation tools, Escherichia coli is always chosen as an ideal host strain for industrial production of l-aspartate family amino acids, such as l-threonine, l-lysine and l-methionine (Li et al. 2017, 2018). Meanwhile, the biosynthetic pathway and regulatory mechanisms of amino acids have been extensively studied in E. coli (Figge 2007; Chen et al. 2018), which provides important clues for its engineering with improved productivity. The synthesis pathway of l-methionine in E. coli is complicated. It consists of three main modules: O-succinyl homoserine synthesis module, l-cysteine synthesis module (sulfur source), and CH3–THF synthesis module (one carbon unit) (Huang et al. 2018; Willke. 2014). Meanwhile, there are corresponding node genes between modules, which play important roles in regulating the expression and activity of the key enzymes involved in the pathway (Kawano et al. 2018; Zhang et al. 2017). Therefore, how to properly handle the balance among branches is extremely important to increase l-methionine production.
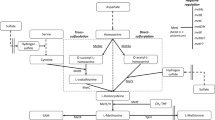
The biosynthesis of l-methionine can be divided into three steps: (1) Glucose is generally absorbed and converted to aspartic acid via glycolysis and TCA cycle, which could be subsequently converted to O-succinyl-homoserine (OSH) by aspartate kinase, aspartate-semialdehyde dehydrogenase, homoserine dehydrogenase and homoserine O-succinyltransferase (Figge et al. 2016), (2) l-cysteine and the resulting OSH are catalyzed by cystathionine γ-synthase (metB) to γ-cystathionine, which is subsequently converted to homocysteine by cystathionine β-lyase (metC/malY) (Shim et al. 2016), (3) Homocysteine and 5-methyltetrahydrofolate (CH3-THF) are finally converted to l-methionine by methionine synthase (metE/metH) (Fig. 1) (Matthews et al. 2008).
Construction of recombinant E. coli for enhanced production of l-methionine. The yellow frame represents the CH3–THF synthesis module, the green frame represents the O-succinyl homoserine synthesis module and the gold frame represents the CH3–THF synthesis module. The red font represented the genes that were overexpressed by replacing the native promoter with the Trc promoter in genome (gcvTHP: encoding glycine cleavage system). The blue font represented the genes that were overexpressed in plasmid (metC/malY: encoding cystathionine β-lyase/l-cysteine desulfhydrase, metH: encoding cobalamin-dependent methionine synthase, glyA: encoding serine hydroxymethyltransferase and metZ: encoding succinylhomoserine sulfhydrylase). OAS: O-acetyl serine, THF: tetrahydrofolate, 5,10-CH2 − THF: 5,10-methylene-tetrahydrofolate, CH3 − THF: 5-methyltetrahydrofolate
Many attempts have been made to improve the l-methionine titer, including deletion of competitive pathway and negative regulatory factor, overexpression of key enzyme and transporters, as well as attenuation of methionine consumption pathway (Huang et al. 2017; Li et al. 2016). In our previous study, the strain E. coli ΔmetJΔmetIΔlysA/pTrcA*H was constructed by deletion of genes metJ, metI and lysA, which encode the negative transcriptional regulator, l-methionine importer, and the enzyme involved in l-lysine biosynthesis pathway, respectively, as well as overexpression of yjeH and metA encoding l-methionine exporter and homoserine O-succinyltransferase, the first enzyme involved in l-methionine biosynthesis in E. coli. As a result, 0.593 g/L of methionine was produced in shake flask fermentation (Huang et al. 2017). By further analyzing the l-methionine synthesis pathway in the engineered E. coli and regulating the balance between the three modules including O-succinyl homoserine synthesis module, l-cysteine synthesis module, and CH3-THF synthesis module, an optimal strain E. coli W3110 IJAHFEBC/PAm was obtained with l-methionine production of 2.8 g/L in shake flask fermentation (Huang et al. 2018). Although the l-methionine production with high titer was achieved, the titer of the reported strains using glucose as carbon source was still lower than the theoretical value (0.52 (C-mol) (C-mol)−1) (Krömer et al. 2006). Thus, it is necessary to rationally analyze the metabolic bottleneck of the strain capable of over-producing l-methionine.
In this study, homocysteine synthesis was first enhanced by expressing O-succinylhomoserine sulfhydrylase (metZ) to provide more l-methionine precursor. The γ-cystathionine lysis and homocysteine methylation were enhanced by strengthening the expression of metC/malY (encoding cystathionine β-lyase/l-cysteine desulfhydrase) and metH (encoding cobalamin-dependent methionine synthase), respectively. Based on the result that the insufficient supply of one carbon unit was the bottleneck limiting l-methionine titer, the CH3-THF supply was enhanced with exogenous addition of l-glycine and improvement of the glycine cleavage system (Fig. 1). By above strategies, the l-methionine titer was finally increased.
Materials and methods
Bacterial strains, plasmids, materials and chemicals
The E. coli ΔIJAHFEBC (M2)/PAm was constructed in our lab previously (Huang et al. 2018) and its characteristics were described in Supplementary Table 1. In this stain, the expression of metH (encoding homocysteine-N5-methyltetrahydrofolate transmethylase), metF (encoding methylenetetrahydrofolate reductase), cysE (encoding serine O-acetyltransferase), serB (encoding phosphoserine phosphatase) and serC (encoding phosphoserine aminotransferase) were enhanced by replacing their original promoters with trc promoter, and the genes metI encoding the methionine importer, metJ encoding the negative transcriptional regulator, and lysA encoding the enzyme involved in lysine biosynthesis pathway were knocked out. The plasmid PAm was constructed by insertion of genes serA (encoding D-3-phosphoglycerate dehydrogenase), metA (encoding homoserine transsuccinylase), and yjeH (encoding l-methionine exporter) in the pTrc99A plasmid.
Construction of plasmids and strains
For the expression of key enzymes, the genes metC (encoding cystathionine β-lyase/l-cysteine desulfhydrase), malY (encoding cystathionine β-lyase), metH (encoding cobalamin-dependent methionine synthase) and glyA (encoding serine hydroxymethyltransferase) were amplified from E. coli W3110 genomic using the corresponding primers (Supplementary Table 2). The metZ (encoding succinylhomoserine sulfhydrylase) was directly synthesized with codon optimization in E. coli host. The obtained DNA fragments metC, malY, metH, glyA and metZ were cloned into the PAm plasmid after the locus of serA gene by onestep clone kit (ClonExpress®), generating PAmC, PAmlY, PAmH, PAmlyA and PAmZ plasmids, respectively (Supplementary Fig. 1). The obtained DNA fragments metH and glyA were further cloned into after the metZ gene, generating PAmZH and PAmZlyA plasmid, respectively. The pTarget plasmid used for gene editing was subjected to site-directed mutagenesis using primers pTarget-gcvTHP-F and pTarget-gcvTHP-R to obtain pTarget-gcvTHP plasmid. The DNA polymerase, plasmid DNA Extraction Kit and One step clone kit were purchased from Vazyme Biotech (Nanjing, China). All the other reagents and chemicals used were of analytical grade and commercially available.
In order to enhance the utilization of l-glycine, the gcvTHP (encoding glycine cleavage) native promoter was replaced with the Trc promoter using the gene editing CRISPR/Cas9. The construction process was according to the method of previous reports (Xia et al. 2016; Xu et al. 2019). A 500-bp homologous arms to native gcvTHP promoter on each side was amplified, respectively. The two homologous arms were further fused together with trc promoter by overlap-extension PCR, generating the donor dsDNA. A total of 400 ng donor dsDNA and 100 ng corresponding pTargetgcvTHP plasmid were transformed into the competent cells by electroporation. Positive colonies were confirmed by PCR and sequencing. The elimination of the pTarget was carried out by cultivating the positive colonies in LB containing 1 mM IPTG for 12–16 h at 30 °C. The pCas was eliminated by cultivating the engineered strain at 37 °C overnight. After elimination of the pTargetgcvTHP and Pcas plasmids, M3 strain was obtained. The PAmZ plasmid was then transformed in M3 strain, to generate the corresponding recombinant strain M3/PAmZ.
Growth conditions
Luria broth (LB) was used for seed cultures. MS medium was used for fermentation cultures to produce l-methionine, which contained 16 g/L (NH4)2SO4, 2 g/L yeast extract, 20 g/L glucose, 1 g/L KH2PO3, 2 g/L Na2S2O3, 1 mg/L Vb12, 0.01 g/L l-lysine, 1 mL/L salty solution and 10 g/L CaCO3. Salty solution was composed of 5 g/L MnSO4·8H2O, 500 g/L MgSO4·7H2O, 5 g/L ZnSO4, 5 g/L FeSO4·7H2O. For shake flask fermentation, the single colony of the engineered E. coli was cultivated in LB medium at 37 °C overnight and then inoculated into 20 mL MS medium with 5% (v/v) inoculum in a 500 mL shake flask. The cultivation was performed at 30 °C on a rotatory shaker at 150 rpm for 48 h.
Analytical methods
To analyze l-methionine, the following protocol was followed: 50 μL cell culture was taken and centrifuged at 12,000 rpm for 1 min. The supernatant was mixed with 50 μL ddH2O, 100 μL NaHCO3 (0.5 M) and 100 μL DNFB (2,4-dinitrofluorobenzene, 1:100 v/v in acetonitrile) for derivatization reaction, which was performed in 60 °C for 1 h and terminated by adding 700 μL phosphate buffer (200 mM, pH 7.0) (Huang et al. 2018). The samples were filtered through a 0.22 μm nylon filter (Jinteng, Tianjin, China) and then analyzed by HPLC (Primaide, Hitachi, Japan) equipped with an ultraviolet detector. The C18 column (5 μm, 250 × 4.6 mm) was used to separate the samples at 40 °C and the detection wavelength was set as 365 nm. The elution buffer was constituted of water (with 2.05 g/L NaAc and 1.5 mL/L acetic acid dissolved) and methyl alcohol in a ratio of 1:1 (v/v). The flow rate was set at 1 mL/min. The retention time for l-methionine was 14 min.
Results and discussion
Conversion of γ-cystathionine for l-methionine production
The M2/PAm strain was constructed in our previous study and its metabolome analysis showed that γ-cystathionine was significantly accumulated, which is the precursor of homocysteine synthesis (Huang et al. 2018). Since homocysteine is the precursor of l-methionine, two different γ-cystathionine lyases including metC and malY were overexpressed in this stain, to convert γ-cystathionine for improved l-methionine production. Out of our expectation, l-methionine production was not increased (Fig. 2). The quantitative RT-PCR for metC and malY was performed in M2/PAm, M2/PAmC and M2/PAmlY strains. It was found that the transcriptional level of metC and malY in M2/PAmC and M2/PAmlY strains were increased by 100, 240 times, respectively (Supplementary Fig. 2), indicating the successful expression of the two enzymes. It was proposed that the metC and malY were involved in conversion of both γ-cystathionine and l-cysteine. Therefore, their overexpression promoted the lysis of γ-cystathionine while decreased the supply of sulfur source for l-methionine biosynthesis at the same time, failing to increase the l-methioninetiter (Figge 2007).
l-methionine production with enhancement of homocysteine synthesis and homocysteine methylation in engineered E. coli. Red bars represent l-methionine titer, blue bars represent the biomass (dry cell weight, DCW). M2/PAm: the original strain, M2/PAmC: overexpression of metC in M2/PAm strain, M2/PAmlY: overexpression of malY in M2/PAm strain, M2/PAmZ: overexpression of metZ in M2/PAm strain, M2/PAmH: overexpression of metH in M2/PAm strain, M2/PAmZH: overexpression of metH in M2/PAmZ strain. Error bars show the standard deviations of triplicate samples
Introduction of O-succinylhomoserine sulfhydrylase (metZ) for converting OSH to homocysteine
Since overexpression of γ-cystathionine lyases in strain M2/PAm did not effectively increase l-methionine production, the γ-cystathionine precursor OSH was designed to be directly converted to the l-methionine precursor homocysteine. MetZ derived from Chromobacterium violaceum was found to catalyze H2S and OSH to form homocysteine (Figge et al. 2016; Shim J et al. 2016) and it was introduced in the M2/PAm strain. The results showed that the l-methionine production level was increased from 2.8 to 3.22 g/L (Fig. 2). The introduction of metZ not only increased the conversion of OSH to l-methionine precursor homocysteine, but also effectively uses H2S as a sulfur source to synthesize l-methionine. Therefore, enhanced l-methionine production was obtained.
Effect of enhancing homocysteine methylation on l-methionine production
The terminal step in l-methionine biosynthesis can be catalyzed by two apparently unrelated proteins, cobalamin-independent methionine synthase (MetE) and cobalamin-dependent methionine synthase (MetH). Both enzymes catalyze the transfer of a methyl group to homocysteine (Hondorp and Matthews 2009). The cobalamin-dependent enzyme catalysis seems to be more conducive to the l-methionine biosynthesis, since the kcat values for MetH are 50–100 times higher than that of MetE (Matthews et al. 2008). Therefore, metH was overexpressed on the PAm and PAmZ plasmid. The results in Fig. 2 indicated that overexpression of metH decreased the production of l-methionine, which was explained by the reason that excessive expression of metH may consume a large amount of CH3-HTF, resulting in imbalance of the metabolism of intracellular one carbon units. Therefore, an attempt was made to increase the activity of metH by adding cobalamin (Vb12), a kind of added coenzyme, but without expression of metH in plasmid. As expected, the l-methionine production in M2/PAm was increased to 3.06 g/L (Fig. 3). However, the strain M2/PAmZ overexpressing metZ did not increase l-methionine production after the addition of cobalamin, which indicated that there was still a new rate-limiting step for this strain in the supply of CH3-THF.
l-methionine production with addition of different concentration of Vb12. Red square represents the strain of M2/PAm, blue circle represents the strain of M2/PAmZ. M2/PAm: the original strain, M2/PAmZ: overexpression of metZ in M2/PAm strain. Error bars show the standard deviations of triplicate samples
Effect of glyA overexpression on l-methionine production
In E. coli, about 75% of the one carbon units required for growth are provided by the l-serine hydroxymethyltransferase (glyA) catalyzed reaction, and the other one carbon units are provided by the glycine cleavage system (gcvTHP) (Zhang et al. 2017). The transfer of methyl group from l-serine to THF is performed by the reaction of glyA, to form 5,10-methylenetetrahydrofolate (5,10-CH2–THF) and l-glycine, and the resulting l-glycine can further produce 5,10-CH2–THF through the glycine cleavage system. Finally, 5,10-CH2–THF is catalyzed by the reaction of 5,10-methylenetetrahydrofolate reductase (metF) to form CH3–THF for the synthesis of l-methionine.
In order to increase the content of one carbon unit, we chose to up-regulate the glyA gene on the PAm and PAmZ plasmid, and the results showed that overexpression of the glyA gene on the plasmid not only resulted in loss of l-methionine production, but also accumulation of l-serine in the fermentation broth (Fig. 4). The reason may be that the reaction catalyzed by glyA is reversible, and an increase in its expression affects the balance of the metabolic reaction, thereby causing the reaction to proceed toward the formation of l-serine.
l-methionine production with overexpression of glyA in engineered E. coli strains. Red bars represent l-methionine titer, yellow bars represent l-serine titer, blue bars represent the biomass (dry cell weight, DCW). M2/PAm: the original strain, M2/PAmZ: overexpression of metZ in M2/PAm strain, M2/PAmlyA: overexpression of glyA in M2/PAm strain, M2/PAmZlyA: overexpression of glyA in M2/PAm strain. Error bars show the standard deviations of triplicate samples
Effect of l-glycine addition on l-methionine production
Since l-glycine can supply as one carbon unit through the glycine cleavage system, different concentrations of l-glycine were added to investigate whether the lack of one carbon unit resulted in the inability to increase l-methionine further. The results showed that a slight addition of l-glycine resulted in l-methionine titer increased to 3.68 g/L, but the l-methionine titer no longer increased when the additive contents reached 1 g/L (Fig. 5). It is speculated that l-glycine addition was necessary as the supply of one carbon unit, but its uptake rate does not meet the needs of l-methionine synthesis, so the titer of l-methionine is no longer increased when the intake rate reaches its maximum. To verify whether the rate of l-glycine transport limits the use of glycine by E. coli, we examined the residual extracellular and intracellular l-glycine after adding different concentrations of l-glycine in the fermentation culture. The results showed that the glycine used by E. coli in the fermentation process was almost the same regardless of whether 2 g/L of l-glycine or 1 g/L of l-glycine was added (Supplementary Fig. 3). Meanwhile, intracellular l-glycine was not detected when different concentrations of l-glycine were added, indicating that the effect of l-glycine addition on l-methionine production is limited by the transportation of l-glycine.
Effect of l-glycine addition concentration on l-methionine production. Red square represents the strain of M2/PAm, blue circle represents the strain of M2/PAmZ. M2/PAm: the original strain, M2/PAmZ: overexpression of metZ in M2/PAm strain. Error bars show the standard deviations of triplicate samples
Strengthening the glycine cleavage system for further enhancement of l-methionine production
Since the addition of high concentration of l-glycine can only slightly increase l-methionine production, it was considered to strengthen the glycine cleavage system in M2 for efficient supply of one carbon units. Therefore, the glycine cleavage system was overexpressed by replacing the gcvTHP native promoter with the Trc promoter, resulting in strain M3. As shown in the Fig. 6, the l-methionine titer was increased to 3.96 g/L when 1 g/L of l-glycine was added. However, no further increase in l-methionine production was observed when the amount of l-glycine added was increased to 2 g/L.
Effect of enhancing one carbon unit synthesis on l-methionine production. Red bars represent l-methionine titer, blue bars represent the biomass (dry cell weight, DCW). M2/PAmZ: overexpression of metZ in M2/PAm strain, M3/PAmZ: replacing the original promoter of gcvTHP with a strong Trc promoter in M2 genome. Error bars show the standard deviations of triplicate samples
The above results indicated that the bottleneck in the engineered strains for enhanced production of l-methionine is mainly the insufficient supply of one-carbon units, but on the other hand, because one-carbon units participate in many complex metabolic pathways in vivo, its regulation is difficult. Efficient supply of one carbon unit is going to be the further research focus for efficient production of l-methionine in the engineered E. coli.
Conclusions
In our previous studies, engineered E. coli strains were constructed for efficient production of l-methionine. However, further increase in l-methionine yield was restricted. The blocked lysis of cystathionine and insufficient supply of one carbon unit was found to be the main limiting factors and different strategies were tried to solve the problems. By introducing the key enzyme involved in thiolation of OSH to form l-methionine precursor homocysteine and strengthening the glycine cleavage system for efficient supply of one carbon unit, the final l-methionine titer reached 3.96 g/L, which was 42% higher than that of the original strain. The results provided important guidance for further construction of engineered strains with enhanced production of l-methionine, and meanwhile the strategies employed were meaningful for rational design of cell factories for similar amino acid biosynthesis.
References
Chen XL, Gao C, Guo L, Hu GP, Luo QL, Liu J, Nielsen J, Chen J, Liu LM (2018) DCEO biotechnology: tools to design, construct, evaluate, and optimize the metabolic pathway for biosynthesis of chemicals. Chem Rev 118(1):4–72
Figge RM (2007) Methionine biosynthesis in Escherichia coli and Corynebacterium glutamicum. In: Wendisch VF (ed) Amino acid biosynthesis pathways, regulation and metabolic engineering. Springer, Berlin
Figge R, Dumon Seignovert L, Vasseur P, Dischert W (2016) Method and microorganism for methionine production by fermentation with improved methionine efflux. European Patent WO 2016034536 A1.
Hondorp ER, Matthews RG (2009) Oxidation of cysteine 645 of cobalamin-independent methionine synthase causes a methionine limitation in Escherichia coli. J Bacteriol 191(10):3407–3410
Huang JF, Liu ZQ, Jin LQ, Tang XL, Shen ZY, Yin HH, Zheng YG (2017) Metabolic engineering of Escherichia coli for microbial production of L-methionine. Biotechnol Bioeng 114(4):843–851
Huang JF, Shen ZY, Mao QL, Zhang XM, Zhang B, Wu JS, Liu ZQ, Zheng YG (2018) Systematic analysis of bottlenecks in a multi-branched and multilevel regulated pathway: The molecular fundamentals of L-methionine biosynthesis in Escherichia coli. ACS Synth Biol 7:2577–2589
Jankowski J, Kubinska M, Zdunczyk Z (2014) Nutritional and immunomodulatory function of methionine in poultry diets—a review. Ann Anim Sci 14(1):17–31
Kawano Y, Suzuki K, Ohtsu I (2018) Current understanding of sulfur assimilation metabolism to biosynthesize L-cysteine and recent progress of its fermentative overproduction in microorganisms. Appl Microbiol Biot 102(19):8203–8211
Krömer JO, Wittmann C, Schröder H, Heinzle E (2006) Metabolic pathway analysis for rational design of L-methionine production by Escherichia coli and Corynebacterium glutamicum. Metab Eng 8(4):353–369
Li H, Wang BS, Li YR, Zhang L, Ding ZY, Gu ZH, Shi GY (2016) Metabolic engineering of Escherichia coli W3110 for the production of L-methionine. J Ind Microbiol Biot 44(1):75–88
Li Y, Wei H, Wang T, Xu Q, Zhang C, Fan X, Ma Q, Chen N, Xie X (2017) Current status on metabolic engineering for the production of L-aspartate family amino acids and derivatives. Bioresource Technol 245:1588–1602
Li T, Zhou W, Bi H, Zhuang Y, Zhang T, Liu T (2018) Production of caffeoylmalic acid from glucose in engineered Escherichia coli. Biotechnol Lett 40(7):1057–1065
Matthews RG, Koutmos M, Datta S (2008) Cobalamin-dependent and cobamide-dependent methyltransferases. Curr Opin Struct Biol 18(6):658–666
Shim J, Shin Y, Lee I, Kim SY (2016) L-methionine production. Amino Acid Fermentation. Springer, Berlin, pp 153–177
Willke T (2014) Methionine production—a critical review. Appl Microbiol Biot 98(24):9893–9914
Xia J, Wang L, Zhu JB, Sun CJ, Zheng MG, Zheng L, Lou YH, Shi L (2016) Expression of Shewanella frigidimarina fatty acid metabolic genes in E. coli by CRISPR/cas9-coupled lambda Red recombineering. Biotechnol Lett 38(1):117–22.
Xu JM, Li JQ, Zhang B, Liu ZQ, Zheng YG (2019) Fermentative production of the unnatural amino acid L-2-aminobutyric acid based on metabolic engineering. Microb Cell Fact 18:43
Zhang Y, Kang P, Liu S, Zhao YJ, Wang ZW, Chen T (2017) glyA gene knock-out in Escherichia coli enhances L-serine production without glycine addition. Biotechnol Bioprocess 22(4):390–396
Zhou HY, Wu WJ, Niu K, Xu YY, Liu ZQ, Zheng YG (2019) Enhanced L-methionine production by genetically engineered Escherichia coli through fermentation optimization. 3 Biotech 9(3):96.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2018YFA0901403) and National Natural Science Foundation of China (31971342 and 31700095).
Supporting Information
Supplementary Table 1—Plasmids and strains used in this study.
Supplementary Table 2—Primers used in this study.
Supplementary Table 3—The nucleotide sequences of codon-optimized genes used in this study.
Supplementary Table 4—Comparison of L-methionine productivity in different engineered strains.
Supplementary Fig. 1—The procedure to construct the recombinant plasmids. (Take the PAmZ plasmid construction as an example).
Supplementary Fig 2—Quantitative RT-PCR results of metC and malY genes in M2/PAm, M2/PAmC and M2/PAmlY, respectively.
Supplementary Fig. 3—Residual amount of extracellular glycine in M2/PAmZ strain after adding different concentrations of L-glycine in the fermentation culture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, XL., Du, XY., Chen, LJ. et al. Enhanced production of l-methionine in engineered Escherichia coli with efficient supply of one carbon unit. Biotechnol Lett 42, 429–436 (2020). https://doi.org/10.1007/s10529-019-02786-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02786-z