Abstract
Objective
Thrombin, platelets, and plasmin are three key factors involved in hemostasis and thrombolysis. Thrombolytic therapy with clinically approved drugs is often followed by recurrent thrombosis caused by thrombin-induced platelet aggregation from the clot debris. In order to minimize these problems, new constructs were designed for the expression of recombinant staphylokinase (rSAK) and also a fusion protein composed of staphylokinase, 20 amino acids containing 2 RGD followed by tsetse thrombin Inhibitor (SAK-2RGD-TTI) in Pichia pastoris.
Result
Modeling the tertiary structure of SAK-2RGD-TTI showed that the linker containing RGD and TTI did not interfere with proper folding of SAK. In laboratory testing, the purified SAK-2RGD-TTI (420 μg/mL) dissolved an average of 45% of the blood clot. The activity of the SAK-2RGD-TTI was also confirmed in various tests including human plasminogen activation assay, fibrin clot lysis assay, well diffusion method, activated partial thromboplastin time and platelet rich clot lysis assay.
Conclusion
Our findings suggest that SAK-2RGD-TTI has improved therapeutic properties preventing reocclussion. It further confirms that it is practicable to assemble and produce a hybrid multifunctional protein that targets hemostatic process at various stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombotic disorders including myocardial infarction and stroke have become a major health issue that imperil human life (Lian et al. 2003; Szemraj et al. 2005). Thrombolytic therapy is a well-established treatment for these disorders in which the fibrinolytic system of the patient is activated by infusing the plasminogen activators (Pulicherla et al. 2013). One is streptokinase which induces systemic plasminogen activation with sever side-effects such as reocclussion and bleeding complications (Apte-Deshpnade et al. 2009). As a frequently occurring problem (Szemraj et al. 2007), reocclussion results from the large amount of thrombin released during thrombolysis, which in turn activates the coagulation system, promotes platelet degranulation, and inhibits fibrinolysis (Kowalski et al. 2009; Pulicherla et al. 2013; Szemraj et al. 2011). The platelets in the secondary thrombi release factors such as platelet plasminogen activator inhibitor-1 (PAI-1) rendering these clots resistant to fibrinolytic agents such as tissue plasminogen activator (t-PA) (Serizawa et al. 1993).
Staphylokinase (SAK) produced by certain lysogenized Staphylococcus aureus strains is a promising thrombolytic agent with high fibrin specificity. Mature SAK includes of 136 amino acid (Nguyen and Quyen 2012b). Its fibrin specificity is indirect as it preferentially forms a 1:1 stoichiometric complex with fibrin-bound plasminogen (Kumar et al. 2010; Lian et al. 2003). SAK-plasmin (Ogen) complex circulating in the blood is rapidly neutralized by the circulatory plasmin inhibitor, α2-antiplasmin (Apte-Deshpnade et al. 2009; Kumar et al. 2010), while the fibrin-bound SAK complex is nearly 100 times more resistant to α2-antiplasmin-mediated inhibition (Pulicherla et al. 2013). Therefore, SAK activates plasminogen on the clot surface without the systemic fibrinolytic activation (Pulicherla et al. 2013). Since SAK is PAI-I resistant, it efficiently mediates fibrinolysis of platelet-rich and retracted secondary clots (Apte-Deshpnade et al. 2009).
Several studies used staphylokinase fused to antiplatelet or antithrombin sequences or both (Kotra et al. 2013; Kowalski et al. 2009; Kumar et al. 2013; Pulicherla et al. 2012, 2013; Szarka et al. 1999; Szemraj et al. 2007, 2011; Van Zyl et al. 1997; Wang et al. 2009). Hirudin-based multifunctional recombinant sthaphylokinases such as PLATSAK (Van Zyl et al. 1997), HE-SAKK (Lian et al. 2003), HV1-SAK and SAK-HVI (Szarka et al. 1999), SAK-Thrombin recognition peptide-HV2 (Zhang et al. 2010), SAK-Hirul (Kotra et al. 2013), SAK-RGD-K2-Hir (Szemraj et al. 2005), SAK-RGD-K2-Hirul (Kowalski et al. 2009), SRH (Pulicherla et al. 2013), were constructed and expressed in different expression systems. Mature tsetse thrombin inhibitor (TTI), with 32 amino acid, is a specific stoichiometric inhibitor peptide of thrombin (Cappello et al. 1998). It does not have any activity against common serine proteases, such as thrombolytic cascade, components of the human coagulation, trypsin and chymotrypsin (Cappello et al. 1996, 1998). TTI not only has intrinsic anticoagulation effects, it is also a potent inhibitor for thrombin-induced platelet aggregation (Cappello et al. 1996, 1998; Rydel et al. 1990). Unlike hirudin, TTI does not require reshuffling the disulfide bonds to show functional effects (Kowalski et al. 2009; Lian et al. 2003).
It was previously demonstrated that the rSAK expressed in Pichia pastoris was a potent thrombolytic product (Faraji et al. 2017). This study aimed to design and express a fusion of SAK with RGD and recently rediscovered TTI, instead hirudin or its derivatives, to investigate their effects on SAK biological activity.
Materials and methods
Design, structure prediction
The protein sequence of SAK was as previously reported (Faraji et al. 2017); the TTI sequence (O97373) was obtained from Uniprot database. The C-terminal mature SAK fragments was fused to a linker (ARASGRGDGGDSGRGDGGRA) followed by TTI. For providing structural flexibility, this linker contains glycine and serine residues flanking RGD motifs. Another linker (ARASLIL) fused C-terminal of TTI to C-Myc and 6 His tag.
Potential effects of the linkers and TTI on SAK was investigate using bioinformatics tools. Secondary structure was predicted using the improved self-optimized prediction method (SOPMA) software (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html). Four conformational states (helices, sheets, turns and coils) of candidate construct were analyzed. Tertiary structure was predicted by I-TASSER server (http://zhanglab.ccmb.med.umich.edu/I-TASSER). It is based on folding recognition or treading, plus homology with other known structures. This software delivers 5 models in PDB format, in which the best model is the one with higher C-score. In order to improve modelled protein structure, the model energy was minimized using Swiss-PDB Viewer software (v. 4.1.0, Basel, Switzerland). Then, the model was validated by Ramachandran Plot using Rampage software (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php).
Expression and purification of the SAK-2RGD-TTI
The preparation of the coding construct, its expression and condition optimization (effect of methanol, pH and temperature on expression and activity) were carried out as previously described (Faraji et al. 2019). A codon-optimized construct for the pPICZαA-SAK-2RGD-TTI was expressed in Pichia pastoris with or without 20 μg/mL tunicamycin (deglycosylating antibiotic) in the expression medium (BMMY). The final product was purified using Ni2+-IDA (iminodiacetic acid) resin and quantified using BCA protein quantification kit (both purchased from Parstous, Mashhad, Iran). The presence of fusion protein and its purity were also assessed by western blotting technique using mouse anti c-Myc (9E10) primary antibody (Santa Cruz Biotechnology, Dallas, TX) and goat anti-mouse IgG conjugated to horseradish peroxidase (HRP) (Santa Cruz) and densitometric analysis of coomassie brilliant blue stained gels (12% (w/v) SDS-PAGE) using gel-pro software (Media Cybernetics Silver Springs, MD). Flow chart of study design was illustrated (Fig. 1).
Human plasminogen activation assay of the SAK-2RGD-TTI
Biological activity of the purified SAK-2RGD-TTI and rSAK was determined by its amidolytic activity as previously described (Hernández et al. 1990) with minor modification. Briefly, the SAK-2RGD-TTI was dissolved in 100 mM phosphorus buffer (pH 7.4). In the test tubes, 50 μL of the SAK-2RGD-TTI (4, 8 and 16 μM) was mixed with 100 μL of plasminogen (2.4 μM) and incubated at 37 °C for 30 min. Subsequently, chromogenic substrate Spectrozyme PL (American Diagnostic Inc., USA) was added to the SAK-2RGD-TTI-plasminogen complex at a final concentration of 0.3 mM and incubated at 37 °C for 30 min. The reaction was stopped by adding 10 μL of 0.4 M acetic acid and chromogenic release of p-nitroanilide was measured at 405 nm. Each experiment was repeated 3 times and compared with the rSAK.
Fibrin clot lysis assay of the SAK-2RGD-TTI
In a 96-well plate, 100 μL of a mixture forming fibrin clot including human thrombin (1 NIH unit/mL) and human fibrinogen (1 mg/mL) (sigma) was prepared and incubated for 3 h at room temperature (Pulicherla et al. 2013). Then 100 μL of different concentrations of the purified SAK-2RGD-TTI or the rSAK, and plasminogen (2.4 µM), pH 7.4, was added to each well and incubated for 5 h. The turbidity in each well was measured at 405 nm. Residual turbidity for different concentrations of the SAK-2RGD-TTI was calculate versus negative control without the SAK-2RGD-TTI. The concentration of SAK-2RGD-TTI required to achieve 50% the clot lysis (a decrease 50% in turbidity) was considered as C50. Each experiment was repeated 3 times and compared with the rSAK. To convert molecular weight to mole, the Russian online site “http://molbiol.edu.ru/eng/scripts/01_04.html” was used.
Fibrinolytic activity assay of the SAK2RGD-TTI using clot lytic method and residual clot weight
The cost effectively clot lytic method, described by Swetha Prasad et al. (Faraji et al. 2017), was used to confirm the fibrinolytic activity of the SAK-2RGD-TTI. In this method, 500 μL of clotted whole blood was mixed with 100 μL of the SAK-2RGD-TTI and incubated at 37 °C for 90 min. The weight of the clot before incubation with the SAK-2RGD-TTI was compared to the after-incubation one (Prasad et al. 2006).
Fibrinolytic activity assay of the SAK-2RGD-TTI using well diffusion method
Fibrinolytic activity of SAK-2RGD-TTI was measured according to well diffusion method, with streptokinase 10000 U (Sigma-Aldrich, St. Louis, MO, USA) as a standard (Faraji et al. 2017). Samples (30 μL) were loaded on plasma LB agar or nutrient agar plate, and then incubated at 37 °C overnight to get the clearance zones (mm2). The specific activity was determined with regard to standard curve.
Assay for thrombin inhibition
The capacity of thrombin inhibition by SAK-2RGD-TTI and rSAK proteins and delay in clot formation was measured using standard test of activated partial thromboplastin time (aPTT) (Cappello et al. 1996; Chanarin 1989; Van Zyl et al. 1997). The normal value is 30–40 s. Briefly, equal volume (100 μL) of normal citrate plasma, various concentrations of SAK-2RGD-TTI and cephaloplastin were mixed at 37 °C for 4 min. Subsequently, 100 μL CaCl2 (placed in water bath, 37 °C for 5 min) was added together with gently agitation probed for clot formation (Cappello et al. 1996; Chanarin 1989). Each experiment was repeated 3 times and compared with the rSAK results.
Platelet rich clot lysis assay
Thrombolytics activity using the RGD sequences (arginine-glycine-aspartic acid) become more accessible to fibrin, via attachment to integrin receptors of platelet available in clot and inhibition of its aggregation. As a result, it leads to more clot lysis. In the other words, platelet–targeted fibrinolysis increases clot lysis along with inhibition of platelet aggregation (Bode et al. 1991). In this study, to confirm antiplatelet activity of the SAK-2RGD-TTI, a simple method without the need for specialized equipment’s, namely the platelet rich clot lysis assay was used (Harrison 2005).
Whole blood was collected with trisodium citrate (3.2% (w/v)), at a ratio 1 to 9, centrifuged at 200 g, for 15 min, to separate the platelets and plasma from the red blood cell. It was followed by spinning the plasma at 400 g for 15 min to prepare platelet enriched plasma (PRP) (Zhang et al. 2010). A mixture (100 μL) of human thrombin (1 NIH unit/mL) and human fibrinogen (1 mg/mL) (sigma), CaCl2 20 mM and PRP was transferred to a 96-well plate and incubated for 3 h at room temperature as explained before (Pulicherla et al. 2013). On the clot, 100 μL of different concentrations of the purified SAK-2RGD-TTI or rSAK was added along with plasminogen (2.4 µM), pH 7.4. The C50 was calculated as described earlier for fibrin clot lysis assay. Each experiment repeated 3 times and results of the SAK-2RGD-TTI were compared to the rSAK.
Statistical analysis
Student’s t test was used where applicable (GraphPad Prism V5.0). The mean values were considered statistically significant if the p value was < 0.05. Data was presented as mean ± SD of three independent experiments.
Results
Structure prediction of the SAK-2RGD-TTI
Analysis of the secondary structure of the protein fusion by SOPMA software showed that 24.31% (w/w) of the protein had an alpha-helix structure, 40.22% random coil, 20.64% extended strand and 14.22% beta-turn (Online Resource 1). Five models were constructed using I-TASSER with C-scores ranging from -2.41 to 3.2. Model-1 with the most favorite C-Score (− 2.41) was considered as the best predicted tertiary structure for the fusion protein of the SAK-2RGD-TTI. Ramachandran Plot Assessment of the selected model revealed more than 87% residues were placed in the favored (69.9%) and allowed (17.6%) regions (Online Resource 2). In this model, all components of the fusion protein (SAK, RGD and TTI) were posed separately without interfering with the others compartments (Fig. 2). As a result, it exhibited that the linker containing RGD and TTI would not influence SAK proper folding.
A model of SAK-2RGD-TTI protein predicted by I-TASSER online software after energy minimization. In this model, all functional domains of the protein fusion (SAK, RGD and TTI) was unveiled separately without burying inside other domains. This model was visualized using the viewer lite 4.2 software (Molecular simulations, Inc., San Diego, CA)
The SAK-2RGD-TTI protein analysis
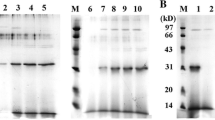
SDS-PAGE analysis of the purified SAK-2RGD-TTI revealed two protein bands of nearly 27 kDa and 24 kDa with intensity ratio 5 to 3, respectively (Fig. 3, lane 2). It was confirmed by western blotting as well (Fig. 3, lane 5). The quantity of the purified protein was 420 µg/mL with more than 99% (w/w) purity. When the protein expressed in presence of tunicamycin, only one protein band was appeared in the SDS-PAGE analysis indicating interruption of glycosylation (Fig. 3, lane 3).
The SDS-PAGE and western blotting analyses of the SAK-2RGD-TTI protein. Lane M: protein marker (10-250 kDa); SDS-PAGE analysis, lane 1: culture supernatant of Pichia pastoris including pPICZαA as negative control, lane 2: elute of the purified SAK-2RGD-TTI, lane 3: culture supernatant of Pichia pastoris including pPICZαA-SAK-2RGD-TTI after treatment with tunicamycin (20 µg/mL), lane 4: elute of the purified rSAK; Western blotting analysis, lane 5: elute of the purified SAK-2RGD-TTI. The SAK-2RGD-TTI protein was probed using mouse anti-cMyc antibody and HRP-conjugated secondary anti-mouse IgG followed by visualization using enhanced chemiluminescence method
Comparing human plasminogen activator activity
The amidolytic activity of human plasmin (ogen) is in parallel with fibrinolytic activity. Thus, to confirm the fibrinolytic properties of the SAK-2RGD-TTI and rSAK, plasminogen activation activity was investigated. It was indicated by the enzyme activities of 1099 ± 113, 2226 ± 413 and 4212 ± 500 U/mL in presence of the SAK-2RGD-TTI at the concentrations of 4, 8 and 16 μM, respectively. Comparatively, the same Concentrations of the purified rSAK resulted in the enzyme activities of 824 ± 163, 1917 ± 290 and 3469 ± 438 U/mL. As shown in Fig. 4, there was no significant difference between plasminogen activator activity of equimolar concentrations of the SAK-2RGD-TTI and rSAK (p value > 0.7).
Human plasminogen activator activity of the purified SAK-2RGD-TTI and rSAK proteins. Different concentrations (4, 8 and 16 μM) of the SAK-2RGD-TTI and rSAK were compared. Optical density (OD) of chromogenic substrate released from Spectrozyme PL at 405 nm was investigated. Data were represented as mean ± SD of three independent experiments (p value = 0.8312)
Comparing the fibrin clot lysis activity
The concentration required for 50% fibrin clot lysis (C50) for the SAK-2RGD-TTI was slightly lower than the rSAK (467 and 544 nM, respectively) (Fig. 5). However, this was not significant difference (p value = 0.3702).
Comparing of fibrinolytic activity using the clot lytic method and residual clot weight
The residual clot weight after exposure to fibrinolytic proteins was determined. The results showed that 100 μL of the purified SAK-RGD-TTI (420 μg/mL) dissolved 45% of the blood clot, while the crude culture supernatant (175 μg/mL) nearly 20% of the clot. Further, the purified rSAK (720 μg/mL) and non-purified one (310 μg/mL) could dissolve approximately 74% and 35% of blood clots, respectively (Faraji et al. 2017). Increase of blood clot lysis from 20 to 41% was demonstrated for the non-purified, deglycosylated SAK-2RGD-TTI.
Comparison of anti-thrombin activity
As shown in Fig. 6, the SAK-2RGD-TTI with a TTI fragment prolonged the activated partial thromboplastin time (aPTT) of normal human plasma in a concentration–dependent manner. Compared to the rSAK, a concentration at least 130 nM of the SAK-2RGD-TTI effectively increased around two 2.3 times the PTT test (52 s) when normal control was 27 s. There was a statistically significant difference between the two groups at the clotting time (p value < 0.0001).
Comparing lysis activity on platelet rich clot
More tendency of the SAK-2RGD-TTI towards platelets of platelet rich clot led to more fibrinolysis along with turbidity reduction. The required concentration of the SAK-2RGD-TTI for 50% platelet rich clot lysis (C50) was 233 nM, which was significantly (p value = 0.0033) less than 778 nM (C50) of the rSAK (Fig. 7). In other words, as compared to the rSAK, the SAK-2RGD-TTI with a lower mole concentration (< 1/3) was caused a 50% platelet rich clot lysis after 5 h.
Discussion
Platelet and thrombin play the critical role in thrombosis development in the body while plasmin dissolves the thrombosis produced (Van Zyl et al. 1997). It is not surprising, therefore, to be designed an antithrombotic agent that can inhibit the action of thrombin and platelet aggregation, while causing a clot lysis. Platelet inhibitors include the synthetic peptide sequences of arginine-glycine-aspartic acid (RGD) (Taylor and Gartner 1992), F(ab ‘)2 fragment of the monoclonal antibody 7E3 (Coller et al. 1989) and snake venom (Savage et al. 1990). Among the thrombin inhibitors, there are hirudin (Markwardt 1970), hirudin portions (Dennis et al. 1990), bivalirudin (hirulog) (Maraganore et al. 1990), hyrogen (Maraganore et al. 1989), synthetic peptides obtained from C-terminal portion of hirudin (Dimaio et al. 1990), fibrinopeptide A (Van Zyl et al. 1997a), dipetalin domains (Icke et al. 2002) and tsetse thrombin inhibitor (Cappello et al. 1996, 1998). Inhibitors of thrombin production include the recombinant tick anticoagulant protein (rTAP) (Neeper et al. 1990), and the recombinant activated protein C (Gruber et al. 1990). Initially, Cohen and Lijnen proposed that the effectiveness of anti-coagulant and antiplatelet agents could be enhanced by fusing them to thrombolytic agents such as staphylokinase (Collen and Lijnen 1995). Continually, various studies used staphylokinase fused to antiplatelet or antithrombin sequences or both (Kotra et al. 2013; Kowalski et al. 2009; Kumar et al. 2013; Pulicherla et al. 2012, 2013; Szarka et al. 1999; Szemraj et al. 2007; 2011; Van Zyl et al. 1997; Wang et al. 2009). In this study, a fusion protein (SAK-2RGD-TTI) was designed and expressed in Pichia pastoris which contained fibrinolytic SAK, 2xRGD platelet aggregation inhibitor and newly tsetse thrombin inhibitor. Modelling the tertiary structure confirmed that the linker containing RGD and TTI did not interfere with SAK proper folding. Following, it was purified and characterized, in vitro. In well diffusion method for determining fibrinolytic activity (Faraji et al. 2017; Jasim et al. 2015; Pulicherla et al. 2012), our purified SAK-2RGD-TTI and rSAK showed a specific activity of 19,616 U/mg and 21,042 U/mg, respectively, after purification. Overview of other studies expressing staphylokinase or its derivatives appeared different results. The specific activity was ranged from very highly 2,197,420 U/mg with S2251 (d-valyl-leucyl-lysine-p-nitroanilide dihydrochloride) as substrate in E. coli (Mandi et al. 2009), very highly activity 102,955 IU/mg reported in the well diffusion method with streptokinase as standard in E. coli (Pulicherla et al. 2013), high activity of 20,658 U/mg with AAS (N-(p-tosyl)-gly-pro-lys 4-nitroanilide acetate salt) as substrate in P. pastoris (Nguyen and Quyen 2012b), 15,175 U/mg protein with AAS as substrate in E. coli (Nguyen and Quyen 2012a), low activity 2.5 U/mg of glycosylated and 95 U/mg of non-glycosylated with S2251 as substrate in P. pastoris (Apte-Deshpnade et al. 2009) to zero U/mg of glycosylated but high level of non-glycosylated with S2251 as substrate in P. pastoris (Miele et al. 1999). PLATSAK, SAK containing RGD as an antiplatelet together with hirudin and segments of fibrinopeptide A as thrombin inhibitors exhibited slightly decrease in fibrinolytic activity (Van Zyl et al. 1997). Further, fibrinolytic activity of the SAK-RGD-Hirulog (Pulicherla et al. 2013) and SAK-hirulog (Kotra et al. 2013) produced in E.coli was 102,730 U/mg and 21,825 U/mL, respectively. Despite of small decrease, there was no significant difference in the fibrinolytic activity between rSAK and its recombinant derivatives (Kotra et al. 2013; Pulicherla et al. 2013). Totally, it was demonstrated added compartments would not have unfavorable effects over fibrinolytic activity of staphylokinase. It was also revealed that adding the Kringle 2 domain (fibrin binding) from the tissue plasminogen activator protein (t-PA) to SAK-RGD-Hirulog or SAK-RGD-Hirudin increased significantly fibrin clot lysis (Kowalski et al. 2009; Szemraj et al. 2005, 2011). In present study, more thrombolytic activity of the SAK-2RGD-TTI compared to the rSAK with equal molar proportions was shown in fibrin clot lysis test and plasminogen activation assay. However, consistence with a similar study (Pulicherla et al. 2013), there was no significant difference between the rSAK and its multifunctional derivative. These indicated that adding antiplatelet and anti-thrombin segments had no adverse effect on the kinetics of SAK. Staphylokinase, alone or as part of a fusion protein, would form a binary complex with plasmin (ratio 1 to 1), led to non-significant difference of its plasminogen activation potential. In addition, the non-significant C50 between the SAK-2RGD-TTI and rSAK in the fibrin clot lysis test reaffirmed that the addition of RGD and TTI would not reduce fibrinolytic activity.
Moreover, the RGD and TTI sequences increased significantly the antiplatelet and antithrombin activities in the SAK-2RGD-TTI compared to the rSAK. Increase of these activities was also reported in previous studies (Icke et al. 2002; Kowalski et al. 2009; Pulicherla et al. 2013; Szemraj et al. 2007). The SAK-2RGD-TTI at a molar concentration less than 1/3 relative to the rSAK led to 50% lysis of platelet rich clot after 5 h. Hence, it demonstrated visibly the high efficacy of the SAK-2RGD-TTI in lysis of platelet-rich clot. It might increase the thrombolytic activity through binding directly the fusion protein to the GPIIb/IIIa receptors of the activated platelet on surface of the clot. In this study, the efficacy of fused TTI was also demonstrated along with the used previously thrombin inhibitors (Icke et al. 2002; Kotra et al. 2013; Kowalski et al. 2009; Pulicherla et al. 2013; Van Zyl et al. 1997; Wang et al. 2009). In this regard, the TTI of fusion protein at a concentration of at least 130 nm increased around 2 times the aPTT test (52 s). Hence, the TTI fragment could access to the thrombin and inhibit its catalytic activity.
In the clot lytic method and residual clot weight, increase of blood clot lysis nearly 2 times (from 20 to 41%) was demonstrated for the non-purified, deglycosylated SAK-2RGD-TTI consistence with changing its specific fibrinolytic activity from 8269 to 18,196 U/mg according to well diffusion method. For the non-purified deglycosylated rSAK, more increase of blood clot lysis (2.3 times, from 35 to 82%) was also confirmed by well diffusion method (Faraji et al. 2017). Induction with tunicamycin did not increase the rSAK expression. Also, fibrinolytic part of the SAK-2RGD-TTI was accessible to fibrin (19,616 U per mg protein with more molecular weight relative to the rSAK). Glycosylation of staphylokinase decreases fibrinolytic activity (Apte-Deshpnade et al. 2009; Miele et al. 1999). The rSAK was mostly glycosylated when expressed in Pichia pastoris (Faraji et al. 2017), while the SAK-2RGD-TTI was shown a mixture of glycosylated and nonglycosylated forms with a ratio of 5:3. As a results, after induction with tunicamycin, the more glycosylated rSAK proteins changed into non-glycosylated than that the SAK-2RGD-TTI.
Future perspectives
In this study, staphylokinase fused to anti-platelet and newly anti-thrombin sequences was designed and after the codon optimization was expressed and purified successfully from Pichia pastoris with improved functional properties. However, there are following suggestions in the article for continuation of the work. They include: separation of glycosylated proteins from non-glycosylated after purification via column chromatography of concavalin A. Evaluation of thrombolytic properties of the SAK-2RGD-TTI and rSAK, in vivo, for instance, thrombosis of the rats tail with carrageenan (Kumar et al. 2013). Evaluation of other pharmacokinetic profiles of the SAK-2RGD-TTI and rSAK such as plasma half-life, renal clearance and immunogenicity, in vivo. Adding additional compartments to the SAK-2RGD-TTI, such as the Kringle 2 domain from t-PA and determining its subsequent effects. Removal of glycosylated sites and also sites with high antigenic potential using site directed mutagenesis approach and determining its subsequent effects.
Conclusions
From the present study is concluded that adding RGD and TTI peptides increased antiplatelet and antithrombin activities to the conventional plasminogen activator of the SAK protein. These changes are expected to improve the therapeutically function of the SAK-2RGD-TTI by preventing reocclussion.
References
Apte-Deshpnade A, Mandal G, Soorapaneni S, Prasad B, Kumar J, Padmanabhan S (2009) High-level expression of non-glycosylated and active staphylokinase from Pichia pastoris. Biotechnol Lett 31:811–817
Bode C et al (1991) Platelet-targeted fibrinolysis enhances clot lysis and inhibits platelet aggregation. Circulation 84:805–813
Cappello M, Bergum PW, Vlasuk GP, Furmidge BA, Pritchard DI, Aksoy S (1996) Isolation and characterization of the tsetse thrombin inhibitor: a potent antithrombotic peptide from the saliva of Glossina morsitans morsitans. Am J Trop Med Hygiene 54:475–480
Cappello M et al (1998) Tsetse thrombin inhibitor: bloodmeal-induced expression of an anticoagulant in salivary glands and gut tissue of Glossina morsitans morsitans. Proc Natl Acad Sci USA 95:14290–14295
Chanarin I (1989) Laboratory haematology: an account of laboratory techniques. Churchill Livingstone, Edinburgh
Collen D, Lijnen H (1995) Molecular basis of fibrinolysis, as relevant for thrombolytic therapy. Thromb Haemost 74:167–171
Coller BS, Folts JD, Smith SR, Scudder LE, Jordan R (1989) Abolition of in vivo platelet thrombus formation in primates with monoclonal antibodies to the platelet GPIIb/IIIa receptor. Correlation with bleeding time, platelet aggregation, and blockade of GPIIb/IIIa receptors. Circulation 80:1766–1774
Dennis S, Wallace A, Hofsteenge J, Stone SR (1990) Use of fragments of hirudin to investigate thrombin-hirudin interaction. FEBS J 188:61–66
Dimaio J, Gibbs B, Munn D, Lefebvre J, Ni F, Konishi Y (1990) Bifunctional thrombin inhibitors based on the sequence of hirudin45-65. J Biol Chem 265:21698–21703
Faraji H, Ramezani M, Sadeghnia HR, Abnous K, Soltani F, Mashkani B (2017) High-level expression of a biologically active staphylokinase in Pichia pastoris. Prep Biochem Biotechnol 47:379–387
Faraji H, Ramezani M, Mashkani B, Sadeghnia HR, Benhangi HM, Hosseini Teshnizi S, Soltani F (2019) Comparison of expression optimization of new derivative of staphylokinase (SAK-2RGD-TTI) with the Rsak. Biotechnol Prog 35(4):e2819
Gruber A, Hanson SR, Kelly AB, Yan BS, Bang N, Griffin JH, Harker LA (1990) Inhibition of thrombus formation by activated recombinant protein C in a primate model of arterial thrombosis. Circulation 82:578–585
Harrison P (2005) Platelet function analysis. Blood Rev 19:111–123
Hernández L, Rodríguez P, Castro A, Serrano R, Rodriguez M, Rubiera R, Estrada M, Perez A, De La Fuente J, Herrera L (1990) Determination of streptokinase activity by quantitative assay. Biotecnol Apl 7:153–160
Icke C, Schlott B, Ohlenschläger O, Hartmann M, Gührs K-H, Glusa E (2002) Fusion proteins with anticoagulant and fibrinolytic properties: functional studies and structural considerations. Mol Pharmacol 62:203–209
Jasim HM, Dellol RA, Hamzah AS (2015) Optimum conditions of staphylokinase production cloned in E. coli Jm109 (DE3). Int J Curr Microbiol Appl Sci 4(12):10–19.
Kotra SR, Peravali J, Yanamadala S, Kumar A, Samba-Siva-Rao K, Pulicherla K (2013) Large scale production of soluble recombinant staphylokinase variant from cold shock expression system using IPTG inducible E. coli BL21 (DE3). Int J Bio-Sci Bio-Technol 5:107–116
Kowalski M et al (2009) Cloning and expression of a new recombinant thrombolytic and anthithrombotic agent—a staphylokinase variant. Acta Biochim Pol 56:41
Kumar A, Pulicherla K, Ram KS, Rao K (2010) Evolutionary trend of thrombolytics. Int J Bio-sci Bio-technol 2:51–68
Kumar A, Pulicherla KK, Mayuren C, Kotra S, Rao KRS (2013) Evaluation of a multifunctional staphylokinase variant with thrombin inhibition and antiplatelet aggregation activities produced from salt-inducible E. coli GJ1158. Can J Physiol Pharmacol 91:839–847
Lian Q, Szarka SJ, Ng KK, Wong S-L (2003) Engineering of a staphylokinase-based fibrinolytic agent with antithrombotic activity and targeting capability toward thrombin-rich fibrin and plasma clots. J Biol Chem 278:26677–26686
Mandi N, Soorapaneni S, Rewanwar S, Kotwal P, Prasad B, Mandal G, Padmanabhan S (2009) High yielding recombinant staphylokinase in bacterial expression system—cloning, expression, purification and activity studies. Protein Expr Purif 64:69–75
Maraganore J, Bourdon P, Jablonski J, Ramachandran K, Fenton Jd (1990) Design and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombin. Biochemistry 29:7095–7101
Maraganore JM, Chao B, Joseph ML, Jablonski J, Ramachandran K (1989) Anticoagulant activity of synthetic hirudin peptides. J Biol Chem 264:8692–8698
Markwardt F (1970) Hirudin as an inhibitor of thrombin. In: Methods in enzymology, vol 19. Elsevier, Amsterdam, pp 924–932
Miele RG, Prorok M, Costa VA, Castellino FJ (1999) Glycosylation of asparagine-28 of recombinant staphylokinase with high-mannose-type oligosaccharides results in a protein with highly attenuated plasminogen activator activity. J Biol Chem 274:7769–7776
Neeper M et al (1990) Characterization of recombinant tick anticoagulant peptide. A highly selective inhibitor of blood coagulation factor Xa. J Biol Chem 265:17746–17752
Nguyen THT, Quyen DT (2012a) Cloning, high-level expression, purification and characterization of a staphylokinase variant, SakøC, from Staphylococcus aureus QT08 in Escherichia coli BL21. Afr J Biotechnolgy 11:5995–6003
Nguyen THT, Quyen DT (2012b) High-level expression, purification and properties of a fully active even glycosylated staphylokinase variant SakfC from Staphylococcus aureus QT08 in Pichia pastoris. Afr J Microbiol Res 6:2129–2136
Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF (2006) Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb J 4:14
Pulicherla K, Seetharam K, Kumar A, Rekha V, Rao KS (2012) Cloning and high level expression of recombinant heterologous fusion protein SAK RGD in methanol inducible Pichia pastoris GS115. Int J Res Pharm Biomed Sci 3:1008–1013
Pulicherla K, Kumar A, Gadupudi G, Kotra SR, Sambasiva Rao K (2013) In vitro characterization of a multifunctional staphylokinase variant with reduced reocclusion, produced from salt inducible E. coli GJ1158. BioMed Res Int 2013:297305.
Rydel TJ, Ravichandran K, Tulinsky A, Bode W, Huber R, Roitsch C, Fenton JW (1990) The structure of a complex of recombinant hirudin and human alpha-thrombin. Science 249:277–280
Savage B, Marzec U, Chao B, Harker L, Maraganore J, Ruggeri ZM (1990) Binding of the snake venom-derived proteins applaggin and echistatin to the arginine-glycine-aspartic acid recognition site (s) on platelet glycoprotein IIb.IIIa complex inhibits receptor function. J Biol Chem 265:11766–11772
Serizawa K, Urano T, Kozima Y, Takada Y, Takada A (1993) The potential role of platelet PAI-1 in t-PA mediated clot lysis of platelet rich plasma. Thromb Res 71:289–300
Szarka S, Sihota E, Habibi H, Wong S-L (1999) Staphylokinase as a plasminogen activator component in recombinant fusion proteins. Appl Environ Microbiol 65:506–513
Szemraj J et al (2007) A new recombinant thrombolytic and antithrombotic agent with higher fibrin affinity—a staphylokinase variant. Thromb Haemost 98:1037–1045
Szemraj J, Walkowiak B, Kawecka I, Janiszewska G, Buczko W, Bartkowiak J, Chabielska E (2005) IN FOCUS: a new recombinant thrombolytic and antithrombotic agent with higher fibrin affinity—a staphylokinase variant. I. In vitro study. J Thromb Haemost 3:2156–2165
Szemraj J, Zakrzeska A, Brown G, Stankiewicz A, Gromotowicz A, Grędziński T, Chabielska E (2011) New derivative of staphylokinase SAK-RGD-K2-Hirul exerts thrombolytic effects in the arterial thrombosis model in rats. Pharmacol Rep 63:1169–1179
Taylor DB, Gartner TK (1992) A peptide corresponding to GPIIb alpha 300–312, a presumptive fibrinogen gamma-chain binding site on the platelet integrin GPIIb/IIIa, inhibits the adhesion of platelets to at least four adhesive ligands. J Biol Chem 267:11729–11733
Van Zyl WB, Pretorius GH, Hartmann M, Kotzé HF (1997) Production of a recombinant antithrombotic and fibrinolytic protein, PLATSAK, in Escherichia coli. Thromb Res 88:419–426
Wang M et al (2009) Construction and characterization of a novel staphylokinase variant with thrombin-inhibitory activity. Biotechnol Lett 31:1923–1927
Zhang G, Zhong G, Wang X, Wang L, Qin Y, Yu A (2010) Optimization of fed-batch fermentation for a staphylokinase-hirudin fusion protein in Escherichia coli BL21. Afr J Biotechnol 9:5078–5083
Acknowledgements
We appreciated Dr. Mohammad Soukhtanloo, Dr. Mostafa Khedri and Dr. Manouchehr Teymouri for their valuable comments.
Supporting information
Supplementary Figure 1—The secondary structure of the SAK-2RGD-TTI protein predicted by SOPMA online software. Amino acid residues of N-terminal, which is indeed a catalytic site for fibrinolytic activity, has more the random coil structure.
Supplementary Figure 2—Ramachandran plot analysis of the SAK-2RGD-TTI using the RAMPAGE online software. It is based on torsional angles (Phi or ф and Psi or ψ) of amino acid residues (A) led to their falling in energetically favoured, allowed or outlier regions. More amino acids in favoured and allowed regions, the model more valid. The conformations and location of amino acids is shown in four individual plots having heading as general (all amino acids except Glycine, Proline and Pre-Pro (amino acid before proline), Glycine, Pre-Pro and Proline. Amino acids marked with red rectangleplace in outlier region.
Funding
This study was supported by the Pharmaceutical Research Center, Buali (Avicenna) Research Institute, Mashhad University of Medical Sciences with Grant No. 921731.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest with the current work or its publication.
Ethical approval
All procedures were performed in accordance with the ethical standards of the local ethics committee of Mashhad University of Medical Sciences (Iran) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (Grant No: 921731).
Informed consent
All authors are aware of and agree to the content of the manuscript.
Research involving human participants and/or animals
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faraji, H., Soltani, F., Ramezani, M. et al. Designing a multifunctional staphylokinase variant (SAK-2RGD-TTI) with appropriate thrombolytic activity in vitro. Biotechnol Lett 42, 103–114 (2020). https://doi.org/10.1007/s10529-019-02748-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02748-5