Abstract
Objective
To evaluate the role and the molecular mechanism of miR-30d in non-small cell lung cancer (NSCLC).
Results
qRT-PCR was used to detect miR-30d expression in NSCLC tissues and cell lines. miR-30d was frequently down-regulated in NSCLC and its expression was associated with clinicopathological features of NSCLCC patients. Over-expression of miR-30d notably inhibited cell migration and invasion in NSCLC cell lines. miR-30d could negatively regulate Nuclear factor I B (NFIB) by directly targeting its 3′-UTR, which was confirmed by luciferase assay. NFIB also reversed miR-30d-mediated suppression on the migration and invasion in NSCLC cell lines.
Conclusion
miR-30d suppressed cell migration and invasion by directly targeting NFIB in NSCLC, and NFIB could partially abrogated the inhibition of biological functions by miR-30d.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 80% lung cancers (Siegel et al. 2015). Despite advancements in NSCLC therapies, the prognosis for patients with advanced NSCLC remains poor (Bansal et al. 2016). Metastasis is the main cause of lung cancer-related deaths (Turajlic and Swanton 2016). Therefore, it is necessary to understand the mechanisms of metastasis in NSCLC and thus identify novel therapeutic targets.
MicroRNAs (miRNAs) are small non-coding, endogenous RNAs that directly bind to the 3′-untranslated(3′-UTR)regions of target mRNAs and lead to the degradation or translational repression of the target mRNAs (Bartel 2009). Deregulation of miRNAs is involved in human tumorigenesis and progression including NSCLC (Li et al. 2017; Zhou et al. 2017). These aberrantly expressed miRNAs function as tumor suppressors or oncogenges in various biological processes of tumorigenesis, such as cell cycle arrest, migration, invasion and proliferation (Sun et al. 2016; Wang et al. 2016). miR-30d is the member of miR-30 family which is involved in various biological and pathological processes, such as metastasis, proliferation and differentiation (Baraniskin et al. 2012; Gaziel-Sovran et al. 2011; Karbiener et al. 2011). Ye et al. (2015) indicated that miR-30d functioned as a suppressor in ovarian cancer by targeting Snail and over-expression of miR-30d blocked TGF-GFctiduced EMT. However, the role of miR-30d in NSCLC and the underlying mechanism remain elusive.
Nuclear factor I B (NFIB) gene is located at 9p24.1 and is one of the members of the nuclear factor I (NFI) family of transcription factors (Gronostajski 2000). NFIB plays critical roles in various cancer (Becker-Santos et al. 2017; Zhang et al. 2015). Several miRNAs, such as miR-1246, miR-92b and miR-365, are involved in the regulation of NFIB (Becker-Santos et al. 2016; Roy et al. 2017; Zhang et al. 2015). Dooley et al. (2011) indicated that NFIB was an oncogene in small cell lung cancer. It will be important, therefore, to investigate the role of NFIB in the development of NSCLC. We have now performed qRT-PCR to analyze miR-30d expression in NSCLC tissues and cell lines and found that miR-30d is significantly down-regulated in NSCLC tissues and cell lines. The biological functions of miR-30d in NSCLC have been evaluated and show that miR-30d inhibit NSCLC cell migration and invasion in vitro. Additionally, we have found that miR-30d regulates the expression of NFIB by targeting the 3′-UTR of NFIB. These findings are appeared and indicate that miR-30d/NFIB axis is a potential novel pathway for therapy of NSCLC.
Materials and methods
Cell culture
Non-small cell lung cancer cell lines (A549, H1299, H358, H460) and a normal lung epithelial cells line (BEAS-2B) were purchased from the Cell Resource Center, Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in RPMI-1640 with 10% (v/v) fetal bovine serum and penicillin and streptomycin. The cells were cultured at 37 °C with 5% (v/v) CO2 in a humidified atmosphere.
Patients
50 pairs of fresh tumor specimens and the matched adjacent non-cancerous tissues from NSCLC patients were obtained at Linyi Central Hospital of Shandong, China. Tissue samples were stored in −80 °C. All patients gave written informed consent. This study was approved by all patients and the Ethics Committee of Linyi Central Hospital of Shandong.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNAs were extracted using cDNA was synthesized with random primers (N6) or miRNA specific primers and MMLV reverse transcriptase (Fermentas, Canada). qRT-PCR analyses were carried out using the Bio-Rad CfX96TM Real-Time PCR System (BioRad) with SYBR Premix Ex Taq (TaKaRa) according to the manufacturer’s instructions. β-Actin was used as the control. The primers for NFIB and β-microglobulin (β2-M) were synthesized from Invitrogen. For miRNA detection, qRT-PCR analyses were performed using TaqMan miRNA assays (Applied Biosystems, USA) and SYBR Premix Ex Taq (TaKaRa). U6 was used as the control. Primers for miR-30d and U6 were synthesized from RiBoBio (Guangzhou, China). The mRNA and miRNA expression was normalized. All of the reactions were conducted in triplicates, and relative quantification was analyzed by the \( 2^{{ - \Delta \Delta {\text{C}}_{\text{t}} }} \) method.
Transfection
miRNA mimics, inhibitor were purchased from RiBoBio (Guangzhou, China). A549 cell lines were transfected with miR-30d mimics or inhibitor using Lipofectamine 2000 (Invitrogen). NFIB over-expression vector and control vector were purchased from Addgene (Cambridge, MA) and X-treme Gene HP transfection reagent (Roche) was used for the transfection of the plasmids. After 48 h, the cell samples were collected for further analysis.
Luciferase reporter assay
Cancer cells were seeded into 24-well plates and co-transfected with luciferase reporters wild-type (WT)/mutant (Mut) NFIB 3′-UTR and miR-30d mimics/control mimics using Lipofectamine 2000. After 48 h, cells were harvested and the luciferase activity of the cell lysates was measured by the Dual Luciferase reporter assay system (Promega). Firefly luciferase activity was normalized to the internal control Renilla.
Western blot analysis
Total proteins were extracted from cells with RIPA lysis buffer (Beyotime, China) supplemented with PMSF. Cell lysates were collected and the protein concentrations were measured by a BCA protein assay kit. The proteins were separated by 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% (w/v) BSA in Tris-buffered saline with Tween 20 (TBST) buffer at room temperature and then incubated with primary antibodies, followed by incubation with secondary antibodies. The protein signals were detected using a chemiluminescence method (ECL, Millipore). GAPDH was used as an internal control.
Cell migration and invasion assays
For migration assay, A549 cells were harvested 48 h post-transfection and re-suspended in serum-free RPMI-1640 medium, and 5 × 104 cells were seeded into the upper transwell chamber (pore size, 8 mm; Corning Life Sciences). For the invasion assay, 105 cells were seeded into the upper chamber coated with Matrigel (BD Biosciences, USA). RPMI-1640 medium containing 20% (v/v) FBS was added to the lower chamber. After 24 h, the cells on the upper membrane were removed with cotton swabs, and the cells on the lower membrane were fixed with methanol, stained with 0.1% (w/v) Crystal Violet and imaged through an IX51 inverted microscope. All experiments were independently repeated three times.
Statistical analysis
Each experiment was performed at least in triplicate. The significant difference of two groups was determined using Student’s t test in SPSS 17.0 (SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
miR-30d is down-regulated and negatively correlated with NFIB in NSCLC
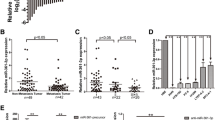
To investigate whether the expression of miR-30d was altered in NSCLC, qRT-PCR was performed on 50 pairs of NSCLC tissues and adjacent non-cancerous tissues, as well as NSCLC cell lines. As shown in Fig. 1a, miR-30d expression was significantly decreased in Fig. 1.
miR-30d is down-regulated and negatively correlated with NFIB in NSCLC. a miR-30d expression level was detected in 50 pairs of NSCLC tissues and adjacent non-cancerous tissues. b miR-30d expression level was detected in NSCLC cell lines (A549, H1299, H358, H460, BEAS-2B). c NFIB expression level was detected in 50 pairs of NSCLC tissues and adjacent non-cancerous tissues. d The correlation analysis between miR-30d and NFIB mRNA levels in NSCLC tissues. Data are presented as the mean ± SD of triplicate determinations. *P < 0.05, **P < 0.01, ***P < 0.001
39/50 (78%) of the NSCLC specimens compared with the matched adjacent non-cancerous tissues (0.99 ± 0.05vs 0.47 ± 0.40, P < 0.001). miR-30d was also down-regulated in the NSCLC cell lines A549, Table 1.
H1299, H358, H460, compared with the normal lung epithelial cells BEAS-2B (Fig. 1b). Furthermore, the clinicopathological features of the 50 patients were retrospected and miR-30d expression was correlated with lymph node metastasis and the TNM stage (Table 1).
Additionally, we detected the mRNA expression of NFIB. The results showed that NFIB was up-regulated in NSCLC tissues in comparison to non-cancerous tissues ((1.49 ± 0.46 vs 0.99 ± 0.03, P < 0.001, Fig. 1c). Interestingly, statistical analysis showed that miR-30d expression was negatively correlated with the expression of NFIB in NSCLL tissues (R2 = 0.6575 and P < 0.001, Fig. 1d).
miR-30d suppresses NSCLC cell migration and invasion
Given that the expression of miR-30d was associated with the TNM stage and lymph node metastasis of NSCLC, we assumed that miR-30d played a crucial part in NSCLC progression. Hence, we explored the biological functions of miR-30d in NSCLC cells. A549 cells were transfected with miR-30d mimics or inhibitor. The transfect efficiency was detected by qRT-PCR (Fig. 2a). The enhanced expression of miR-30d remarkably suppressed the migration and invasion abilities of A549 cells, whereas miR-30d knockdown dramatically promoted cell migration and invasion, as determined by transwell assay (Fig. 2b, c). These data demonstrated that miR-30d suppressed the cell migration and invasion of NSCLC in vitro.
miR-30d inhibits NSCLC cell migration and invasion. a miR-30d expression was detected in A549 cell lines which transfected with miR-30d mimics or inhibitor using qRT-PCR. b Transwell assay was used to evaluate the migration and invasion in A549 cell lines. c Quantitative analysis of the migration and invasion number in A549 cells. *P < 0.05, **P < 0.01,*P < 0.001
miR-30d directly targets NFIB 3′-UTR and decreases NFIB expression
To understand the underlying mechanism of miR-30d in NSCLC cell migration and invasion, TargetScan and Miranda were used to predict potential targets of miR-30d. NFIB was one of the potential target of miR-30d and the predicted binding sites for miR-30d were exhibited at 5538–5544 bp in the 3′-UTR of NFIB (Fig. 3a). To confirm whether NFIB was a direct target of miR-30d, we constructed the wild-type (WT) or mutated (Mut) NFIB 3′-UTR luciferase Fig. 3.
miR-30d directly targets NFIB 3′-UTR and decreases NFIB expression. a The putative binding site in the 3′-UTR of NFIB. Mutation was generated in the complementary sites for the seed regions in miR-30d. b Luciferase acitivity. A549 cells were co-transfected with miR-30d mimics and NFIB 3′-UTR luciferase reporter vector. Firefly luciferase activity was normalized to Renilla luciferase activity. Western blot analysis (c) and qRT-PCR analysis (d) of NFIB expression in A549 cells transfected with miR-30d mimics or inhibitor. *P < 0.05, **P < 0.01
reporter vector. Luciferase reporter assay was performed in A549 cells. Our results indicate that miR-30d decreased wild-type NFIB 3′-UTR luciferase reporter activity and had no significant effect on the mutant NFIB 3′-UTR luciferase reporter activity (Fig. 3b).
We next assessed the effect of miR-30d on the expression of NFIB at mRNA and protein levels. miR-30d mimics and inhibitor were transfected into A549 cells, respectively, and NFIB expression was measured using qRT-PCR and western blot. Over-expression of miR-30d significantly inhibited endogenous NFIB expression at the mRNA (0.22 ± 0.02 vs 1.01 ± 0.04, P < 0.01) and protein levels in NSCLC cells, while silencing miR-30d by the miR-30d inhibitor increased NFIB expression (For mRNA level, 1.61 ± 0.04 vs 0.98 ± 0.02, P < 0.01, Fig. 3c, d). These results suggested that miR-30d directly regulated the expression of NFIB by binding to its 3′-UTR.
NFIB is involved in miR-30d mediated suppression of cell migration and invasion
To further investigate the role of NFIB in miR-30d mediated suppression of cell migration and invasion,rescue assays were performed. NFIB over-expression vector or control vector were transfected into A549 cells which contained miR-30d mimics. The transfection efficiency was verified using qRT-PCR (Fig. 4a). Transwell assays were used to detect the cell migration and invasion. As shown in Fig. 4b, c, the inhibition in cell migration and invasion mediated by miR-30d was partially rescued by the NFIB over-expression vector.
NFIB is involved in miR-30d mediated suppression of cell migration and invasion. a Relative expression of NFIB was detected by qRT-PCR in A549 cells with different transfection treatment. b Over-expression of NFIB abrogated miR-30d-mediated inhibition of cell migration and invasion in A549 cells. c Quantitative analysis of the migration and invasion number in A549 cells. *P < 0.05, **P < 0.01
Discussion
Dysregulation of miRNAs is associated with various cancers, including NSCLC (Zhou et al. 2016). Deregulation of miRNAs is involved in cell proliferation, metastasis and invasion of cancer. A number of miRNAs, such as miR-590, miR-346, miR-574-5p, participate in the development and progression of NSCLC (Sun et al. 2016; Wang et al. 2016; Zhou et al. 2016). miR-30d is up-regulated in hepatocellular carcinoma and promote tumor invasion and metastasis (Yao et al. 2010). However, we found that miR-30d was significantly down-regulated in NSCLC tissues and cell lines. The expression of miR-30d was associated with clinicopathological features. Additionally, over-expression of miR-30d inhibited NSCLC cell migration and invasion.
To determine the underlying mechanisms of miR-30d in NSCLC cell migration and invasion, we used different biological databases and identified NFIB was a candidate target of miR-30d in NSCLC. miR-30d decreased the expression of NFIB by directly binding to its 3′-UTR in A549 cell line. NFIB belongs to the members of the nuclear factor I (NFI) family of transcription factors that function both in mammalian development and adenoviral DNA replication (Gronostajski 2000). Genetic inactivation of NFIB in mice was critical for lung development (Grunder et al. 2002; Steele-Perkins et al. 2005). NFIB played critical roles in regulating proliferation and epithelial differentiation during lung maturation (Hsu et al. 2011). NFIB was a putative target of onco fetal miRNAs and was associated with tumor aggressiveness in lung adenocarcinoma (Becker-Santos et al. 2016). Dooley et al. (2011) suggested that NFIB was an oncogenic transcription factor in human small cell lung cancer (SCLC) and promoted murine cell viability, proliferation during cellular transformation. NFIB accelerated both expansive proliferation and extensive metastasis of tumors in mouse models of SCLC. NFIB is frequently overexpressed in human metastatic neuroendocrine lung tumors (Semenova et al. 2016). NFIB is essential for lung development and the pathogenesis of several lung-related tumors.And its role in NSCLC is not established. We have demonstrated that NFIB is a direct target gene of miR-30d in NSCLC and partially attenuates the inhibition of cellular migration and invasion capacities caused by miR-30d over-expression.
miR-30d is down-regulated in NSCLC tissues and cell lines. Over-expressing miR-30d suppresses NSCLC cell migration and invasion by inhibiting NFIB expression. In addition, NFIB can partly rescue the compromised migration and invasion caused by miR-30d over-expression. Therefore, the miR-30d/NFIB axis provided a novel insight into the pathogenesis of NSCLC, especially in migration and invasion aspects, thereby representing a novel target for therapy of NSCLC.
References
Bansal P, Osman D, Gan GN, Simon GR, Boumber Y (2016) Recent advances in targetable therapeutics in metastatic non-squamous NSCLC. Front Oncol 6:112
Baraniskin A, Birkenkamp-Demtroder K, Maghnouj A, Zollner H, Munding J, Klein-Scory S, Reinacher-Schick A, Schwarte-Waldhoff I, Schmiegel W, Hahn SA (2012) MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis 33:732–739
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Becker-Santos DD, Thu KL, English JC, Pikor LA, Martinez VD, Zhang M, Vucic EA, Luk MT, Carraro A, Korbelik J, Piga D, Lhomme NM, Tsay MJ, Yee J, MacAulay CE, Lam S, Lockwood WW, Robinson WP, Jurisica I, Lam WL (2016) Developmental transcription factor NFIB is a putative target of oncofetal miRNAs and is associated with tumour aggressiveness in lung adenocarcinoma. J Pathol 240:161–172
Becker-Santos DD, Lonergan KM, Gronostajski RM, Lam WL (2017) Nuclear Factor I/B: a master regulator of cell differentiation with paradoxical roles in cancer. EBiomedicine 22:2–9
Dooley AL, Winslow MM, Chiang DY, Banerji S, Stransky N, Dayton TL, Snyder EL, Senna S, Whittaker CA, Bronson RT, Crowley D, Barretina J, Garraway L, Meyerson M, Jacks T (2011) Nuclear factor I/B is an oncogene in small cell lung cancer. Genes Dev 25:1470–1475
Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, de Miera EVS, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, Hernando E (2011) miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 20:104–118
Gronostajski RM (2000) Roles of the NFI/CTF gene family in transcription and development. Gene 249:31–45
Grunder A, Ebel TT, Mallo M, Schwarzkopf G, Shimizu T, Sippel AE, Schrewe H (2002) Nuclear factor I-B (Nfib) deficient mice have severe lung hypoplasia. Mech Dev 112:69–77
Hsu YC, Osinski J, Campbell CE, Litwack ED, Wang D, Liu S, Bachurski CJ, Gronostajski RM (2011) Mesenchymal nuclear factor I B regulates cell proliferation and epithelial differentiation during lung maturation. Dev Biol 354:242–252
Karbiener M, Neuhold C, Opriessnig P, Prokesch A, Bogner-Strauss JG, Scheideler M (2011) MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol 8:850–860
Li C, Ge Q, Liu J, Zhang Q, Wang C, Cui K, Chen Z (2017) Effects of miR-1236-3p and miR-370-5p on activation of p21 in various tumors and its inhibition on the growth of lung cancer cells. Tumour Biol 39:1010428317710824
Roy S, Bantel H, Wandrer F, Theres Schneider A, Gautheron J, Vucur M, Tacke F, Trautwein C, Luedde T, Roderburg C (2017) miR-1224 inhibits cell proliferation in acute liver failure by targeting the antiapoptotic gene Nfib. J Hepatol. doi:10.1016/j.jhep.2017.06.007
Semenova EA, Kwon MC, Monkhorst K, Song JY, Bhaskaran R, Krijgsman O, Kuilman T, Peters D, Buikhuisen WA, Smit EF, Pritchard C, Cozijnsen M, van der Vliet J, Zevenhoven J, Lambooij JP, Proost N, van Montfort E, Velds A, Huijbers IJ, Berns A (2016) Transcription factor NFIB is a driver of small cell lung cancer progression in mice and marks metastatic disease in patients. Cell Rep 16:631–643
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Steele-Perkins G, Plachez C, Butz KG, Yang G, Bachurski CJ, Kinsman SL, Litwack ED, Richards LJ, Gronostajski RM (2005) The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol Cell Biol 25:685–698
Sun CC, Li SJ, Yuan ZP, Li DJ (2016) MicroRNA-346 facilitates cell growth and metastasis, and suppresses cell apoptosis in human non-small cell lung cancer by regulation of XPC/ERK/Snail/E-cadherin pathway. Aging 8:2509–2524
Turajlic S, Swanton C (2016) Metastasis as an evolutionary process. Science 352:169–175
Wang FF, Wang S, Xue WH, Cheng JL (2016) microRNA-590 suppresses the tumorigenesis and invasiveness of non-small cell lung cancer cells by targeting ADAM9. Mol Cell Biochem 423:29–37
Yao J, Liang L, Huang S, Ding J, Tan N, Zhao Y, Yan M, Ge C, Zhang Z, Chen T, Wan D, Yao M, Li J, Gu J, He X (2010) MicroRNA-30d promotes tumor invasion and metastasis by targeting Galphai2 in hepatocellular carcinoma. Hepatology 51:846–856
Ye Z, Zhao L, Li J, Chen W, Li X (2015) miR-30d blocked transforming growth factor beta1-induced epithelial-mesenchymal transition by targeting snail in ovarian cancer cells. Int J Gynecol Cancer 25:1574–1581
Zhang Q, Cao LY, Cheng SJ, Zhang AM, Jin XS, Li Y (2015) p53-induced microRNA-1246 inhibits the cell growth of human hepatocellular carcinoma cells by targeting NFIB. Oncol Rep 33:1335–1341
Zhou R, Zhou X, Yin Z, Guo J, Hu T, Jiang S, Liu L, Dong X, Zhang S, Wu G (2016) MicroRNA-574-5p promotes metastasis of non-small cell lung cancer by targeting PTPRU. Sci Rep 6:35714
Zhou Y, Li S, Li J, Wang D, Li Q (2017) Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol Biochem 42:1431–1446
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y., Zhang, J., Hou, S. et al. Non-small cell lung cancer: miR-30d suppresses tumor invasion and migration by directly targeting NFIB. Biotechnol Lett 39, 1827–1834 (2017). https://doi.org/10.1007/s10529-017-2428-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2428-9