Abstract
Objective
The aim of this study was to investigate the developmental competence of oocytes parthenogenetically activated by an electric pulse (EP) and treated with anisomycin and to determine whether this method is applicable to somatic cell nuclear transfer (SCNT).
Results
Embryos derived from porcine oocytes parthenogenetically activated by an EP and treatment with 0.01 µg/mL anisomycin had a significantly improved in vitro developmental capacity. Furthermore, 66.6% of blastocysts derived from these embryos had a diploid karyotype. The blastocyst formation rate of cloned embryos was similar between oocytes activated by an EP and treated with 2 mM 6-dimethylaminopurine for 4 h and those activated by an EP and treated with 0.01 µg/mL anisomycin for 4 h. The level of maturation-promoting factor was significantly decreased in oocytes activated by an EP and treated with anisomycin. Finally, the mRNA expression levels of apoptosis-related genes (Bax and Bcl-2) and pluripotency-related genes (Oct4, Nanog, and Sox2) were checked by RT-PCR.
Conclusion
Our results demonstrate that porcine oocyte activation via an EP in combination with anisomycin treatment can lead to a high blastocyst formation rate in parthenogenetic activation and SCNT experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian follicular oocytes can be matured in vitro. During oocyte maturation, meiosis arrests at metaphase II (MII) until insemination or parthenogenetic activation (PA) occurs. This process is mainly regulated by cytoplasmic maturation-promoting factor (MPF), a complex of cyclin and p34cdc2, which is stabilized by cytostatic factor (Kubiak et al. 1993; Whitaker 1996).
A common method to activate MII oocytes is exposure to an electric pulse (EP) followed by treatment with a protein synthesis inhibitor, such as cycloheximide (CHX). Protein synthesis inhibitors prevent synthesis of cyclin B, the regulatory subunit of MPF, and induce a subsequent decrease in the MPF level, thereby allowing oocytes to progress into interphase. This method is widely used to activate bovine oocytes (Presicce and Yang 1994). 6-Dimethylaminopurine (6-DMAP), a protein kinase inhibitor, inhibits MPF activity by blocking protein phosphorylation, which causes chromosomal abnormalities and increases the activation rate of pig oocytes (De and King 1998; Velde et al. 1999). In addition, cyclin-dependent kinase inhibitors such as butyrolactone I selectively inhibit the catalytic subunit of MPF and can be used to achieve a high formation rate of good-quality blastocysts (Dinnyés et al. 1999).
Anisomycin, a protein synthesis inhibitor, effectively activates newly matured bovine oocytes (Felmer and Arias 2015). In comparison with 6-DMAP and CHX treatment, bovine oocyte activation via an EP and treatment with 1 μg/mL anisomycin for 5 h significantly improves the in vitro developmental competence of embryos in PA and somatic cell nuclear transfer (SCNT) experiments, reduces the occurrence of chromosomal abnormalities, and improves the quality of blastocysts. However, it is not known if anisomycin can effectively activate porcine oocytes.
The goal of this study was to investigate the development of porcine oocytes parthenogenetically activated by an EP in combination with anisomycin treatment. The optimal concentration and duration of anisomycin treatment were determined. Then, a MPF assay, karyotype analysis, and RT-PCR analysis of several apoptosis- and pluripotency-related genes in embryos were conducted. We also compared the developmental competence of SCNT embryos between the anisomycin- and 6-DMAP-treated groups.
Materials and methods
Chemicals
All chemicals used in this study were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA), unless otherwise indicated. All experimental procedures were approved by the Ethics Committee of Yanbian University.
In vitro maturation
Ovaries were obtained from a local abattoir, transported to the laboratory within 2 h, and collected in physiological saline at 39 °C. Cumulus–oocyte complexes (COCs) were aspirated from follicles with a diameter of 2–7 mm using a ten gauge needle. The selected good-quality COCs were washed three times in Tyrode’s lactate Hepes-buffered solution containing 0.1% polyvinyl alcohol (PVA), cultured in NCSU-37 medium supplemented with 10% porcine follicular fluid, 0.6 mM cysteine, 0.1 IU/mL human chorionic gonadotropin (hCG), and 0.1 IU/mL pregnant mare serum gonadotropin (PMSG) for 20–22 h, and then cultured for another 22 h in medium lacking PMSG and hCG at 38.5 °C in a humidified atmosphere of 5% CO2.
PA of oocytes and in vitro culture (IVC)
All matured oocytes with an extruded first polar body were exposed to a direct current pulse (1.5 kV/cm, 60 µs) in 0.28 mol/L mannitol containing 0.1 mM MgSO4, 0.05 mM CaCl2, and 0.1% PVA. After PA, oocytes were washed with NCSU-37 medium supplemented with 4 mg/mL bovine serum albumin and incubated in culture medium containing anisomycin and cytochalasin B (CB) or CB alone.
After PA, oocytes were cultured in 0.75 mL of IVC medium at 38.5 °C in a humidified atmosphere with 5% CO2 for 2 days to record their morphology and cleavage rate. All cleaved embryos were cultured for another 5 days in IVC medium at 38.5 °C in a humidified atmosphere with 5% CO2. The blastocyst formation rate was recorded on day 7.
Measurement of MPF levels
A total of 240 oocytes were divided into two groups and treated with 5 μg/mL CB alone or together with anisomycin, respectively. Oocytes were washed several times in phosphate-buffered saline (PBS) and placed in a droplet of PBS. Oocytes were repeatedly blown with a fine needle whose diameter was smaller than that of the oocytes until they ruptured. The samples were centrifuged at 1200 rpm for 15 min at 4 °C. The supernatant was collected, and the level of MPF was determined using the Porcine MPF ELISA Kit (Kexing, Shanghai, China) following the manufacturer’s protocol. In brief, 10 µL of the oocyte extract was added to each well, followed by 40 µL of dilution buffer (i.e., the sample was diluted fivefold). Plates were incubated at 37 °C in the dark for 30 min. The wells were washed thoroughly with buffer and supplemented with 50 µL per well of a horseradish peroxidase conjugate reagent, except for the blank. Samples were incubated at 37 °C for 30 min and then washed. TMB substrate solution was added to each well, the plate was incubated for 15 min at 37 °C in the dark, and the reaction was terminated by adding 50 μL of sulfuric acid. The color change was spectrophotometrically measured at 450 nm within 15 min of the reaction being terminated. The concentration of MPF in the sample was determined using a standard curve.
Chromosomal analysis
Blastocysts derived from the anisomycin plus CB-treated group were subjected to chromosomal analysis. The blastocysts were pre-treated with 0.2 µg/mL demecolcine for 4 h in 5% CO2 at 37 °C to block cell division at metaphase and then incubated in a hypotonic solution (0.075 M KCl) for 20 min at 37 °C. Swollen blastocysts were placed on a clean glass slide immersed in stationary liquid (methanol:acetic acid = 3:1). After drying, slides were stained with 10% Giemsa solution (Invitrogen, Carlsbad, CA) for 10 min. The stained chromosomes were imaged using oil immersion optics (Nikon) to determine the chromosome number.
Nuclear transfer
Nuclear transfer was performed as described by Yin et al. (2002). In vitro matured oocytes with the first polar body were cultured in medium supplemented with 0.05 mol/L sucrose and 0.4 μg/mL demecolcine for 1 h. Sucrose was used to enlarge the perivitelline space of oocytes. Treated oocytes with a protruding membrane were transferred to medium supplemented with 5 μg/mL CB and 0.4 μg/mL demecolcine. Protrusions were removed by aspirating with a glass pipette with an inner diameter of 15 μm. A single donor cell was inserted into the perivitelline space of each oocyte, which were then electrically fused using two direct pulses of 150 V/mm for 50 μs in 0.28 mol/L mannitol supplemented with 0.1 mM MgSO4 and 0.01% PVA (vol/vol). Fused oocytes were cultured for 1 h in medium containing 0.4 μg/mL demecolcine before electroactivation. The reconstructed oocytes were activated by two direct pulses of 100 V/mm for 20 μs in 0.28 mol/L mannitol supplemented with 0.1 mM MgSO4 and 0.05 mM CaCl2. Activated oocytes were divided into three groups and cultured in NCSU-37 medium containing 5 μg/mL CB, 0.01 μg/mL anisomycin plus 5 μg/mL CB, and 2 mmol/L 6-DMAP, respectively, for 4 h. Thereafter, the embryos were cultured in 500 μL of NCSU-37 medium supplemented with 4 mg/mL bovine serum albumin and 0.6 mM cysteine under paraffin oil on a plastic Petri dish for 7 days at 38.5 °C in 5% CO2 and air without a medium change. The inner cell mass of blastocysts at day 7 (168 h) was stained.
Gene expression in porcine blastocysts derived from parthenogenetically activated oocytes
The mRNA expression levels of apoptosis-related genes (Bax and Bcl-2) and pluripotency-related genes (Sox2, Oct4, and Nanog) were measured, together with ribosomal protein L19 (RPL19) as an endogenous reference gene. Total RNA was extracted from cumulus cells of 40 blastocysts using the Dynabeads® mRNA DIRECT™ Kit (Life Technologies AS, Oslo, Norway) according to the manufacturer’s instructions. The RNA concentration was determined using a UV–vis spectrophotometer (Shimadzu, UV-2450, Tokyo, Japan). cDNA was immediately synthesized from extracted RNA using the SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, US), according to the manufacturer’s directions. The primers used for RT-PCR analysis are shown in Supplementary Table S1. The expected PCR product sizes of Bax, Bcl-2, Sox2, Oct4, Nanog, and RPL19 were 189, 196, 103, 136, 214, and 79 bp, respectively. For PCR, platinum Taq DNA polymerase (Invitrogen) was added to the cDNA mixture (each cDNA sample was mixed with PCR mix containing 1 × PCR buffer, 0.1 mM dNTP mixture, 1.5 mM MgCl2, and 0.25 μM of each primer) and denatured. The mixture was subjected to PCR in a thermal cycler (T100™ Thermal Cycle; Bio-Rad Laboratories, Inc., CA, USA). The PCR cycle was as follows: 95 °C for 5 min, followed by 46 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, and a final extension of 72 °C for 7 min. PCR products were electrophoresed in 1.5% agarose gels. Ready-load 100 bp DNA ladder (Invitrogen) was used as a molecular weight marker. Stained gels were imaged with a digital fluorescence recorder (GelDoc-It® TS Imaging System, UVP, Upland, CA, USA). The mRNA expression levels were determined by measuring the intensity of each band.
Statistical analysis
Each experiment was repeated at least three times. The data were expressed as mean values ± standard deviation. The data were analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 19.0, IBM Corp., Armonk, NY). After arcsine transformation of proportional data, a one-way analysis of variance was performed to determine whether there were any statistical differences in the cleavage rate, blastocyst formation rate, and number of cells per blastocyst among the groups. P < 0.05 was considered significant.
Results
In a preliminary experiment, exposure to an EP and treatment with anisomycin did not inhibit second polar body exposure in matured oocytes; therefore, the experimental group was treated with anisomycin plus 5 μg/mL CB and the control group was treated with 5 μg/mL CB alone.
Effects of treatment with different concentrations of anisomycin on PA of porcine oocytes and karyotype analysis
A total of 524 oocytes were parthenogenetically activated by exposure to an EP and divided into four groups, which were treated with CB plus 0, 0.01, 0.1, and 1 μg/mL anisomycin, respectively. The percentage of embryos that developed to the blastocyst stage was significantly higher in the groups treated with 0.01 and 0.1 µg/mL anisomycin than in those treated with 0 and 1 µg/mL anisomycin (31.8 and 27.1 vs. 16.7 and 0%, P < 0.05; Table 1). There were no significant differences in the number of cells per blastocyst among the groups treated with 0, 0.01, and 0.1 µg/mL anisomycin. The blastocyst formation rate was higher in the group treated with 0.01 µg/mL anisomycin for 4 h than in the groups treated with 0.01 µg/mL anisomycin for 2 and 6 h; however, this was not significant (39.5, 33.3, and 29.2%, respectively; Table 2). Blastocysts were subjected to karyotype analysis (Fig. 1b). After treatment with 0.01 µg/mL anisomycin for 4 h, 66.6% (20/30) of blastocysts were classified as diploid (Fig. 1a).
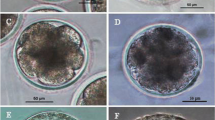
a Porcine parthenogenetically activated of blastocysts of Day 7 derived from the anisomycin-treated group. b An image of a Day 7 embryo treated with anisomycin after parthenogenetic activation stained with Hoechst 33342 and used ultraviolet (UV) light excitation. Bars 100 μM. c Porcine cloned blastocysts of Day 7 derived from the Anisomycin-treated group. d An image of a Day 7 cloned embryo treated with anisomycin and stained with Hoechst 33342, then used ultraviolet (UV) light excitation. Bars 100 μM
Comparison of the in vitro developmental competence of SCNT embryos derived from oocytes activated by an EP and treated with anisomycin or 6-DMAP
After SCNT, the percentage of embryos that developed to the blastocyst stage was significantly higher in the anisomycin- and 6-DMAP-treated groups than in the control group (18.3 and 13.0 vs. 4.2%, P < 0.05; Table 3). The cleavage rate (86.6, 72.5, and 76.1%, respectively) and total number of cells per blastocyst (42.0 ± 4.7, 42.0 ± 6.0, and 42.3 ± 7.8, respectively) in SCNT embryos were similar in the anisomycin-, 6-DMAP-, and CB-treated groups (Table 3).
Levels of MPF and gene expression in porcine embryos derived from oocytes parthenogenetically activated by an EP and anisomycin treatment
Matured porcine oocytes were activated by an EP and then treated with anisomycin or the control. Thereafter, the level of MPF was determined. The level of MPF was significantly lower in the anisomycin-treated group (254.2 pg/mL) than in the control group (361.3 pg/mL) (P < 0.05; Fig. 2).
The effect of anisomycin treatment on the expression of pluripotency-related genes (Oct4, Sox2, and Nanog) and apoptosis-related genes (Bcl-2 and Bax) in blastocysts was assessed. The mRNA expression levels of these genes did not differ between the control and anisomycin-treated groups (Fig. 3).
Discussion
PA and SCNT in domestic animals have contributed to basic research in developmental biology, medicine, and agriculture (Blelloch et al. 2006). However, cloning productivity in vitro remains low in most animals (Koo et al. 2005; Sepulveda-Rincon et al. 2016). MII oocytes contain MPF, which can cause dissolution of the nuclear envelope, condensation of donor chromatin, and dispersion of nucleoli in the donor nucleus, and this may be essential for nuclear reprogramming (Choi and Campbell 2015). Although MPF may play a pivotal role during oocyte aging, its mechanisms on oocyte developmental potential are not fully understood (Lee et al. 2016). Our experiments confirmed that an EP alone is not sufficient to initiate embryonic development in porcine species, and MPF activity may be reduced using an inhibitor of protein synthesis or histone kinases (Nanassy et al. 2007). Anisomycin, a protein synthesis inhibitor, was previously reported to efficiently increase the bovine blastocyst formation rate after PA and SCNT by reducing MPF activity (Felmer and Arias 2015). This is the first report of the effect of anisomycin on the in vitro development of porcine oocytes (Fig. 4).
We tested the effects of treatment with anisomycin at various concentrations and for various durations on PA of porcine oocytes. The highest cleavage and blastocyst formation rates of parthenogenetically activated porcine oocytes were achieved by treatment with 0.01 μg/mL anisomycin for 4 h. This is in contrast to bovine oocytes, in which treatment with 1 μg/mL anisomycin for 5 h is optimal; treatment with this concentration did not affect porcine oocyte activation and no blastocysts were observed. Cell death (apoptosis) must have occurred in porcine oocytes treated with this high concentration of anisomycin. This might be related to the interactions of epigenetic factors within the conceptus, environmental factors, and several epigenetic mechanisms that operate in pregnancy (Ostrup et al. 2011). The development of bovine SCNT embryos is more effective when activated oocytes are treated with anisomycin than when they are treated with 6-DMAP (Felmer and Arias 2015). There were no significant differences in the developmental competence of porcine SCNT embryos between the anisomycin- and 6-DMAP-treated groups in the present study. In addition, 6-DMAP inhibits extrusion of the second polar body from oocytes, resulting in a high formation rate of diploid parthenotes that are more developmentally competent than haploid parthenotes (Liu et al. 1998; Szöllösi et al. 1993). Exposure to an EP and treatment with anisomycin alone cannot inhibit second polar body extrusion in porcine oocytes. To maintain diploidy in the future embryo, extrusion of the second polar body must be avoided by co-culture with a microfilament inhibitor such as CB (Han and Gao 2013). Karyotype analysis in previous studies revealed an increased incidence of chromosomal abnormalities in 6-DMAP-treated bovine and porcine embryos (Bhak et al. 2006; Milazzotto et al. 2008; Hao et al. 2005). However, the percentage of embryos with chromosomal abnormalities was decreased in the anisomycin-treated group in the present study.
Members of the Bcl-2 gene family play a major role in regulation of apoptosis. Bcl-2 has an anti-apoptotic function and promotes cell survival, whereas Bax is proapoptotic and promotes cell death (Cui et al. 2011). Oct4, Sox2, and Nanog, which are important early transcription factors and major reprogramming factors for regulation of stem cell pluripotency, are expressed in embryos (Sommer et al. 2009). To determine whether anisomycin affects expression of these genes during porcine blastocyst formation, we examined their mRNA levels in blastocysts by RT-PCR. The expression levels of these genes did not differ between the control and anisomycin-treated groups.
Conclusion
In summary, this study demonstrated that treatment with 0.01 μg/mL anisomycin for 4 h significantly improved the in vitro development of embryos derived from parthenogenetically activated oocytes. However, there were no significant differences in SCNT embryos between the anisomycin- and 6-DMAP-treated groups. Anisomycin treatment significantly reduced the MPF level in porcine oocytes, increased the percentage of blastocysts with a normal diploid karyotype, and had no significant effects on expression of pluripotency- and apoptosis-related genes. These results suggest that anisomycin is a safe alternative to activate porcine oocytes in vitro. Our results increase knowledge of reproductive technology and embryonic development.
References
Bhak JS, Lee SL, Ock SA et al (2006) Developmental rate and ploidy of embryos produced by nuclear transfer with different activation treatments in cattle. Anim Reprod Sci 92:37–49
Blelloch R, Wang Z, Meissner A et al (2006) Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells 24:2007–2013
Choi I, Campbell KHS (2015) The role of protein kinases in reprogramming and development of SCNT embryos. J Embryo Transf 30:33–43
Cui MS, Liu ZX, Wang XL et al (2011) Relationship between differential expression of Bax and Bcl-2 genes and developmental differences of porcine parthenotes cultured in PZM-3 and NCSU-23. Agric Sci China 10:1772–1780
De LFR, King WA (1998) Developmental consequences of karyokinesis without cytokinesis during the first mitotic cell cycle of bovine parthenotes. Biol Reprod 58(4):952–962
Dinnyés A, Hirao Y, Nagai T (1999) Parthenogenetic activation of porcine oocytes by electric pulse and/or butyrolactone I treatment. Cloning 1:209–216
Felmer R, Arias ME (2015) Activation treatment of recipient oocytes affects the subsequent development and ploidy of bovine parthenogenetic and somatic cell nuclear transfer (SCNT) embryos. Mol Reprod Dev 82:441–449
Han B, Gao J (2013) Effects of chemical combinations on the parthenogenetic activation of mouse oocytes. Exp Ther Med 5:1281–1288
Hao YH et al (2005) Developmental competence of porcine parthenogenetic embryos relative to embryonic chromosomal abnormalities. Mol Reprod Dev 1:77–82
Koo DB, Chae JI, Kim JS et al (2005) Inactivation of MPF and MAP kinase by single electrical stimulus for parthenogenetic development of porcine oocytes. Mol Reprod Dev 72:542–549
Kubiak JZ, Weber M, De PH et al (1993) The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J 12:3773–3778
Lee HS, Kim KH, Kim EY et al (2016) Obox4-silencing-activated STAT3 and MPF/MAPK signaling accelerate nuclear membrane breakdown in mouse oocytes. Reproduction 151:369–378
Liu L, Ju JC, Yang X (1998) Differential inactivation of maturation-promoting factor and mitogen-activated protein kinase following parthenogenetic activation of bovine oocytes. Biol Reprod 59:537–545
Milazzotto M, Feitosa W, Coutinho A et al (2008) Effect of chemical or electrical activation of bovine oocytes on blastocyst development and quality. Reprod Domest Anim 43:319–322
Nanassy L, Lee K, Javor A et al (2007) Changes in MPF and MAPK activities in porcine oocytes activated by different methods. Theriogenology 68:146–152
Ostrup E, Hyttel P, Ostrup O (2011) Embryo-maternal communication: signalling before and during placentation in cattle and pig. Reprod Fert Dev 23:964–975
Presicce GA, Yang X (1994) Parthenogenetic development of bovine oocytes matured in vitro for 24 hr and activated by ethanol and cycloheximide. Mol Reprod Dev 38:380–385
Sepulveda-Rincon LP, Solanas ED, Serrano-Revuelta E et al (2016) Early epigenetic reprogramming in fertilized, cloned, and parthenogenetic embryos. Theriogenology 86:91–98
Sommer CA, Stadtfeld M, Murphy GJ et al (2009) Induced pluripotent stem cell generation using a single Lentiviral stem cell cassette. Stem Cells 27:543–549
Szöllösi MS, Kubiak JZ, Debey P et al (1993) Inhibition of protein kinases by 6-dimethylaminopurine accelerates the transition to interphase in activated mouse oocytes. J Cell Sci 104:861–872
Velde AVD, Liu L, Bols PEJ et al (1999) Cell allocation and chromosomal complement of parthenogenetic and IVF bovine embryos. Mol Reprod Dev 54:57–62
Whitaker M (1996) Control of meiotic arrest. Rev Reprod 1:127–135
Yin XJ, Tani T, Yonemura I et al (2002) Production of cloned pigs from adult somatic cells by chemically assisted removal of maternal chromosomes. Biol Reprod 67:442–446
Acknowledgements
This work was supported by the State Key Development Program for Basic Research of China (Grant No. 20150622005JC) and the Institute for Basic Science (Grant No. IBS-R021-D1-2015-a02).
Supporting information
Supplementary Table 1—Primer sequences used for gene expression analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yu-Chen Zhang, Long Jin, Hai-Ying Zhu have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, YC., Jin, L., Zhu, HY. et al. The developmental competence of oocytes parthenogenetically activated by an electric pulse and anisomycin treatment. Biotechnol Lett 39, 189–196 (2017). https://doi.org/10.1007/s10529-016-2249-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2249-2