Abstract
Objectives
To characterize a novel endoglucanase, Celal_2753, from the psychrophilic bacterium Cellulophaga algicola IC166T.
Results
Celal_2753 was purified to homogeneity with a yield of 81 % and with a molecular weight of 40 kDa on SDS-PAGE. It had maximum hydrolytic activity towards carboxymethyl cellulose at 40 °C and pH 6. It showed 33 % of the maximum activity at 10 ºC. Its activity increased to 272–316 % in the presence of 0.25–2 M NaCl and KCl at 40 °C. Celal_2753 was stable in the presence of 10 % (v/v) Tween 20, 10 % (v/v) Triton X-100, 16 mM SDS, 6 M urea or 2 M guanidine hydrochloride. Celal_2753 that had been boiled for 5 min recovered 55 % of its initial activity by incubating at 30 °C for 60 min.
Conclusion
Because of its cold-adapted, thermotolerant and denaturant-stable properties, endoglucanase Celal_2753 is promising in detergent industry and bioethanol production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most abundant renewable carbohydrate polymer (Annamalai et al. 2013). Endoglucanases (3.2.1.4) can attack the cellulose chain randomly and play a key role in its degradation (Lynd et al. 2002). Endoglucanases have attracted interest because of their application in various industrial processes, including textiles, laundries, agriculture, pulp and paper manufacture (Bhat 2000; Kuhad et al. 2011). There is also interest in using endoglucanases to convert lignocellulosic materials for the production of bioethanol (Yennamalli et al. 2013).
Although endoglucanases have been isolated from bacteria, as well as from fungi, plants and animals, marine extremophilic microorganisms offer an attractive resource for new enzymes that have good thermostability, cold-adaption, acid-stability, alkaline-stability, salt tolerance and detergent stability to withstand harsh conditions (Kasana and Gulati 2011; Mai et al. 2014; Wu et al. 2015). Cellulophaga algicola IC166T was isolated from the surface of the chain-forming sea-ice diatom, Melosira, collected from the Eastern Antarctic coastal zone (Bowman 2000). It can grow between −2 and 28 °C in the presence of 0.5–10 % (w/v) NaCl, and produces an extracellular cold-adapted protease, β-galactosidase and α-amylase (Nichols et al. 1999). Strain IC166T can also hydrolyze carboxymethyl cellulose (CMC). Its genome sequence revealed the presence of an endoglucanase, Celal_2753, that belongs to glycoside hydrolase family 5 (GH-5) (Abt et al. 2011).
In this study, Celal_2753 has been cloned, expressed in Escherichia coli, purified and characterized. The cold-adpation, thermotolerance, salt-activation and denaturant-stability of this enzyme suggest that it may have potential use in industrial applications.
Materials and methods
Bacterial strain and culture condition
Cellulophaga algicola type strain IC166T (=DSM 14237) was obtained from DSM and cultured at 15 °C in Zobell 2216E medium.
Construction of expression plasmid
The genomic DNA of C. algicola IC166T was isolated using MiniBEST Bacterial Genomic DNA Extraction Kit (TaKaRa). The sequence of celal_2753 was amplified by PCR using the forward primer F (5′-GGAATTCCATATGGAGCCTGAAATTATTGACCCTG-3′) and reverse primer R (5′-CCGCTCGAGGTAGGTAGGATTTTTTTCTAACAT-3′) incorporated NdeI and XhoI restriction sites (underlined letters). The PCR product was purified from the agarose gel, digested with NdeI and XhoI, and ligated into a similarly digested plasmid pET-24a(+) (Novagen). The resulting plasmid, pET24-Celal_2753, was transformed into Escherichia coli BL21(DE3).
Expression and purification of Celal_2753
Escherichia c oli BL21(DE3) cells containing plasmid pET24-Celal_2753 were grown at 37 °C in lysogeny broth (LB) supplemented with kanamycin (30 μg/ml). When OD600 reached 0.5, the cells were induced by 1 mM IPTG at 25 °C for 20 h. Cells were harvested, resuspended in 20 mM phosphate buffer (pH 7.0) and disrupted by sonication. After centrifugation at 10,000×g for 15 min at 4 °C, the supernatant was loaded on a HisTrap HP column (GE Healthcare), washed with buffer I (20 mM phosphate buffer, 500 mM NaCl, pH 7) followed by buffer I containing 25 mM imidazole, then eluted with buffer I containing 50 mM imidazole. The homogeneity and molecular weight of the purified endoglucanase were determined by SDS-PAGE. Protein bands were visualized by Coomassie Brilliant Blue R-250. Protein concentration was measured by the method of Lowry using bovine serum albumin (BSA) as the standard.
Endoglucanase activity assay
Endoglucanase activity was determined by measuring the amount of reducing sugar by the 3,5-dinitrosalicylic acid (DNS) assay. Standard assay conditions were as follows: 100 μl enzyme solution was mixed with 900 μl 10 g carboxymethyl cellulose (CMC)/l in 50 mM phosphate buffer (pH 7.0), and incubated at 40 °C for 10 min. One unit (U) of endoglucanase activity was defined as the amount of enzyme required to liberate 1 μmol reducing sugar per min under the above conditions.
Effect of temperature, pH, metal ions and denaturants
The optimal temperature of Celal_2753 was determined by assaying its activity at 0–70 ºC in 50 mM phosphate buffer (pH 6). The thermostability was evaluated by measuring the residual activity under standard conditions immediately after the enzyme was incubated at 0–70 ºC for 1 h in 50 mM phosphate buffer (pH 6). The optimal pH was determined in 50 mM Na2HPO4/citric acid buffer (pH 3–8), 50 mM sodium phosphate buffer (pH 6–8), 50 mM Tris–HCl buffer (pH 7–9), 50 mM glycine/NaOH buffer (pH 8.6–10.6). The pH stability was analyzed by measuring the residual activity under standard conditions after the enzyme was pretreated in the above buffers at 4 °C for 24 h. Effect of metal ions on Celal_2753 activity was investigated at 1 mM except Na+ (0–2 M) and K+ (0–2 M) at 40 °C. The influence of denaturants on the stability of Celal_2753 was assessed by measuring the residual activity under standard conditions after the enzyme was incubated at 25 °C for 30 min with guanidine hydrochloride (0–4 M), urea (0–8 M), ionic surfactant SDS (0–16 mM), non-ionic surfactants Tween 20 and Triton X-100 (0–10 %, v/v).
Results and discussion
Expression and purification of Celal_2753
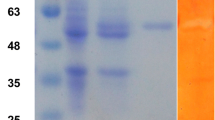
Recombinant Celal_2753 was purified to homogeneity with an overall yield of 81 %. It showed a single band with an apparent molecular weight of 40 kDa on SDS-PAGE which is close to its theoretical molecular weight (Fig. 1).
Characterization of recombinant Celal_2753
Recombinant Celal_2753 was most active at 40 °C and showed 33 % of the highest activity at 10 °C (Fig. 2a). It retained more than 90 % of its original activity after incubation for 1 h below 40 °C (Fig. 2b). Its optimal pH was pH 6 in phosphate buffer (Fig. 2c). The enzyme was stable between pH 5–9. 5 (Fig. 2d).
Effects of temperature and pH on activity and stability of Celal_2753. a The optimal temperature was determined by assaying the activity at 0–70 °C in 50 mM phosphate buffer (pH 6). b The thermostability was evaluated by measuring the residual activity after the enzyme was incubated at 0–70 °C for 1 h in 50 mM phosphate buffer (pH 6). Residual activities were measured at 40 °C. c The optimal pH was determined by measuring the activity in 50 mM Na2HPO4/citric acid buffer (pH 3–8, cross), sodium phosphate buffer (pH 6–8, closed triangle), Tris/HCl buffer (pH 7–9, open square), glycine-NaOH buffer (pH 8.6–10.6, closed diamond). d The pH stability was analyzed by measuring the residual activity after the enzyme was pretreated in the above buffers at 4 °C for 24 h
The effects of metal ions on the activity of Celal_2753 are shown in Table 1. Although the enzyme was active without NaCl or KCl, its activity increased to 272–316 % in the presence of 0.25–2 M NaCl and KCl. The effects of various denaturants on the stability of Celal_2753 are shown in Table 2. Celal_2753 was stable in the presence of 10 % (v/v) Tween 20, 10 % (v/v) Triton X-100, 16 mM SDS, 6 M urea or 2 M guanidine hydrochloride that denature most proteins (Gudiksen et al. 2006; Ma et al. 2013). Traditionally, lignocellulose are pretreated with ionic liquids and hydrolyzed with cellulases (Joseph et al. 2010). Since ionic liquids are strong denaturing agents for most cellulases, the pretreated cellulose has to be separated and rinsed with water before being hydrolysed. However, because Celal_2753 is halophilic and denaturant-stable, it has a potential use in the direct conversion of ionic liquids pretreated cellulose without cellulose recovery. Detergent-stable Celal_2753 could also be used in the textile and laundry industries.
Heat inactivation and activity recovery of Celal_2753
Celal_2753 was boiled for 5 min. After heat treatment, the enzyme was immediately incubated for 10 min at various temperatures (0–50 °C). The activity of Celal_2753 recovered and reached 32.7–55.3 % after incubation between 0 and 40 °C for 10 min (Fig. 3). After the heat-treated enzyme was incubated at 30 °C for 60 min, a maximum recovery of activity (55.3 %) was obtained (Fig. 4). Prolonged incubation time (up to 20 h) resulted in 1.1–2.5 % decrease of recovered activity (data not shown). The characteristics of recovered Celal_2753 were similar to non-heated enzyme (data not shown). The thermotolerant Celal_2753 could facilitate its storage and transportation since the heat-inactivated enzyme could recover its activity after incubation between 0 and 40 °C.
Effect of incubation temperature on the activity recovery of heat-inactivated Celal_2753. The enzyme was boiled for 5 min, then immediately incubated for 10 min at various temperatures (0–50 °C). The recovered enzymatic activities were measured under standard conditions. The non-heated enzyme was taken as control (100 %). Values given are the average of three replications, the data were expressed as mean ± standard deviation
Effect of incubation time on the activity recovery of heat-inactivated Celal_2753. The enzyme was boiled for 5 min, then immediately incubated at 30 °C for 60 min. Aliquots were withdrawn at set time intervals to measure the recovered enzymatic activity under standard conditions. The non-heated enzyme was taken as control (100 %). Values given are the average of three replications, the data were expressed as mean ± standard deviation
References
Abt B, Lu M, Misra M, Han C et al (2011) Complete genome sequence of Cellulophaga algicola type strain (IC166). Stand Genomic Sci 4:72–80
Annamalai N, Rajeswari MV, Elayaraja S, Balasubramanian T (2013) Thermostable, haloalkaline cellulase from Bacillus halodurans CAS 1 by conversion of lignocellulosic wastes. Carbohydr Polym 94:409–415
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Bowman JP (2000) Description of Cellulophaga algicola sp. nov., isolated from the surfaces of Antarctic algae, and reclassification of Cytophaga uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Cellulophaga uliginosa comb. nov. Int J Syst Evol Microbiol 50:1861–1868
Binder Joseph B, Raines Ronald T (2010) Fermentable sugars by chemical hydrolysis of biomass. Proc Natl Acad Sci USA 107:4516–4521
Gudiksen KL, Gitlin I, Whitesides GM (2006) Differentiation of proteins based on characteristic patterns of association and denaturation in solutions of SDS. Proc Natl Acad Sci USA 103:7968–7972
Kasana RC, Gulati A (2011) Cellulases from psychrophilic microorganisms: a review. J Basic Microbiol 51:572–579
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzym Res 2011:280696
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Ma S, Tan YL, Yu WG, Han F (2013) Cloning, expression and characterization of a new ι-carrageenase from marine bacterium, Cellulophaga sp. Biotechnol Lett 35:1617–1622
Mai Z, Su H, Yang J, Huang S, Zhang S (2014) Cloning and characterization of a novel GH44 family endoglucanase from mangrove soil metagenomic library. Biotechnol Lett 36:1701–1709
Nichols D, Bowman J, Sanderson K, Nichols CM, Lewis T, McMeekin T, Nichols PD (1999) Developments with antarctic microorganisms: culture collections, bioactivity screening, taxonomy, PUFA production and cold-adapted enzymes. Curr Opin Biotechnol 10:240–246
Wu G, Zhang X, Wei L, Wu G, Kumar A, Mao T, Liu Z (2015) A cold-adapted, solvent and salt tolerant esterase from marine bacterium Psychrobacter pacificensis. Int J Biol Macromol 81:180–187
Yennamalli RM, Rader AJ, Kenny AJ, Wolt JD, Sen TZ (2013) Endoglucanases: insights into thermostability for biofuel applications. Biotechnol Biofuels 6:136
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31000361), Natural Science Foundation of Shandong Province (ZR2015CM024) and the Fundamental Research Funds for the Central Universities (201013008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Yu, W. & Han, F. Expression and characterization of a cold-adapted, thermotolerant and denaturant-stable GH5 endoglucanase Celal_2753 that withstands boiling from the psychrophilic bacterium Cellulophaga algicola IC166T . Biotechnol Lett 38, 285–290 (2016). https://doi.org/10.1007/s10529-015-1971-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1971-5