Abstract
Objectives
To obtain an ethanolamine plasmalogen (PlsEtn)-hydrolyzing enzyme and to develop an assay that would help determine PlsEtn concentrations in human serum as an indicator of Alzheimer-type dementia and of arteriosclerosis.
Results
Phospholipase A1s, SaPLA1 and SvPLA1 from, respectively, Streptomyces albidoflavus NA297 and S. avermitilis JCM5070—but not phospholipase B from Streptomyces sp. NA684, PLA2-Nagase from S. avermitilis, PLA2IIL from S. violaceoruber nor LIPOMOD 699L (porcine phospholipase)—hydrolyzed choline plasmalogen (PlsCho) and PlsEtn (PlsCho preferred over PlsEtn). Using a combination of SaPLA1, lysoplasmalogen-specific phospholipase D (LyPls-PLD), with amine oxidase, an end-point assay was developed for measuring serum PlsEtn concentration. The standard curve, generated using various amounts of PlsEtn in this assay, was linear between 0 and 0.2 mM. PlsEtn concentrations in forty-seven serum samples, determined independently by this enzyme-based assay and 125I-HPLC method, exhibited a linear relationship, indicating that the assay is suitable for fast and accurate measurement of serum PlsEtn concentration.
Conclusions
An assay, developed using SaPLA1, LyPls-PLD, and AOX, selectively measured PlsEtn levels in blood samples. This assay could be a useful diagnostic tool for early stage detection of diseases such as Alzheimer-type dementia and arteriosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasmalogens (Pls), which are vinyl ether-type phospholipids, may be associated with several diseases such as Alzheimer-type dementia and arteriosclerosis. Wood et al. (2010) showed that ethanolamine plasmalogen (PlsEtn) levels in the serum declined in the early stage of Alzheimer-type dementia. Maeba et al. (2007) showed a positive correlation between Pls and high-density lipoprotein and also demonstrated that serum Pls levels decrease with aging. Nishimukai et al. (2014) reported a strong association of serum choline plasmalogen (PlsCho), particularly those containing oleic acid (18:1) in the sn−2 position, with a wide range of risk factors for metabolic syndrome/atherosclerosis. These results suggested that serum Pls might serve as a reliable biomarker for these diseases, which have increasingly gained worldwide attention.
As of now, the concentration of Pls in the serum is measured either by HPLC using 125I3 −, 125I-HPLC (Maeba and Ueta 2004; Maeba et al. 2012) or by LC–MS (Nishimukai et al. 2014). Each methods, however, is either time-consuming (complicated, long sample processing procedures and extensive analysis time) or expensive. Thus, a new method, possibly using a Pls-specific enzyme, is warranted for the development of a diagnostic tool that could easily and rapidly determine the concentration of Pls. To the best of our knowledge, there are no prior reports of any Pl-hydrolyzing enzyme. There are, however, only two reports on enzymatic hydrolysis of lysoplasmalogen (LyPls): Wu et al. (2011) showed that rat liver microsomal lysoplasmalogenase (called TMEM86b) catalyzed the hydrolysis of the vinyl ether bond of LyPls, and Wykle and Schremmer (1974) reported on a lysophospholipase D in brain microsomes. We have identified a LyPls-specific phospholipase D (LysPls-PLD) from Thermocrispum sp. strain RD004668 (manuscript in preparation). Thus, we reasoned that the Pls level in blood can be selectively determined if an enzyme that could hydrolyze Pls to LyPls is combined with LyPls-PLD and amine oxidase (AOX) or choline oxidase (COX). Therefore, we explored whether phospholipases A1, A2, and B (PLA1, PLA2, and PLB), that are known to hydrolyze glycerophospholipids into free fatty acids (FFAs) and lysophospholipids or glycerol-3-phosphodiesters, could also hydrolyze Pls.
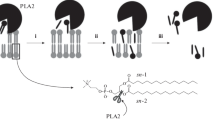
PLA1 and PLA2 have sn-1 and sn-2 positional selectivity, respectively. We have previously shown that SaPLA1 hydrolyzed POPC and, at reaction equilibrium, the molar ratios of released fatty acids were ~70:30 (sn-1/sn-2) (Sugimori et al. 2012). We unexpectedly, however, found that SaPLA1 from Streptomyces albidoflavus NA297 (Sugimori et al. 2012) and SvPLA1 from Streptomyces avermitilis JCM5070 can hydrolyze sn-2 ester bond of Pls (Fig. 1). Here, we report that SaPLA1 and SvPLA1 hydrolyzed Pls. We also describe the mechanism of Pls hydrolysis by SaPLA1 and report developing a novel enzymatic method for the determination of Pls concentration by using a combination of three enzymes SaPLA1, LyPls-PLD, and AOX.
Materials and methods
Chemicals
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1-hexadecyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphoethanolamine (PAF PE16-18:1), 1-(1Z-octadecenyl)-2-oleoyl-sn-glycero-3-phosphoethanolamine (PlsEtn18-18:1), 1-(1Z-octadecenyl)-2-arachidonoyl-sn-glycero-3-phosphoethanolamine (PlsEtn18-20:4), 1-(1Z-octadecenyl)-2-oleoyl-sn-glycero-3-phosphocholine (PlsCho18-18:1), 1-(1Z-octadecenyl)-2-arachidonoyl-sn-glycero-3-phosphocholine (PlsCho18-20:4), and 1-O-1′-(Z)-octadecenyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (LyPlsEtn) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). N,N-Bis-3-methylaniline, disodium salt (TODB) was purchased from Dojindo Laboratories (Kumamoto, Japan). 4-Aminoantipyrine (4-AA) was from Nacalai Tesque, Inc. (Kyoto, Japan). All other chemicals were of highest available pure grade or analytical grade.
Enzymes and bacterial strains
PLA2IIL (20120 U ml−1) of Streptomyces violaceoruber and recombinant SvPLA1 (0.1 U ml−1) were obtained from Asahi Kasei Pharma Corp. (Shizuoka, Japan). PLA2-Nagase (12710 U ml−1) of S. avermitilis was purchased from Nagase Chemtex Corp. (Osaka, Japan). LIPOMOD 699L (≥10000 U ml−1) of porcine pancreas was purchased from Biocatalyst Ltd. (Wales, UK). Recombinant SaPLA1 (rSaPLA1, 57 U ml−1) from S. albidoflavus NA297 (Sugimori et al. 2012) and recombinant PLB (rPLB684, 38 U ml−1) from Streptomyces sp. NA684 (Matsumoto et al. 2013) were prepared as previously reported. His6-tagged recombinant LyPls-specific phospholipase D (rLyPls-PLD) from Thermocrispum sp. RD004668 was expressed in E. coli using pET24a(+) expression vector, and the expressed protein was purified by affinity column chromatography (manuscript in preparation). His6-tagged recombinant AOX from Syncephalastrum racemosum (rSrAOX) was also expressed in E.coli using pET24a(+) and the expressed rSrAOX protein was purified by affinity column chromatography (manuscript submitted). Yeast extract and peroxidase (POD) were from Oriental Yeast Co., Ltd. (Tokyo, Japan). Streptomyces albidoflavus NA297 (Sugimori et al. 2012) and Streptomyces sp. NA684 (Matsumoto et al. 2013) were previously isolated by us, and these strains are deposited as NITE BP-1014 and NITE BP-1015, respectively, in the NITE Patent Microorganisms Depositary (NPMD) (Chiba, Japan). Thermocrispum sp. strain RD004668 was obtained from Biological Resource Center, NITE (NBRC; Chiba, Japan).
Hydrolysis of PlsEtn18-18:1 by phospholipases
The composition of the phospholipase assay reaction mixture was: 120 mM Tris/HCl buffer (pH 7.5), 2 mM PlsEtn18-18:1, 2 mM CaCl2 and 10 U enzyme ml−1. The reaction was initiated by adding the enzyme and the mixture was incubated at 37 °C for 10 min. The reaction was stopped by extracting with an equivalent volume of chloroform/methanol (2:1, v/v). The organic layer was used for the analysis of residual lipid using TLC, which was carried out on a silica gel 60 (Merck) TLC plate, and a mobile phase of chloroform/methanol/water (65:25:4, by vol.). The spot of the residual PlsEtn18-18:1 was detected by spraying the plate with ninhydrin and the concentration of residual PlsEtn18-18:1 was estimated by densitometric scanning of the spot with a CS-9000 dual wavelength flying spot scanner (Shimadzu). The hydrolysis rate was expressed as 100 % when 2 mM PlsEtn18-18:1 (substrate) was completely hydrolyzed by the enzyme to LysPlsEtn in 10 min.
Determination of substrate specificity
The standard assay mixture containing 100 mM Tris/HCl (pH 7.2), 0.5 % (w/v) substrate, 1 % (w/v) Triton X-100, and 0.157 ± 0.036 U SaPLA1 ml−1 was incubated at 37 °C for 5 min. The enzyme reaction was stopped by heating the mixture at 100 °C for 5 min. After centrifugation (21,800×g, 5 min), the supernatant was collected and the concentration of FFAs released in the supernatant were determined using an NEFA C Kit (Wako Pure Chemical Industries Ltd.) and oleic acid as the standard. Assay was performed according to the instructions provided by the manufacturer. One unit (U) of enzyme activity was defined as the amount of enzyme that released 1 μmol free fatty acids from a given substrate per min. Relative activity was determined by defining the activity of the enzyme for PlsCho18-18:1 as 100 %. Three independent experiments were carried out. β-Acetyl-γ-O-hexadecyl-L-α-phosphatidylcholine was not tested as a substrate because the NEFA C Kit was not suitable for acetate determination.
Analysis of reaction products
The reaction products in the organic layer, obtained after extracting the phospholipase assay mixture with chloroform/methanol (see above), were analyzed using a Synapt G2 Mass Spectrometer equipped with an Acquity UPLC system (Waters Corp., Milford, MA, USA). One μl of the organic layer was chromatographed on an Acquity UPLC HSS column C18 (1.8 μm, 2.1 mm × 50 mm) with the UPLC system. The column was at 40 °C, and ultrapure water containing 0.1 % (v/v) formic acid (A) and 2-propanol (B) were employed as mobile phases. A typical 15 min sample run consisted of using a solvent A gradient from 80 to 0 % over 10 min, maintaining 0 % solvent A for 3 min and then increasing its concentration to 80 % over 2 min. A flow rate of 0.3 ml min−1 was used and the eluate was monitored at 220 nm. The UPLC system was interfaced by electrospray ionization (ESI+) to Synapt G2 in positive ion mode. Accurate mass LC–MS/MS DDA data were acquired in the centroid mode from 100 to 1500 m/z.
Docking simulation
Docking simulations between SaPLA1 (PDB-ID: 4HYQ) and palmitate or 1,2-dipentanoyl-sn-glycero-3-phosphoethanolamine (C5PE) were performed using AutoDock (Trott and Olson 2010) to find circumstance under which 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA) would bind to SaPLA1.
Enzymatic measurement of PlsEtn
We focused on PlsEtn because of its possible association with Alzheimer-type dementia. To generate a standard curve for quantifying the PlsEtn level, the reaction mixture, containing 20 mM BES/NaOH buffer (pH 7.5), 0–200 μM PlsEtn18-20:4, 2 mM CaCl2, 5 U rSaPLA1 ml−1, was incubated at 37 °C for 5 min. The assay was started by addition of 5 U rLyPls-PLD ml−1 and followed by adding 5 U POD ml−1, 0.02 % (w/v) TODB, 0.755 U ml−1 rSrAOX, 0.03 % (w/v) 4-AA, 10 mM EDTA and further incubation (10 min, 37 °C). PlsEtn18-20:4 concentration was measured from the amount of H2O2 produced, which was determined by measuring A550 using a Multiskan FC microplate reader (Thermo Fisher Sientific K.K., Yokohama, Kanagawa, Japan). Sera from 47 patients were used as test samples and physiological saline (PS) was used as a control. In addition to measuring the PlsEtn concentration in all sera samples by this enzymatic assay, PlsEtn concentration in samples was also independently measured by the 125I-HPLC method (Maeba et al. 2007; Nishimukai et al. 2014). Sufficient PLA1 for the complete hydrolysis of substrate was added into the assay reaction mixture as PLA1 can hydrolyze lysophosphatidylethanolamine (LPE) (Sugimori et al. 2012). Moreover, PE and LPE cannot interfere the PlsEtn quantification, because they are not substrates for LyPls-PLD (manuscript in preparation).
Results and discussion
Surprisingly, both SaPLA1 and SvPLA1 not only hydrolyzed Pls but also hydrolyzed PAF PE 16-18:1 (Table 1; Fig. 2). PLB and PLA2s, however, exhibited almost no hydrolytic activity for any of the substrates tested. This observation was in contrast to the observation obtained from an earlier study where it was reported that PLA1 from Serratia sp. MK1 exhibited no activity toward Pls (Kim et al. 1999). Thus, it is possible that the actinomycete PLA1, but not the bacterial PLA1, possesses universal hydrolytic activity toward Pls. Alternatively, the substrate recognition mechanisms, (i.e., the size and shape of the binding pocket and cleft) of these two enzymes might differ from each other. To our knowledge, this is the first report demonstrating that PLA1 hydrolyzed Pls.
Substrate specificity of SaPLA1. Activity of SaPLA1 was assayed as described in "Materials and methods" section. Data shown represent average mean ± standard deviation (n = 3)
Both SaPLA1 and SvPLA1 hydrolyzed Pls in the presence and absence of Ca2+, suggesting that the hydrolysis process was not dependent on Ca2+. MS analysis of reaction products revealed that hydrolysis of PlsEtn18-18:1 (m/z, 730.55) by SaPLA1 produced LyPlsEtn (m/z, 466.31).
As shown in Fig. 2, the relative hydrolyzing activity of SaPLA1 for PlsEtn18-18:1 and PlsEtn18-20:4 was somewhat less than that for POPE. The relative activity of SaPLA1 for hydrolyzing PlsCho18-18:1 was highest among all the substrates tested. However, SaPLA1 was unable to hydrolyze DOPE and PlsCho18-20:4. The sn-2 positional selectivity (~70:30, sn-1/sn-2) of SaPLA1 (Sugimori et al. 2012) is lower than that of PLB684 (Matsumoto et al. 2013) and PLA2. Nevertheless, SaPLA1 hydrolyzed the sn-2 acyl ester bond of Pls.
We also examined the substrate recognition mechanism of SaPLA1. We have previously determined the crystal structure of SaPLA1 at 1.75 Å resolution (PDB-ID: 4HYQ) and showed that the hydrophobic pocket and cleft for lipid binding were adjacent to the active site (Murayama et al. 2013). The hydrophobic pocket was ~13–15 Å deep, sufficiently deep enough for accepting a C14-C16 aliphatic chain (Fig. 3b). Our analysis also showed that the span of the lipid-binding cleft was 14–16 Å, and the amino acid residue Trp210 blocked the end of the cleft. SaPLA1 preferred choline-type substrates over ethanolamine-type substrates (Fig. 2). Docking simulations using structural models of SaPLA1and DPPA revealed the binding features of SaPLA1, indicating specificity for diacylglycerophospholipid as a substrate (Fig. 3a), which may explain its sn-1 positional preference. Docking simulations also suggested that the cleft of SaPLA1 could not accommodate both oleate (18:1) and C18 aliphatic chains of DOPE, PlsEtn18-18:1, or PlsEtn18-20:4. It is, however, possible to accommodate a lipid substrate that contained an oleate (18:1) chain and an arachidonate (20:4) chain such that the arachidonate (20:4) chain at sn-2 position is placed into the hydrophobic pocket and the C18 aliphatic chain at sn-1 position is placed into the cleft (Fig. 3b). SaPLA1 preferred PAF PE 16-18:1 over POPE, suggesting that the cleft possibly preferred ether bond at sn-1. However, why PAF PE 16-18:1 was preferred by SaPLA1 is not clear at this point. It also appears that the structure–activity correlation results obtained using PlsEtn18-20:4 and PlsCho18-20:4 were somewhat inconsistent. It is expected that the pocket, binding cleft and head group stabilization should play some roles in the substrate recognition process. It is also possible that some other unknown mechanism might be involved in substrate recognition. Further work is needed to fully elucidate the mechanism.
Model showing substrate bound at the cleft and hydrophobic pocket of SaPLA1. The structure of SaPLA1 is shown using the ribbon model. The secondary structural elements are colored in blue (helices) and pink (strands). a 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA) bound model. b Illustrative representation of putative binding of PlsEtn to the enzyme binding site
Herein, we have developed an enzymatic assay system for determining the concentration of PlsEtn in human sera because PlsEtn is attractive potential biomarker for Alzheimer-type dementia. In this end point assay using four enzymes (rSaPLA1, rLyPls-PLD, rSrAOX, and POD), the concentration of PlsEtn18-20:4 could be directly extrapolated from the ethanolamine concentration, which is measured from the amount of H2O2 produced in the reaction mixture. As can be seen from Fig. 4a, two calibration curves, [data points for which were obtained using this enzyme-based assay and sample solutions containing various concentrations of PlsEtn18-20:4 in either physiological saline (open circles) or human serum (closed circles)] showed linearity between 0 and 0.2 mM PlsEtn18-20:4. Linear regression analysis showed that the data points could be best fitted to the equation y = 2.28 × 10−3 × −0.0547 (R 2 = 0.999) for the physiological saline solutions and to the equation y = 2.10 × 10−3 × +0.165 (R 2 = 0.996) for the serum solutions. In each serum sample, the concentration of PlsEtn was higher than the added PlsEtn amount (by ~17 μM), suggesting that extra PlsEtn probably came from the serum that was used in sample preparation. Both straight lines, however, exhibited similar slopes. In addition, results obtained using dilutions of PlsEtn in physiological saline suggested that the lower limit for measuring the PlsEtn concentration using this assay is ~50 µM.
a Standard curves for PlsEtn assay. Data points were generated using rSaPLA1, rLyPLs-PLD, rSrAOX and POD as described in "Materials and methods" section. Physiological saline (open circles) and serum (closed circles) supplemented with known concentrations of PlsEtn 18-20:4 were used as samples for generating these standard curves. The regression lines shown were defined by the equations y = 2.28 × 10−3 × −0.0547; R 2 = 0.999 (open circles) and y = 2.10 × 10−3 × +0.165; R 2 = 0.996 (closed circles). b PlsEtn concentrations in forty-seven serum samples were measured using the enzymatic assay described in this study and also by the 125I-HPLC method. The regression line shown was defined by the equation y = 0.781 × +16.772; r = 0.7357
Pls concentration in human serum is between ~0.1 and 0.3 mM and the content ratio of PlsCho to PlsEtn ranged from 0.5 to 1.5 (Maeba et al. 2007). At high PlsEtn concentrations, results obtained using this enzyme-based method were lower than those obtained using the HPLC method, suggesting that the enzyme probably did not completely hydrolyze the substrate PlsEtn. Thus, to obtain more accurate concentration of PlsEtn in the serum sample, it may be necessary to dilute the sample appropriately. Since the plasma concentration of PlsEtn is 50–100 μM, as assessed by 125I-HPLC method (Maeba et al. 2007; Nishimukai et al. 2014), we believe that the method described here is sufficiently sensitive for measuring PlsEtn concentration in plasma. It may be possible to implement this method for developing a high-throughput assay for measuring PlsEtn in crude blood samples. In a similar manner, one could also determine the plasma concentration of PlsCho by using COX in place of AOX.
We found a linear correlation (y = 0.781 × +16.772; r = 0.7357) between the PlsEtn concentrations obtained using the enzyme-based assay described in this study and PlsEtn concentrations determined using the 125I-HPLC method (Fig. 4b), suggesting that PlsEtn concentration measured using this enzyme-based assay method is, at least, as accurate as that measured using the HPLC method. The measured concentrations of PlsEtn in serum sample were 63.9 ± 18.4, 67.0 ± 16.5 and 66.6 ± 16.8 μM by the 125I-HPLC method, LC–MS method and our enzyme-based method, respectively. Although 125I-HPLC and LC–MS methods could determine Pls concentration in serum accurately, both of them require longer sample processing time (such as, preparation of serum, lipid extraction, and drying of sample under N2 etc.), expensive instrumentation and longer sample analysis time. By contrast, the enzyme method does not involve any complicated and time consuming step, and extensive analysis time, so that the concentration of Pls in large number of samples can be easily and rapidly determined.
Finally, we believe that our enzymatic method could be used in high-throughput assays for screening serum samples with altered Pls levels, even though human serum contains a mixture of phospholipids. This is the first report describing enzymatic measurement of Pls level in human serum. Thus, this method could be easily used for assessing Pls as a biomarker for important diseases such as Alzheimer-type dementia and arteriosclerosis, and thereby may help in detecting these diseases at an early stage. We are currently exploring the practical applications of our assay system as a diagnostic tool.
References
Kim JK, Chung GH, Rhee JS (1999) Concentration of bovine plasmalogen using phospholipase A1 in two-phase system. Biotechnol Tech 13:589–593
Maeba R, Ueta N (2004) Determination of choline and ethanolamine plasmalogens in human plasma by HPLC using radioactive triiodide (1-) ion (125I3-). Anal Biochem 331:169–176
Maeba R, Maeda T, Kinoshita M, Takao K, Takenaka H, Kusano J, Yoshimura N, Takeoka Y, Yasuda D, Okazaki T (2007) Plasmalogens in human serum positively correlate with high-density lipoprotein and decrease with aging. J Atherosclero Thromb 14:12–18
Maeba R, Yamazaki Y, Nezu T, Okazaki T (2012) Improvement and validation of 125I-high-performance liquid chromatography method for determination of total human serum choline and ethanolamine plasmalogens. Ann Clin Biochem 49:86–93
Matsumoto Y, Mineta S, Murayama K, Sugimori D (2013) A novel phospholipase B from Streptomyces sp. NA684: purification, characterization, gene cloning, extracellular production, and prediction of the catalytic residues. FEBS J 280:3780–3796
Murayama K, Kano K, Matsumoto Y, Sugimori D (2013) Crystal structure of phospholipase A1 from Streptomyces albidoflavus NA297. J Struct Biol 182:192–196
Nishimukai M, Maeba R, Ikuta A, Asakawa N, Kamiya K, Yamada S, Yokota T, Sakakibara M, Tsutsui H, Sakurai T, Takahashi Y, Hui S-P, Chiba H, Okazaki T, Hara H (2014) Serum choline plasmalogens—those with oleic acid in sn−2—are biomarkers for coronary artery disease. Clin Chim Acta 437:147–154
Sugimori D, Kano K, Matsumoto Y (2012) Purification, characterization, molecular cloning and extracellular production of a phospholipase A1 from Streptomyces albidoflavus NA297. FEBS Open BioL 2:318–327
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, Flax J, Goodenowe DB (2010) Circulating plasmalogen levels and Alzheimer disease assessment scale–cognitive scores in Alzheimer patients. J Psychiat Neurosci 35:59–62
Wu L-C, Pfeiffer DR, Calhoon EA, Madiai F, Marcucci G, Liu S, Jurkowitz MS (2011) Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen. J Biol Chem 286:24916–24930
Wykle RL, Schremmer JM (1974) A Lysophospholipase D pathway in the metabolism of ether-linked lipids in brain microsomes. J Biol Chem 249:1742–1746
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant Number 15K05557, a grant from the Adaptable and Seamless Technology Transfer Program through target-driven R&D, JST (Grant No. AS251Z00099P), a grant from Research for Promoting Technological Seeds” from Innovation Plaza Miyagi, Japan Science and Technology Agency, and also by a research grant funding from the Takahashi Industrial and Economic Research Foundation to D. S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
The study was approved by the institutional ethics committees of Tokyo Metropolitan Institute of Gerontology and Teikyo University School of Medicine. Sera from patients at Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology were collected after receiving their written informed consents.
Rights and permissions
About this article
Cite this article
Sakasegawa, Si., Maeba, R., Murayama, K. et al. Hydrolysis of plasmalogen by phospholipase A1 from Streptomyces albidoflavus for early detection of dementia and arteriosclerosis. Biotechnol Lett 38, 109–116 (2016). https://doi.org/10.1007/s10529-015-1955-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1955-5