Abstract
Objective
Bacillus subtilis BS2, which can produce tetramethylpyrazine (TTMP) from glucose, was engineered by knockout of the 2,3-butanediol (2,3-BD) dehydrogenase gene (bdhA) and then regulated through the addition of 2,3-BD to enhance the TTMP yield.
Results
The bdhA of B. subtilis BS2 was disrupted to construct a TTMP-producing strain termed BSA. In microaerobic flask fermentation, the BSA strain produced 27.8 g TTMP/l. This was 6 g/l higher than that produced by the initial strain. Compared with that in BS2, the maximum yield of acetoin, which is a TTMP precursor, also increased from 11.3 to 16.4 g/l in BSA. The TTMP production by BS2 was enhanced by 2,3-BD supplemented to the fermentation medium. The maximum TTMP and acetoin yields were improved from 21.8 to 29.7 g/l and from 11.3 to 15.4 g/l, respectively, as the 2,3-BD concentration increased from 0 to 3 g/l. Conversely, the yields did not increase when the 2,3-BD concentration in the matrix was ≥4 g/l.
Conclusions
This study provides valuable information to enhance the TTMP productivity of mutagenic strains through gene manipulation and fermentation optimization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetramethylpyrazine (TTMP) is a heterocyclic, nitrogen-containing compound responsible for different food flavors, such as nutty and roasty (Muller and Rappert 2010; Xiao et al. 2006). It is therefore used as a food additive to enhance flavor. TTMP is also an important aromatic compound in some Chinese liquors (Fan et al. 2007). Moreover, TTMP is a key component of Ligusticum chuanxiong Hort to cure cardiovascular and cerebrovascular diseases (Ai-ping et al. 1986). Pharmacological studies have confirmed that TTMP can dilate blood vessels, increase coronary blood flow, and inhibit platelet aggregation. TTMP serves as a platform compound commonly applied in many industries other than the food and beverage industry.
TTMP can be produced through chemical or biological synthesis (Amrani-Hemaimi et al. 1995; Zhu et al. 2010). Kosuge et al. (1962) provided the first evidence that microorganisms can synthesize pyrazines; showing that TTMP can be produced by Bacillus subtilis. TTMP in liquor mainly comes from microbial metabolism and not from the Maillard reaction (Yan et al. 2011). A newly proposed theory is related to the TTMP synthesis in Chinese liquor through solid-state fermentation with enzyme/thermal dynamic coupling catalysis to synthesize TTMP (Jianfeng and Yan 2014). Bacillus sp. can produce a high TTMP yield via the precursor acetoin by using an endogenous precursor screening strategy (Zhu et al. 2010). Compared with chemical synthetic and enzyme conversion methods, the microbial fermentation route provides several advantages, such as environmentally friendly and cost-effective processes.
Although B. subtilis has been engineered to produce acetoin it has yet to be developed to produce TTMP through gene modification. Our current interest focuses on the genetic alterations of carbon flux into the acetoin biosynthesis pathway by blocking the degradation and competing pathways; thus, acetoin can accumulate to enhance the TTMP yield. The final titer and TTMP yield from glucose of the engineered strain proved higher than those of the parent strain. This study also developed a novel method with 2,3-butanediol (2,3-BD) supplementation during fermentation to improve TTMP production at a low cost.
Materials and methods
Strains, plasmids, primers and medium
The strains, plasmids, and their relevant genotypes used in this study are listed in Supplementary Table 1. The PCR primers used in this study are listed in Supplementary Table 2. DNA was manipulated by using standard protocols. Escherichia coli was grown at 37 °C in lysogeny broth (LB) (composed of 10 g NaCl/l, 10 g tryptone/l, and 5 g yeast extract/l) supplemented with 100 μg ampicillin/ml to select positive E. coli transformants. In tetramethylpyrazine (TTMP) and acetoin fermentation, 70 g glucose/l was added to LB, and 5 ml supplement (1 mg glucose/ml) was added every 12 h. Furthermore, LB plates were supplemented with 100 μg filter-sterilized kanamycin/ml to select B. subtilis transformants harboring the KanMX gene. The solid media contained 20 g agar/l.
Construction of bdhA knockout mutants
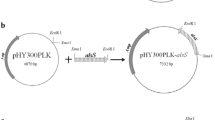
The oligonucleotides used to construct plasmids are listed in Supplementary Table 2. A series of plasmids was constructed (Fig. 1) to establish a genome integration cassette. First, a 423 bp promoter region of 2,3-BD dehydrogenase gene (bdhA) was amplified from the genomic DNA of B. subtilis BS2 by using the primer pair bdhA -AU/bdhA -AD. The PCR product was cut with EcoRI/KpnI, and then inserted into pUC19 (Invitrogen, China) cut with EcoRI/KpnI to yield pUC-bSA. Likewise, a 511 bp terminator region of bdhA was amplified through PCR from the genomic DNA of B. subtilis BS2 by using the primer pair bdhA -BU/bdhA -BD, cut with BamHI/HindIII, and inserted into pUC-bSA cut with BamHI/HindIII to yield the pUC-bSAB plasmid. Next, the kanamycin (Kan) resistance cassette was amplified from the pET28a plasmid by using the primer pair kan-up/kan-down. The PCR product with a size of approximately 1249 bp was purified, digested with BamHI/KpnI and inserted into pUC-bSAB cut with the same enzyme pair to yield pUC-bSABK.
TTMP production of the wild-type and mutant strains
A single colony of B. subtilis BS2 cells was transferred into 5 ml LB containing the corresponding antibiotic as required. Cultivations were performed at 37 °C. After 12 h shaking at 200 g, a 2 % (v/v) inoculum was added to 50 ml LB with 10 g glucose/l in a 250 ml shake-flask and cultivated for 12 h. This culture was inoculated at 4 % (v/v) into 200 ml LB with 70 g glucose/l in a 500 ml flask and shaken at 200 g for 8 days. The initial OD600 was ~0.05. Five ml of supplement (1 mg glucose/ml) was added every 12 h. pH was controlled at 7.5 by adding 10 M NaOH.
2,3-BD addition to the TTMP production
At the beginning of fermentation, 2,3-BD was added to the medium at initial concentrations of 1, 2, 3, 4 and 5 g/l.
Analytical methods
The biomass of the fermentation broth was determined from the OD600 value. Glucose concentration of the fermentation broth was determined by using a SBA-40C biosensor.
The TTMP concentration was determined through headspace solid-phase micro-extraction and GC-nitrogen, as reported previously. The concentrations of acetoin and 2,3-BD were determined by GC (Gao et al. 2014; Shi et al. 2014).
Results and discussion
Characterization of the bdhA-disrupted transformants
The 2,3-butanediol (2,3-BD) synthesis pathway was blocked by disrupting the 2,3-BD dehydrogenase gene (bdhA), and acetoin, which is the tetramethylpyrazine (TTMP) precursor (Fig. 2), can accumulate to increase the yield of TTMP. Compared with the acetoin yield of the initial strain (B. subtilis BS2), the maximum acetoin yield increased by 45 % (w/w) from 11.3 to 16.4 g/l; the maximum TTMP yield also increased by 27.5 % (w/w) from 21.8 to 27.8 g/l in the bdhA-disrupted mutant strain (B. subtilis BSA). By contrast, 2,3-BD production decreased by 72.4 % (w/w) (Fig. 3 a).
Tetramethylpyrazine (TTMP) biosynthetic pathway and other overflow metabolism pathways in Bacillus subtilis BS2. AlsS, ALDC, and bdhA encode acetolactate synthase, acetolactate decarboxylase, and 2,3-butanediol (2,3-BD) dehydrogenase, respectively. PPP Pentose phosphate pathway; TCA tricarboxylic acid cycle; NOD oxidative decarboxylation of non-enzymatic catalysis. Disrupted pathway steps are indicated by arrow breaks
TTMP, acetoin, and 2,3-BD concentrations of BS2, BSA, and 2,3-BD-regulated BS2 (BS2R3) cultivated with 200 ml of LB in 500 ml flask shaken at 200 g and 37 °C for 8 days. a Effects of bdhA disruption on TTMP, acetoin and 2,3-BD production by B. subtilis BSA. b Effects of 2,3-BD addition on TTMP, acetoin and 2,3-BD production by B. subtilis BS2
Compared with those in BS2, the TTMP and acetoin production in the BSA strain increased by 27.5 % (w/w) and 45 % (w/w), respectively, when the strains were cultivated for 168 h and 144 h, respectively (Fig. 3a); this increase was mainly due to (i) the abolished 2,3-BD production in the early stationary phase and (ii) the accumulated precursor acetoin in the early stationary phase. BS2 accumulated more than 9 g 2,3-BD/l when cultivated for 72 h, and the BSA strain accumulated <3 g 2,3-BD/l when cultivated for 72 h in the medium with 1 g residual glucose/l. The amount of 2,3-BD decreased in the stationary phase (Fig. 3a). This result is consistent with that described in a previous study, which suggested the degradation pathway of 2,3-BD via acetoin as an intermediate (Thanh et al. 2010).
The amount of acetoin accumulated by BS2 decreased at 120 h (Fig. 3a) because of acetoin degradation. Acetoin is mainly used as a precursor of TTMP or as a carbon source for growth (Huang et al. 1999). A high acetoin concentration accumulates in the BSA strain; as a result, a high TTMP concentration is produced; nevertheless, acetoin is metabolized more slowly by BSA than by BS2 (Silbersack et al. 2006).
Effect of 2,3-BD supplemented to the medium
The effect of 2,3-BD on the TTMP and acetoin production by BS2 was investigated by adding 1, 2, 3, 4, and 5 g/l of 2,3-BD to the medium. TTMP production was affected by 2,3-BD supplemented to the fermentation medium. The TTMP and acetoin production improved when 1–3 g/l of 2,3-BD was added to the culture medium; by contrast, this supplementation slightly influenced cell growth (Table 1), residual glucose concentration, and 2,3-BD production by BS2 (Fig. 4). TTMP increased as the 2,3-BD dosage increased, but no increase was observed when the supplemented 2,3-BD in matrix was ≥4 g/l (Table 1). Compared with those of BS2 (no dosage), the maximum TTMP and acetoin yields increased by 36.2 % (w/w) and 36.3 % (w/w) in BS2R3 (BS2 with 3 g 2,3-BD/l), respectively; this increase was observed when the strains were cultivated for 144 h and 168 h, respectively (Fig. 3b). In general, 3 g 2,3-BD/l gives the maximum production.
The TTMP and acetoin yields improved from 21.8 to 29.7 g/l and from 11.3 to 15.4 g/l, respectively, as the 2,3-BD dosage increased from 0 to 3 g/l. Thus, 2,3-BD can dose-dependently promote the TTMP and acetoin yields. The maximum TTMP and acetoin yields were reached at 3 g 2,3-BD/l in the culture medium. These results suggested that the methodology is a cost-effective option for the TTMP and acetoin production. Nevertheless, the mechanism by which acetoin synthesis is improved by 2,3-BD supplementation is poorly understood. AR/BDH likely catalyzes the conversion between AC and BD (Juni and Heym 1956); the initial 2,3-BD causes feedback regulation to catalyze the conversion from BD to AC; as a result, the concentration of AC increases and the concentration of BD decreases (Fig. 3b). After 2,3-BD was added, BS2 accumulates a high concentration of acetoin and produces a high amount of TTMP (Fig. 3b); 2,3-BD is slightly conducive to cell growth (Fig. 4). Enzyme activities reach the peak in the presence of high carbohydrate concentrations and rapidly decrease after carbon sources are depleted (Skory 2000); thus, the initial suppression on AR/BDH by 2,3-BD causes a very low activity and impedes the synthesis of 2,3-BD when sugars are almost completely exhausted at the end of fermentation. Furthermore, 2,3-BD can be used as a carbon source to generate acetoin when the initial concentration of the carbon source in the medium is low (Zhang et al. 2011). When 2,3-BD is added at an inhibitory concentration, cell growth is adversely affected and product synthesis is inhibited (Table 1). However, the inhibitory mechanism of 2,3-BD remain unclear. This mechanism is a possible result of enzymes activation or inhibition by 2,3-BD during synthesis; this mechanism is also a possible consequence of the transfer induced by different enzymes. Therefore, the inhibitory mechanism of 2,3-BD should be further investigated.
Conclusion
An engineered BSA strain giving a high yield of tetramethylpyrazine (TTMP) was constructed. The alteration of carbon flux into the acetoin biosynthetic pathway was demonstrated as a direct and effective strategy to enhance the TTMP yield. Acetoin catabolic and competing pathways were blocked by disrupting the 2,3-butanediol (2,3-BD) dehydrogenase gene (bdhA), which abolished 2,3-BD production and accumulated the precursor acetoin accumulation in the early stationary phase. In flask fermentation, the TTMP and acetoin yields respectively increased by 27.5 % (w/w) and 45 % (w/w) in BSA compared with BS2. Different concentrations of 2,3-BD were supplemented to the fermentation medium to improve the TTMP and acetoin production by BS2. The supplementation of 2,3-BD to the substrate is a novel and efficient method to improve the TTMP and acetoin production. Our results showed that the TTMP and acetoin yields respectively increased by 36.2 % (w/w) and 36.3 % (w/w) acetoin in BSA compared with BS2. Nevertheless, the proposed mechanism should be further investigated.
References
Ai-ping Z, Di-xun W, Feng W (1986) Effect of tetramethylpyrazine on acute and chronic hypoxic pulmonary hypertension of the rat. J Tongji Med Univ 6:133–137
Amrani-Hemaimi M, Cerny C, Fay LB (1995) Mechanisms of formation of alkylpyrazines in the maillard reaction. J Agric Food Chem 43:2818–2822
Fan W, Xu Y, Zhang Y (2007) Characterization of pyrazines in some Chinese liquors and their approximate concentrations. J Agric Food Chem 55:9956–9962
Gao S, Guo W, Shi L, Yu Y, Zhang C, Yang H (2014) Characterization of acetoin production in a budC gene disrupted mutant of Serratia marcescens G12. J Ind Microbiol Biotechnol 41:1267–1274
Huang M, Oppermann-Sanio FB, Steinbuchel A (1999) Biochemical and molecular characterization of the Bacillus subtilis acetoin catabolic pathway. J Bacteriol 181:3837–3841
Jianfeng W, Yan X (2014) Formation mechanism of tetramethylpyrazine produced with B. subtilis S12 under the fermentation condition simulated bacterial qu preparation used for Chinese liquor brewing. J Food Sci Biotechnol 33
Juni E, Heym GA (1956) A cyclic pathway for the bacterial dissimilation of 2,3-butanediol, acetylmethyl carbinol and diacetyl II. The synthesis of diacetylmethylcarbinol from diacetyl, a new diphosphothiamin catalyzed reaction. J Bacteriol 72:746–753
Kosuge T, Adachi T, Kamiya H (1962) Isolation of tetramethylpyrazine from culture of bacillus natto, and biosynthetic pathways of tetramethylpyrazine. Nature 195:1103
Muller R, Rappert S (2010) Pyrazines: occurrence, formation and biodegradation. Appl Microbiol Biotechnol 85:1315–1320
Shi L, Gao S, Yu Y, Yang H (2014) Microbial production of 2,3-butanediol by a newly-isolated strain of Serratia marcescens. Biotechnol Lett 36:969–973
Silbersack J, Jürgen B, Hecker M, Schneidinger B, Schmuck R, Schweder T (2006) An acetoin-regulated expression system of Bacillus subtilis. Appl Microbiol Biotechnol 73:895–903
Skory CD (2000) Isolation and expression of lactate dehydrogenase genes from Rhizopus oryzae. Appl Environ Microbiol 66:2343–2348
Thanh T, Jurgen B, Bauch M, Liebeke M, Lalk M, Ehrenreich A, Evers S, Maurer K-H, Antelmann H, Ernst F, Homuth G, Hecker M, Schweder T (2010) Regulation of acetoin and 2,3-butanediol utilization in Bacillus licheniformis. Appl Microbiol Biotechnol 87:2227–2235
Xiao ZJ, Xie NZ, Liu PH, Hua DL, Xu P (2006) Tetramethylpyrazine production from glucose by a newly isolated Bacillus mutant. Appl Microbiol Biotechnol 73:512–518
Yan X, Qun W, Wenlai F, Bingfeng Z (2011) The discovery & verification of the production pathway of Tetramethylpyrazine (TTMP) in chiese liquor. Liquor-Making Sci Technol 7:37–40
Zhang X, Yang T, Lin Q, Xu M, Xia H, Xu Z, Li H, Rao Z (2011) Isolation and identification of an acetoin high production bacterium that can reverse transform 2,3-butanediol to acetoin at the decline phase of fermentation. World J Microbiol Biotechnol 27:2785–2790
Zhu B-F, Xu Y, Fan W-L (2010) High-yield fermentative preparation of tetramethylpyrazine by Bacillus sp. using an endogenous precursor approach. J Ind Microbiol Biotechnol 37:179–186
Acknowledgments
This work was supported by National High Technology Research and Development Program of China (863 Program) (Grant No. 2012AA022108) and The Science and Technology Development Plan Project of Shan dong Province (Grant No. 2014GSF121008).
Supporting information
Supplementary Table 1—Bacterial strains and plasmids used.
Supplementary Table 2—Primers used.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, W., Wang, R. & Xiao, D. Metabolic engineering of Bacillus subtilis to enhance the production of tetramethylpyrazine. Biotechnol Lett 37, 2475–2480 (2015). https://doi.org/10.1007/s10529-015-1950-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1950-x