Abstract
The modification of N6-methyladenosine (m6A), primarily orchestrated by the reader protein YTHDF1, is a pivotal element in the post-transcriptional regulation of genes. While its role in various biological processes is well-documented, the specific impact of m6A-YTHDF1 on the regulation of GRIN2D, a gene implicated in cancer biology, particularly in the context of bladder cancer, is not thoroughly understood. Utilizing a series of bioinformatics analyses and experimental approaches, including cell culture, transfection, RT-qPCR, and western blotting, we investigated the m6A modification landscape in bladder cancer cells. The relationship between m6A-YTHDF1 and GRIN2D expression was examined, followed by functional assays to assess their roles in cancer progression and glycolytic activity. Our analysis identified a significant upregulation of m6A modification in bladder cancer tissues. YTHDF1 was found to regulate GRIN2D expression positively. Functionally, GRIN2D was implicated in promoting bladder cancer cell proliferation and enhancing aerobic glycolysis. Inhibition of the m6A-YTHDF1-GRIN2D axis resulted in the suppression of cancer progression and metabolic alterations. Through this research, we have elucidated the significant influence of the m6A-YTHDF1 axis on the modulation of GRIN2D expression, which in turn markedly impacts the progression of bladder cancer and its metabolic pathways, particularly aerobic glycolysis. Our findings uncover critical molecular dynamics within bladder cancer cells, offering a deeper understanding of its pathophysiology. Furthermore, the insights gained from this study underscore the potential of targeting the m6A-YTHDF1-GRIN2D pathway for the development of innovative therapeutic strategies in the treatment of bladder cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer, a common yet complex urinary tract malignancy, carries a significantly dire prognosis in its muscle-invasive form. Definitive therapeutic options (including surgery, radiotherapy and chemotherapy), though intended to be curative, often come with significant recurrence and can profoundly impact the quality of life. This understandably leads to patient reluctance towards such interventions (Gild et al. 2019). This stark reality accentuates the pressing need to delve into the molecular underpinnings of the disease and to identify viable targets for therapeutic intervention.
To identify potential targets that could contribute to the progression of bladder cancer, an in-depth analysis was performed comparing gene expression in bladder cancer tissues to adjacent non-tumor samples, utilizing high-throughput sequencing from GEO datasets (GSE236932, GSE190079, GSE130598). The NMDA receptor, a glutamate-gated ion channel critical for neural signaling, has been implicated in various malignancies, including bladder cancer, highlighting its potential as a therapeutic target (Chen et al. 2023a; Gallo et al. 2023; Wang et al. 2023). Notably, GRIN2D, a subunit of the NMDA receptor, has been identified as overexpressed in several cancers and linked to enhanced glycolysis, an attribute essential for cancer cell survival and proliferation, providing a promising avenue for future therapeutic strategies (Deutsch et al. 2014).
Aerobic glycolysis, the preferred metabolic pathway in cancer cells, contributes significantly to an immunosuppressive tumor microenvironment. The Warburg effect, characterized by increased glucose uptake and lactate production, is known to modulate the behavior of tumor-associated macrophages. It drives macrophages towards a phenotype that not only suppresses T-cell activation but also promotes tumor progression. In this milieu, GRIN2D emerges as a gene of interest due to its role in cancer cell metabolism and potential influence on the tumor microenvironment (Pham et al. 2019; Zhong et al. 2023). Given these insights, the role of GRIN2D in bladder cancer merits further investigation. Modulating GRIN2D could alter the metabolic reprogramming of cancer cells, potentially reviving the effectiveness of immune responses and leading to stronger immunotherapeutic strategies (Ferguson, et al. 2016; Sánchez Lázaro et al. 2020; Zhang et al. 2020). Thus, we posit that GRIN2D could serve as a prospective target gene in bladder cancer, potentially playing a role in the facilitation of aerobic glycolysis.
Recent advances in the field of oncology have unveiled the intricate relationship between epigenetic modifications and cancer progression. Central to this development is the N6-methyladenosine (m6A) modification, which has been recognized as a predominant RNA modification mechanism in mammalian systems, influencing various aspects of RNA metabolism, including stability, splicing, and translation (Xiong et al. 2022; You et al. 2023). This modification is dynamically regulated by ‘writers,’ ‘erasers,’ and ‘readers’ that add, remove, or interpret the m6A mark, respectively. In particular, the m6A ‘reader’ YTHDF1 and 'eraser' RBM15 have been identified as upregulated in a range of cancers, suggesting a role in oncogenesis and potential as prognostic biomarkers (Wang et al. 2021; Zhang et al. 2022a). The overexpression of m6A ‘reader’ YTHDF1 and ‘eraser’ RBM15 are correlated with diverse cancer outcomes and responses to immunotherapy, positing them as pan-cancer biomarkers that could be pivotal in the prognosis and therapeutic targeting of malignancies (Hu et al. 2021; Zhu et al. 2023a). The roles of RBM15 and YTHDF1 in bladder cancer are gaining interest due to their involvement in m6A RNA modification. While the specific role of RBM15 in bladder cancer is not fully established, studies have shown its overexpression in various cancers correlates with poor prognosis, suggesting a potential similar impact in bladder cancer (Zhao et al. 2022). YTHDF1, known for promoting bladder cancer cell proliferation via specific molecular pathways, has been linked to increased cancer cell growth and drug resistance (Zhu et al. 2023a; Chen et al. 2023b). These findings highlight the importance of RBM15 and YTHDF1 in bladder cancer research, underscoring their potential as targets for therapy and prognosis.
Based on the interaction of m6A RNA modification and its regulatory proteins, we hypothesized that the transcription and translation of GRIN2D were regulated by m6A modification, thus promoting the development of bladder cancer and aerobic glycolysis, not only promoting the development of bladder cancer, but also participating in aerobic glycolytic pathway, which is the key feature of cancer cell metabolism.
Methods and Materials
Clinical Sample Collection
In this study, a total of 50 clinical specimens were gathered. Stringent adherence to established clinical ethical principles and the acquisition of informed consent from all participants were ensured prior to sample acquisition. Participants received comprehensive information regarding the nature and objectives of the research. Sample inclusion and exclusion criteria were scrupulously defined, encompassing factors such as prior pharmacological interventions, ensuring the selection of a homogeneous cohort. Detailed records were maintained regarding the medical history of the participants, including medication history. The collected clinical samples were meticulously preserved under controlled conditions to maintain their integrity and stability. This includes maintaining a constant temperature of − 80 °C in ultra-low temperature freezers and using cryogenic vials specifically designed for long-term biostorage, ensuring the optimal preservation of sample quality.
Bladder Cell Culture
Following the procurement process, bladder cancer cell lines T24 (Company: ATCC, Product No: HTB-4) and UMUC3 (Company: ATCC, Product No: CRL-1749) were acquired for the study. The cell was subsequently resuspended in DMEM Medium supplemented with FBS (Gibco, Cat#: 26140079) and Penicillin–Streptomycin (Gibco, Cat#: 15140122). The bladder cells were then seeded onto collagen-coated plates at a density determined via a hemocytometer. These cultures were maintained in a 37 °C incubator with a 5% CO2 atmosphere. Medium changes were performed every 2–3 days to provide essential nutrients and eliminate waste products. Cell health and morphology were routinely monitored under a phase-contrast microscope.
Transfection of Bladder Cells
For the transfection of bladder cells with sh-GRIN2D#1 and sh-GRIN2D#2, were synthesized by Shanghai GenePharma Co., Ltd. The following protocol was employed: Cells were grown to 70–80% confluence in collagen-coated plates. Prior to transfection, the medium was replaced with antibiotic-free DMEM Medium supplemented with FBS (Gibco, Cat#: 26140079). Transfection was performed using Lipofectamine 3000 Reagent (Invitrogen, Cat#: L3000015) according to the manufacturer’s instructions. Briefly, sh-GRIN2D#1 and sh-GRIN2D#2 were diluted in Opti-MEM I Reduced Serum Medium (Gibco, Cat#: 31985070). In parallel, Lipofectamine 3000 was also diluted in Opti-MEM I Reduced Serum Medium. After a 5 min incubation at room temperature, the diluted sh-GRIN2D#1/sh-GRIN2D#2 was combined with the diluted Lipofectamine 3000. Following a 20 min incubation to allow complex formation, the mixture was added to the cells. The cells were then returned to the 37 °C incubator with a 5% CO2 atmosphere. After 6 h, the transfection medium was replaced with the regular DMEM medium supplemented with FBS and Penicillin–Streptomycin. The transfected cells were further incubated for 48–72 h prior to downstream applications. Transfection efficiency was evaluated by monitoring the knockdown of the GRIN2D gene at the mRNA and protein levels.
RNA Isolation and RT-qPCR
RNA was extracted from the transfected bladder cells using the RNeasy Mini Kit (Qiagen, Cat#: 74104) according to the manufacturer’s instructions. The concentration and purity of the extracted RNA were determined using a NanoDrop spectrophotometer. For cDNA synthesis, 1 µg of total RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Cat#: 4368814) following the manufacturer’s protocol. Quantitative PCR (qPCR) was performed using the PowerUp SYBR Green Master Mix (Applied Biosystems, Cat#: A25742) on a QuantStudio 5 Real-Time PCR System (Applied Biosystems). The specific primers for GRIN2D and the housekeeping gene GAPDH (specific product numbers to be inserted) were used. The relative expression levels of GRIN2D were calculated using the 2^-ΔΔCT method, with β-actin serving as the internal control.
Cell Counting Kit-8 (CCK8)
To determine the viability of cells, we conducted the CCK8 assay. The HT22 cells were placed in a 96-well plate with a density of 5000 cells per well and were permitted to attach overnight. After the necessary treatment, the cells were exposed to CCK-8 reagent (CK04, Dojindo Molecular Technologies) diluted in a complete growth medium at a final concentration of 10% for 2 h at 37 °C. Using a microplate reader (SpectraMax M5, Molecular Devices), the absorbance was measured at 450 nm while subtracting the background absorbance at 650 nm from the readings. To calculate the cell viability, the absorbance of treated cells was compared to that of control cells. The experiment was carried out in triplicate, and the results were presented as mean ± standard deviation.
Clonogenic Cell Survival Assay
Cells were seeded in a 96-well plate at a density of 5000 cells per well. After incubation for 24 h, the medium was replaced with fresh medium containing various concentrations of the test compound. After incubation for 24–72 h, the medium was removed, and the cells were washed with PBS. The cells were then fixed with methanol and stained with crystal violet. Colonies were counted using a microscope.
Transwell Assay
The Transwell migration assay was performed to assess cell migration. Cells were seeded on the upper chamber of a Transwell insert (8 µm, 24-well format, product number ECM508, MilliporeSigma) at a density of 5000 cells per well and allowed to attach overnight. The lower chamber was filled with medium containing the chemoattractant. After incubation for the appropriate time, the cells that had migrated to the lower side of the membrane were stained and counted. The absorbance was measured at 450 nm using a microplate reader (SpectraMax M5, Molecular Devices), with the background absorbance at 650 nm subtracted from the readings. The cell migration rate was calculated by comparing the absorbance of the migrated cells to that of the control cells.
Western Blot
Proteins were extracted from the cells and quantified. Equal amounts of protein were separated by SDS-PAGE and transferred to a PVDF membrane. The membrane was blocked to prevent non-specific binding and then incubated with primary antibodies. After washing, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody. The signal was developed using an enhanced chemiluminescence (ECL) substrate, and the bands were visualized and quantified using a chemiluminescence imaging system. Antibody information is shown in Table 1.
Extracellular Acidification Rate Analyzer
The Seahorse XFe96 Extracellular Flux Analyzer (Agilent Technologies) was used to analyze the extracellular acidification rate (ECAR). Cells were seeded in the assay wells of a custom 96-well XF microplate at a density typically between 0.5 × 104 and 4 × 104 cells per well. The Seahorse XFe96 Analyzer measures the oxygen consumption rate (OCR) and ECAR of live cells in a multiwell plate, interrogating key cellular functions such as mitochondrial respiration and glycolysis. The ECAR is measured in real-time, providing a direct assessment of glycolysis. The ECAR is an indicator of the rate at which the cells are producing and exporting protons, which is directly related to the rate of glycolysis.
MeRIP-qPCR
The MeRIP-qPCR method was used to detect and quantify m6A modification in specific genes. Total RNA was extracted from the cells and chemically fragmented into 100 nucleotides or smaller fragments. The fragmented RNA was then subjected to immunoprecipitation using a monoclonal antibody against m6A, which was included in the Magna MeRIP m6A Kit (Catalog No. 17-10499, MilliporeSigma). Following immunoprecipitation, the isolated RNA fragments were reverse transcribed into cDNA. The cDNA was then used as a template for qPCR, with primers designed specifically for the genes of interest. The RT-qPCR was performed in triplicate, and the Ct values were used to calculate the level of m6A modification in each gene.
Bioinformatics Analysis
GSE236932, GSE190079 and GSE130598 were downloaded from the Gene Expression Comprehensive Database (GEO, https://www.ncbi.nlm.nih.gov/geo/). The differential genes were analyzed by R Limma software package, and the volcano map and Venn map were made by R language.
Quantification of m6A RNA Methylation
The EpiQuik m6A RNA Methylation Quantification Kit (Colorimetric) (Product No: P-9005-48, EpigenTek) was utilized for quantifying m6A modifications in RNA. Initially, total RNA was extracted from the cells and chemically fragmented into fragments of 100 nucleotides or smaller. The fragmented RNA was then subjected to immunoprecipitation using the monoclonal antibody against m6A included in the kit. Following immunoprecipitation, the isolated RNA fragments were reverse transcribed into cDNA. The cDNA was then used as a template for RT-qPCR, with primers designed specifically for the genes of interest. Each RT-qPCR was performed in triplicate, and the Ct values were used to calculate the level of m6A modification in each gene.
Statistical Analysis
The experiments were conducted three times in each cell line, and the results are presented as the mean and standard deviation of three distinct experiments. Statistical analyses were performed using GraphPad Prism 5 software from La Jolla, California, U.S.A. One-way analysis of variance, followed by Dunnett’s test, was used to determine the significance relative to the control.
Results
Identification and Validation of GRIN2D Expression Level for Bladder Cancer Progression
Grounded in the current understanding that dysregulation of gene expression plays a pivotal role in cancer progression, as substantiated by various studies (Hai et al. 2021; Zboril et al. 2021), we hypothesized that certain genes would be differentially expressed in bladder cancer tissues, thereby contributing to the malignancy's advancement.
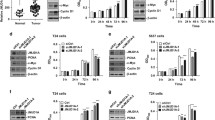
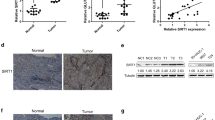
Leveraging extensive gene expression datasets from the GEO repository specifically aimed at sequencing analysis of bladder cancer, we conducted a comprehensive differential expression analysis. Our analysis utilized datasets GSE236932, GSE190079, and GSE130598. The initial examination of GSE236932 revealed a differential expression pattern involving 38 genes, markedly categorized into upregulated and downregulated groups. In the case of GSE190079, a significant differential expression was noted in 2491 genes, while GSE130598 exhibited this pattern in 186 genes. Both of these datasets demonstrated a clear distinction between genes that were upregulated and those that were downregulated, underscoring the complexity and heterogeneity in bladder cancer gene expression profiles. This initial exploration provided a panoramic view of the gene expression alterations in bladder cancer and was encapsulated in the volcanic plots (Fig. 1A). To distill this information further, we applied a Venn diagram analytical approach, overlaying the differentially expressed genes from the three datasets. This intersectional analysis identified two genes, GRIN2D and FAM83A, as shared candidates across the datasets, with GRIN2D being consistently upregulated (Fig. 1B). The emergence of GRIN2D as a commonality in gene expression elevation across diverse data sets underscored its potential significance in the pathogenesis of bladder cancer. The elevation of GRIN2D was further scrutinized through quantitative PCR and Western Blot assays in a cohort consisting of Normal and Tumor tissue samples (n = 50). These methodologies confirmed an increased expression of GRIN2D in tumor samples, suggesting a possible role in tumorigenesis (Fig. 1C). We extended this examination to a cellular model, comparing GRIN2D expression across different bladder cancer cell lines: SV-HUC-1, UMUC3, T24, and EJ. The analyses yielded results that were in alignment with our previous findings, with a marked upregulation of GRIN2D in T24 and UMUC3 cell lines, corroborating its potential as a biomarker for bladder cancer (Fig. 1D). Furthermore, we quantified GRIN2D expression levels across the different cell lines. The results were unequivocal, showing a marked upsurge in GRIN2D expression particularly in the T24 and UMUC3 cell lines. This heightened expression aligns with the aggressive phenotypes commonly associated with these cell lines and indicates the gene's potential involvement in tumor aggressiveness and possibly in the mechanisms of metastasis (Fig. 1E and F). The expression analysis of GRIN2D in bladder cancer reveals consistent upregulation in both tumor tissues and various bladder cancer cell lines.
Comparative gene expression analysis and identification of differentially expressed genes in bladder cancer versus adjacent non-cancerous tissues. A Volcano plots displaying differential gene expression and Western blot showing protein levels between bladder cancer tissues and adjacent non-cancerous tissues from the GSE236932 dataset. B Venn diagram illustrating the overlap of differentially expressed genes identified in three independent GEO datasets. C Quantitative PCR analysis of GRIN2D expression in normal and tumor tissue samples from a cohort of 50 patients. D Western blot analysis confirming the differential expression of GRIN2D protein in bladder cancer cell lines compared to control. *P < 0.05, **P < 0.01, ***P < 0.001
GRIN2D Knockdown Impairs Bladder Cancer Cell Proliferation, Migration, and Aerobic Glycolysis
Building on our previous findings that GRIN2D is upregulated in bladder cancer, we further investigated the functional consequences of GRIN2D knockdown on bladder cancer cell proliferation, migration, and aerobic glycolysis. In our experimental design, we transfected T24 and UMUC3 bladder cancer cell lines with two different shRNA constructs targeting GRIN2D (sh-GRIN2D#1 and sh-GRIN2D#2) and included a non-targeting control (sh-NC). Initial qPCR analysis confirmed the knockdown efficiency, with sh-GRIN2D#1 exhibiting a more pronounced reduction in GRIN2D expression. Subsequently, Western blot analysis was performed to verify the knockdown at the protein level, demonstrating that both sh-GRIN2D constructs effectively reduced GRIN2D protein expression (Fig. 2A). This knockdown construct was then selected for further experimentation (Fig. 2A). To assess the impact of GRIN2D silencing on cell viability, we performed CCK-8 assays. The results indicated a significant suppression of cell viability in the sh-GRIN2D group compared to the sh-NC group, suggesting that GRIN2D plays a role in supporting the proliferative capacity of bladder cancer cells (Fig. 2B). Consistent with the CCK-8 results, the number of colonies formed by the sh-GRIN2D transfectants was substantially lower than those in the negative control and control groups, highlighting the growth-inhibitory effect of GRIN2D knockdown (Fig. 2C). Transwell assays revealed that bladder cancer cells with GRIN2D knockdown (sh-GRIN2D) exhibited significantly reduced migration compared to controls, suggesting a crucial role of GRIN2D in facilitating cell migration in bladder cancer (Fig. 2D). Lastly, we investigated the effect of GRIN2D silencing on the metabolic profile of bladder cancer cells by analyzing the extracellular acidification rate (ECAR) using the Seahorse XFe96 Extracellular Flux Analyzer. The sh-GRIN2D group exhibited a significant decrease in ECAR, indicative of impaired glycolytic function. This finding suggests that GRIN2D may be involved in the metabolic reprogramming of bladder cancer cells to support their high proliferative and invasive behaviors (Fig. 2E).
Suppression of bladder cancer cell proliferation and metabolic activity by GRIN2D knockdown. A PCR and Western blot were used to analyze the expression of GRIN2D in sh-GRIN2D transfected cells. B Cell viability assessment using CCK-8 assays in bladder cancer cell lines following GRIN2D knockdown. C Quantitative representation of colony formation ability in bladder cancer cells post GRIN2D silencing. D Transwell migration assays depicting the invasive potential of bladder cancer cells after GRIN2D knockdown. E ECAR measured using the Seahorse XFe96 Extracellular Flux Analyzer to evaluate metabolic changes following GRIN2D knockdown in bladder cancer cell lines. *P < 0.05, **P < 0.01, ***P < 0.001
Overall, the silencing of GRIN2D resulted in a pronounced reduction in bladder cancer cell proliferation, migration, and glycolytic activity, suggesting that GRIN2D is not merely a bystander in cancer progression but may actively contribute to the malignant phenotype of bladder cancer cells.
Interplay Between RBM15 and YTHDF1 Dictates m6A-Dependent Regulation of GRIN2D mRNA Stability in Bladder Cancer Pathogenesis
To provide a bioinformatic prediction of m6A methylation sites within the GRIN2D mRNA sequence, a confidence score distribution was drawn for predicted m6A sites along the GRIN2D mRNA sequence, with peaks representing sites of very high confidence where methylation is most likely to occur (Supplemental Fig. 1A). Supplemental Fig. 1B and C provide a detailed breakdown of these sites, listing their nucleotide positions and context within the sequence. Notably, several sites are highlighted with very high confidence scores, signaling strong candidates for methylation, and the highlighted positions further pinpoint these sites for subsequent empirical investigation. High scores, especially those labeled as “Very high confidence,” indicate strong potential for m6A modification at the corresponding nucleotide position. It is noteworthy that the positions marked in yellow highlight particularly significant sites that could be of interest for further functional studies.
Our analysis revealed that GRIN2D mRNA exhibited a significant m6A modification, with a tenfold increase observed (Fig. 3A). RBM15 modulation resulted in a pronounced change in these m6A levels: a decrease by nearly half with RBM15 knockdown (Fig. 3B) and a doubling with overexpression (Fig. 3C). Correspondingly, GRIN2D mRNA levels decreased by 60% upon RBM15 knockdown (Fig. 3D) and increased by 120% with overexpression. YTHDF1’s interaction with GRIN2D mRNA showed a 40% decrease in mRNA levels upon YTHDF1 knockdown (Fig. 3G) and a 150% increase with overexpression (Fig. 3H). Inhibition of YTHDF1 led to a 60% reduction in m6A levels on GRIN2D mRNA (Fig. 3I), while overexpression resulted in over a twofold increase (Fig. 3J). Disruption of RBM15 reduced YTHDF1’s binding to TMBIM6 mRNA by about 70% (Fig. 3K), indicating RBM15’s broader regulatory impact.
MeRIP assay to detect m6A modifications on GRIN2D mRNA. A MeRIP assay to detect m6A modifications on GRIN2D mRNA. B, C Immunoprecipitation of m6A-modified RNA followed by qRT-PCR to assess the effect of RBM15 expression levels on m6A modification of GRIN2D mRNA. D, E The effects of RBM15 knockdown and overexpression on RBM15 and GRIN2D mRNA levels. F RIP-qPCR to detect the enrichment of YTHDF1 binding at m6A-modified sites on GRIN2D mRNA. G, H Analysis of YTHDF1 and GRIN2D mRNA levels upon knockdown and overexpression of YTHDF1. I, J Immunoprecipitation and qRT-PCR analysis to evaluate the impact of YTHDF1 suppression and overexpression on m6A modifications of GRIN2D mRNA. K RIP-qPCR to investigate the potential interaction between RBM15 and the direct binding of YTHDF1 to TMBIM6 mRNA. *P < 0.05, **P < 0.01, ***P < 0.001
Upregulation of RBM15 and YTHDF1 in Bladder Cancer Tissues and Cells
m6A modification sites specifically recognized by RBM15 [GCAG] and YTHDF1 [GGAC], as identified in prior research (Wang et al. 2022), were found on the GRIN2D mRNA sequence, falling within the ‘Very high confidence’ category. The presence of these sites, along with reports of overexpression of RBM15 and YTHDF1 in various cancers, including bladder cancer, guided our further investigation into their potential regulatory interplay with GRIN2D in tumorigenesis (Supplemental Figs. 2B and C).
In the clinical assessment, we quantitatively assessed RBM15 and YTHDF1 in a set of 50 normal and tumor bladder tissue samples. The results demonstrated an upsurge in the levels of both proteins within the tumor tissues (Fig. 4A). This elevation in expression suggests a possible oncogenic role for RBM15 and YTHDF1 and posits them as potential contributors to the tumor microenvironment's pathology. Complementing the tissue-based findings, we explored the expression patterns in a cellular context. Data from bladder cancer cell lines, particularly the aggressive T24 and UMUC3 lines, showed a similar trend of upregulation for both RBM15 and YTHDF1 (Fig. 4B). The expression profiles in the less aggressive SV-HUC-1 and EJ cell lines (Figs. 4C and D) further substantiated these observations. Although RBM15 and YTHDF1 were overexpressed relative to normal controls, the magnitude of expression was comparatively lower than that observed in T24 and UMUC3 cells. These variations in expression levels across different cell lines may reflect a gradient of aggressiveness in bladder cancer phenotypes.
Elevated expression of RBM15 and YTHDF1 in bladder cancer tissues and cell lines. A Expression analysis of RBM15 and YTHDF1 in normal and tumor bladder tissue samples using quantitative PCR and Western blot. B Evaluation of RBM15 and YTHDF1 protein levels in aggressive bladder cancer cell lines T24 and UMUC3, compared to control cell lines. C qPCR assessment of RBM15 and YTHDF1 mRNA expression in less aggressive bladder cancer cell lines SV-HUC-1 and EJ. D The protein expression profiles of RBM15 and YTHDF1 across a panel of bladder cancer cell lines. **P < 0.01, ***P < 0.001
Reversal of GRIN2D Silencing-Induced Tumor Suppression by Overexpression of RBM15 or YTHDF1 in Bladder Cancer Cells
Next, to directly test our hypothesis, we conducted rescue experiments in the same cell lines by silencing GRIN2D and simultaneously overexpressing RBM15. This approach aimed to elucidate the interplay between GRIN2D suppression and the compensatory effects of RBM15 overexpression within the m6A pathway, particularly in relation to cancer cell viability, proliferation, and migration.
Upon shGRIN2D-mediated knockdown of GRIN2D, the expression levels of YTHDF1 and RBM15 remained unchanged. Conversely, co-transfection with shGRIN2D and overexpression vectors for YTHDF1 or RBM15 resulted in an upregulated expression of GRIN2D, suggesting a compensatory effect by these m6A-modifying proteins (Fig. 5A, B). Moreover, we determined the impact of YTHDF1 or RBM15 overexpression on cell proliferation independently (Supplemental Fig. 2A, B). Overexpression of YTHDF1 does not affect RBM15 expression and vice versa. Additionally, cell viability and proliferation assays demonstrate that cells overexpressing either YTHDF1 or RBM15 exhibit an increase in proliferation, suggesting that both proteins may contribute to cellular growth when overexpressed (Supplemental Fig. 2C, D). In the cell viability assays depicted in (Fig. 5C), T24 and UMUC3 cells transfected with sh-GRIN2D exhibited a notable reduction in cellular activity when compared to the sh-NC (non-targeting control) group. The subsequent overexpression of RBM15 or YTHDF1 in sh-GRIN2D-transfected cells demonstrated a reversal of this effect, with a measurable restoration of cell viability, suggesting that the loss of GRIN2D-induced suppression can be mitigated by these m6A-modifying proteins. In the Colony formation assay, where cells with silenced GRIN2D formed significantly fewer colonies than those in the control groups, indicating reduced cell proliferation (Fig. 5D). This suppressive effect on the colony count was counteracted by the overexpression of RBM15 or YTHDF1, which led to an increase in colony numbers, approaching those observed in the control groups. The cell migration assay results revealed that GRIN2D knockdown resulted in a decreased number of migrating cells, highlighting the inhibitory effect of GRIN2D silencing on cell motility. However, this reduction in migration was notably reversed upon overexpression of RBM15 or YTHDF1, as evidenced by the increased migratory cell counts, which approached or exceeded the levels seen in the sh-NC group (Fig. 5E). Lastly, the knockdown of GRIN2D led to a significant reduction in ECAR, indicative of impaired glycolytic function. This metabolic suppression was reversed with the overexpression of RBM15 or YTHDF1, which restored ECAR levels closer to those observed in control cells (Fig. 5F).
Analysis of GRIN2D, YTHDF1, and RBM15 in Bladder Cancer Cell Lines Following shRNA and Overexpression Treatments. A qPCR analysis for GRIN2D, YTHDF1, and RBM15 expression. B Western blot analysis for GRIN2D, YTHDF1, and RBM15 protein levels. C CCK-8 assay for cell viability. D Plate colony formation assay for cell proliferation. E Transwell assay for cell migration. F Seahorse XFe96 analysis of ECAR. *P < 0.05, **P < 0.01, ***P < 0.001
In addition, RBM15 knockdown significantly decreases its own and GRIN2D expression levels in both T24 and UMUC3 cell lines, while the expression of YTHDF1 remains unaffected (Supplemental Fig. 3A). The compensatory effect of GRIN2D is evident when its overexpression in the RBM15-silenced cells restores its levels, suggesting a possible feedback mechanism that does not involve YTHDF1. Cell proliferation assays, reflected by the CCK-8 assay over a 72 h period (Supplemental Fig. 3B), and colony formation assays show a clear decrease in cell growth following RBM15 silencing. However, overexpression of GRIN2D in RBM15-silenced cells reverses this suppressive effect, bringing the proliferation rates back to baseline, which underscores the pivotal role of GRIN2D in cell proliferation despite RBM15 knockdown (Supplemental Fig. 3C, D). Furthermore, metabolic assays depicted reveal a marked reduction in glycolysis upon RBM15 silencing, as evidenced by the lower extracellular acidification rates (ECAR). Overexpression of GRIN2D in RBM15-deficient cells not only restores glycolysis but also suggests that GRIN2D may compensate for the loss of RBM15, indicating a regulatory role in cellular energy metabolism (Supplemental Fig. 3E).
To summarize, the quantitative changes observed across the assays provide a compelling narrative that the tumor-suppressive effects of GRIN2D knockdown, spanning from reduced cell proliferation and migration to impaired glycolysis, can be effectively reversed by the upregulation of either RBM15 or YTHDF1.
Discussion
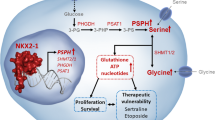
Bladder cancer research is evolving with a focus on the heterogeneity and the development of molecular subtypes to predict chemotherapy response and survival. Muscle-invasive bladder cancers (MIBCs) are molecularly diverse, with subtypes showing different clinical courses and chemotherapy sensitivities (Choi et al. 2014; Helal et al. 2023). These studies are pivotal in the current understanding of bladder cancer, providing a backdrop against which the findings of this study are particularly salient. Our investigation has contributed to this landscape by demonstrating the upregulation of m6A methylation regulators in bladder cancer, a novel layer of complexity in its molecular pathology. The differential expression of GRIN2D and its regulation by RBM15 and YTHDF1 (Fig. 1), the functional impact of GRIN2D silencing on cancer cell phenotypes (Fig. 2), and the intricate control of GRIN2D mRNA stability via m6A modifications (Fig. 3) together refine the molecular character of bladder cancer. These findings could inform future therapeutic strategies, targeting the m6A methylation pathway to counteract the aggressive traits of bladder cancer cells (Figs. 4 and 5).
The upregulation of GRIN2D in bladder cancer, as evidenced in our study, aligns with its emerging role in various cancer types. For instance, in pancreatic ductal adenocarcinoma (PDAC), GRIN2D has been identified as a novel therapeutic target, underscoring its potential in promoting tumor growth and liver metastasis. This is particularly relevant given the limited effective therapies for PDAC, a disease driven by genetic aberrations such as KRAS, TP53, CDKN2A, and SMAD4 mutations (Ferguson et al. 2016; Wang et al. 2023). In colorectal cancer (CRC), GRIN2D expression is specific to tumor vessels, as demonstrated by RTqPCR and IHC analysis, and its expression correlates with improved survival outcomes. This suggests a role for GRIN2D in endothelial function and angiogenesis, key processes in tumor development and metastasis (Zhang et al. 2022b). Similarly, in liver cancer, GRIN2D, along with other neural-related genes, has been proposed as a potential biomarker for prognosis and immune response, indicating its involvement in the nerve-cancer crosstalk and immune infiltration within the tumor microenvironment. Furthermore, the glutamate receptor, of which GRIN2D is a subunit, is overexpressed in multiple cancers, including triple-negative breast cancer. GRIN2D in these cancers is linked to its function in dysregulated signaling pathways crucial for cancer cell growth, adding another layer of complexity to its involvement in tumorigenesis (Li et al. 2022). In the context of bladder cancer, our findings that GRIN2D is consistently upregulated in tumor tissues and certain aggressive cell lines provide a new perspective on its role in this malignancy. The observed effects of GRIN2D knockdown on bladder cancer cell proliferation, migration, and glycolysis further reinforce its functional significance in cancer progression. These results suggest that GRIN2D might be involved in similar signaling pathways and mechanisms in bladder cancer as it is in other cancer types, potentially contributing to tumor aggressiveness and metastatic capacity. Furthermore, the overexpression of GRIN2D has been implicated in the modulation of endothelial function and angiogenesis, indicating its significance in tumor vascularization and potential as a therapeutic target.
Recent advances in cancer research have illuminated the significance of m6A RNA modification, particularly through the actions of the m6A reader protein YTHDF1, in various cancers. As a pivotal player in RNA methylation, m6A is known to play crucial roles in the progression of several human cancers. The YTHDF1 protein, in particular, has been reported to accelerate the translation of m6A-modified mRNAs in the cytoplasm, thus impacting gene expression and subsequently influencing tumor biology (Zhu et al. 2023b). This aligns with our findings that show a direct interaction between YTHDF1 and m6A-modified GRIN2D mRNA, influencing its stability in bladder cancer. Numerous studies underscore the importance of YTHDF1 in tumor biology. YTHDF1 mediates protein translation of key genes and affects the expression of factors involved in vital cell signaling pathways (Chen et al. 2021). This broad impact of YTHDF1 on gene expression is further highlighted by its versatility as the most powerful m6A reader protein, capable of mediating target gene expression and participating in disease processes (Xu et al. 2021; Ren et al. 2023). In the context of bladder cancer, as revealed in our research, the interplay between RBM15 and YTHDF1 emerges as a critical determinant in the m6A-dependent regulation of GRIN2D mRNA stability. The intricate regulatory mechanism wherein RBM15 modulates m6A levels on GRIN2D mRNA and YTHDF1 recognizes these m6A sites to influence mRNA stability aligns with the growing body of literature emphasizing the epigenetic regulation by m6A modification and YTH family proteins in cancer. This m6A modification, being the most prevalent and reversible modification in mammalian cells, plays a pivotal role in both physiological and pathological conditions, including the initiation and progression of different cancer types (Ma et al. 2019; Miller et al. 2023). Our findings, which demonstrate the impact of modulating RBM15 and YTHDF1 levels on GRIN2D mRNA stability, are consistent with the established roles of these proteins in cancer. By elucidating this molecular mechanism in bladder cancer, we contribute to a deeper understanding of the m6A-YTHDF1 pathway's role in tumorigenesis and progression.
The interplay between GRIN2D, RBM15, and YTHDF1 within the tumor microenvironment (TME) of bladder cancer represents a multifaceted mechanism impacting both tumor cell intrinsic characteristics and the immunological landscape. YTHDF1, a pivotal m6A reader protein, has been implicated in modulating immune evasion and antigen processing, particularly through its influence on lysosomal gene translation and MHC-I antigen presentation (Lin et al. 2023). This role extends to shaping the immune milieu, as evidenced by significant correlations between YTHDF1 expression and tumor immune-infiltrated cells (TILs), underscoring its influence in urogenital cancers, including bladder cancer (Liu et al. 2022). The expression of RBM15, another key player in m6A RNA methylation, exhibits a positive correlation with immune cell infiltration, as observed in pancreatic adenocarcinoma, suggesting its potential role in modulating the immune contexture in bladder cancer as well (Zhao et al. 2022). Additionally, YTHDF1’s involvement in the cross-presentation of tumor antigens and the activation of CD8 + T cells highlights its crucial function in the antitumor immune response (Cell 2021). The dual impact of these proteins, through their regulatory roles on mRNA stability and translation, extends to the formation of an antitumor immune microenvironment, contributing to the complexity of bladder cancer progression and therapy response (Boo and Kim 2020).
One significant limitation of our study is the lack of in-depth exploration into the relationship between glycolysis, immune evasion, and the m6A-YTHDF1-GRIN2D axis in bladder cancer. While we have established a correlation and functional roles of these components, the detailed mechanisms, particularly how GRIN2D influences tumor progression and metabolic reprogramming in the context of immune response, remain unexplored. Future research should delve deeper into this aspect, investigating the interplay between glycolysis, immune evasion, and the m6A pathway, using advanced techniques and in vivo models to unravel the intricate dynamics and their implications for bladder cancer therapy.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the corresponding author, without undue reservation, to any qualified researcher.
References
Boo SH, Kim YK (2020) The emerging role of RNA modifications in the regulation of mRNA stability. Exp Mol Med 52:400–408. https://doi.org/10.1038/s12276-020-0407-z
Chen Z, Zhong X, Xia M, Zhong J (2021) The roles and mechanisms of the m6A reader protein YTHDF1 in tumor biology and human diseases. Mol Ther Nucleic Acids 26:1270–1279. https://doi.org/10.1016/j.omtn.2021.10.023
Chen Z, Song Y, Li P, Gao W (2023a) GRIN2D knockdown suppresses the progression of lung adenocarcinoma by regulating the E2F signalling pathway. Cell Signal 107:110685. https://doi.org/10.1016/j.cellsig.2023.110685
Chen L et al (2023b) N6-methyladenosine reader YTHDF family in biological processes: structures, roles, and mechanisms. Front Immunol 14:1162607. https://doi.org/10.3389/fimmu.2023.1162607
Choi W et al (2014) Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol 11:400–410. https://doi.org/10.1038/nrurol.2014.129
Deutsch SI, Tang AH, Burket JA, Benson AD (2014) NMDA receptors on the surface of cancer cells: target for chemotherapy? Biomed Pharmacother 68:493–496. https://doi.org/10.1016/j.biopha.2014.03.012
Ferguson HJ et al (2016) Glutamate dependent NMDA receptor 2D is a novel angiogenic tumour endothelial marker in colorectal cancer. Oncotarget 7:20440–20454. https://doi.org/10.18632/oncotarget.7812
Gallo S, Vitacolonna A, Crepaldi T (2023) NMDA receptor and its emerging role in cancer. Int J Mol Sci. https://doi.org/10.3390/ijms24032540
Gild P et al (2019) Contemporary survival rates for muscle-invasive bladder cancer treated with definitive or non-definitive therapy. Clin Genitourin Cancer 17:e488–e493. https://doi.org/10.1016/j.clgc.2019.01.009
Hai R, He L, Shu G, Yin G (2021) Characterization of histone deacetylase mechanisms in cancer development. Front Oncol 11:700947. https://doi.org/10.3389/fonc.2021.700947
Helal DS, Darwish SA, Awad RA, Ali DA, El-Guindy DM (2023) Immunohistochemical based molecular subtypes of muscle-invasive bladder cancer: association with HER2 and EGFR alterations, neoadjuvant chemotherapy response and survival. Diagn Pathol 18:11. https://doi.org/10.1186/s13000-023-01295-y
Hu J et al (2021) YTHDF1 is a potential pan-cancer biomarker for prognosis and immunotherapy. Front Oncol 11:607224. https://doi.org/10.3389/fonc.2021.607224
Li Q, Gu Z, Tan Q, Ren L, Chen S (2022) MicroRNA-129-1-3p represses the progression of triple-negative breast cancer by targeting the GRIN2D gene. Biomed Res Int 2022:1549357. https://doi.org/10.1155/2022/1549357
Lin W et al (2023) Tumor-intrinsic YTHDF1 drives immune evasion and resistance to immune checkpoint inhibitors via promoting MHC-I degradation. Nat Commun 14:265. https://doi.org/10.1038/s41467-022-35710-7
Liu XS et al (2022) Comprehensive analysis of YTHDF1 immune infiltrates and ceRNA in human esophageal carcinoma. Front Genet 13:835265. https://doi.org/10.3389/fgene.2022.835265
Ma C, Liao S, Zhu Z (2019) Crystal structure of human YTHDC2 YTH domain. Biochem Biophys Res Commun 518:678–684. https://doi.org/10.1016/j.bbrc.2019.08.107
Miller LG, Demny M, Tamamis P, Contreras LM (2023) Characterization of epitranscriptome reader proteins experimentally and in silico: current knowledge and future perspectives beyond the YTH domain. Comput Struct Biotechnol J 21:3541–3556. https://doi.org/10.1016/j.csbj.2023.06.018
Pham KTH et al (2019) Health inequality between migrant and non-migrant workers in an industrial zone of Vietnam. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16091502
Ren W et al (2023) The function and clinical implication of YTHDF1 in the human system development and cancer. Biomark Res 11:5. https://doi.org/10.1186/s40364-023-00452-1
Sánchez Lázaro JA, Fernández Hernández Ó, Madera González F (2020) Arthroscopic contribution of synthetic graft in tibiotalocalcaneal arthroscopic fusions. Cureus 12:e12334. https://doi.org/10.7759/cureus.12334
Wang X et al (2021) RBM15 facilitates laryngeal squamous cell carcinoma progression by regulating TMBIM6 stability through IGF2BP3 dependent. J Exp Clin Cancer Res 40:80. https://doi.org/10.1186/s13046-021-01871-4
Wang S et al (2022) N6-methyladenosine reader YTHDF1 promotes ARHGEF2 translation and RhoA signaling in colorectal cancer. Gastroenterology 162:1183–1196. https://doi.org/10.1053/j.gastro.2021.12.269
Wang J et al (2023) Identification of GRIN2D as a novel therapeutic target in pancreatic ductal adenocarcinoma. Biomark Res 11:74. https://doi.org/10.1186/s40364-023-00514-4
Xiong J et al (2022) Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell 82:1660–1677. https://doi.org/10.1016/j.molcel.2022.02.033
Xu Y et al (2021) YTH domain proteins: a family of m(6)A readers in cancer progression. Front Oncol 11:629560. https://doi.org/10.3389/fonc.2021.629560
You Q et al (2023) m(6) A reader YTHDF1-targeting engineered small extracellular vesicles for gastric cancer therapy via epigenetic and immune regulation. Adv Mater 35:e2204910. https://doi.org/10.1002/adma.202204910
Zboril E, Yoo H, Chen L, Liu Z (2021) Dynamic interactions of transcription factors and enhancer reprogramming in cancer progression. Front Oncol 11:753051. https://doi.org/10.3389/fonc.2021.753051
Zhang X et al (2020) P-selectin glycoprotein ligand 1 deficiency prevents development of acute pancreatitis by attenuating leukocyte infiltration. World J Gastroenterol 26:6361–6377. https://doi.org/10.3748/wjg.v26.i41.6361
Zhang Z, Mei Y, Hou M (2022a) Knockdown RBM15 inhibits colorectal cancer cell proliferation and metastasis via N6-methyladenosine (m6A) modification of MyD88 mRNA. Cancer Biother Radiopharm 37:976–986. https://doi.org/10.1089/cbr.2021.0226
Zhang YG, Jin MZ, Zhu XR, Jin WL (2022b) Reclassification of hepatocellular cancer with neural-related genes. Front Oncol 12:877657. https://doi.org/10.3389/fonc.2022.877657
Zhao Z, Ju Q, Ji J, Li Y, Zhao Y (2022) N6-methyladenosine methylation regulator RBM15 is a potential prognostic biomarker and promotes cell proliferation in pancreatic adenocarcinoma. Front Mol Biosci 9:842833. https://doi.org/10.3389/fmolb.2022.842833
Zhong J et al (2023) Circ_0000235 targets MCT4 to promote glycolysis and progression of bladder cancer by sponging miR-330-5p. Cell Death Discov 9:283. https://doi.org/10.1038/s41420-023-01582-z
Zhu J et al (2023a) YTHDF1 promotes bladder cancer cell proliferation via the METTL3/YTHDF1-RPN2-PI3K/AKT/mTOR Axis. Int J Mol Sci. https://doi.org/10.3390/ijms24086905
Zhu Y et al (2023b) The potential role of m6A reader YTHDF1 as diagnostic biomarker and the signaling pathways in tumorigenesis and metastasis in pan-cancer. Cell Death Discov 9:34. https://doi.org/10.1038/s41420-023-01321-4
Acknowledgements
Sincere appreciation is expressed to the reviewers for the helpful feedback they provided.
Funding
This study is funded by the Hunan Development and Reform Commission (No. [2021]212-22).
Author information
Authors and Affiliations
Contributions
Meixian Le did the investigation, clinical and experimental studies, data acquisition and analysis of the manuscript. Meiying Qing did the investigation, experimental studies, statistical analysis of the manuscript. Xiangju Zeng did the investigation, data acquisition and analysis of the manuscript. Shunhua Cheng is the guarantor of the work, who did study concepts and design, preparation and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10528_2024_10875_MOESM1_ESM.tif
Supplementary file1 (TIF 2822 kb)—Supplemental Fig. 1 Prediction score distribution along the query sequence. A. Graphical representation of predicted m6A methylation sites across the GRIN2D mRNA sequence, indicating confidence levels of potential modification sites. B. Table listing the predicted m6A sites within GRIN2D mRNA, including sequence context and structural features, accompanied by confidence scores. C. Detailed table displaying additional predicted m6A sites on GRIN2D mRNA, with corresponding scores for each site's likelihood of methylation. *P < 0.05, **P < 0.01, ***P < 0.001.
10528_2024_10875_MOESM2_ESM.tif
Supplementary file2 (TIF 2869 kb)—Supplementary Fig. 2 Effects of YTHDF1 and RBM15 overexpression on cell proliferation. A, B: qPCR and Western blot analysis assessing the expression levels of RBM15 and YTHDF1 upon overexpression. C. Cell viability assessed using the CCK-8 assay. D. Colony formation assay to evaluate cell proliferation. **P < 0.01, ***P < 0.001
10528_2024_10875_MOESM3_ESM.tif
Supplementary file3 (TIF 19857 kb)—Supplementary Fig. 3 Compensatory effects of GRIN2D overexpression on the phenotypic consequences of RBM15 knockdown. A. Western blot analysis of RBM15 and GRIN2D expression following RBM15 knockdown and subsequent GRIN2D overexpression. B. Cell proliferation assessed using the CCK-8 assay. C. Migration assay measuring cell motility. D. Additional cell proliferation assay under different experimental conditions. E. Measurement of glycolytic function through the extracellular acidification rate (ECAR). *P < 0.05, **P < 0.01, ***P < 0.001
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le, M., Qing, M., Zeng, X. et al. m6A-YTHDF1 Mediated Regulation of GRIN2D in Bladder Cancer Progression and Aerobic Glycolysis. Biochem Genet (2024). https://doi.org/10.1007/s10528-024-10875-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10528-024-10875-6