Abstract
SET domain-containing 5 (SETD5), a member of protein lysine methyltransferase family, is expressed in multiple cancers, making it potential therapeutic targets. However, the role of SETD5 in colorectal cancer remains largely unknown. The expression of SETD5 in the 30 pairs colorectal cancer tissues samples and cell lines were determined by qRT-PCR. The functions of SETD5 was detected by knocked-down or overexpression in colorectal cancer cell lines SW480 and HCT116 cells. Cell proliferative activity, cell death, and stemness characteristics were assessed. BEZ235, a PI3K/AKT/mTOR pathway inhibitor, was used to perform rescue experiment to analyze whether SETD5 exerted its effects through activating PI3K/AKT/mTOR pathway. SETD5 was substantially upregulated in colorectal cancer, and correlated to metastasis and clinical stage of patients. Knockdown of SETD5 inhibited SW480 and HCT116 cell growth, as evidenced by the inhibition of cell viability and clone-forming. Moreover, Knockdown of SETD5 suppressed the capability of tumor sphere formation of SW480 and HCT116 cells, and reduced the expression of stemness-related proteins Nanog and Sox2. Further western blot analysis revealed that SETD5 knockdown inhibited the phosphorylation of proteins associated with the PI3K/AKT/mTOR pathway. In contrast, overexpression of SETD5 exerted the opposite effects. Mechanistically, by blocking PI3K/AKT/mTOR pathway with BEZ235, the effects of SETD5 overexpression on cell viability and Nanog and Sox2 protein expression were reversed. Our results substantiated that SETD5 functioned as an oncogene by promoting cell growth and stemness in colorectal cancer cells through activating the PI3K/AKT/mTOR signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is one of the malignancy of alimentary system (Bock et al. 2023; Xiong et al. 2023). According to statistical data from 2020, colorectal cancer accounts for 10.0% and 9.4% of the overall cancer incidence rate and mortality rate, respectively, ranking third most prevalent cancer in incidence and the second in mortality. Currently, surgical resection, followed by chemotherapy and radiotherapy perioperatively and other comprehensive treatments, are the main therapies for colorectal cancer. Despite advancements in treatment, many colorectal cancer patients still require intense chemotherapy and radiation regimens (Riesco-Martinez et al. 2022; Wang et al. 2021; Oh et al. 2021). Therefore, finding new targets and methods for colorectal cancer therapeutic is a very hot topic.
SET domain-containing 5 (SETD5), a recently discovered histone methyltransferase, plays important roles in epigenetic regulation (Wang et al. 2020; Sessa et al. 2019). Recent studies have reported that SETD5 expression was altered in many cancers, and played essential roles in tumor progression (Yang et al. 2021; Jiang et al. 2022; Park et al. 2022). SETD5 exerts profound effects on cellular activities, including proliferation, differentiation, stemness, and metastasis, and also functions as an important signaling pathway regulator in tumors (Nakagawa et al. 2020; Iwagawa et al. 2023). Although the role of SETD5 in cancer development has attracted widespread concern. Nevertheless, the role of SETD5 in the colorectal cancer remains unclear.
The involvement of Phosphatidylinositol 3-kinase (PI3K) in mediating the effects of growth factors on cellular signaling pathways is widely recognized (Noorolyai et al. 2019). Activation of PI3K leads to the production of PIP3, which subsequently facilitates the recruitment of PDK1 and AKT proteins to the plasma membrane which prompts PDK1 to phosphorylate AKT protein, thereby inducing partial activation of AKT and activated AKT can then directly act on mTOR and regulate cell growth and proliferation (Ersahin et al. 2015; Xu et al. 2020). Abnormal PI3K/AKT/mTOR pathway activity contributes to the development of cancers, including colorectal cancer (Narayanankutty 2019). Current research has found that SETD5 controls cancer stem cell characteristics in non-small cell lung cancer and esophageal squamous cell carcinoma by modulating the PI3K/AKT/mTOR pathway (Chen et al. 2021; Piao et al. 2020). In this study, we investigated whether SETD5 affects colorectal cancer cell growth and stemness via regulating PI3K/AKT/mTOR pathway in order to provide new ideas for colorectal cancer treatment.
Materials and Methods
Patient Sample
Colorectal cancer tissues were collected from 30 patients who were diagnosed with colorectal cancer and suffered from surgical resection at the Nanjing Gaochun People’s Hospital, along with adjacent normal tissues gathered as controls. Tissues samples were collected during the operation, snap frozen in liq. N2, and kept at – 80 °C. This study was approved by the Nanjing Gaochun People’s Hospital Ethics Committee, wherein every patient delivered the signed informed consent. Clinical information were listed in Table 1.
Cell Culturing
ATCC (Shanghai, China) provided human colorectal cancer cell lines HCT116 and SW480 cells. Cells were maintained in DMEM medium (Sigma-Aldrich, St Louis, MO, USA) containing 10% FBS (Sigma-Aldrich) with 1% penicillin/streptomycin (Solarbio, Beijing, China).
Quantitative Real-Time PCR (qRT-PCR)
RNA was extracted from tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA), and then the Takara reverse transcription kit (Dalian, China) was employed to synthesize cDNA from 150 ng of RNA. Following this, a qRT-PCR was conducted with the cDNA using SYBR Green method (Applied Biosystems, Shanghai, China) on an ABI7500 system (Applied Biosystems). Relative gene expression, normalized to β-actin, was assessed using 2−ΔΔct method. Primers employed were listed in Table 2.
Immunohistochemistry (IHC) Staining
SETD5 expression in colorectal cancer tissue samples was examined by IHC staining. Formalin-fixed tissue samples were embedded in paraffin, and cut into sections. After dewaxed, rehydrated, and blocked in 10% BSA, the sections were kept overnight with anti-SETD5 primary antibody at 4 °C. Next, the secondary antibody was used for 2 h at room temperature, and diaminobenzidine and hematoxylin were applied.
Cell Transfection
To knock down SETD5, two siRNAs targeting SETD5 (si-SETD5#1 and si-SETD5#2) were designed by GenePharma (Shanghai, China), and a non-sense siRNA served as a control (NC). What’s more, a SETD5 overexpression vector (SETD5) or empty vector was constructed by GenePharma to overexpression SETD5. Lipofectamine 2000 (Invitrogen) was used for transfection, and after 48 h, the western blot was used to assess the efficiency of knockdown or overexpression.
Western Blot
Western blotting was conducted according to the already reported protocol (Xiao et al. 2023). In short, RIPA buffer was used to extract protein from the SW480 and HCT116 cells. Proteins were then separated by electrophoresis, and transferred to PVDF membrane. The secondary antibodies were applied after the primary antibodies as incubated. Protein bands were visualized by ECL kit (Millipore, Darmstadt, Germany). The following antibodies against SETD5 (ab204363, 1:400, Abcam), Nanog (ab21624, 1:1000, Abcam), Sox2 (ab137385, 1:1000, Abcam), phosphorylated PI3K (p-PI3K, Tyr458, 4228, 1:500, Cell Signaling Technology), β-actin (ab6276, 1:1000, Abcam), mTOR (ab134903, 1:10,000, Abcam), phosphorylated mTOR (ab137133, 1:1000, Abcam), AKT (4691, 1:1000, Cell Signaling Technology), phosphorylated AKT (p-AKT, Ser473, 1:2000, Cell Signaling Technology), and PI3K (sc-23962, 1:2000, Cell Signaling Technology) were used.
Cell Counting Kit-8 (CCK-8) Assay
For CCK-8 assay, 5 × 104 cells/well transfected with siRNA-SETD5 or SETD5 overexpression vector were plated in 96-well plates, and treated with or without BEZ235 for 24 h. Next, CCK-8 reagent (Dojindo, Laboratories, Kumamoto, Japan) was used at designated time points and incubated for another 2 h. Then, viability measurements were conducted at 450 nm wavelength.
Trypan Blue Staining
Staining by Trypan blue was conducted to quantify the dead cells. Concisely, 0.4% Trypan blue probe was utilized to incubate with cells for 2 min. Then, images were captured, and the number of dead cells (Trypan blue-positive cells) was counted.
Colony Formation Assay
1000 cells/well were plated into 6-well plates and cultured for 7–10 days. The media was changed every 3 days. Colony numbers were counted after cells stained with crystal violet.
Sphere-Forming Assay
The sphere-forming capability of SW480 and HCT116 cells following SETD5 knockdown or overexpression was determined by sphere-forming assay. The transfected cells were harvested, followed by resuspension DMEM medium containing 20 ng/mL of bEGF and bFGF, and then seeded into low attachment 24-well plates with 500cells/well. Following 7 days of culture, the sphere-formation of cells was observed under a micrography, and the diameter of spheroid was calculated.
Statistical Analysis
The data were presented as mean ± SD (mean ± standard deviation) of at least three duplicates per experiment. The differential analysis for the two groups was performed using Student’s t test. The one-way analysis of variance (ANOVA), and Tukey’s post hoc analyses were conducted for various comparisons. Chi-square test were utilized to analyze the enumeration data expressed in percentage. The statistical significance was set at P < 0.05.
Results
SETD5 Upregulation in Colorectal Cancer
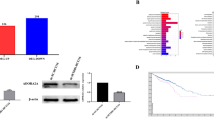
An in-depth analysis of TCGA data on TIMER, Ualcan, and GEPIA platform was conducted to evaluate the SETD5 expression patterns in colorectal cancer. The results revealed the higher expressions of SETD5 in colorectal cancer cases compared with normal samples (Fig. 1a–c). Furthermore, we collected 30 pairs of colorectal cancer samples and neighboring tissue samples for SETD5 expression analysis by IHC and qRT-PCR, and results showed that SETD5 was upregulated in tumor compared with the adjacent tissue, which was concordance with the database analysis (Fig. 1d, e). In addition, we analyzed link of SETD5 expression with clinical features in 30 colorectal cancer patients, and observed that tumor metastasis and clinical stage were correlated with SETD5 expression (Table 1). These data indicated that high SETD5 expression was associated with the malignancy of colorectal cancer.
SETD5 was highly expressed in colorectal cancer. a SETD5 expressions in colorectal cancer tissue and normal tissue were obtained from TIMER database. b SETD5 expressions in colorectal cancer tissue and normal tissue were obtained from Ualcan database. c SETD5 expressions in colorectal cancer tissue and normal tissue were obtained from GEPIA database. d SETD5 mRNA expressions in 30 pairs of colorectal cancer and neighboring normal tissue were measured via qRT-PCR. e SETD5 expressions in 30 pairs of colorectal cancer and neighboring normal tissue were examined through IHC. ***P < 0.001
SETD5 Promotes Colorectal Cancer Cells Growth
SETD5 functions were investigated by transfecting siRNA-SETD5 or pcDNA-SETD5 overexpression vector into SW480 and HCT116 cells. SETD5 protein and mRNA levels were decreased upon SETD5 knockdown and increased after SETD5 overexpression (Fig. 2a and b). Subsequently, CCK8 assay results showed that the cell viability was reduced by SETD5 knockdown while enhanced by SETD5 overexpression (Fig. 2c). Trypan blue staining showed that the number of dead cells increased after SETD5 knockdown and decreased after SETD5 overexpression (Fig. 2d). Moreover, the results of colony formation revealed that SETD5 knockdown decreased the capacity of SW480 and HCT116 cells to form colonies, while SETD5 overexpression exhibited opposite effects (Fig. 2e). These data substantiated that SETD5 promotes cell growth in colorectal cancer.
SETD5 knockdown inhibited, while overexpression promoted colorectal cancer cell proliferation. a The SETD5 protein level in SW480 and HCT116 cells was analyzed via the Western blotting after SETD5 knockdown or overexpression. b The SETD5 mRNA expression in SW480 and HCT116 cells was analyzed via qRT-PCR. After SETD5 knockdown or overexpression, the proliferation of SW480 and HCT116 cells was assessed by CCK-8 assay (c), Trypan blue staining (d), and clone formation (e). *P < 0.05, **P < 0.01, ***P < 0.001
SETD5 Promotes Cell Stemness in Colorectal Cancer
Next, a sphere-forming experiment was conducted to determine the stemness of SW480 and HCT116 cells, and the results showed that SETD5 deletion reduced the number of colorectal cancer spheres formed by SW480 and HCT116 cells, while SETD5 overexpression promoted SW480 and HCT116 sphere formation ability (Fig. 3a). Nanog and Sox2, cancer stem cell markers, are believed to promote tumorigenesis (Sun et al. 2020; Dianat-Moghadam et al. 2020). Western blotting was employed to further analyze the expressions of Nanog and Sox2, and the results revealed that the protein levels of Nanog and Sox2 were decreased in SW480 and HCT116 cells after SETD5 knockdown and increased upon overexpression of SETD5 (Fig. 3b). Nanog and Sox2 mRNA levels mirrored the protein findings (Fig. 3c). These data indicated that SETD5 facilitated cell stemness characteristic in colorectal cancer.
SETD5 knockdown inhibited, while overexpression promoted colorectal cancer cell sphere formation ability. a Representative images (left) and quantification of spheroid number (right) in sphere formation assay in SW480 amd HCT116 cells. b Expressions of Nanog and Sox2 proteins in HCT116 and SW480 cells were examined via the western blotting. c Expressions of Nanog and Sox2 mRNA in HCT116 and SW480 cells were detected by qRT-PCR. **P < 0.01, ***P < 0.001
SETD5 Exerts Its Promotion on Colorectal Cancer Cell Growth and Stemness by Activating PI3K/AKT/mTOR Pathway
PI3K/AKT/mTOR pathway is important for cell proliferation and maintaining stemness of cancer cells (Kashyap et al. 2018; Hassan et al. 2020). Herein, the western blotting depicted reduced phosphorylated PI3K, AKT, and mTOR levels in SETD5 knockdown SW480 and HCT116 cells, and elevated in SETD5 overexpression cells (Fig. 4a–c). To confirm whether SETD5 promoted cell proliferation and stemness features of colorectal cancer through PI3K/AKT/mTOR pathway, we used BEZ235, a PI3K/AKT/mTOR pathway inhibitor, to conduct rescue experiment. Through CCK-8 assay, we found that BEZ235 reversed the promoting of SETD5 overexpression on cell proliferation (Fig. 4d). Furthermore, Nanog and Sox2 protein expressions were examined when the SETD5 overexpressed cells were treated with or without BEZ235 by western blot. Findings exhibited that BEZ235 rescued the effects of SETD5 overexpression on Nanog and Sox2 protein levels (Fig. 4e). These finding indicated that PI3K/AKT/mTOR pathway was required for the proliferation and stemness mediated by SETD5.
SETD5 accelerated colorectal cancer cell proliferation and stemness by activating PI3K/AKT/mTOR pathway. a–c Phosphorylated PI3K, AKT, mTORand total PI3K, AKT, and mTOR protein levels were analyzed via western blot. d HCT116 and SW480 cells overexpressed SETD5 were treated with or not BEZ235 and then subjected to the CCK8 assay. e Sox2 and Nanog protein levels were monitored via the western blotting. *P < 0.01, **P < 0.01, ***P < 0.001
Discussion
Statistically, colorectal cancer is globally ranked as 3rd among all cancers, and its mortality is increasing yearly (Patel et al. 2022; Baidoun et al. 2021). Despite the fact that current treatment strategies have improved the clinical efficacy of colorectal cancer, the prognosis for patients, especially those with metastatic colorectal cancer, is still unsatisfactory (Biller and Schrag 2021; Jin and Frankel 2018). Therefore, exploring new therapeutic targets and finding better therapeutic methods are very necessary.
SETD5, one of the SET domain-containing proteins, has been reported to play diverse roles in regulating tumor progression (Li et al. 2023). For example, lack of SETD5 suppressed hepatocellular carcinoma cell proliferation and invasion, at least partly through reducing glycolysis (Park et al. 2022). SETD5 promoted the cell migration and invasion of non-small cell lung cancer (NSCLC) and was related to tumor stage and metastasis of patients (Yu et al. 2019). Another study showed that SETD5 facilitated the cancer stem cell feature of NSCLC by inhibiting PI3K/AKT/mTOR activation (Chen et al. 2021). In addition, a previous study confirmed that downregulation of SETD5 in esophageal squamous cell carcinoma cells prevented tumor spheroid formation and cell proliferation by inhibiting stemness-related proteins and the PI3K/AKT pathway (Piao et al. 2020). Herein, for the first time, it was discovered that SETD5 suppressed cell growth and stemness of colorectal cancer cells.
Cancer stem cells are capable of self-renew, and are responsible for tumor growth (Relation et al. 2017). Like embryonic stem cells, the stem maintenance of tumor stem cells also relies on transcription factors Nanog, SOX3, and OCT4 (Walcher et al. 2020). Prior studies have demonstrated that aberrant activation of Nanog promotes tumor growth through the regulation of tumor stemness and tumor cells proliferation (Yuan et al. 2021; Zhang et al. 2022). Herein, our results demonstrated that SETD5 deficiency inhibited colorectal cancer cell proliferation activity. Further, SETD5 depletion suppressed cells’ sphere-forming ability, as manifested by the reduced SOX2 and Nanog expressions. However, overexpression of SETD5 had the opposite effects. PI3K/AKT/mTOR, the classical cancer-related pathway, is regarded as an attractive therapeutic target of multiple cancers (Yu et al. 2022). Herein, our results further showed that SETD5 suppressed the PI3K/AKT/mTOR pathway activity. This pathway regulates many cellular processes, including cell growth, survival, apoptosis, and stemness (Karami Fath et al. 2022; Stanciu et al. 2022). Over the years, researchers have attempted to inhibit tumor cell proliferation and survival by targeting various nodes of this pathway to achieve purpose of tumor therapy (Asati et al. 2016; Mardanshahi et al. 2021). However, the current effects of the inhibitor of this pathway in cancer treatment is unsatisfactory because of toxic side effects and single therapeutics (Alzahrani 2019). Hence, a more sophisticated strategy of multi-target intervention strategy is required to be explored. Herein, we found that after treatment with BEZ235, the inhibitor for PI3K and mTOR, the promoting effects of SETD5 on colorectal cancer cell viability and stemness characteristics were eliminated, suggesting that promoting mTOR, AKT, and PI3K phosphorylation is the mechanism by which SETD5 acts as an oncogene in colorectal cancer (Fig. 5).
In conclusion, our results demonstrated that SETD5 exerted its oncogenic effects through promoting cell growth and stemness by regulating PI3K/AKT/mTOR pathway activation.
Data Availability
The accumulated or analyzed data in the course of this study have been incorporated in this manuscript. The corresponding author on prior request will provide datasets employed and/or analyzed in the current work.
References
Alzahrani AS (2019) PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol 59:125–132
Asati V, Mahapatra DK, Bharti SK (2016) PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem 109:314–341
Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A (2021) Colorectal cancer epidemiology: recent trends and impact on outcomes. Curr Drug Targets 22(9):998–1009
Biller LH, Schrag D (2021) Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA 325(7):669–685
Bock S, Henley S, O’Neil M, Singh S, Thompson T, Wu M (2023) Cancer distribution among Asian, native Hawaiian, and pacific islander subgroups—United States, 2015–2019. MMWR Morb Mortal Wkly Rep 72(16):421–425
Chen Q, Sun Z, Li J, Zhang D, Guo B, Zhang T (2021) SET domain-containing protein 5 enhances the cell stemness of non-small cell lung cancer via the PI3K/Akt/mTOR pathway. J Environ Pathol Toxicol Oncol 40(2):55–63
Dianat-Moghadam H, Azizi M, Eslami SZ, Cortés-Hernández LE, Heidarifard M, Nouri M, Alix-Panabières C (2020) The role of circulating tumor cells in the metastatic cascade: biology, technical challenges, and clinical relevance. Cancers (Basel) 12(4):867
Ersahin T, Tuncbag N, Cetin-Atalay R (2015) The PI3K/AKT/mTOR interactive pathway. Mol Biosyst 11(7):1946–1954
Hassan G, Du J, Afify SM, Seno A, Seno M (2020) Cancer stem cell generation by silenced MAPK enhancing PI3K/AKT signaling. Med Hypotheses 141:109742
Iwagawa T, Kawabata R, Fukushima M, Kuribayashi H, Watanabe S (2023) Setd5, but not Setd2, is indispensable for retinal cell survival and proliferation. FEBS Lett 597(3):427–436
Jiang Z, Zhao J, Zou H, Cai K (2022) CircRNA PTPRM promotes non-small cell lung cancer progression by modulating the miR-139-5p/SETD5 axis. Technol Cancer Res Treat 21:15330338221090090
Jin M, Frankel WL (2018) Lymph Node Metastasis in Colorectal Cancer. Surg Oncol Clin N Am 27(2):401–412
Karami Fath M, Ebrahimi M, Nourbakhsh E, Zia Hazara A, Mirzaei A, Shafieyari S, Salehi A, Hoseinzadeh M, Payandeh Z, Barati G (2022) PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol Res Pract 237:154010
Kashyap T, Pramanik KK, Nath N, Mishra P, Singh AK, Nagini S, Rana A, Mishra R (2018) Crosstalk between Raf-MEK-ERK and PI3K-Akt-GSK3β signaling networks promotes chemoresistance, invasion/migration and stemness via expression of CD44 variants (v4 and v6) in oral cancer. Oral Oncol 86:234–243
Li M, Hou Y, Zhang Z, Zhang B, Huang T, Sun A, Shao G, Lin Q (2023) Structure, activity and function of the lysine methyltransferase SETD5. Front Endocrinol (Lausanne) 14:1089527
Mardanshahi A, Gharibkandi NA, Vaseghi S, Abedi SM, Molavipordanjani S (2021) The PI3K/AKT/mTOR signaling pathway inhibitors enhance radiosensitivity in cancer cell lines. Mol Biol Rep 48(8):1–14
Nakagawa T, Hattori S, Nobuta R, Kimura R, Nakagawa M, Matsumoto M, Nagasawa Y, Funayama R, Miyakawa T, Inada T et al (2020) The autism-related protein SETD5 controls neural cell proliferation through epigenetic regulation of rDNA expression. Science 23(4):101030
Narayanankutty A (2019) PI3K/Akt/mTOR pathway as a therapeutic target for colorectal cancer: a review of preclinical and clinical evidence. Curr Drug Targets 20(12):1217–1226
Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B (2019) The relation between PI3K/AKT signalling pathway and cancer. Gene 698:120–128
Oh C, Jo Y, Sim S, Yun S, Jeon S, Chung W, Yoon S-H, Lim C, Hong B (2021) Post-operative respiratory outcomes associated with the use of sugammadex in laparoscopic colorectal cancer surgery: a retrospective, propensity score matched cohort study. Signa Vitae 17(1):117–123
Park M, Moon B, Kim JH, Park SJ, Kim SK, Park K, Kim J, Kim SY, Kim JH, Kim JA (2022) Downregulation of SETD5 suppresses the tumorigenicity of hepatocellular carcinoma cells. Mol Cells 45(8):550–563
Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR (2022) The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol 7(3):262–274
Piao L, Li H, Feng Y, Yang Z, Kim S, Xuan Y (2020) SET domain-containing 5 is a potential prognostic biomarker that promotes esophageal squamous cell carcinoma stemness. Exp Cell Res 389(1):111861
Relation T, Dominici M, Horwitz EM (2017) Concise review: an (Im)penetrable shield: how the tumor microenvironment protects cancer stem cells. Stem Cells 35(5):1123–1130
Riesco-Martinez M, Modrego A, Espinosa-Olarte P, La Salvia A, Garcia-Carbonero R (2022) Perioperative chemotherapy for liver metastasis of colorectal cancer: lessons learned and future perspectives. Curr Treat Options Oncol 23(9):1320–1337
Sessa A, Fagnocchi L, Mastrototaro G, Massimino L, Zaghi M, Indrigo M, Cattaneo S, Martini D, Gabellini C, Pucci C et al (2019) SETD5 regulates chromatin methylation state and preserves global transcriptional fidelity during brain development and neuronal wiring. Neuron 104(2):271-289.e213
Stanciu S, Ionita-Radu F, Stefani C, Miricescu D, Stanescu S II, Greabu M, Ripszky Totan A, Jinga M (2022) Targeting PI3K/AKT/mTOR signaling pathway in pancreatic cancer: from molecular to clinical aspects. Int J Mol Sci 23(17):10132
Sun L, Han T, Zhang X, Liu X, Li P, Shao M, Dong S, Li W (2020) PRRX1 isoform PRRX1A regulates the stemness phenotype and epithelial-mesenchymal transition (EMT) of cancer stem-like cells (CSCs) derived from non-small cell lung cancer (NSCLC). Transl Lung Cancer Res 9(3):731–744
Walcher L, Kistenmacher AK, Suo H, Kitte R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S, Kossatz-Boehlert U (2020) Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol 11:1280
Wang H, Li X, Peng R, Wang Y, Wang J (2021) Stereotactic ablative radiotherapy for colorectal cancer liver metastasis. Semin Cancer Biol 71:21–32
Wang Z, Hausmann S, Lyu R, Li TM, Lofgren SM, Flores NM, Fuentes ME, Caporicci M, Yang Z, Meiners MJ et al (2020) SETD5-coordinated chromatin reprogramming regulates adaptive resistance to targeted pancreatic cancer therapy. Cancer Cell 37(6):834-849.e813
Xiao H, Za Z, Wei C, Peng D, Guo Y (2023) CENPF facilitates endometrial cancer cell progression through PI3K/AKT/mTOR pathway. Eur J Gynaecol Oncol 44(1):106–114
Xiong X, Wang C, Cao J, Gao Z, Ye Y (2023) Lymph node metastasis in T1–2 colorectal cancer: a population-based study. Int J Colorectal Dis 38(1):94
Xu F, Na L, Li Y, Chen L (2020) Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 10(1):54
Yang Z, Zhang C, Che N, Feng Y, Li C, Xuan Y (2021) Su(var)3–9, enhancer of zeste, and trithorax domain-containing 5 facilitates tumor growth and pulmonary metastasis through up-regulation of AKT1 signaling in breast cancer. Am J Pathol 191(1):180–193
Yu H, Sun J, Zhao C, Wang H, Liu Y, Xiong J, Chang J, Wang M, Wang W, Ye D et al (2019) SET domain containing protein 5 (SETD5) enhances tumor cell invasion and is associated with a poor prognosis in non-small cell lung cancer patients. BMC Cancer 19(1):736
Yu L, Wei J, Liu P (2022) Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol 85:69–94
Yuan Y, Zhang M, Yan G, Ma Q, Yan Z, Wang L, Yang K, Guo D (2021) Nanog promotes stem-like traits of glioblastoma cells. Front Biosci 26(3):552–565
Zhang M, Peng R, Wang H, Yang Z, Zhang H, Zhang Y, Wang M, Wang H, Lin J, Zhao Q et al (2022) Nanog mediated by FAO/ACLY signaling induces cellular dormancy in colorectal cancer cells. Cell Death Dis 13(2):159
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Xiaohua Zhou and Wenqiang Chen designed the study, completed the experiment and supervised the data collection, Duanming Zhuang analyzed the data, interpreted the data, Guangqi Xu, Yongqiang Puyang and Hongqing Rui prepare the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no conflicts of interest for disclosure.
Ethical Approval
Ethics Committee of Nanjing Gaochun People’s Hospital Hospital approved the study ethics.
Informed Consent
A legally authorized representative(s) for anonymized patient information provided the written informed consent for its publishing in the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, X., Chen, W., Zhuang, D. et al. Knockdown of SETD5 Inhibits Colorectal Cancer Cell Growth and Stemness by Regulating PI3K/AKT/mTOR Pathway. Biochem Genet (2024). https://doi.org/10.1007/s10528-024-10766-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10528-024-10766-w