Abstract
Recent research has shown that Doublecortin-like kinase 1 (DCLK1) is overexpressed in different types of cancer. It has recently been described as a cancer stem cells (CSCs) marker, is associated with carcinogenesis, and positively correlates with infiltration of multiple immune cell types in some cancers. However, studies focused on assessing DCLK1 expression in HCC are limited, and the role of DCLK1 in HCC tumor immunity remains to be determined. In this study, we used a modified model of the resistant hepatocyte (MRHM) to evaluate DCLK1 expression in HCC. Furthermore, DCLK1 expression in HCC was analyzed using TIMER 2.0, UALCAN, GEPIA, GEO, and HPA web-based tools. Correlations between DCLK1 expression and clinicopathological factors in patients were analyzed using the UALCAN web-based tool. Finally, correlations between DCLK1 and immune infiltrates were investigated using the TIMER 2.0 and TISIDB web-based tools. The results showed that DCLK1 is significantly overexpressed during progression of the HCC carcinogenic process in the MRHM. DCLK1 is overexpressed in HCC according to multiple publics web-based tools, and its overexpression is associated with cancer stage. Furthermore, DCLK1 expression was correlated with infiltration levels of multiple immune cells, immunomodulatory factors, immunoinhibitors, MHC molecules, chemokines, receptors, and immune cell-specific markers. These results suggest that DCLK1 is a potential prognostic biomarker that determines cancer progression and correlates with immune cell infiltration in HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer worldwide and accounts for approximately 80–90% of cases (Chidambaranathan-Reghupaty et al. 2021; Llovet et al. 2021). In 2020, the International Agency for Research on Cancer reported that liver cancer was the sixth most common cancer worldwide and the third leading cause of cancer-related death globally (Sung et al. 2021). The main risk factors associated with the development of HCC include chronic Hepatitis B virus (HBV) or Hepatitis C virus (HCV) infection, which accounts for approximately 80% of all HCC cases worldwide (Chidambaranathan-Reghupaty et al. 2021; Yang et al. 2019). Non-alcoholic fatty liver disease (NAFLD), chronic alcohol consumption, and exposure to aflatoxins also represent risk factors for the development of HCC (Llovet et al. 2021; Yang et al. 2019). The pathogenesis of HCC has been described as a complex multistage process involving different altered cellular and molecular mechanisms (Chidambaranathan-Reghupaty et al. 2021; Llovet et al. 2021; Ogunwobi et al. 2019). Although the mechanisms that favor its progression may vary depending on the underlying etiology, it has been established that the usual sequence is liver damage, chronic inflammation, fibrosis, cirrhosis, and finally, culminating in the establishment of HCC (Chidambaranathan-Reghupaty et al. 2021; Sánchez et al. 2021). Due to its rapid tumor progression, the absence of characteristic symptoms during the early stages, and the scarcity of early diagnostic markers, the vast majority of patients are usually diagnosed in advanced stages (Cao et al. 2020a; Ren et al. 2020). To date, and despite recent advances, HCC is still considered one of the most complicated cancers to treat (Liu et al. 2015; Ren et al. 2020). Available treatment includes chemotherapy, surgical resection of the tumor, and liver transplantation, which is considered the most efficient option for patients diagnosed at an early stage of the disease (Anwanwan et al. 2020; Liu et al. 2015). However, in most cases, HCC is diagnosed during an advanced stage, limiting these patients’ treatment options (L. Cao et al. 2020a, b). In addition, available drugs such as sorafenib show low efficacy and a low positive impact on patient’s survival rate (Anwanwan et al. 2020; Liu et al. 2015; Ren et al. 2020). Therefore, developing new treatment strategies specific to HCC and identifying reliable biomarkers for early diagnosis remains an unmet need.
Cancer stem cells (CSCs) represent a subpopulation of cells that maintain similar characteristics to normal stem cells, including self-renewal and differentiation potential, and CSCs have been described as having properties that are associated with tumor severity and cancer recurrence after therapy, such as high tumorigenic, metastatic, chemotherapy- and radiation-resistant capacities (Khalaf et al. 2021; Y.-C. Liu et al. 2020). During tumor development, formation of an immunosuppressive tumor microenvironment (TME) enriched in various soluble factors that facilitate the recruitment of tumor-associated macrophages (TAMs) and other immune regulatory cells, is favored (Bayik and Lathia 2021; Cao et al. 2020a, b). In this regard, CSCs have been shown to play an essential role in TME shaping, through regulation of TAMs and the immune checkpoint (Cao et al. 2020a, b).
Doublecortin-like kinase 1 (DCLK1) is a member of the protein kinase superfamily and the doublecortin family (Fan et al. 2017). It is mainly associated with the regulation of microtubule polymerization and neurogenesis (Broner et al. 2021; Fan et al. 2017). Initially, it was described in the developing rodent brain as a brain-specific protein (Vijai et al. 2021). However, DCLK1 is overexpressed in different cancer types such as pancreatic cancer (Li et al. 2018), colorectal cancer (Gagliardi et al. 2012), breast cancer (Lv et al. 2017), clear cell renal carcinoma (Weygant et al. 2014), head and neck squamous cell carcinoma (Broner et al. 2021), lung adenocarcinoma (Panneerselvam et al. 2020), and HCC (Fan et al. 2017), and has recently been described as a CSCs marker (Fan et al. 2017; Li et al. 2018). Therefore, overexpression of DCLK1 in cancer has been studied for its close relationship with various biological processes associated with cancer development, such as angiogenesis, epithelial-mesenchymal transition (EMT), metastasis, multidrug resistance, self-renewal of CSCs, and regulation of the tumor immune microenvironment (Cao et al. 2020a, b; Fan et al. 2017). For example, overexpression of DCLK1 is associated with a significant increase in metastatic capacity in breast cancer cell lines and CRC cell lines (Gao et al. 2016; Liu et al. 2019). In contrast, deletion of DCLK1 inhibits metastasis-associated features such as EMT, migration, and invasion in breast cancer and non-small cell lung cancer cell lines (Liu et al. 2019; Panneerselvam et al. 2020). Recently, it has been reported that DCLK1 is overexpressed in gastric and colon cancer patients and correlates with a worse clinical prognosis because it is positively associated with infiltration of multiple immune cell types, especially TAMs and regulatory T cells, suggesting that DCLK1 may contribute to TAMs-mediated inhibition of CD8+T cells (Wu et al. 2020). However, few studies have focused on evaluating the role of DCLK1 in HCC tumor immunity.
In this study, we explored the expression of DCLK1 during the progression of the carcinogenic process of HCC in the modified resistant hepatocyte model (MRHM). In addition, we used multiple public web-based tools to investigate DCLK1 expression in human HCC. We analyzed the relationship between DCLK1 expression and clinicopathological parameters of HCC and levels of tumoral immune cell infiltration. Our results revealed the prognostic value of DCLK1 in HCC and provided new insights into the correlation and possible mechanism between DCLK1 expression and immune infiltration. We believe that this study may expand the knowledge about DCLK1 role in HCC and provide background for new lines of research.
Materials and Methods
Animals and Experimental Protocol
Male Fischer-344 rats (180-200 g weight, two months of age) were obtained from the Unit for Production and Experimentation of Laboratory Animals of the Center for Research and Advanced Studies of the National Polytechnic Institute (UPEAL-CINVESTAV-IPN; CDMX, Mexico). Performed animal handling and experimentation according to the regulations of the institutional committee for the use and care of laboratory animals. The experimental protocol was previously reviewed and approved by the local Animal Experimentation Committee. During the testing procedures, rats were fed ad libitum, and all rats were maintained under temperature conditions of 21 °C, relative humidity of 50%, in a controlled environment with 12 h light/dark cycles.
The HCC study was conducted using the modified resistant hepatocyte model (MRHM), as previously reported (Vásquez-Garzón et al. 2015). The MRHM consisted of three unique procedures. First, a single dose of the initiating agent DEN, a carcinogen and mutagen, is administered by intraperitoneal administration (200 mg/kg) (N0258, Sigma Aldrich, St. Louis, MO, USA). Second, three consecutive administrations of the promoting agent 2-acetylaminofluorene (2-AAF), a mutagenic derivative of fluorene, starting on day 7 after initiation by intragastric administration (20 mg/kg) (A7015, Sigma-Aldrich, St. Louis, MO, USA) at this stage the initiated hepatocytes undergo proliferation under the promotion of the mitogenic stimulus provided by 2-acetylaminofluorene. At the same time, this promotion inhibits uninitiated hepatocytes. Third, on day 10 after initiation, rats were subjected to a 70% partial hepatectomy to subject them to a proliferative stimulus that accelerates the progression of the carcinogenic process. The rats were sacrificed, and livers were recovered at different times after DEN administration: 24 h and 7 days (initiation stage), 11 and 16 days (promotion stage), and 30 days, 5, 9, 12, and 18 months (progression stage). These time points allowed us to examine the initial changes in liver tissue, understand early effects of the carcinogen agent, demonstrate the carcinogenic process of 2-AAF and its development, and observe the morphological and molecular changes of preneoplastic lesions and their long-term progression to established tumors. The negative control was livers from untreated rats. After sacrifice, one segment of each liver was preserved in 4% formaldehyde for dehydration and paraffin embedding and used for immunohistochemistry. The remaining liver segments were flash-frozen in 2-methyl-butane using liquid nitrogen and stored at -70 °C. The frozen liver segments were used for histochemical determination of the tumor marker gamma-glutamyl-transpeptidase (GGT) and subsequent RNA and protein extraction.
Histochemistry of GGT
Frozen liver tissue Sect. 20 μm thick were mounted on previously gelatinized slides and fixed with absolute ethanol for 10 min at -20 °C. Then, they were incubated for 30 min at room temperature with a solution of 125 mg/ml g-glutamyl-1,4-methoxy-2-naphthylamide (GMNA) (Sigma Aldrich, St. Louis, MO, USA), 0.5 mg/ml glycyl-glycine (Sigma Aldrich, St. Louis, MO, USA) and 0.5 mg/ml fast blue (Sigma Aldrich, St. Louis, MO, USA) in 100mM Tris buffer solution. Finally, they were washed with physiological saline, and the red precipitates of enzyme activity were fixed with 100nM copper sulfate (CuSO4) solution (Sigma Aldrich, St. Louis, MO, USA), hen pictures were taken. The formation of the red precipitates due to the enzymatic activity of the tumor marker GGT determines the preneoplastic lesions.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from the nodular, non-nodular, tumor, and non-tumor areas of rat liver tissue at 0 and 24 h, at 7, 11, 16, and 30 days and 5, 9, 12, and 18 months after receiving carcinogenic treatment, using TriPure isolation reagent (Roche, Berlin, Germany) according to the manufacturer’s instructions. RNA quality-verified purity and quantity with the NanoDropR 1000 Uv/Visible spectrophotometer (NanoDrop Technologies, Inc.), and purity and integrity were determined by a 1% agarose gel run. Samples that presented 260/280 nm coefficients greater (higher) than 1.8 and showed integrity on agarose gels were converted to cDNA. Total RNA (2 µg) was reverse transcribed using Oligo Random Primers (Invitrogen, Waltham, MA, USA), dNTPs mix (Applied Biosystems, Foster City, CA, USA), and SuperScript II Reverse Transcriptase (Invitrogen, Waltham, MA, USA). Expression levels of the DCLK1 target gene were measured by qRT-PCR amplification using specific TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) probes on StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The results were evaluated according to the comparative Ct method.
Western Blot
Total proteins were isolated from the nodular, non-nodular, tumor, and non-tumor areas of rat liver tissue at 0 and 24 h, at 7, 11, 16, and 30 days and 5, 9, 12, and 18 months after receiving carcinogenic treatment, using TriPure Isolation Reagent (Roche, Berlin, Germany) according to the manufacturer’s instructions. The Lowry Assay Kit (BioRad, Richmond, California, USA) was used to determine protein concentrations and was purchased from. 50 µg of the protein extract from each sample was separated by 12% SDS-PAGE gel electrophoresis. Proteins were transferred to a PVDF membrane. The membranes were then incubated with the primary anti-DCLK1 and anti-actin antibodies overnight at 4 °C. Subsequently, the membranes were incubated with a horseradish peroxidase-conjugated rabbit anti-IgG secondary antibody for 1 h at room temperature. A chemiluminescence reaction with luminol detected the bands. Performed densitometry analysis with ImageJ (Image Processing and Analysis in Java) software.
Immunohistochemistry
Tissue samples were fixed in 4% formaldehyde, dehydrated, and finally embedded in paraffin. Made histological sections of 3 μm thickness from paraffin-embedded tissue, deparaffinized, and gradually hydrated. Unmasked antigens by immersing the histological sections in citrate buffer at pH 6 in the express pot for 15 min. Slides were blocked with methanol-H2O2 for 20 min, avidin for 10 min, biotin, and CAS-BLOCK (Invitrogen, Paisley, UK) for 10 min at room temperature. Slides were incubated with primary antibody anti-DCLK1 (1:100; Abcam, Cambridge, MA, USA), and anti-GST-P (1:50; Dako, Carpinteria, California, USA) overnight at 4 °C, the next day they were incubated with universal specific secondary antibody (1 drop) for 1 h at 37 °C. Then, the streptavidin-HRP complex was added for 30 min at 37 °C. Subsequently, the DAB kit revealed the immunostaining signal (Invitrogen, California, USA). The sections were stained with Harris hematoxylin, dehydrated, and mounted. Obtained representative images on an Olympus visible light inverted microscope (Olympus Europa GmbH, Hamburg, Germany).
Statistical Analysis
All experiments were performed on at least four animals per treatment group. Differences between control and treatment groups were evaluated with Student’s t-test, and a p-value < 0.05 was considered statistically significant.
GEO Database Analysis
The datasets (GSE36376, GSE112790, and GSE121248) (Lim et al. 2013; Shimada et al. 2019; Wang et al. 2007) were obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds) and analyzed for differential DCLK1 mRNA expression between HCC and control samples. Differences between groups were assessed with Student’s t-test, and a p-value < 0.05 was considered statistically significant.
Analysis UALCAN
UALCAN (http://ualcan.path.uab.edu/) is a comprehensive web-based tool that provides access to The Cancer Genome Atlas (TCGA) database and facilitates the visualization and analysis of OMICS data in cancer, allowing users to quickly identify mRNA gene expression of interest and assess its correlation with clinicopathological parameters (Chandrashekar et al. 2017). This study analyzed DCLK1 expression by age, gender, race, weight, cancer stage, tumor grade, histopathological subgroups, nodal metastasis status, and TP53 mutation status. The correlation was considered statistically significant when p-values were < 0.05.
Analysis GEPIA
GEPIA (http://gepia.cancer-pku.cn) is a web-based tool for visualizing and analyzing RNA sequencing expression data of different cancer subtypes from TCGA and genotype-tissue expression (GTEx) projects through standard processing (Tang et al. 2017). Therefore, this web-based tool offers various functions, such as analyzing the expression of genes of interest in normal/tumor tissues in different types of cancer. In this research, the GEPIA web-based tool was used to compare the expression of DCLK1 between normal liver tissue and HCC tissue using a threshold of p-value < 0.05.
Analysis HPA
The HPA (www.proteinatlas.org/pathology) is a comprehensive web-based tool that provides researchers with highly relevant information on immunohistochemistry results for human proteins in normal human tissues and different types of human tumours (Uhlén et al. 2015). In this research, the HPA web-based tool was used to compare DCLK1 protein expression between normal liver tissue and HCC tissue by immunohistochemical staining.
Immune Infiltration Analysis
The Tumour Immune Estimation Resource (TIMER 2.0) (http://timer.cistrome.org/) is a comprehensive web-based tool for the analysis of immune infiltrates in various cancer types from the TCGA database (Li et al. 2020). This study first used TIMER 2.0 to analyze DCLK1 expression in various tumors and adjacent normal tissues. Subsequently, we evaluated the correlations between DCLK1 expression, immune cell infiltrations, and multiple immune markers. TISIDB is a comprehensive web-based tool for high-throughput analysis of genetic data in oncoimmunology to identify and predict the association of specific genes with tumor immune cell infiltration (Ru et al. 2019). In this study, TISIDB web-based tool (http://cis.hku.hk/TISIDB) was used to comprehensively study the correlation between DCLK1 expression and tumor-infiltrating lymphocytes (TILs) and immunostimulators, immunoinhibitors, MHC molecules, chemokines, and receptors. The correlation was considered statistically significant when p-values were < 0.05.
Results
DCLK1 is Overexpressed in Different Types of Human Cancers, Including HCC
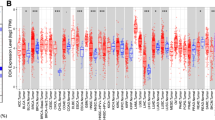
We analyzed DCLK1 mRNA expression levels in HCC with the help of multiple web-based tools. Initially, we performed an analysis in TCGA and GTEx database with TIMER 2.0, UALCAN and GEPIA web-based tool. As shown in Fig. 1A, DCLK1 mRNA expression was significantly increased in 6 cancer types, including Liver Hepatocellular Carcinoma (LIHC), Cholangiocarcinoma (CHOL), Kidney Renal Clear Cell Carcinoma (KIRC), Kidney Renal Papillary Cell Carcinoma (KIRP), Lung Adenocarcinoma (LUAD), Pheochromocytoma and Paraganglioma (PCPG) than in their corresponding normal tissues. In contrast, DCLK1 was down-regulated in 14 cancer types, including Bladder Urothelial Carcinoma (BLCA), Breast Invasive Carcinoma (BRCA), Cervical and Endocervical Cancer (CESC), Colon Adenocarcinoma (COAD), Esophageal Carcinoma (ESCA), Glioblastoma Multiforme (GBM), Head and Neck Cancer (HNSC), Kidney Chromophobe (KICH), Pancreatic Adenocarcinoma (PAAD), Prostate Adenocarcinoma (PRAD), Rectum Adenocarcinoma (READ), Stomach Adenocarcinoma (STAD), Thyroid Carcinoma (THCA) and Uterine Corpus Endometrial Carcinoma (UCEC).
Furthermore, the data obtained from UALCAN web-based tool showed that DCLK1 mRNA was significantly higher in HCC tissues (371 cases) than in normal liver tissues (50 cases) (p < 0.05), as shown in Fig. 1B. Meanwhile, data obtained from GEPIA web-based tool showed that DCLK1 mRNA was significantly higher in tissues with HCC (369 cases) than in normal liver tissues (160 cases) (p < 0.05) as shown in Fig. 1C. Subsequently, we evaluated DCLK1 mRNA expression levels in 3 HCC datasets from GEO databases, the results obtained showed that DCLK1 mRNA was statistically significantly overexpressed in liver cancer tissues relative to normal liver tissues (Fig. 1D-F).
In addition, we analyzed DCLK1 protein expression levels in HCC with the help of HPA web-based tool. immunohistochemical results obtained from HPA indicated that DCLK1 showed low expression in normal liver tissue samples (Fig. 1G-I) and high expression in HCC tissues (Fig. 1J-L). Our results demonstrated that DCLK1 expression at both mRNA and protein levels was higher in HCC patients than in healthy controls, suggesting that DCLK1 may play a role in HCC.
DCLK1 expression levels in hepatocellular carcinoma and different cancers in TIMER 2.0, UALCAN, GEPIA, and HPA web-based tools. A DCLK1 expression levels in different types of cancer tissues from TCGA database by analysis in TIMER 2.0 web-based tool. B Box plot of DCLK1 expression in hepatocellular carcinoma compared with normal tissues using UALCAN web-based tool. C Box plot of DCLK1 expression in hepatocellular carcinoma compared with normal tissues using GEPIA web-based tool. D–F Box plot of DCLK1 expression in hepatocellular carcinoma compared to normal tissues using 3 GEO data sets (GSE36376, GSE112790, and GSE121248). G–L Representative images of immunohistochemical staining for DCLK1 in normal liver tissues and HCC tissues from HPA, black arrows indicate DCLK1-positive areas. Note: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001
DCLK1 is Overexpressed in Rat Liver Tumors
To obtain more reliable results, we validated DCLK1 expression at the mRNA and protein level in the MRHM model of HCC. Initially, we analyzed the expression profile of tumor marker GGT to assess the development of nodules and tumors in rat liver. GGT is a protein with enzymatic activity that is overexpressed in various liver cancer models and is considered a marker for early detection of carcinogenic development (Carrasco-Torres et al. 2017). Nodular and tumor areas were detected by the presence of red-colored areas (GGT+) in liver sections (Fig. 2A). GGT+lesions are observed 30 days after initiation of DEN treatment. At 5 months, remodeling occurs within the liver of treated rats. Some tissue lesions disappear; others remain. The latter are called persistent preneoplastic lesions. At 9 months, first tumors begin to appear. At 12 months, tumors in the rats are well defined, and at 18 months, tumors occupy most of the liver (Fig. 2A). After identifying preneoplastic lesions, tumors, and non-tumor tissue, the expression of DCLK1 messenger RNA by RT-PCR and DCLK1 protein by Western blot was determined. In Fig. 2B, we can see that, in all groups, DCLK1 messenger expression is increased concerning the control. The highest expression of DCLK1 messenger is observed in 12-month tumors in livers of fully treated rats followed by neoplastic lesions at 30 days. (p ≤ 0.05, n = 3). The increase in DCLK1 expression occurs early in the carcinogenic process (30d) and is maintained throughout the progression stage (9 m, 12 m, 18 m) in the MRHM. In Fig. 2C-D, we observe DCLK1 expression at the protein level in the different stages of the MRHM. DCLK1 is overexpressed in the livers of treated rats compared with their untreated controls. The overexpression of DCLK1 is statistically significant at times 7d, 11d, 16d, 30dN, 30dNN, 18mT, and 18mNT. These results show significant DCLK1 mRNA and protein overexpression during liver cancer progression.
DCLK1 expression during HCC progression in the MRHM. A Histochemical analysis of rat tissue samples from the MRHM. Preneoplastic lesions and HCC development were detected by GGT activity (red area). B Evaluation of gene expression by RT-qPCR of DCLK1. C Densitometric analysis of western blot signals. D Western blot of DCLK1 expression. d = day, m = month, NN = no nodule, N = nodule, NT = no tumor, T = tumor, C = control. Note: ∗p < 0.05, ∗∗p < 0.01
We utilized immunohistochemical analysis to visualize and determine specific DCLK1 expression in rat liver under normal conditions and after exposure to carcinogenic treatment. To identify preneoplastic and tumor lesions, we used GST-p as a marker (Fig. 3G-L). The results of our study unveiled the basal expression of DCLK1 in the normal adult rat liver, with positive staining predominantly observed in the bile duct areas (Fig. 3A, indicated by black arrows, signifying DCLK1-positive regions). Hepatocytes, on the other hand, did not exhibit immunoreactivity against DCLK1 (Fig. 3A). Furthermore, the results demonstrated a distinct expression pattern of DCLK1 in the livers of rats exposed to the carcinogen, particularly at the 30-day, 5, 9, 12, and 18-month time points in the murine model. Positive staining for DCLK1 was predominantly observed in tumor cells present in preneoplastic lesions and established tumors in the murine model (Fig. 3A-F, black arrows indicate DCLK1-positive areas). Notably, the highest expression of DCLK1 was observed at the 18-month time point following the onset of carcinogenic damage (Fig. 3F).
Relationship Between DCLK1 Expression and Clinicopathological Features of HCC Patients
We then analyzed clinical information from the TCGA-LIHC dataset samples in the UALCAN web-based tool to explore the relationship between DCLK1 mRNA expression and clinicopathological features of LIHC patients. The results indicated that DCLK1 mRNA expression was statistically significantly increased in the 21–40 and 61–80 age groups but not significantly for the 41–60 and 81–100 age groups compared to normal tissues (Fig. 4A). Analyses showed that DCLK1 expression was higher in gender female tissues subgroup compared to normal tissues (Fig. 4B). In addition, analysis of clinical subgroups showed that DCLK1 expression was higher in the Caucasian group; however, no significant increase was observed in the African-American and Asian subgroups compared to normal tissues (Fig. 4C). As shown in Fig. 4D, DCLK1 mRNA level was higher concerning patient weight, with a statistically significant increase observed in the extreme weight, obese, and extreme obese subgroups compared to normal tissues. Concerning the cancer stage, the results showed that DCLK1 had a statistically significant increase in stage 1, 2, and 4 subgroups, but no statistically significant increase was observed in the stage 3 subgroup (Fig. 4E).
On the other hand, concerning tumor grade, the results indicated that DCLK1 had a statistically significant increase in the grade 3 tumor subgroups, but no statistically significant increase was observed for the grade 1, 2, and 5 tumor subgroups (Fig. 4F). Furthermore, histopathological subgroups showed a statistically significant increase in the HCC and hepatocholangio carcinoma subgroups but not in the fibrolamellar carcinoma subgroup (Fig. 4G). A statistically significant increase was observed in patients with HCC without regional lymph node metastases but not in patients with metastases (Fig. 4H). DCLK1 showed a significant increase in the subgroups of patients with mutant and non-mutant TP53 status (Fig. 4I). Taken together, these results suggest that high DCLK1 expression may be closely associated with tumor progression.
Association between DCLK1 mRNA expression and clinicopathological features of HCC patients using UALCAN web-based tool. A Box plot of the relative expression of DCLK1 based on patients’ age. B Box plot of the relative expression of DCLK1 concerning the gender of the patients. C Box plot of the relative expression of DCLK1 concerning the race of the patients. D Box plot of the relative expression of DCLK1 concerning the weight of the patients. E Box plot of the relative expression of DCLK1 concerning cancer stages. F Box plot of the relative expression of DCLK1 concerning tumor grade. G Box plot of relative DCLK1 expression concerning histological subtypes. H Box plot of relative DCLK1 expression concerning nodal metastasis status. I Box plot of the relative expression of DCLK1 about TP53 mutation status. Note: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001
The Association of DCLK1 Expression and Immune Infiltration in HCC
Because DCLK1 has recently been reported to be overexpressed in different cancer types and correlates with the infiltration of multiple immune cell types (Wu et al. 2020), we further analyzed the correlation between DCLK1 expression and immune infiltration levels using the TIMER 2.0 and the TISIDB web-based tool. The results obtained from TIMER 2.0 showed that DCLK1 expression was significantly correlated with immune cell infiltration, including B cells, CD8 + T cells, CD4 + T cells, macrophages, neutrophils, dendritic cells, and myeloid-derived suppressor cells (Fig. 5).
We also assessed the relationship between DCLK1 expression and the abundance of 28 tumor-infiltrating lymphocytes (TILs) through the TISIDB web-based tool. As shown in the heat map in Fig. 6A, DCLK1 maintained a positive correlation with TILs in different cancer types. Specifically, in LIHC, DCLK1 expression was significantly correlated with 26 of the 28 TILs, including Activated CD8 T cell (Act_CD8), Central memory CD8 T cell (Tcm_CD8), Effector memory CD8 T cell (Tem_CD8), Activated CD4 T cell (Act_CD4), Central memory CD4 T cell (Tcm_CD4), Effector memory CD4 T cell (Tem_CD4), T follicular helper cell (Tfh), Type 1 T helper cell (Th1), Type 17 T helper cell (Th17), Type 2 T helper cell (Th2), Regulatory T cell (Treg), Activated B cell (Act_B), Immature B cell (Imm_B), Memory B cell (Men_B), Natural killer cell (NK), CD56bright natural killer cell (CD56bright), CD56dim natural killer cell (CD56dim), Myeloid derived suppressor cell (MDSC), Natural killer T cell (NKT), Activated dendritic cell (Act_DC), Plasmacytoid dendritic cell (pDC), Macrophage, Eosinophil, Mast cell (Mast), Monocyte, and Neutrophil (Fig. S1).
In addition, we also employed the TIMER 2.0 web-based tool to explore the correlation between DCLK1 and immune cell biomarker expression. As shown in Table 1, we observed that biomarkers of CD8+T cell (CD8A and CD8B), T cell (CD3D, CD3E, and CD2), B cell (CD19 and CD79A), Monocyte (CD86 and CD115), TAM (CCL2, CD68 and IL 10), M1 macrophages (INOS, IRF5 and COX2), M2 macrophages (CD163, VSIG4, MS4A4A), Neutrophils (CD11b, and CCR7), Dendritic cell (HLA − DPB1, HLA − DQB1, HLA − DRA, HLA − DPA1, BDCA − 1, BDCA − 4, and CD11c), Th1 (T − bet, STAT4, STAT1, and IFN − γ), Th2 (GATA3, STAT6, STAT5A, and IL13) Tfh (BCL6), Th17 (STAT3 and IL17A), Treg ( FOXP3, CCR8, STAT5B, and TGFβ) and T cell exhaustion (PD − 1, CTLA4, and TIM − 3) were significantly associated with DCLK1 expression. These results, therefore, suggest that DCLK1 is closely correlated with immune cell infiltration in HCC, leading us to hypothesize that DCLK1 plays an essential role in the tumor microenvironment and immune response during HCC development.
Correlation of DCLK1 expression with TILs, immunostimulators, immunoinhibitors, MHC molecules, chemokines, and receptors in HCC by TISIDB web-based tool. A Correlation between DCLK1 expression with TILs. B Correlation between DCLK1 expression and immunostimulators. C Correlation between DCLK1 expression and immunoinhibitors. D Correlation between DCLK1 expression and MHC molecules. E Correlation between DCLK1 expression and chemokines. F Correlation between DCLK1 expression and receptors
The Association of DCLK1 Expression and Immunostimulators, Immunoinhibitors, MHC Molecules, Chemokines, and Receptors in HCC
We used the TISIDB web-based tool to explore the relationship between DCLK1 and immunostimulators, immunoinhibitors, MHC molecules, chemokines, and receptors in HCC. As shown in the heat maps in Fig. 6B-F, DCLK1 maintained a positive correlation with immunostimulators, immunoinhibitors, MHC molecules, chemokines, and receptors in different cancer types. Specifically, in HCC, DCLK1 expression correlated positively with 29 of 45 immunostimulators, including C10orf54, CD27, CD276, CD28, CD40LG, CD48, CD80, CD86, CXCL12, CXCR4, ENTPD1, ICOS, IL2RA, IL6, KLRK1, LTA, TMEM173, TNFRSF17, TNFRSF18, TNFRSF25, TNFRSF4, TNFRSF8, TNFRSF9, TNFSF13, TNFSF13B, TNFSF15, TNFSF4, TNFSF9 and ULBP1 (Fig. S2). Furthermore, relative to HCC, DCLK1 expression correlated positively with 16 of 24 immunoinhibitors, including ADORA2A, BTLA, CD244, CD274, CD96, CSF1R, CTLA4, HAVCR2, KDR, LGALS9, PDCD1, PVRL2, TGFB1, TGFBR1, TIGIT and VTCN1 (Fig. S3). In the same vein, DCLK1 expression was positively correlated with 12 of the 21 MHC molecules, including B2M, HLA-DMA, HLA-DMB, HLA-DOA, HLA-DOB, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DQA2, HLA-DQB1, HLA-DRA, and HLA-DRB1 (Fig. S4). Likewise, concerning HCC, DCLK1 expression correlated positively with 22 of the 41 chemokines, including CCL2, CCL3, CCL4, CCL5, CCL11, CCL13, CCL14, CCL18, CCL19, CCL20, CCL21, CCL22, CCL23, CCL26, CX3CL1, CXCL1, CXCL9, CXCL12, CXCL13, CXCL14, CXCL16, and XCL2 (Fig. S5). Finally, it was observed that concerning HCC, DCLK1 expression was positively correlated with 14 of the 18 receptors, including CCR1, CCR2, CCR4, CCR5, CCR6, CCR7, CCR10, CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, and CX3CR1 (Fig. S6). Based on these results, we hypothesize that DCLK1 plays an essential role in immune infiltration and regulation in different human cancers, including HCC.
Discussion
HCC is one of the most common cancers worldwide, with a high mortality rate (Sung et al. 2021). Despite significant advances in the study of HCC, it remains one of the most challenging cancers to treat; therefore, prognosis for these patients is often devastating (Liu et al. 2015; Ren et al. 2020). Early detection of tumors represents the only chance for patients to have long-term survival; however, due to rapid progression of the disease, absence of characteristic symptoms that manifest during early stages, and lack of early diagnostic markers, the vast majority of patients are usually diagnosed at an advanced stage of HCC (L. Cao et al. 2020a, b; Ren et al. 2020). Therefore, the discovery of genes and proteins related to HCC progression, the identification of new therapeutic targets, and the detection of new early, specific and non-invasive diagnostic markers to improve the detection rate of HCC remain an unmet need.
Several investigations have supported the essential role of CSCs in the development, progression, and metastasis of a variety of cancers (Lee et al. 2022). In this sense, focusing on the study and understanding CSCs biological role, their driver genes and associated proteins to identify key molecules in cancer development, is a promising strategy to find new therapeutic targets and biomarker candidates for early diagnosis of HCC.
Therefore, in this study, we explored and evaluated expression levels of DCLK1, a putative CSCs marker during progression of HCC carcinogenic process of in the MRHM. In addition, using various web-based tools, we investigated expression levels of DCLK1 in human HCC. Then, we performed a comprehensive investigation regarding DCLK1 expression levels and their correlation with various clinicopathological parameters of HCC. Finally, we analyzed the correlation between DCLK1 expression and levels of immune cell infiltration in the tumor.
Previous studies have reported a link between DCLK1 overexpression and development of various cancers (Gagliardi et al. 2012; Liu et al. 2018, 2019; Lv et al. 2017; Panneerselvam et al. 2020; Weygant et al. 2014). For example, DCLK1 is overexpressed in different types of gastrointestinal cancers such as colon cancer, stomach cancer, and primary neuroendocrine tumors of the liver, gallbladder, and pancreas; furthermore, its overexpression is associated with a poor prognosis (Fan et al. 2020; Gagliardi et al. 2012; Wu et al. 2020). The results obtained in our study demonstrate that DCLK1 is overexpressed at the mRNA and protein level during the carcinogenic process of the MRHM, one of the most accepted rodent models for HCC study (Carrasco-Torres et al. 2017; Vásquez-Garzón et al. 2013). These results suggest that DCLK1 is overexpressed in the rat model of HCC, and therefore, the MRHM is indicated to further study DCLK1 biological role in HCC carcinogenic development.
To acquire more detailed information on DCLK1 expression in HCC progression, we performed a step-by-step bioinformatics analysis to evaluate the mRNA expression profile of DCLK1 using TIMER 2.0, UALCAN, GEPIA, GEO, and HPA web-based tools. The results obtained showed that, compared to normal tissues, DCLK expression in HCC was significantly overexpressed. In addition, results obtained from UALCAN web-based tool showed that DCLK1 expression is associated with cancer stages, histological subtypes, TP53 mutation status, and nodal metastasis status. However, even though an increase in DCLK1 expression was observed in patients with node metastases and without node metastases, this increase was only statistically significant in HCC patients without node metastases, but not in patients with nodal metastases is probably associated with the low number of samples (n = 4) for patients without nodal metastases; therefore, these results suggest further study of the relationship between DCLK1 expression and the metastatic status of HCC. Moreover, these results coincide with our findings in the MRHM rat model. Therefore, these results suggest that DCLK1 may play an essential role in the initiation and progression of HCC. Recent research has shown that DCLK1 is overexpressed in pancreatic cancer tissue, and its expression is associated with cancer progression and poor prognosis. Furthermore, they showed that silencing DCLK1 expression significantly abolished pancreatic cancer cells’ migratory and invasive capacities. Silencing DCLK1 in pancreatic cancer cells suppresses EMT by up-regulating the epithelial marker E-cadherin and down-regulating Bmi-1 and the mesenchymal markers Snail and Vimentin (Li et al. 2018). Previous studies have evaluated DCLK1 expression in tissue and plasma of HCC patients, observed a statistically significant increase in DCLK1 expression in HCC compared to controls, and revealed that increased DCLK1 expression was associated with poor prognosis in HCC patients (Fan et al. 2017; Sureban et al. 2015). Therefore, these results suggest that DCLK1 may participate in and contribute to the development and progression of HCC and represents a candidate biomarker for its diagnosis and assessment of cancer progression.
In the tumor microenvironment (TME) of HCC, infiltrating immune cells play a significant role in tumor development and are linked to poor prognosis (Cao et al. 2020a, b; Guo et al. 2022). Recent research has associated DCLK1 with immune cell infiltration in various cancers, such as gastric and colon cancer, particularly with TAM and Treg infiltration. Additionally, DCLK1 has been proposed to correlate with an increase in CD8+T-cell inhibitors like TGFB1, CXCL12, and its receptors (Wu et al. 2020). However, little is known about the correlation between DCLK1 and immune cell infiltration in HCC. Our analysis using TIMER 2.0 revealed a positive correlation between high DCLK1 expression and infiltration levels of B cells, CD8+T cells, CD4+T cells, macrophages, neutrophils, dendritic cells, and myeloid-derived suppressor cells in HCC. Similarly, correlation analysis between DCLK1 and 28 tumor-infiltrating lymphocytes (TILs) in TISIDB demonstrated a positive correlation between DCLK1 expression and 26 TILs in HCC. Furthermore, DCLK1 expression showed positive correlations with numerous immunostimulators, immunoinhibitors, MHC molecules, chemokines, receptors, and a significant number of immune cell marker genes in HCC.
Recent research in renal cell carcinoma (RCC) revealed that overexpression of DCLK1 is associated with increased M2 immunosuppressive macrophages and decreased infiltration of CD8+cytotoxic T cells. Treatment of RCC cells with a specific DCLK1 inhibitor (DCLK1-IN-1) reduced the expression of the immune checkpoint ligand PD-L1 and promoted immune-mediated tumor cell killing, both alone and in combination with anti-PD-L1 therapy (Ding et al. 2021). Similarly, in pancreatic cancer cell lines, overexpression of DCLK1 correlated with increased PD-L1 expression at mRNA and protein levels, while suppressing DCLK1 led to reduced PD-L1 expression (Yan et al. 2020). Consistent with these findings, our analysis using the TISIDB web-based tool showed a positive correlation between PD-L1 (CD274) expression and DCLK1 expression levels (Spearman correlation test: rho = 0.172, p = 0.000849). These results suggest that DCLK1 may actively participate in the tumor immune microenvironment and holds potential as a therapeutic target for HCC. This intriguing mechanism merits further investigation in future research.
Previous studies have suggested that FOXP3+Treg lymphocytes represent a prominent suppressor cell population within the TME and are associated with tumor progression, invasiveness and metastasis in HCC. These Treg lymphocytes play a crucial role in inhibiting the immune response, which impedes tumor cell killing and promotes cancer growth and spread (Granito et al. 2021). Of particular interest, the analysis conducted using the TIMER 2.0 web tool revealed significant correlations between DCLK1 expression and typical Treg lymphocyte markers. Specifically, a positive correlation was observed between DCLK1 and markers such as FOXP3 (Cor = 0.178, p value = 9e-04), CCR8 (Cor = 0.5, p value = 3.16e-23), STAT5B (Cor = 0.315, p value = 2.29e-09), and TGFB1 (Cor = 0.563, p value = 3.12e-30).
Increased DCLK1 expression has been linked to elevated infiltration of Treg lymphocytes in gastric and colon cancers, correlating with poor prognosis (Wu et al. 2020). Nevertheless, the prognostic roles of FOXP3+Treg lymphocytes remain controversial in various cancer types (Shang et al. 2015). For instance, in breast cancer, cervical cancer, renal cancer, melanoma, HCC, gastric cancer, and pancreatic cancer, higher numbers of tumor infiltrating FOXP3+Treg lymphocytes have been associated with higher tumor grade and worse prognosis (FUKUI et al. 2020; Mahmoud et al. 2011; Shang et al. 2015). Conversely, in estrogen receptor (ER)-negative breast cancer, ovarian cancer, esophageal cancer, and head and neck cancer, increased FOXP3+Treg lymphocyte infiltration has been linked to a more favorable prognosis (Leffers et al. 2009; Shang et al. 2015; West et al. 2013). A comprehensive understanding of the role of FOXP3+Treg lymphocytes and their correlation with DCLK1 expression across different cancer types is essential for deciphering tumor immunity and developing more effective immunotherapies.
Furthermore, DCLK1 has been implicated in suppressing tumor-specific cytotoxic T-cell activity and has been proposed to play a significant role in recruiting myeloid suppressor cells (MDSCs) via the CXCL1-CXCR2 pathway. This interaction fosters an immunosuppressive microenvironment within the tumor, dampening the antitumor immune response (Yan et al. 2023). These findings offer insights into the immunological mechanisms that enable tumors to evade immune surveillance and underscore the importance of DCLK1 as a key regulator in immune suppression. Understanding this pathway holds significant implications for developing targeted therapies that reverse immunosuppression in cancer, thus enhancing the effectiveness of immunotherapy and presenting new opportunities for treating cancers like HCC.
In summary, we evaluated DCLK1 expression levels during the progression of the HCC carcinogenic process in MRHM and analyzed the DCLK1 expression profile in HCC in multiple publics web-based tools Furthermore, we evaluated the correlation between DCLK1 expression and some clinicopathological features of patients and finally identified its correlation with immune cell infiltration in HCC. The findings indicate that DCLK1 is overexpressed in HCC, correlates with immune cell infiltration, and is associated with tumor progression. However, there are some limitations to our study. Namely, this study was based on the analysis of publics web-based tools and a rat model of HCC; therefore, there is a need to validate these results in samples from different cohorts of patients diagnosed with HCC. In addition, the biological function of DCLK1 expression in vitro and in vivo systems needs to be fully elucidated. This study provided information for future research on DCLK1 and the development and progression of HCC.
Conclusion
In conclusion, our study demonstrated that DCLK1 is overexpressed in HCC and was associated with clinicopathological parameters of the disease. Furthermore, high DCLK1 expression was positively correlated with the levels of infiltrating immune cells. Our research highlights the essential role of DCLK1 in the carcinogenic process, tumor immunity, and prognosis in HCC.
Data Availability
The datasets generated and/or analyzed during the present study are available on the TIMER 2.0, TISIDB, UALCAN, GEPIA, and HPA web-based tool, and are available upon request from the corresponding author.
References
Anwanwan D, Singh SK, Singh S, Saikam V, Singh R (2020) Challenges in liver cancer and possible treatment approaches. Biochim et Biophys acta Reviews cancer 1873(1):188314–188314. https://doi.org/10.1016/j.bbcan.2019.188314
Bayik D, Lathia JD (2021) Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer 21(8):526–536. https://doi.org/10.1038/s41568-021-00366-w
Broner EC, Trujillo JA, Korzinkin M, Subbannayya T, Agrawal N, Ozerov IV, Izumchenko E (2021) Doublecortin-like kinase 1 (DCLK1) is a Novel NOTCH Pathway Signaling Regulator in Head and Neck Squamous Cell Carcinoma. Front Oncol 11:677051
Cao L, Cheng H, Jiang Q, Li H, Wu Z (2020a) APEX1 is a novel diagnostic and prognostic biomarker for hepatocellular carcinoma. Aging 12(5):4573–4591. https://doi.org/10.18632/aging.102913
Cao Z, Weygant N, Chandrakesan P, Houchen CW, Peng J, Qu D (2020b) Tuft and cancer stem cell marker DCLK1: a new target to enhance anti-tumor immunity in the tumor microenvironment. Cancers (Basel) 12(12):3801. https://doi.org/10.3390/cancers12123801
Carrasco-Torres G, Monroy-Ramírez HC, Martínez-Guerra AA, Baltiérrez-Hoyos R, Romero-Tlalolini MdlÁ, Villa-Treviño S, Vásquez-Garzón VR (2017) Quercetin reverses Rat Liver Preneoplastic lesions Induced by Chemical Carcinogenesis. Oxidative Med Cell Longev 2017:4674918. https://doi.org/10.1155/2017/4674918
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S (2017) UALCAN: a portal for facilitating Tumor Subgroup Gene expression and survival analyses. Neoplasia 19(8):649–658. https://doi.org/10.1016/j.neo.2017.05.002
Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D (2021) Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res 149:1–61. https://doi.org/10.1016/bs.acr.2020.10.001
Ding L, Yang Y, Ge Y, Lu Q, Yan Z, Chen X, Weygant N (2021) Inhibition of DCLK1 with DCLK1-IN-1 suppresses renal cell Carcinoma Invasion and Stemness and promotes cytotoxic T-Cell-mediated Anti-tumor Immunity. Cancers (Basel) 13(22):5729. https://doi.org/10.3390/cancers13225729
Fan M, Qian N, Dai G (2017) Expression and prognostic significance of doublecortin-like kinase 1 in patients with hepatocellular carcinoma. Oncol Lett 14(6):7529–7537. https://doi.org/10.3892/ol.2017.7082
Fan M, Yan H, Gou M, Qian N, Dai G (2020) Divergent expression of DCLK1 in gastrointestinal neuroendocrine tumors and primary hepatic, gallbladder, and pancreatic neuroendocrine tumors. Int J Clin Exp Pathol 13(9):2249–2258
Fukui R, Fujimoto Y, Watanabe T, Inoue N, Miyoshi Bunahiguchit Y (2020) Association between FOXP3/CD8 lymphocyte ratios and Tumor infiltrating lymphocyte levels in different breast Cancer subtypes. Anticancer Res 40:2141–2150
Gagliardi G, Goswami M, Passera R, Bellows CF (2012) DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol 5:35–42. https://doi.org/10.2147/CEG.S30281
Gao T, Wang M, Xu L, Wen T, Liu J, An G (2016) DCLK1 is up-regulated and associated with metastasis and prognosis in colorectal cancer. J Cancer Res Clin Oncol 142(10):2131–2140. https://doi.org/10.1007/s00432-016-2218-0
Granito A, Muratori L, Lalanne C, Quarneti C, Ferri S, Guidi M, Muratori P (2021) Hepatocellular carcinoma in viral and autoimmune liver diseases: role of CD4 + CD25 + Foxp3 + regulatory T cells in the immune microenvironment. World J Gastroenterol 27(22):2994–3009. https://doi.org/10.3748/wjg.v27.i22.2994
Guo Y, Yang J, Ren K, Tian X, Gao H, Tian X, Kan Q (2022) The heterogeneity of immune cell infiltration landscape and its immunotherapeutic implications in hepatocellular carcinoma. Front Immunol 13:861525
Khalaf K, Hana D, Chou JT-T, Singh C, Mackiewicz A, Kaczmarek M (2021) Aspects of the Tumor Microenvironment involved in Immune Resistance and Drug Resistance. Front Immunol 12:656364–656364. https://doi.org/10.3389/fimmu.2021.656364
Lee TK, Guan XY, Ma S (2022) Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol 19(1):26–44. https://doi.org/10.1038/s41575-021-00508-3
Leffers N, Gooden MJM, de Jong RA, Hoogeboom B-N, ten Hoor KA, Hollema H, Nijman HW (2009) Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother 58(3):449–459. https://doi.org/10.1007/s00262-008-0583-5
Li J, Wang Y, Ge J, Li W, Yin L, Zhao Z, Wang H (2018) Doublecortin-like kinase 1 (DCLK1) regulates B Cell-Specific Moloney murine leukemia virus insertion site 1 (Bmi-1) and is Associated with Metastasis and Prognosis in Pancreatic Cancer. Cell Physiol Biochem 51(1):262–277. https://doi.org/10.1159/000495228
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Liu XS (2020) TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res 48(W1):W509–w514. https://doi.org/10.1093/nar/gkaa407
Lim H-Y, Sohn I, Deng S, Lee J, Jung SH, Mao M, Xu J, Wang K, Shi S, Joh JW, Choi YL, Park C-K (2013) Prediction of Disease-free Survival in Hepatocellular Carcinoma by Gene Expression Profiling. Ann Surg Oncol 20(12):3747–3753. https://doi.org/10.1245/s10434-013-3070-y
Liu CY, Chen KF, Chen PJ (2015) Treatment of Liver Cancer. Cold Spring Harb Perspect Med 5(9):a021535. https://doi.org/10.1101/cshperspect.a021535
Liu X, Long X, Liu W, Zhao Y, Hayashi T, Yamato M, Ikejima T (2018) Type I collagen induces mesenchymal cell differentiation into myofibroblasts through YAP-induced TGF-β1 activation. Biochimie 150:110–130. https://doi.org/10.1016/j.biochi.2018.05.005
Liu H, Wen T, Zhou Y, Fan X, Du T, Gao T, An G (2019) DCLK1 plays a metastatic-promoting role in human breast cancer cells. Biomed Res Int 2019:1061979. https://doi.org/10.1155/2019/1061979
Liu Y-C, Yeh C-T, Lin K-H (2020) Cancer Stem Cell functions in Hepatocellular Carcinoma and Comprehensive therapeutic strategies. Cells 9(6):1331. https://doi.org/10.3390/cells9061331
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Finn RS (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7(1):6. https://doi.org/10.1038/s41572-020-00240-3
Lv Y, Song G, Wang R, Di L, Wang J (2017) Doublecortin-like kinase 1 is a novel biomarker for prognosis and regulates growth and metastasis in basal-like breast cancer. Biomed Pharmacother 88:1198–1205. https://doi.org/10.1016/j.biopha.2017.01.082
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR (2011) An evaluation of the clinical significance of FOXP3 + infiltrating cells in human breast cancer. Breast Cancer Res Treat 127(1):99–108. https://doi.org/10.1007/s10549-010-0987-8
Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, Tan Y, Nguyen MT (2019) Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol 25(19):2279–2293. https://doi.org/10.3748/wjg.v25.i19.2279
Panneerselvam J, Mohandoss P, Patel R, Gillan H, Li M, Kumar K, Chandrakesan P (2020) DCLK1 regulates Tumor Stemness and Cisplatin Resistance in Non-small Cell Lung Cancer via ABCD-Member-4. Mol Therapy - Oncolytics 18:24–36. https://doi.org/10.1016/j.omto.2020.05.012
Ren Z, Ma X, Duan Z, Chen X (2020) Diagnosis, therapy, and prognosis for hepatocellular carcinoma. Anal Cell Pathol 2020:8157406. https://doi.org/10.1155/2020/8157406
Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, Zhang J (2019) TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics 35(20):4200–4202. https://doi.org/10.1093/bioinformatics/btz210
Sánchez PS, Rigual MD, Djouder N (2021) Inflammatory and non-inflammatory mechanisms Controlling Cirrhosis Development. Cancers (Basel) 13(20):5045
Shang B, Liu Y, Jiang SJ, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3 + regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5:15179. https://doi.org/10.1038/srep15179
Shimada S, Mogushi K, Akiyama Y, Furuyama T, Watanabe S, Ogura T, Ogawa K, Ono H, Mitsunori Y, Ban D, Kudo A, Arii S, Tanabe M, Wands JR, Tanaka S (2019) Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 40:457–470. https://doi.org/10.1016/j.ebiom.2018.12.058
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Sureban SM, Madhoun MF, May R, Qu D, Ali N, Fazili J, Houchen CW (2015) Plasma DCLK1 is a marker of hepatocellular carcinoma (HCC): Targeting DCLK1 prevents HCC tumor xenograft growth via a microRNA-dependent mechanism. Oncotarget 6(35):37200–37215. https://doi.org/10.18632/oncotarget.5808
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45(W1):W98–W102. https://doi.org/10.1093/nar/gkx247
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Pontén F (2015) Proteomics. Tissue-based map of the human proteome. Science 347(6220):1260419. https://doi.org/10.1126/science.1260419
Vásquez-Garzón VR, Macias-Pérez JR, Jiménez-García MN, Villegas V, Fattel-Fazenta S, Villa-Treviño S (2013) The chemopreventive capacity of quercetin to induce programmed cell death in hepatocarcinogenesis. Toxicol Pathol 41(6):857–865. https://doi.org/10.1177/0192623312467522
Vásquez-Garzón VR, Beltrán-Ramírez O, Salcido-Neyoy ME, Cervante-Anaya N, Villa-Treviño S (2015) Analysis of gene expression profiles as a tool to uncover tumor markers of liver cancer progression in a rat model. Biomed Rep 3(2):167–172. https://doi.org/10.3892/br.2014.411
Vijai M, Baba M, Ramalingam S, Thiyagaraj A (2021) DCLK1 and its interaction partners: an effective therapeutic target for colorectal cancer. Oncol Lett 22(6):850–850. https://doi.org/10.3892/ol.2021.13111
Wang SM, Ooi LL, Hui KM (2007) Identification and validation of a novel gene signature associated with the recurrence of human hepatocellular carcinoma. Clin Cancer Res 13(21):6275–6283. https://doi.org/10.1158/1078-0432.CCR-06-2236
West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, Watson PH (2013) Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer 108(1):155–162. https://doi.org/10.1038/bjc.2012.524
Weygant N, Qu D, May R, Tierney RM, Berry WL, Zhao L, Houchen CW (2014) DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget 6(4):2193
Wu X, Qu D, Weygant N, Peng J, Houchen CW (2020) Cancer stem cell marker DCLK1 correlates with tumorigenic Immune infiltrates in the Colon and gastric adenocarcinoma microenvironments. Cancers (Basel) 12(2):274. https://doi.org/10.3390/cancers12020274
Yan R, Li J, Zhou Y, Yao L, Sun R, Xu Y, An G (2020) Inhibition of DCLK1 down-regulates PD-L1 expression through Hippo pathway in human pancreatic cancer. Life Sci 241:117150. https://doi.org/10.1016/j.lfs.2019.117150
Yan R, Li J, Xiao Z, Fan X, Liu H, Xu Y, Ge Y (2023) DCLK1 suppresses tumor-specific cytotoxic T lymphocyte function through recruitment of MDSCs via the CXCL1-CXCR2 Axis. Cell Mol Gastroenterol Hepatol 15(2):463–485. https://doi.org/10.1016/j.jcmgh.2022.10.013
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16(10):589–604. https://doi.org/10.1038/s41575-019-0186-y
Funding
This research was funded by the Consejo Nacional de Ciencia y Tecnología CONACyT, grant No. CF2019-53358 to Verónica Rocío Vásquez-Garzón, and Saúl Villa-Treviño.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and data analysis were performed by Juan Manuel Velázquez-Enríquez, Renata Cerna, Olga Beltrán-Ramírez, Carolina Piña-Vázquez, Saúl Villa-Treviño, and Verónica Rocío Vásquez-Garzón. The first draft of the manuscript was written by Juan Manuel Velázquez-Enríquez, Renata Cerna. Critical reading and correction of the manuscript was performed by Juan Manuel Velázquez-Enríquez, Renata Cerna, Olga Beltrán-Ramírez, Carolina Piña-Vázquez, Saúl Villa-Treviño, and Verónica Rocío Vásquez-Garzón. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All experimental animal studies were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of CINVESTAV-IPN, protocol 0168 − 15.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Velázquez-Enríquez, J.M., Cerna, R., Beltrán-Ramírez, O. et al. DCLK1 is Overexpressed and Associated with Immune Cell Infiltration in Hepatocellular Carcinoma. Biochem Genet (2024). https://doi.org/10.1007/s10528-024-10667-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10528-024-10667-y