Abstract
Existing research has confirmed the dysregulation of circular RNA (circRNA) in a wide variety of human diseases. Thus, in this study, we explored the potential mechanism of circRNA_0088196 in preeclampsia (PE). We performed quantitative real-time PCR to examine circRNA_0088196 expression and verified the function of circRNA_0088196 in vitro using CCK-8, TUNEL, flow cytometry, and Western blotting analyses. Additionally, we studied the mechanism using dual-luciferase reporter gene experiments. The results of our research revealed the up-regulation of circRNA_0088196 in PE patients’ placentas and Heat Shock 70 kDa Protein 5 (HSPA5)-stimulated trophoblast (HTR-8/SVneo) cells. An investigation of the mechanism also showed that there was a binding between miR-379-5p and circRNA_0088196. Additionally, circRNA_0088196 inhibited HTR-8/SVneo cell proliferation and promoted cell apoptosis via the miR-337-3p/HSPA5 axis, thereby facilitating PE. In vivo experiments indicated that circRNA_0088196 regulated HTR-8/SVneo cell production through miR-379-5p. Overall, the findings of this study illustrate that circRNA_0088196 interference promotes cell apoptosis and inhibits HTR-8/SVneo proliferation via the miR-379-5p/HSPA5 axis, thereby accelerating the development of PE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact Statement
Increasing evidence expounds that the dysregulation of circRNAs is associated with the progress of various human diseases. Nevertheless, it is currently unclear whether circRNA_0088196 acts as a ceRNA in the progress of PE. This study expounded that the interference with circRNA_0088196 restrained HTR-8/SVneo proliferation and boosted cell apoptosis through the miR-379-5p/HSPA5 axis, eventually accelerating the progress of PE.
Introduction
Preeclampsia (PE) refers to a complex systemic disease that involves clinical features such as proteinuria and new-onset hypertension. It affects approximately 3–5% of women and can lead to maternal mortality and fetal morbidity (Chappell et al. 2021). However, this condition has a complex background and unknown etiology. The exact cause of PE is still unknown, but it has significant implications for both short-term and long-term maternal and fetal health. Early prediction of PE is crucial in order to implement preventive measures and reduce the risk. However, diagnosing PE can be challenging due to its diverse initial symptoms (Vidaeff et al. 2021). Currently, termination of pregnancy is the most effective treatment for PE. However, the decision to terminate should consider the potential benefits and risks to both mother and infant, as well as predicting disease severity and adverse outcomes. Timely administration of appropriate treatment, including determining when to terminate the pregnancy, is essential. After the onset of PE, various pathological factors directly or indirectly affect trophoblasts—cells that play a critical role in placental development. These factors include suppressing inflammatory responses and promoting trophoblast apoptosis, progress (Zhang et al. 2021; Cheng et al. 2019) as emerging evidence indicates. Accordingly, studying trophoblast proliferation mechanisms as well as understanding how apoptosis influences PE progression is an important area for further research with significant implications for managing this condition effectively.
Circular RNA (circRNA) belongs to a special endogenous non-coding molecule class and is the primary focus of current RNA research. Unlike linear RNA, circRNA molecules have no 5′ end cap or 3′ end polyadenylation tail, and they exhibit tissue-specific expression and a stable structure (Szabo and Salzman 2016; Memczak et al. 2013). According to a growing number of reports, circRNA dysregulation is closely associated with the progression of a wide range of human diseases. Ye et al. illustrated that PE placental tissue raises circ-AK2 levels, and high circ-AK2 expression can boost apoptosis and restrain trophoblast proliferation (Ye et al. 2021). Besides, Hu et al. discovered that circ_0015382 overexpression impedes PE progression by inhibiting human trophoblast cell growth, migration, and invasion (Hu et al. 2021). In this study, we focused on circRNA_0088196, specifically known as circ-TNC, which is upregulated in PE cases (Liu et al. 2019), suggesting a possible connection between PE occurrence and circRNA_0088196. Additionally, growing evidence suggests that the binding sites of miRNA contain a considerable amount of circRNA, affecting downstream target mRNA expression through competing endogenous RNA (ceRNA) interaction with miRNA (Hansen et al. 2013; Zheng et al. 2016). Notably, circRNA_0088196 is derived from the exon 15 and 16 regions. Nevertheless, it remains unclear whether circRNA_0088196 is a ceRNA in PE development.
MicroRNA (miRNA) refers to a type of single-strand noncoding RNA with a length of 20–22 nucleotides. It exists extensively in eukaryotic organisms and can be combined with 3′UTR (untranslated region) of the target mRNA through complete or incomplete complementary pairing, resulting in the degradation of target genes or inhibition of protein translation. On that basis, a wide variety of biological processes are regulated, such as cell apoptosis, tissue differentiation, and organ development (Sheikh et al. 2016). Besides, existing research has suggested a correlation between PE development and abnormal miRNA expression (Brodowski et al. xxxx; Rokni et al. 2019). Further studies have also suggested that miR-30b-5p undergoes abnormal up-regulation after PE occurs, and the PE symptoms in rat models are reduced by suppressing miR-30b-5p expression and supplementing ferroptosis inhibitors (Zhang et al. 2020). Besides, Chen et al. reported that miR-203a-3p plays an anti-inflammatory role in pregnant women with PE through the down-regulation of IL24 (Ma et al. 2020). Notably, our previous research suggested the down-regulation of miR-379-5p in PE placental tissue (Inno et al. 2021). Nevertheless, the mechanism of miR-379-5p in PE requires further investigation.
HSPA5 is also known as glucose-regulated protein 78 (GRP78) or the immunoglobulin heavy chain binding protein. It belongs to the heat shock protein 70 (Hsp70) family and principally exists in the endoplasmic reticulum (ER). Besides, HSPA5 participates in the genesis and development of numerous ailments (Lee 2014) and is also a therapeutic target for various diseases (Booth et al. 2015; Rezanezhad et al. 2013). The ER signal sequence at the N end and the KDEL search sequence at the C end of HSPA5 are structural features that are unique from other Hsp70 proteins. This distinctive structure enables HSPA5 to transfer to the ER and maintain the form of an ER protein (Wang et al. 2017). Moreover, HSPA5 regulates endoplasmic reticulum homeostasis, mediates endoplasmic reticulum-related cell apoptosis, controls the inflammatory signal pathway, and performs other functions related to the pathogenesis of PE. The results of bioinformatics analysis have indicated that the target molecule hsa-mir-379-5p binds to hsa-circ_0088196, while hsa-mir-379-5p has the potential to exert the negative targeted regulation of HSPA5.
The expressions of circRNAs were screened in healthy controls and patients with PE. We established that hsa-circ_0088196 was the most significantly up-regulated circRNA, and RT-qPCR further verified that it was up-regulated by a factor of approximately four in the placentas of patients with PE. This suggested that hsa-circ_0088196 may participate in the regulation of the PE pathophysiological process. Subsequently, after we transfected trophoblast cells with adenovirus-encapsulated circRNA_0088196, cell apoptosis increased while the proliferation capacity fell drastically. In contrast, using shRNA to knock down circRNA_0088196 significantly promoted the proliferation of trophoblast cells and inhibited the number of apoptotic cells. Thus, circRNA_0088196 may be a new target for regulating the proliferation and apoptosis of trophoblast cells. However, the molecular signal pathway for regulating the function of trophoblast cells still requires further clarification.
Materials and Methods
Patients and Tissue Samples
The Tongde Hospital of Zhejiang Province provided a total of 20 participants between January 2020 and January 2021, including ten patients with preeclampsia (Group I) and ten healthy pregnancy controls (term delivery) (Group II). The demographic characteristics of participants are shown in Table 1. Preeclampsia refers to systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg on at least two occasions after the 20-week gestation period, as well as significant proteinuria (> 2 g/24 h and/or 2+ on dipstick testing) or evidence of multiorgan problems, such as central nervous system perturbations, liver dysfunction, thrombocytopenia, oliguria, seizures, or pulmonary edema (Brown et al. 2018).
All participants in the study opted for cesarean section delivery. The exclusion criteria included HELLP syndrome, fetal malformation, thrombophilic conditions, autoimmune diseases, heart diseases, chronic hypertension, chronic nephritis, diabetes, and multiple pregnancies. When the placenta was extracted from the uterus, the excision of placental tissue pieces (~ 1.0 cm3) was performed in random regions, such that areas of infarction, calcification, or vessels were avoided. Sterilized saline water was utilized to wash the samples three times, then the samples were kept at − 80 ℃ for subsequent application. Informed consent was obtained from all placenta donors and approval was given by the Ethics Committee of the Tongde Hospital of Zhejiang Province.
RNA Extraction and Reverse Transcription-Quantitative Real-Time PCR (RT-qPCR)
The isolation of RNA from placental tissue specimens or HTR-8/SVNEO cells was achieved using TRIzol reagent (#10606ES60; Yeasen, Shanghai, China). The thermal conditions used for RT-qPCR are as follows. Amplification: 40 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. Extension at 72 °C for 5 min and termination. Subsequently, the synthesis of complementary DNA (cDNA) was conducted with First-Strand cDNA Synthesis Master Mix (#11123ES60; Yeasen, Shanghai, China). Besides, qPCR was carried out using SYBR Green Master Mix (#11202ES08; Yeasen, Shanghai, China). The fold change was examined by applying the \(2^{{ - \Delta \Delta C_{{\text{t}}} }}\) method with U6 acting as an internal control of circRNA_0088196 and miR-379-5p or GAPDH as an internal reference of HSPA5. We designed the primers in the primer 3.0 (https://primer3.ut.ee/), t he specific primer sequences were shown in Table 2.
About quantification of miR-379-5p expression, \(2^{{ - \Delta \Delta C_{{\text{t}}} }}\) method was applied to represent the relative expression. Firstly, ΔCt was calculated by subtracting the Ct value of U6 from the Ct value of miR-379-5p. Then, subtract the average ΔCt of healthy pregnant subjects from the ΔCt value of every sample to obtain ΔΔCt value. Finally, calculate the \(2^{{ - \Delta \Delta C_{{\text{t}}} }}\) value to represent the relative expression of miR-379-5p.
Western Blotting
The extraction of total protein was conducted using radioimmunoprecipitation assay (RIPA) buffer (#P0013B; Beyotime, Beijing, China). Also, 30 μm protein was utilized for SDS-PAGE, followed by transference to a polyvinylidene fluoride (PVDF) membrane. The non-specific protein signal was blocked with 5% skim milk for 1 h. The primary antibodies (HSPA5; #11,587-1-AP/66574-1-Ig; 1/3000; Proteintech, Wuhan, China; Cyclins; #4656T; 1/2000; CST, China; and GAPDH; #ab186930; 1/5000; Abcam, USA) were incubated at 4 ℃ overnight. Next, the protein was held for 1 h at 37 ℃ with HRP-conjugated goat anti-rabbit secondary antibodies (#SA00001-2; 1/5000; Proteintech, Wuhan, China). ECL reagent was used to capture the protein band image and the gray value was examined using ImageJ software.
ELISA
The placenta samples of both Group I (N = 10) and Group II (N = 10) were quantitatively analyzed using a sandwich enzyme-linked immunosorbent assay (ELISA) for pro-inflammatory HSPA5. A quantikine analysis of HSPA5 was performed according to the protocols of the ELISA kit manufacturer (Xiamen Lunchangshuo Biotechnology Co., Ltd; catalog no. ED-10036). The minimum detectable dose for HSPA5 was typically < 10 pg/ml.
Dual-Luciferase Report
The amplification of specific circRNA_0088196 and HSPA5 3′-untranslated region (3′-UTR) sequences harboring miR-379-5p binding sites was realized. They were then cloned as the pmirGLO vectors for the production of the corresponding wild-type luciferase reporter system (circRNA_0088196-WT or HSPA5-WT). Mutation of the putative binding site sequences was achieved, and these sequences were introduced into pGL3-basic to build a mutated luciferase reporter system (circRNA_0088196-MUT and HSPA5-MUT). Thereafter, 50 ng pGL3 vectors, 10 ng pRL-TK Renilla plasmids, and 50 nM miR-379-5p mimics were co-transfected into HTR8 cells. To examine relative luciferase activity, the Dual-Luciferase Reporter Assay System (Promega, USA) was applied.
Cell Culture
Roswell Park Memorial Institute-1640 (RPMI-1640) medium with 100 μg/ml streptomycin, 100 U/ml penicillin, and 10% fetal bovine serum (FBS) was used to maintain the HTR-8/SVNEO human trophoblast cells. A 5% CO2 incubator was employed to grow cells at 37 ℃.
Transfection of Oligonucleotides and Overexpression Plasmids
The specific oligonucleotides and plasmids of circRNA_0088196 and HSPA5 were sourced from GenePharma (Shanghai, China). The specific small interfering RNAs (siRNAs) at a final concentration of 50 nM targeting circRNA_0088196 and HSPA5 were shown in Table 3. Full-length circRNA_0088196 and HTRA1 were separately cloned into pLX2 and pVL3 overexpression vectors. Moreover, GenePharma (Shanghai, China) provided mimics and inhibitors to match the negative controls (NCs) of miR-379-5p. Using Lipofectamine® 2000 reagent (Thermo Fisher, USA), the above plasmids and oligonucleotides were transfected into the HTR-8/SVNEO cell line. After 48 h, the transfection effects were estimated using RT-qPCR.
Cell Proliferation Assay
Based on the manufacturer’s guidelines, Cell Counting Kit-8 (CCK-8) kits were utilized to assess cell proliferation. To achieve this, the HTR-8/SVNEO cell line was seeded onto 96-well plates and incubated for 48 h. Next, 10 μm CCK-8 solution was added to the respective wells and the cells were incubated for another 3 h. A microplate reader was employed to examine optical density values at 450 nm.
Terminal Deoxynucleotidyl Transferase-Mediated Nick End Labeling (TUNEL) Assay
Cell apoptosis was evaluated with a TUNEL kit (#ATK00001; AtaGenix, Wuhan, China). After PBS was used to wash the HTR-8/SVneo cells, the cells were fixed with 4% paraformaldehyde and ethanol was used to permeabilize the cells. Subsequently, 50 μl TUNEL assay solution was administrated to the HTR-8/SVneo cells for 60 min at 37 ℃ without light, and then anti-fluorescence quenching liquid was used to seal the cells. A fluorescence microscope was used to acquire the TUNEL results, with 515–565 nm emission light and 450–500 nm excitation light. The apoptotic cells were determined in five random regions, and the experiments were performed in triplicate.
Cell Cycle Assay
The HTR-8/SVNEO cells were cleaned with PBS, and then 0.25% trypsin was added to digest the cells. When the cells adhering to the wall were rounded or detached from the wall, the digestion process was terminated by adding complete culture medium. Then, centrifugation of cells was performed for 5 min at 1500 × g and PBS was used to wash the cells at 4 ℃. The precipitates were resuspended with pre-cooled anhydrous ethanol and fixed overnight. Next, the cells were placed in a centrifuge at 350 × g for 5 min, before the supernatant was removed. The cells were exposed to PI solution (#421301; Biolegend, Beijing, China) and RNase A solution (#B500474; Sangon Biotech, Shanghai, China) at ambient temperature for 30 min without light. The cell cycle was estimated with FACScan flow cytometry.
Transwell Assay
For the preparation of the cell suspension (3 × 105 cells/mL), HTR-8/SVNEO cells were trypsinized and administrated with serum-free RPMI 1640 medium. Next, 200 μl cell suspension was pipetted into the upper chamber of a 24-well transwell with a precoated Matrigel matrix (#356234; BD Biocoat, Beijing, China). Following that, 600 μl RPMI 1640 medium and 10% FBS were introduced into the lower chamber of each respective well. A 5% CO2 cell incubator was used to incubate the transwell chamber at 37 ℃ for 24 h. Afterward, the chamber was removed and a cotton swab was used to wipe the non-migrated cells from the upper layer. Next, the chamber was washed three times with sterile PBS, and 4% paraformaldehyde was used to fix the cells for 20 min (#P0099; Beyotime, Shanghai, China). Subsequently, we used 0.1% crystal violet for 15-min staining of the cells (#C0121; Beyotime, Shanghai, China). Finally, we calculated the invaded cell number at five randomly selected locations.
Statistical Analysis
SPSS 23.0 was used to perform the statistical analysis. The normality of the data for the quantitative variables was assessed using the Shapiro–Wilk test. If the data followed a normal distribution, the mean and standard deviation were used to represent the data. For the comparison between two groups, an independent samples t-test was employed. For the comparison among multiple groups, a one-way analysis of variance (ANOVA) was conducted. Post hoc comparisons were performed using the least-significant difference (LSD) test. The results were considered statistically significant when p < 0.05.
Results
CircRNA_0088196 and HSPA5 are Overexpressed and miR379-5p is Reduced in PE Placental Tissue
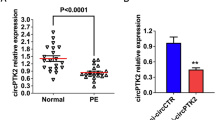
The expression of circRNA_0088196 (chr9:117800539-117819704), miR379-5p, and HSPA5 was measured by RT-qPCR in PE placental tissue from ten patients with PE and ten healthy pregnant subjects. Compared to the healthy controls, circRNA_0088196 expression was considerably up-regulated in PE patients (Fig. 1A). Conversely, there was significant down-regulation of miR379-5p expression in PE patients (Fig. 1B). Furthermore, HSPA5 expression was substantially up-regulated in PE placental tissue (Fig. 1C). HSPA5 expression was further verified by western blotting and ELISA, The western blotting and ELISA results were consistent with the trend of RT-qPCR (Fig. 1D–F). Generally, circRNA_0088196 and HSPA5 were abnormally expressed in patients with PE. The correlation of the expression of circRNA_0088196, miR-379-5p, and HSPA5 was presented in Fig. 1G.
Circ-0088196 and HSPA5 are overexpressed, and miR-397-5p is down-regulated in PE placental tissues. A–C RT-qPCR analysis of the expression of A circ-0088196, B miR-397-5p, and C HSPA5 in placental tissue specimens from PE patients and healthy pregnancy subjects. D Levels detection of HSPA5 expression levels in PE patients and healthy pregnancy subjects using ELISA. E, F Western blotting of HSPA5 expression in placental tissue specimens from PE patients and healthy pregnancy subjects. G The correlation of the expression of circRNA_0088196, miR-379-5p, and HSPA5. The statistical analysis was conducted using Independent-Sample t-test. Mean ± MES, n = 3; *p < 0.05; **p < 0.01; and ***p < 0.001
CircRNA_0088196 Enables the Sponging of miR379-5p to Up-regulate HSPA5
Dual-luciferase reporter experiments were conducted to verify the above interactions between circRNA_0088196 and miR379-5p and between miR379-5p and HSPA5. The relative luciferase activity fell markedly following the co-transfection of circRNA_0088196-WT and miR379-5p mimics but not the co-transfection of circRNA_0088196-MUT or miR379-5p (Fig. 2A, B). This suggested that circRNA_0088196 exerted a sponge effect on miR379-5p. Moreover, there was a notable decrease in relative luciferase activity after the co-transfection of HSPA5-WT and miR379-5p mimics (Fig. 2C, D). However, relative luciferase activity did not change significantly after the co-transfection of HSPA5-MUT and miR379-5p mimics, confirming that miR379-5p was directly bound to HSPA5. In summary, circRNA_0088196 enabled the up-regulation of HSPA5 expression by sponging miR379-5p.
Circ-0088196 sponges miR-397-5p to up-regulate HSPA5. A Schematic diagram of the binding sites of circ-0088196 with miR-397-5p. B Relative luciferase activity in HTR-8/SVNEO cells when co-transfection of circ-0088196-WT or circ-0088196-MUT with miR-397-5p mimics. C Schematic diagram of the binding sites between miR-397-5p and HSPA5. D Relative luciferase activity in HTR-8/SVneo cells when co-transfection of HSPA5-WT or HSPA5-MUT and miR-397-5p mimics. The statistical analysis was conducted using one-way ANOVA and LDS test. Mean ± MES, n = 3; ***p < 0.001; ****p < 0.0001
MiR379-5p Interacts with CircRNA_0088196 and HSPA5 in Trophoblast Cells
In order to explore up-and-down relationships of circRNA_0088196, miR-379-5p, and HSPA5, the specific overexpression plasmids and oligonucleotides of circ-0088196 were separately transfected into HTR-8/SVNEO cells to overexpress or silence circ-0088196. RT-qPCR suggested that circ-0088196 expression was notably elevated by overexpression plasmids (Fig. 3A) and was notably silenced by two specific siRNAs targeting circ-0088196 (Fig. 3B). Moreover, we transfected specific overexpression plasmids and three oligonucleotides of HSPA5 into HTR-8/SVNEO cells. As shown in Fig. 3C, D, compared to the empty, HSPA5 expression was substantially up-regulated in OE-HSPA5. Western blotting indicated that three oligonucleotides of HSPA5 reduced the levels of HSPA5 (Fig. 3E, F).
Overexpress or silence circ-0088196 and HSPA5 in the trophoblast cell line. A, B RT-qPCR analysis of the expression of circ-0088196 in HTR-8/SVNEO cells transfected with specific overexpression plasmids or oligonucleotides of circ-0088196. C–F Western blotting analysis of HSPA5 expression in HTR-8/SVneo cells transfected with specific overexpression plasmids or oligonucleotides of HSPA5. The statistical analysis was conducted using one-way ANOVA and LDS test. Mean ± MES, n = 3; *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001
To further demonstrate that miR-379-5p is involved in crossregulation between circ-0088196 and HSPA5, we evaluated the expression of HSPA5. We transfected specific overexpression plasmids and oligonucleotides of circ-0088196 into HTR-8/SVNEO cells to overexpress and knock down circ-0088196 (Fig. 4A). Compared to NC mimics group, OE-circ_0088196 reduced the level of miR-379-5p expression, while si-cir-0088196 increased the level of miR-379-5p expression (Fig. 4B). As depicted in Fig. 4C–E, miR-379-5p mimics inhibited HSPA5 expression, and its expression was elevated when transfection with miR-379-5p inhibitor. Whereas, OE-circ_0088196 increased the level of HSPA5 expression. In contrast, Knockdown of circ_0088196 suppressed the mRNA and protein expression of HSPA5 in HTR-8/SVNEO cells. In general, circ-0088196 enabled to elevate HSPA5 expression through sponging miR-379-5p in trophoblast cells.
Circ-0088196 enables to sponge miR-397-5p to up-regulate HSPA5 in trophoblast cells. A RT-qPCR analysis of the expression of circ-0088196 in HTR-8/SVneo cells transfected with specific overexpression plasmids or oligonucleotides of circ-0088196. B RT-qPCR analysis of the expression of miR-379-5p in HTR-8/SVneo cells. C RT-qPCR analysis of the expression of HSPA5 in HTR-8/SVneo cells. D, E Western blotting analysis of the expression of HSPA5 in HTR-8/SVneo cells with specific overexpression plasmids and oligonucleotides. The statistical analysis was conducted using one-way ANOVA and LDS test. Mean ± MES, n = 3;*p < 0.05; **p < 0.01; and ****p < 0.0001
CircRNA_0088196 Weaken Proliferation and Facilitates Trophoblast Cell Apoptosis Through miR-397-5p/HSPA5 in Trophoblast Cells
To assess whether CircRNA_0088196 could affect trophoblast cells growth, the CCK8 assay was performed after treatment. In Fig. 5A, overexpressed circ-0088196 or HSPA5 notably inhibited proliferation of HTR-8/SVNEO cells, with opposite effects when circ-0088196 or HSPA5 was knocked out. Moreover, miR-379-5p mimics prominently enhanced proliferation of HTR-8/SVNEO cells, with opposite effects when miR-379-5p inhibitors were transfected. TUNEL staining analysis was conducted to observe the functions of circ-0088196, miR-379-5p, and HSPA5 on trophoblast cell apoptosis. As illustrated in Fig. 5B, C, both overexpressed circ-0088196 and HSPA5 significantly strengthened HTR-8/SVneo cell apoptosis, while knockdown of both significantly inhibited HTR-8/SVneo cell apoptosis. Furthermore, HTR-8/SVNEO cell apoptosis was suppressed by miR-379-5p mimics, while transfection with miR-379-5p inhibitors yielded opposite results. In summary, circ-0088196 enabled restraint of proliferation and facilitated trophoblast cell apoptosis through miR-379-5p/HSPA5 signaling.
Circ-0088196 weaken proliferation and facilitates trophoblast cell apoptosis through miR-397-5p/HSPA5 signaling. A CCK-8 analysis of the proliferation of HTR-8/SVneo cells transfected with specific overexpression plasmids or oligonucleotides of circ-0088196 or HSPA5, or miR-397-5p mimics or inhibitors. B, C TUNEL staining analysis of HTR-8/SVneo cells transfected with specific overexpression plasmids or oligonucleotides of circ-0088196 or HSPA5, or miR-397-5p mimics or inhibitors. Scale bar 50 μm; and magnification × 200. The statistical analysis was conducted using one-way ANOVA and LDS test. Mean ± MES, n = 3; *p < 0.05; and **p < 0.01
CircRNA_0088196 Induces G0/G1 Phase Arrest in Trophoblast Cells via miR379-5p/HSPA5 Signaling
The effects of circRNA_0088196, miR379-5p, and HSPA5 on the cell cycle of trophoblast cells were further evaluated. The results of this study demonstrated that both overexpressed circRNA_0088196 and HSPA5 significantly prolonged the G0/G1 phase of HTR-8/SVNEO cells, with the opposite effect when circRNA_0088196 and HSPA5 were knocked out (Fig. 6A, B). Nevertheless, miR379-5p mimics significantly shortened the G0/G1 phase of HTR-8/SVneo cells, while its inhibitors prolonged the G0/G1 phase. According to Fig. 6C, the S phase of HTR-8/SVneo cells was markedly shortened by the overexpression of circRNA_0088196 or HSPA5 and was prolonged by the knockdown of circRNA_0088196 or HSPA5. The effect of miR379-5p on the cell cycle of HTR-8/SVNEO cells was the opposite of circRNA_0088196 and HSPA5. However, circRNA_0088196, miR379-5p, and HSPA5 did not affect the G2/M phase of HTR-8/SVneo cells (Fig. 6D). Besides that, western blotting analysis of CyclinD1 protein levels in HTR8 cells was consistent with flow cytometry (Fig. 6E, F). Therefore, circRNA_0088196 triggered G0/G1 phase arrest in trophoblast cells by modulating miR379-5p/HSPA5 signaling.
Circ-0088196 induces G0/G1 phase arrest in trophoblast cells via miR-397-5p/HSPA5 signaling. A Cell cycle analysis of HTR-8/SVneo cells transfected with specific overexpression plasmids or oligonucleotides of circ-0088196 or HSPA5, or miR-397-5p mimics or inhibitors. B–D Evaluation of B G0/G1, C S and D G2/M phases of HTR-8/SVneo cells with specific overexpression plasmids or oligonucleotides of circ-0088196 or HSPA5, or miR-397-5p mimics or inhibitors. E, F Western blotting analysis of CyclinD1 protein levels in HTR8 cells. The statistical analysis was conducted using one-way ANOVA and LDS test. Mean ± SD, n = 3; Ns: no significance; ***p < 0.001; and ****p < 0.0001
CircRNA_0088196 Restrains Trophoblast Cell Invasion Through miR379-5p/HSPA5 Signaling
Further analysis demonstrated that overexpression of circRNA_0088196 or HSPA5 inhibited HTR-8/SVneo cell invasion (Fig. 7A, B). In contrast, circRNA_0088196 or HSPA5 knockdown enhanced HTR-8/SVneo cell invasion. Moreover, the invasive capacity of HTR-8/SVneo cells was noticeably strengthened by miR379-5p mimics, while the opposite occurred with miR379-5p inhibitor transfection. Hence, circRNA_0088196 restrained trophoblast cell invasion by regulating miR379-5p/HSPA5 signaling.
Circ-0088196 restrains trophoblast cell invasion through miR-397-5p/HSPA5 signaling. A, B Transwell analysis of the invasion of the HTR-8/SVneo cell line that was transfected with specific overexpression plasmids or oligonucleotides of circ-0088196 or HSPA5, or miR-397-5p mimics or inhibitors. Scale bar 50 μm; and magnification × 200. The statistical analysis was conducted using one-way ANOVA and LDS test. Mean ± SD, n = 3;*p < 0.05; and ***p < 0.001
Discussion
There are few effective strategies for treating PE (Phipps et al. 2019). Therefore, to investigate effective therapy options, there is an urgent need to investigate the underlying molecular mechanisms that enhance PE progress and it is necessary to identify new biological targets. In this study, we discovered that circRNA_0088196 was a key up-regulator of circRNAs, which are linked to the progression of PE. Additionally, further research revealed that circRNA_0088196 exerted a vital role in trophoblast cell proliferation, cell cycle progression, invasion, and apoptosis by modulating miR379-5p/HSPA5 signaling. Therefore, the findings of this study suggested that circRNA_0088196, miR379-5p, and HSPA5 were potential biomarkers and targets for the clinical diagnosis and treatment of PE.
In contrast to the controls, circRNA_0088196 expression was distinctly up-regulated and miR379-5p was significantly down-regulated in PE placental tissue. However, the expression and function of circRNA_0088196 and miR379-5p have already been widely reported. The HSPA5 gene (i.e., GRP78) encodes the Hsp70 chaperone binding immunoglobulin protein within the endoplasmic reticulum (Wang et al. 2017). Moreover, it exhibits substantial up-regulation in PE placental tissue compared to the controls, which is consistent with previous research (Du et al. 2017). Circulating HSPA5 may be an early predictive biomarker of PE in pregnant women (Laverriere et al. 2009). Results from the dual-luciferase reporter assay in this study suggested that circRNA_0088196 enabled the sponging of miR379-5p to up-regulate HSPA5 expression in trophoblast cells. By combining our findings with previous evidence, circRNA_0088196 may act as a sponge for miR379-5p and up-regulate endoplasmic reticulum stress by elevating HSPA5 during PE. Besides that, HSPA5 overexpression reversed the inhibitory action of miR379-5p on the biological behaviors of trophoblast cells. Du et al. argued that placental protein expression of the endoplasmic reticulum (ER) stress-related markers, including HSPA5, was higher in PE, suggesting that the exaggerated ER stress response was associated with increased apoptosis in placenta of PE patients (Du et al. 2017). Abdollahi et al. found miR-379 plays an important role in high-fat diet (HFD)-induced obesity through increased adipose inflammation, mitochondrial dysfunction, and ER stress as well as impaired adipogenesis and angiogenesis (Abdollahi et al. 2022). In PE, Xu’s team found miR-379 was significantly downregulated in severe PE placentas, compared with normal pregnant controls (Xu et al. 2014). Thus, we assumed that HSPA5 could regulate miR-379 through ER stress, and it was proved that miR-379-5p directly bound to circRNA_0088196 in trophoblast cells. By quantitatively assessing apoptosis in the placenta through electron microscopy, placental apoptosis rose noticeably as pregnancy progressed (Smith et al. 2000). Cytotrophoblast cell apoptosis in PE patients was considerably higher than that in full-term pregnancies with normal blood pressure (Longtine et al. 2012). Thus, an enhanced apoptotic level of trophoblast cells is a prominent pathological feature of PE. Our findings revealed that circRNA_0088196 facilitated apoptosis by modulating miR379-5p/HSPA5 signaling. Furthermore, circRNA_0088196 inhibited trophoblast cell proliferation and facilitated G0/G1 phase arrest through miR379-5p/HSPA5 signaling, implying that targeting circRNA_0088196 may exert a protective role on trophoblast cells.
The invasion of trophoblasts into the endometrium and vasculature is a crucial step in human placenta formation (Abbas et al. 2020). Patients with PE exhibit superficial trophoblast invasion and unconverted narrow spiral arteries (Zha et al. 2020). Trophoblasts develop from the trophectoderm, forming the blastocyst wall and creating both villous and extra-villous trophoblasts (Jia et al. 2017). From a physiological perspective, extra-villous trophoblasts attach the placenta to the uterine wall, thereby reshaping the spiral artery. Here, the interstitial trophoblasts destroy the middle layer of the artery by surrounding the spiral artery (Peng et al. 2019). Subsequently, the trophoblasts in the blood vessels move down the lumen of the artery from the cytotrophoblast shells, forming a loose embolus in the first trimester and temporarily congregating in the artery (Zhang et al. 2020). In the first half of pregnancy, the invasive abilities of trophoblasts are strictly modulated (Sun et al. 2021). In the tenth week, the basal decidua includes numerous extra-villous trophoblasts, extending into the myometrium during the 15th week (Su et al. 2021). In normal pregnancies, extra-villous trophoblasts further fuse to multinucleated giant cells, stopping in the inner third of the myometrium (Chen et al. 2020). However, the invasive capacity of trophoblasts falls considerably in pathological pregnancies, such that insufficient conversion of the spiral artery and blood flow disorders occur in the intervillous space (Ridder et al. 2019). CircRNA_0088196 and HSPA5 both inhibit the invasion of HTR-8/SVneo cells, while miR379-5p enhances the invasive capacity, implying that targeting circRNA_0088196 represents a potential strategy for improving the invasive capacity of trophoblasts in PE. Although we conducted a comprehensive series of studies to dissect the role of circRNA_0088196, miR379-5p, and HSPA5 in the pathogenesis of PE, further research is still necessary. In our future research, we will investigate the functions of circRNA_0088196, miR379-5p, and HSPA5 in distinct cell locations within the trophoblasts. Additionally, we will establish in vivo models to verify the function of circRNA_0088196, miR379-5p, and HSPA5 in PE and conduct a larger epidemiological survey to evaluate clinical diagnoses and the therapeutic significance of circRNA_0088196, miR379-5p, and HSPA5.
Conclusion
In summary, our study provides the first evidence that circRNA_0088196 is highly expressed in human PE placental tissue, and it restricts proliferation and invasion while triggering the apoptosis of trophoblast cells through miR379-5p/HSPA5 signaling. Our findings imply that circRNA_0088196 can be a promising therapeutic target for therapeutic interventions in PE.
References
Abbas Y, Turco MY, Burton GJ, Moffett A (2020) Investigation of human trophoblast invasion in vitro. Hum Reprod Update 26(4):501–513. https://doi.org/10.1093/humupd/dmaa017
Abdollahi M, Kato M, Lanting L, Tunduguru R, Wang M, Wang YM et al (2022) miR-379 mediates insulin resistance and obesity through impaired angiogenesis and adipogenesis regulated by ER stress. Mol Ther Nucleic Acids 30:115–130. https://doi.org/10.1016/j.omtn.2022.09.015
Booth L, Roberts JL, Cash DR, Tavallai S, Jean S, Fidanza A et al (2015) GRP78/BiP/HSPA5/Dna K is a universal therapeutic target for human disease. J Cell Physiol 230(7):1661–1676. https://doi.org/10.1002/jcp.24919
Brodowski L, Schroder-Heurich B, von Hardenberg S, Richter K, von Kaisenberg CS, Dittrich-Breiholz O et al (2021) MicroRNA profiles of maternal and neonatal endothelial progenitor cells in preeclampsia. Int J Mol Sci. https://doi.org/10.3390/ijms22105320
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S et al (2018) The hypertensive disorders of pregnancy: ISSHP classification, diagnosis and management recommendations for international practice. Pregnancy Hypertens Int J Womens Cardiovasc Health 13:291–310. https://doi.org/10.1016/j.preghy.2018.05.004
Chappell LC, Cluver CA, Kingdom J, Tong S (2021) Pre-eclampsia. Lancet (lond Engl) 398(10297):341–354. https://doi.org/10.1016/s0140-6736(20)32335-7. (Epub 31 May 2021)
Chen Q, Jiang SJ, Liu HH, Gao Y, Yang XX, Ren ZL et al (2020) Association of lncRNA SH3PXD2A-AS1 with preeclampsia and its function in invasion and migration of placental trophoblast cells. Cell Death Dis. https://doi.org/10.1038/s41419-020-02796-0
Cheng S-B, Nakashima A, Huber WJ, Davis S, Banerjee S, Huang Z et al (2019) Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. https://doi.org/10.1038/s41419-019-2162-4
Du L, He F, Kuang L, Tang W, Li Y, Chen D (2017) eNOS/iNOS and endoplasmic reticulum stress-induced apoptosis in the placentas of patients with preeclampsia. J Hum Hypertens 31(1):49–55. https://doi.org/10.1038/jhh.2016.17
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388. https://doi.org/10.1038/nature11993
Hu Z, Dong C, Dong Q (2021) Circ_0015382 is associated with preeclampsia and regulates biological behaviors of trophoblast cells through miR-149-5p/TFPI2 axis. Placenta 108:73–80. https://doi.org/10.1016/j.placenta.2021.03.005
Inno R, Kikas T, Lillepea K, Laan M (2021) Coordinated expressional landscape of the human placental miRNome and transcriptome. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2021.697947
Jia YH, Li T, Huang XJ, Xu XH, Zhou XY, Jia LY et al (2017) Dysregulated DNA methyltransferase 3A upregulates IGFBP5 to suppress trophoblast cell migration and invasion in preeclampsia. Hypertension 69(2):356. https://doi.org/10.1161/HYPERTENSIONAHA.116.08483
Laverriere A, Landau R, Charvet I, Irion O, Bischof P, Morales M et al (2009) GRP78 as a marker of pre-eclampsia: an exploratory study. Mol Hum Reprod 15(9):569–574. https://doi.org/10.1093/molehr/gap037
Lee AS (2014) Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer 14(4):263–276. https://doi.org/10.1038/nrc3701
Liu S, Xie X, Lei H, Zou B, Xie L (2019) Identification of key circRNAs/lncRNAs/miRNAs/mRNAs and pathways in preeclampsia using bioinformatics analysis. Med Sci Monit 25:1679–1693. https://doi.org/10.12659/MSM.912801
Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM (2012) Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta 33(5):352–359. https://doi.org/10.1016/j.placenta.2012.01.017
Ma HY, Cu W, Sun YH, Chen X (2020) MiRNA-203a-3p inhibits inflammatory response in preeclampsia through regulating IL24. Eur Rev Med Pharmacol Sci 24(10):5223–5230
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441):333–338. https://doi.org/10.1038/nature11928
Peng W, Tong C, Li L, Huang CY, Ran YX, Chen XH et al (2019) Trophoblastic proliferation and invasion regulated by ACTN4 is impaired in early onset preeclampsia. FASEB J 33(5):6327–6338. https://doi.org/10.1096/fj.201802058RR
Phipps EA, Thadhani R, Benzing T, Karumanchi SA (2019) Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 15(5):275–289. https://doi.org/10.1038/s41581-019-0119-6
Rezanezhad L, Zolghadri J, Gharesi-Fard B (2013) Importance of anti-GRP78 antibody in pre-eclampsia. Iran J Immunol 10(4):238–246
Ridder A, Giorgione V, Khalil A, Thilaganathan B (2019) Preeclampsia: the relationship between uterine artery blood flow and trophoblast function. Int J Mol Sci. https://doi.org/10.3390/ijms20133263
Rokni M, Salimi S, Sohrabi T, Asghari S, Teimoori B, Saravani M (2019) Association between miRNA-152 polymorphism and risk of preeclampsia susceptibility. Arch Gynecol Obstet 299(2):475–480. https://doi.org/10.1007/s00404-018-4979-y
Sheikh AM, Small HY, Currie G, Delles C (2016) Systematic review of micro-RNA expression in pre-eclampsia identifies a number of common pathways associated with the disease. PLoS ONE. https://doi.org/10.1371/journal.pone.0160808
Smith SC, Leung TN, To KF, Baker PN (2000) Apoptosis is a rare event in first-trimester placental tissue. Am J Obstet Gynecol 183(3):697–699. https://doi.org/10.1067/mob.2000.106555
Su MT, Tsai PY, Wang CY, Tsai HL, Kuo PL (2021) Aspirin facilitates trophoblast invasion and epithelial–mesenchymal transition by regulating the miR-200-ZEB1 axis in preeclampsia. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2021.111591
Sun MN, Gao JL, Meng T, Liu SH, Chen HY, Liu Q et al (2021) Cyclin G2 upregulation impairs migration, invasion, and network formation through RNF123/Dvl2/JNK signaling in the trophoblast cell line HTR8/SVneo, a possible role in preeclampsia. FASEB J. https://doi.org/10.1096/fj.202001559RR
Szabo L, Salzman J (2016) Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet 17(11):679–692. https://doi.org/10.1038/nrg.2016.114
Vidaeff AC, Saade GR, Sibai BM (2021) Preeclampsia: the need for a biological definition and diagnosis. Am J Perinatol 38(09):976–982. https://doi.org/10.1055/s-0039-1701023
Wang J, Lee J, Liem D, Ping P (2017) HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 618:14–23. https://doi.org/10.1016/j.gene.2017.03.005
Xu P, Zhao YY, Liu M, Wang YQ, Wang H, Li YX et al (2014) Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 63(6):1276–1284. https://doi.org/10.1161/HYPERTENSIONAHA.113.02647
Ye Y, Li M, Chen L, Li S, Quan Z (2021) Circ-AK2 is associated with preeclampsia and regulates biological behaviors of trophoblast cells through miR-454-3p/THBS2. Placenta 103:156–163. https://doi.org/10.1016/j.placenta.2020.10.023
Zha WH, Guan S, Liu N, Li Y, Tian Y, Chen Y et al (2020) Let-7a inhibits Bcl-xl and YAP1 expression to induce apoptosis of trophoblast cells in early-onset severe preeclampsia. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.139919
Zhang H, He Y, Wang JX, Chen MH, Xu JJ, Jiang MH et al (2020) miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. https://doi.org/10.1016/j.redox.2019.101402
Zhang Y-C, Qin X-L, Ma X-L, Mo H-Q, Qin S, Zhang C-X et al (2021) CLDN1 regulates trophoblast apoptosis and proliferation in preeclampsia. Reproduction 161(6):623–632. https://doi.org/10.1530/REP-20-0677
Zheng QP, Bao CY, Guo WJ, Li SY, Chen J, Chen B et al (2016) Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. https://doi.org/10.1038/ncomms11215
Funding
Funding was provided via the following Grants: Basic Public Welfare Research Program of Zhejiang Province, China (No: LGF20H040003).
Author information
Authors and Affiliations
Contributions
ZX and HZ played a guiding role in carrying out the studies, collecting data and drafting the manuscript. SP helped to draft the manuscript. QW and WW was responsible for revision of the paper and the finalization of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Ethical Approval
All patients signed an informed consent with approval from the Institutional Ethic Review Committee of Tongde Hospital of Zhejiang Province.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, Z., Wang, Q., Pei, S. et al. CircRNA_0088196 Regulates Trophoblast Proliferation and Apoptosis in Preeclampsia Through the miR-379-5p/HSPA5 Axis. Biochem Genet 62, 1742–1761 (2024). https://doi.org/10.1007/s10528-023-10506-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-023-10506-6