Abstract
Several spiny leguminous tree species within the genus Neltuma Raf. (formerly Prosopis L.) (Fabaceae) occur as widespread invasive alien plants in South Africa, exerting severe negative socio-economic and ecological impacts. Given these impacts, South Africa recently released the leaf-tying moth Agnippe sp. #1 (syn. Evippe sp. #1) (Lepidoptera: Gelechiidae) as a biological control agent against invasive Neltuma species in 2021. The widespread invasion of Neltuma spp. across a vast and climatically diverse range of South Africa has led to concerns regarding the establishment and impact of the agent. Therefore, this study aimed to assess the constraints posed by climate to the potential establishment and efficacy of Agnippe sp. #1 using both climatic matching (CLIMEX) and thermal-physiology assessments. Climatic analyses revealed relatively high (71%) and moderate (66%) matches of South Africa to the native (Argentina) and introduced (Australia) ranges of Agnippe sp. #1 respectively. Thermal assessments of Agnippe sp. #1, particularly the 4th instar larvae, determined a CTmin = 0.9 ± 0.3 °C and LLT50 = −11.1 ± 0.4 °C, which suggest the moth is suited mainly to warmer regions of South Africa. Overall, these assessments propose that the establishment and performance of Agnippe sp. #1 is likely to be constrained by climate in parts of South Africa, particularly within the cold semi-arid and temperate provinces of the country. Promisingly, these climatic comparisons suggest that Agnippe sp. #1 may become more widely established in the hottest parts of the Northern Cape province, which remains a major biological control target region for Mesquite in South Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous leguminous species within the genus formerly described as Prosopis L. (Fabaceae: Caesalpinioideae) were intentionally introduced as ‘multi-purpose’ trees into several countries worldwide, and, in many cases, have become naturalised and invasive (van Klinken 2012; Shackleton et al. 2015). This genus has now been disintegrated (Hughes et al. 2022), with invasive ‘Prosopis’ species introduced into Africa, predominantly belonging to the genus Neltuma Raf., more commonly referred to as ‘Mesquite’. These trees were introduced into South Africa from the Americas during the late 1800s due to their perceived benefits in terms of shade, fuelwood, fodder (via pod production), and timber, and were consequently widely promoted, distributed, and planted in arid and semi-arid regions up until the 1960s (Zachariades et al. 2011; Shackleton et al. 2015). Since then, these Neltuma spp. have become widespread invasive trees within the Northern Cape and to a lesser extent in the Western Cape, Eastern Cape, Free State and North West provinces (Henderson and Wilson 2017; Henderson 2020). The trees grow rapidly, often forming dense impenetrable stands with little to no fodder value. The seeds are typically dispersed by animals (endozoochorously) or through flooding events, which facilitates the rampant spread of these trees (estimated at ~ 8% annually) (Shackleton et al. 2016; van Wilgen and Wilson 2018). As a result, Neltuma currently exists as one of the most abundant invasive plant genera in South Africa, second only to the Australian tree genus, Acacia, and is estimated to cover some 6–8 million ha of the country (Shackleton et al. 2017; van Wilgen and Wilson 2018; Fig. 1). These Neltuma invasions now exert severe negative socio-ecological impacts, reducing ground water availability and aquafer recharge, depleting arable and pastoral land, impacting biodiversity, and hindering ecosystem services and consequently human livelihoods in South Africa (see Dzikiti et al. 2013; Shackleton et al. 2015, 2017; Reynolds et al. 2020).
Recognising these threats, South Africa has spent over R 1 billion (~ 56 million US$) in attempting to control invasive stands of Neltuma spp. since the mid-1990s (van Wilgen et al. 2012; Shackleton et al. 2017). Despite this substantial expenditure, both mechanical and chemical clearing efforts have been ineffective in managing the Mesquite invasion at a large scale, leading to calls for increased support for and usage of biological control (Shackleton et al. 2017; Kleinjan et al. 2021; van Wilgen et al. 2022). Biological control efforts were first initiated against Neltuma spp. in South Africa during the 1980’s, and as to retain the useful attributes of Mesquite these early efforts focused on reducing the rate of spread, using only seed-feeding agents (Impson et al. 1999; Zachariades et al. 2011). Three seed-feeding bruchids (Chrysomelidae: Bruchinae) were released, two of which have become established (Zachariades 2021). However, their impact has been limited (Impson et al. 1999; Moran et al. 2021; van Wilgen et al. 2022), and in 2014 the decision was made to investigate more damaging candidates targeting any part(s) of Mesquite (Kleinjan et al. 2021). Among these candidates an undescribed species of leaf-tying moth, known as Agnippe sp. #1 (Gelechiidae: Lepidoptera) (syn. Evippe sp. #1), was considered one of the most promising (Zachariades et al. 2011; van Klinken 2014). Following its importation from Australia in 2014, and subsequent host-specificity testing, Agnippe sp. #1 was approved for release in December 2020, with the first releases of the moth undertaken in South Africa during February 2021 (Kleinjan et al. 2021). The release of Agnippe sp. #1 in South Africa was highly anticipated given substantial defoliation of Neltuma spp. by the moth soon after its introduction into parts of Australia (van Klinken et al. 2003a; van Klinken 2012).
In its native range (Argentina) Agnippe sp. #1 is oligophagous, feeding and developing on several species of Neltuma, with its fundamental host range restricted to Neltuma spp. native to the Americas (section: Algarobia) (van Klinken and Heard 2000; van Klinken 2012). The biology of Agnippe sp. #1 predisposes the species to rapid increases in population size, with egg to adult development completed in as few as 34 days (van Klinken et al. 2003a). Adult moths are short-lived, typically surviving no longer than three weeks, with females laying up to ~ 75 eggs, which are oviposited into cracks and fissures of the tree’s bark (van Klinken and Heard 2000). Eggs hatch within a few days and the 1st instar larvae begin feeding by creating mines within the leaves, subsequently forming a series of leaf-ties within which they feed, progress through the remaining three instars and later pupate (van Klinken and Heard 2000). Under adverse conditions, Agnippe sp. #1 enters a facultative diapause during the colder winter months, mainly triggered by reduced daylength, and overwinters as 4th instar larvae and pupae within the leaf ties from late autumn to mid-spring.
Generally, the rapid and widespread establishment of Agnippe sp. #1 across Australia and its defoliation of several Neltuma spp. and their hybrids, is highly promising (van Klinken et al. 2003a, 2009). However, the abundance of Agnippe sp. #1 populations and their subsequent biological control impact on Neltuma spp. remains variable, being strongly linked to climate, particularly temperature (van Klinken 2012; Winston et al. 2014). Agnippe sp. #1 appears to thrive under hot arid conditions, evidenced by the moth’s consistently high population density and associated level of damage in the Pilbara region of Western Australia (van Klinken 2012, 2014; Winston et al. 2014). Climatically, the Pilbara is characterised by exceedingly hot summers, often averaging > 30 °C, with mild and predominantly frost-free winters, which rarely drop below 20 °C, and variable but low levels of rainfall (Sudmeyer 2016). Contrastingly, exceedingly low levels of leaf-tying by Agnippe sp. #1, typically < 10% of foliage, have been recorded in the cooler, semi-arid and temperate regions of the moth’s introduced range in Australia, particularly within New South Wales (van Klinken et al. 2003a). This suggests that both the abundance and impact of Agnippe sp. #1 may be climatically constrained in South Africa, particularly within the country’s cooler semi-arid and temperate inland regions invaded by Neltuma spp.
Climate is widely accepted as an important factor in the success of weed biological control programmes, influencing not only the establishment but the abundance and effectiveness of released agents (Byrne et al. 2004; Robertson et al. 2008; Heimpel and Mills 2017). Agents sourced from climatically similar regions to those in which they are to be released, are assumed to be more likely to establish and offer more effective levels of control (Robertson et al. 2008; Harms et al. 2021), whereas mismatches in these abiotic variables, often termed climatic unsuitability, have been shown to hinder biological control efforts, either restricting the establishment, spread, proliferation and/or survival of insect agents within their introduced ranges (see Byrne et al. 2002; Cowie et al. 2016). As a result, ‘climate matching’ procedures remain a common practice employed in biological control programmes and are frequently used to identify climatically well-suited regions for the survey and collection of candidate agents in their native range or areas suitable for the release of agents on invasive plant populations in the introduced range (Senaratne et al. 2006; Kriticos et al. 2015). In the case of Agnippe sp. #1, climatic matching was used to predict the moth’s likely establishment in Australia, based on occurrences in its native range in Argentina. However, this did not accurately predict that the agent would be most successful in the hottest parts of Australia (i.e., the Pilbara) (van Klinken et al. 2003a, 2009). Therefore, to more accurately infer whether Agnippe sp. #1 will be ‘successful’ in South Africa and the areas in which the moth would most likely establish and be damaging, occurrence data from the native range should be bolstered with the inclusion of data from the introduced range in Australia along with thermal physiology studies on the moth itself.
The abundant and widespread distribution of Neltuma spp. across a diverse range of climatic regions within South Africa is likely to influence the establishment and subsequent impacts of Agnippe sp. #1 and there are concerns that climatic unsuitability may limit this agent’s efficacy in the country (Kleinjan et al. 2021). Several years of sustained damage by Agnippe sp. #1 would be required before notable reductions in the Mesquite invasion become evident, and it would be beneficial to predict the role this agent is likely to play in the future, so that it may be included in a long-term national management plan for Mesquite control. If Agnippe sp. #1 is unlikely to be successful in any part of South Africa, then control efforts should be redirected to other biocontrol agents or suitable management interventions. If it is only likely to provide control localised to certain parts of the country, then alternative strategies will be required in the unsuitable areas. Predicting where Agnippe sp. #1 is likely to be most damaging should also guide release efforts so that releases occur in areas where establishment is most likely, and where the abundance and efficacy of the agent will be greatest. This would improve the chances of successful control and limit wasteful expenditure, particularly in remote areas. Therefore, the aim of this study was to assess the potential constraints posed by climate to (1) the likelihood of establishment of the leaf-tying moth Agnippe sp. #1 and its potential efficacy in South Africa, and (é) to identifying the most suitable release areas in South Africa using climatic matching and thermal-physiology assessments.
Material and methods
Assessment of climatic suitability (climate matching)
To assess the likely establishment and efficacy of the leaf-tying moth Agnippe sp. #1, the climate prediction-modelling program DYMEX simulator (CLIMEX: version 4.3) was used (Kriticos et al. 2015). Initial comparison between South Africa and the moth’s native range in Argentina, using the La Rioja and Santiago del Estero provinces, was done to assess the potential establishment of Agnippe sp. #1 in South Africa. Whereas comparisons between South Africa and the Australian states in which the moth was previously released, namely New South Wales, Northern Territory, Queensland, and Western Australia (the Pilbara), were used to gain insight into the anticipated levels of damage likely to be exerted by Agnippe sp. #1 as an introduced insect (i.e., biological control agent). In the case of South Africa, only the five provinces currently invaded by Neltuma spp., namely the Eastern Cape, Free State, North West, Northern Cape, and Western Cape (Fig. 1), were used for climatic comparisons to both the native (Argentina) and introduced range (Australia) of Agnippe sp. #1.
For a comprehensive comparison, climatic data were selected from all available sites (weather stations) present within CLIMEX (Kriticos et al. 2015) for areas known to be invaded or in close proximity to invasion by Neltuma spp. (SAPIA 2018; GBIF 2023) and then averaged per province/state in Argentina (La Rioja and Santiago del Estero), Australia, and South Africa (Supplementary Table S1). CLIMEX comparisons between South Africa and Argentina as well as South Africa and Australia were made based on the mean annual rainfall, rainfall seasonal pattern, minimum, average and maximum temperatures, RH and soil moisture. All climatic parameters were weighted equally (= 1), excluding annual rainfall and rainfall seasonality, which were weighted at half their effect (= 0.5), given the findings of van Klinken et al. (2003a) regarding the limited effect of rainfall on Agnippe sp. #1.
Overall, the basis on which these climate comparisons between regions/localities is the use of the composite match indices, in which 0 indicates no match between the compared regions/localities and 100 indicates an identical match (Kriticos et al. 2015). Broadly, these indices may be categorised to simplify the comparisons and offer basic inferences into the likelihood of a species establishing in an introduced range. Indices with a value of > 70 are generally considered suitably matched and suggest that the likelihood of an introduced species’ establishment should be ‘high’ (Kriticos et al. 2015; Phillips et al. 2018), whereas composite match indices of ≤ 50 are considered poorly matched and are unsuitable for a species’ establishment. The 50–70 range is variable in terms of the establishment likelihood and often remains species-specific. However, for the purpose of this biological control study indices of: (1) 51–55% were considered marginally matched offering little to no likelihood of establishment, (2) 56–60 were considered poorly matched offering a low likelihood of establishment and (3) indices between 60 and 70 were considered moderately matched and offered a moderate likelihood of the species establishing.
Thermal physiology assessments of Agnippe sp. #1
Agnippe sp. #1 used in these experiments were reared on caged Neltuma sp. plants (~ 0.5 m tall) in a temperature-controlled glasshouse section of the University of the Witwatersrand Insectary Facility (Wits Insectary), Johannesburg, South Africa during mid-summer. The initial Agnippe sp. #1 adults were sourced from a culture currently mass-reared at the Agricultural Research Council—Plant Health and Protection (ARC-PHP) in Roodeplaat, Pretoria, South Africa. The origin of this culture was from field collected individuals from the Pilbara region of Western Australia, first imported during 2014 (Kleinjan et al. 2021). The glasshouse receives full sunlight (∼1800 μmol−1 P.A.R) and follows natural (ambient) day–night light patterns. Temperatures in which Agnippe sp. #1 were cultured averaged 25.9 ± 2.1 °C during the day (12 h) and 19.3 ± 1.5 °C at night (12 h), with a RH of between 50 and 70%.
Critical thermal limits
The critical thermal limits, namely minima (CTmin) and maxima (CTmax), of Agnippe sp. #1 were determined using a double jacket system, connected to a programmable water bath (Julabo F32-ME, Seebach, Germany) (see Cowie et al. 2016). Both adults (moth) and larvae (4th instar caterpillar) were used in the assessments to offer greater insight into the overall thermal capacity and limitations of this species. Moths/caterpillars were randomly selected from the culture and individually sealed within clear glass polytop vials (size No.1: 5 ml) and submerged into the water bath set at 25 °C and allowed a 15 min thermal equilibration period. Thereafter, the water was cooled at a rate of 0.2 °C min−1 for the assessment of CTmin and heated at the same rate (0.2 °C min−1) for CTmax assessments. CTmin/CTmax was recorded, per individual, as the temperature at which a loss of locomotory function (inability to self-right) occurred as well as the failure to show a coherent response to a stimulus (fine bristle paint brush: size 5). Individuals that failed to self-right or showed no locomotory function, whilst in the water bath, were then removed and their response to the stimulus tested immediately. Individuals which were removed but showed a coherent response to the stimulus were disregarded and no data were recorded. Likewise, no data were recorded for individuals which were removed, showed no coherent response but failed to recover (survive).
Lethal temperatures
Upper and lower lethal temperatures (ULT50 and LLT50) were assessed following a similar water bath procedure to the critical thermal limits. Different batches of moths and caterpillars (n = 10 individuals per batch) were individually sealed in glass polytope vials and submerged at 25 °C, allowed a 15 min equilibration period, and the water then cooled or heated (0.2 °C min−1) to the target temperature. Once reached, the target temperature was maintained for a 2 h period, immediately after which all vials were removed, and all insects (per batch) were placed onto fresh leafy Neltuma sp. bouquets and allowed a 24 h recovery period. Target temperatures used for ULT50 assessments of both adults and larvae were: 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 °C. In the case of LLT50 assessments of adult and larval Evippe sp., target temperatures of 0, −1, −2, −3, −4, −5, −6, −7, −8, −9, −10, −11, −12, −13, −14, −15 were used.
Releases of Agnippe sp. #1 in South Africa
Releases of Agnippe sp. #1 were conducted during 2021 and 2022 at several sites within South Africa. These releases were carried out following a protocol largely similar to that of van Klinken et al. (2003a) in Australia. Large styrofoam boxes (22–30 l) containing leaf-ties, with pupae on the verge of emerging as adult moths, were placed directly into the field. Release boxes were marked and affixed to Neltuma trees with several exit holes made to allow for unencumbered dispersal of the newly emerged adult moths. Revisits to the release sites were conducted when possible and typically employed a ~ 30 min active search, starting at the initial release tree working outward, to inspect for any signs of Agnippe sp. #1 (i.e., leaf-mining or tying of the foliage). Sites that were revisited in the following spring/summer seasons were deemed to have been successful in terms of establishment, provided they showed signs of Agnippe sp. #1.
Data analysis
The suitability of climatic variables was quantified, using composite match indices generated using the ‘Match Climates’ feature in CLIMEX (Kriticos et al. 2015) and compared between both the native (Argentina) and introduced (Australia and South Africa) ranges of Agnippe sp. #1. Linear Mixed Effects Models (LMER), with repeated measures, were used to compare mean monthly minimum, average, and maximum temperatures between the South African Agnippe sp. #1 release provinces and the sites in which Agnippe sp. #1 has performed the best and worst in Australia, namely the Pilbara (Western Australia) and New South Wales, respectively. LMERs had temperature set as the response variable, locality (site) set as the fixed effect and time (month) set as the random effect. Differences in larval vs. adult critical thermal minima and maxima (CTmin/CTmax) were assessed using a Student’s t-test (equal variance). Upper and lower lethal temperatures (ULT50/LLT50), at which 50% of the population were predicted to experience mortality, were calculated, and determined as per Cowie et al. (2016), using a binomial Generalized Linear Model (GLM), with survival set as the response variable, temperature as the fixed variable and a probit link function for both the larval (4th instar) and adult (moth) stages of Agnippe sp. #1. All statistical analyses were carried out using R (R Core Team 2022) via the R studio interface (RStudio Team 2022).
Results
Assessment of climatic suitability (climate matching)
Overall, the climatic parameters of the South African provinces, invaded by Mesquite, showed mean match of 71% to the native range of Agnippe sp. #1 in Argentina, particularly La Rioja province (Table 1). In terms of comparison between South Africa and the moth’s introduced range in Australia, a mean climatic match of 66% was calculated and highlighted that most of the South African provinces, namely the Eastern Cape, Free State, North West, and Western Cape, were climatically similar to the cooler semi-arid state of New South Wales (Table 1), whereas the Northern Cape was found to be the most strongly matched South African province to Australia overall (71%), particularly to the warmer semi-arid state of Queensland (75%) (Table 1). In addition, climatic matches of South Africa to the best performing site of Agnippe sp. #1 in Australia (the Pilbara) displayed a moderate match of 61%. This climatic match was projected, along with the release records of Agnippe sp. #1, and showed that the Northern Cape maintained the best match overall, followed by sections of the North West and lastly by the Eastern Cape, Western Cape and Free State provinces (Fig. 1).
Further comparisons of the South African provinces in which Agnippe sp. #1 was released (Fig. 2) to those in Australia where the moth has performed ‘best’ (i.e., the Pilbara) and ‘worst’ (i.e., New South Wales), highlighted notable trends regarding temperature. When compared to the Pilbara, all South African release sites experienced significantly cooler minimum temperatures throughout the year (F4,44 = 453.1; P < 0.001), with the Northern Cape being the least cold, followed by the North West and Free State provinces. Moreover, the North West and Free State provinces were the coldest overall being significantly colder than New South Wales (Fig. 3a). A similar trend was present in terms of average temperature, with all South African release sites found to be significantly colder than the Pilbara (F4,44 = 294.9; P < 0.001) and the North West and Free State provinces aligning most closely to the temperatures experienced in New South Wales (Fig. 3b). Lastly, maximum temperatures experienced in all South African release sites were significantly cooler than the Pilbara throughout the year (F4,44 = 284.3; P < 0.001). However, both the Northern Cape and North West provinces were significantly warmer than New South Wales (Fig. 3c).

(Data adapted from CLIMEX: Kriticos et al. 2015)
Projected climatic match of Neltuma spp. invaded regions in South Africa to Agnippe sp. #1 release sites in Western Australia (the Pilbara). Resolution of grid cells is at a quarter degree square (QDS: ~ 25 km × 25 km)
Mean monthly minimum (a), average (b) and maximum (c) temperatures for the Australian and South African release sites (Free State, North West and Northern Cape) of Agnippe sp. #1. Climatic data were generated from CLIMEX (Kriticos et al. 2015), averaged and plotted using a spline function. Lowercase superscript letters indicate significant differences (P < 0.05) in temperatures between sites. F- and P-values indicate overall differences in site temperature using repeated measures linear mixed effects model (LMER)
Thermal physiology assessments of Agnippe sp. #1
The temperatures at which Agnippe sp. #1 individuals were unable to self-right and respond to stimuli differed between the larval and adult developmental stages at both high and low temperatures (Fig. 4). In the case of low temperatures, Agnippe sp. #1 larvae displayed a significantly lower mean CTmin = 0.9 ± 0.3 °C, compared to that of the adults which had a mean CTmin = 1.3 ± 0.2 °C (t38 = 3.73; P < 0.001; Fig. 4a). Similarly, larvae displayed a significantly greater tolerance to higher temperatures, with a mean CTmax = 46.8 ± 0.5 °C, compared to that of the adult moths, averaging a CTmax = 46.0 ± 0.4 °C (t38 = 4.42; P < 0.001; Fig. 4b).
Critical thermal minima (CTmin) (a) and critical thermal maxima (CTmax) (b) for larval (4th instar caterpillar) and adult (moth) Agnippe sp. #1 (n = 20 individuals per life stage). t- and P-values indicate differences (P < 0.05) between adult and larval stages for CTmin and CTmax temperatures. Boxplots show mean, median, 10th, 25th, 75th and 90th percentiles as well as outliers (grey circles) for the differing life stage thermal minima/maxima
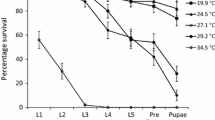
Lethal temperatures calculated for Agnippe sp. #1 appeared to differ between life stages, with larvae displaying a greater thermal tolerance when compared to the adult moths. Fourth instar larvae displayed a lower lethal temperature (LLT50) of −11.1 ± 0.4 °C which was ~ 2 °C lower than that of the adult life stage with an LLT50 of −8.8 ± 0.3 °C (Fig. 5a, b). Likewise, the upper lethal temperature (ULT50) for adult Agnippe sp. #1 was calculated at 43.6 ± 0.3 °C, ~ 1.2 °C lower than that of the fourth instar larvae at 44.8 ± 0.3 °C (Fig. 6a, b).
Larval (4th instar caterpillar) (a) and adult (moth) (b) Agnippe sp. #1 survival (%) after 2-hour exposure to low temperatures of 0, −1, −2, −3, −4, −5, −6, −7, −8, −9, −10, −11, −12, −13, −14, and −15 °C (n = 10 individuals; per temperature treatment) and a 24 h recovery period. Survival data were fitted with a probit function (GLM) to determine the lower lethal temperatures (LLT50)
Larval (4th instar caterpillar) (a) and adult (moth) (b) Agnippe sp. #1 survival (%) after 2 h exposure to high temperatures of 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 and 50 °C and a 24 h recovery period (n = 10 individuals per temperature treatment). Survival data were fitted with a probit function (GLM) to determine the upper lethal temperatures (ULT50)
Release of Agnippe sp. #1 in South Africa
During 2021 and 2022, approximately 17,000 Agnippe sp. #1 individuals were released at seven sites across South Africa (Table 2). The majority of these release sites were located within the predominately hot and arid regions of the Northern Cape province (n = 4), followed by the cold and semi-arid North West (n = 2) and Free State (n = 1) provinces. Although Agnippe sp. #1 appeared to persist initially at the majority of sites after release, to date establishment of the moth has only been recorded at two localities, namely the Kenhardt and Meerkat SKA (Carnarvon) release sites, both within the Northern Cape (Table 2).
Discussion
The preliminary establishment of Agnippe sp. #1 within the Northern Cape remains promising and can largely be attributed to the generally hot arid climate of the region (Beck et al. 2018). Post-release studies on Agnippe sp. #1 as a biological control agent in Australia highlight that optimal establishment occurs in arid areas experiencing exceedingly hot summers as well as warm winters (van Klinken et al. 2003a, 2009; van Klinken 2012). Although Agnippe sp. #1. is also able to establish at temperate semi-arid sites, as recorded in New South Wales (Australia) and even from single relatively small releases (van Klinken et al. 2003b, 2009), this was not the case in South Africa. The failure of Agnippe sp. #1 to establish at sites within the North West and Free State provinces, despite multiple releases at some sites, is likely due to the substantially colder temperatures experienced, particularly during the coldest winter months (June–July) where minimum temperatures frequently drop below 0 °C (Schulze 1997; Kriticos et al. 2015). Ultimately, this suggests that climate is an important factor for consideration when releasing Agnippe sp. #1 in South Africa, as from these climatic assessments it appears that the moth will not become widely established across the entirety of the Mesquite invasion. Rather, the moth’s establishment is likely to predominate within the hotter arid regions of the Northern Cape province. Therefore, Agnippe sp. #1 releases should be prioritised, at least initially, for the most climatically suitable areas within the Northern Cape, to promote wider establishment.
Beyond these initial establishment predictions, biocontrol researchers are also frequently interested in the anticipated levels of damage of recently released biological control agents (Heimpel and Mills 2017; Hill et al. 2020; Muskett et al. 2020). However, as suggested by van Klinken et al. (2003a; b), caution should be exercised when solely using climatic data to make these types of inferences regarding agent performance (Senaratne et al. 2006), and hence the choice of comparison to a similar introduced range of the moth in Australia, rather than using the native range in Argentina. Promisingly, these climatic comparisons with Australia suggest that Agnippe sp. #1 should become established in parts of the Northern Cape province, which remains a major biological control target region since it suffers the highest levels of Mesquite invasion and impact (Shackleton et al. 2015; Henderson 2020; Reynolds et al. 2020). Climatically, much of the Northern Cape resembles that of Queensland in Australia, where high levels of leaf-tying by Agnippe sp. #1 have been recorded. Warmer seasonal temperatures are associated with a greater number of Agnippe sp. #1 generations, resulting in larger populations with a higher abundance of leaf-ties, damaging up to 50–90% of Neltuma spp. foliage (see van Klinken et al. 2003a). Although this offers good prospects for biological control efforts within the Northern Cape, inferences made from climatic data may be somewhat tenuous, as previously noted for Agnippe sp. #1 in Australia (van Klinken et al. 2003a, b) and ground truthing of these suggested outcomes, should Agnippe sp. #1 become more widely established, is strongly advised (e.g., Muskett et al. 2020). In addition, the expectation of an agent like Agnippe sp. #1 to be equally damaging across such a vast and climatically diverse and variable area such as the Northern Cape is unrealistic (van Klinken et al. 2003b; Harms et al. 2021). The large extent of the Mesquite invasion, coupled with the pronounced seasonal variations in temperature (Kriticos et al. 2015) and variable but high levels of leaf drop (50–90%) by Neltuma spp. during winter, are all likely to influence and affect Agnippe sp. #1 populations and their subsequent levels of damage experienced in the province.
Given the importance of temperature on the abundance and damage of Agnippe sp. #1, the inclusion of physiological, particularly thermal, assessments offer further insight to broad scale climate matching (van Klinken et al. 2003b; Muskett et al. 2020). Thermally, Agnippe sp. #1 appears best suited to Mesquite invasions occurring within the Northern Cape. The species’ low CTmin and LLT50 display promising levels of cold tolerance, particularly the 4th instar larvae which is the overwintering life stage. Although the diapause of larvae within leaf-ties may offer some thermal buffering against cold temperatures, there is still a high risk of cold stress accumulation in certain regions of South Africa. Cold semi-arid and temperate inland sections of the Free State, North West and southern parts of the Northern Cape where winter temperatures frequently drop below the moth’s CTmin (Schulze 1997; Kriticos et al. 2015), should be avoided and likely account for the failed establishment at these sites. Although cold winter temperatures appear to preclude the moth’s establishment outside of the Northern Cape at present, this may not persist into the future. There remains the potential for Agnippe sp. #1 to display a degree of phenotypic plasticity or even local adaptation to climatic conditions over the longer term, as seen in other South African biocontrol programmes facing similar climatic constraints (Griffith et al. 2019). Contrastingly, the high temperatures experienced in South Africa are unlikely to pose any hinderances to adult moths or their larvae. The high CTmax and ULT50 should allow the moth to persist readily, even in the hottest regions of South Africa where temperatures average > 32 °C throughout summer (e.g., Vioolsdrif) (Schulze 1997). Moreover, the development of larvae entirely within leaf-ties and the residence of adult moths within the foliage may offer further thermal refuge. Therefore, these thermal physiology assessments support the notion that Agnippe sp. #1 releases should be carried out, at least initially, in the most climatically well-suited regions to prompt greater establishment, increases in population size and subsequently higher levels of damage.
Although climate exists as one of the main constraints to classical biological control programmes (Harms et al. 2021), secondary factors known to hinder the establishment, abundance and efficacy of agents should not be overlooked. Predation, and more particularly parasitism, of various endophytic and lepidopteran agents released in South Africa is well documented and may be a cause for concern (Hill and Hulley 1995), warranting further investigation locally. Promisingly, parasitism of Agnippe sp. #1 in Australia has remained exceedingly low, averaging < 2%, despite the larval and pupal stages attracting a diversity of parasitoids (see van Klinken and Burwell 2005; van Klinken 2012). Similarly, the genetic facets of biological control programmes should not be ignored given the longstanding, historical invasion of Neltuma spp. in South Africa potentially allowing for unique Neltuma genotypes (Ward et al. 2008). Likewise, the likelihood of genetic bottlenecking should be explored if establishment issues arise in climatically well-suited areas, given the collection of Agnippe sp. #1 from a few Australian sites as well as its prolonged laboratory culturing period prior to release (~ six years) (Taylor et al. 2011; Harms et al. 2021). Although Agnippe sp. #1 is known to be damaging across several Neltuma spp. (van Klinken et al. 2003a; Kleinjan et al. 2021), many of which are believed to be present in South Africa, recent studies suggest that the majority of the Neltuma spp. prevalent and invading South Africa represent a ‘hybrid swarm’ (Mazibuko 2012; Richardson et al. 2020) which may influence the performance of Agnippe sp. #1 regardless of any other underlying abiotic or biotic limitations.
In conclusion, the release and preliminary establishment of the leaf-tying moth, Agnippe sp. #1, remains highly promising and an important step towards increasing and sustaining the impact of ongoing biological control efforts (Hill et al. 2020) against notoriously invasive and damaging Neltuma spp. in southern Africa. Climatic assessments comparing South Africa to Argentina and Australia suggest that establishment of the moth is likely to be constrained by climatic factors, particularly low winter temperatures in cold semi-arid inland regions. Parts of the introduced distribution in South Africa are unlikely to be suitable for the agent, but areas of the Northern Cape, where some of the worst infestations of Neltuma spp. persist, are likely to be suitable for establishment. Agnippe sp. #1 is most likely to establish within the Northern Cape, offering moderate to potentially high levels of damage, albeit restricted predominately to the hottest arid regions of the province. Therefore, ongoing biological control efforts should focus releases primarily in the most climatically well-suited areas of the Northern Cape to promote greater establishment of the moth. More broadly, this research highlights the importance of climatic considerations in biological control programmes, particularly to other amenable countries, such as Kenya and Ascension Island, which are currently considering Agnippe sp. #1 as a possible agent for invasive Neltuma spp.
References
Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF (2018) Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci Data 5:180214
Byrne MJ, Currin S, Hill MP (2002) The influence of climate on the establishment and success of the biocontrol agent Gratiana spadicea, released on Solanum sisymbriifolium in South Africa. Biol Control 24(2):128–134
Byrne MJ, Coetzee J, McConnachie AJ, Parasram W, Hill MP (2004) Predicting climate compatibility of biological control agents in their region of introduction. In: Cullen JM, Briese DT, Kriticos DJ (eds) Proceedings of the XI international symposium on biological control of weeds. CSIRO Entomology, Canberra, pp 28–35
Cowie BW, Venturi G, Witkowski ET, Byrne MJ (2016) Does climate constrain the spread of Anthonomus santacruzi, a biological control agent of Solanum mauritianum, in South Africa? Biol Control 101:1–7
Dzikiti S, Schachtschneider K, Naiken V, Gush M, Moses G, Le Maitre DC (2013) Water relations and the effects of clearing invasive Prosopis trees on groundwater in an arid environment in the Northern Cape, South Africa. J Arid Environ 90:103–113
GBIF (2023) Global Biodiversity Information Facility. Prosopis L. GBIF.org (29 January 2023) GBIF Occurrence Download. https://doi.org/10.15468/dl.dvgbbp
Griffith TC, Paterson ID, Owen CA, Coetzee JA (2019) Thermal plasticity and microevolution enhance establishment success and persistence of a water hyacinth biological control agent. Entomol Exp Appl 167(7):616–625
Harms NE, Knight IA, Pratt PD, Reddy AM, Mukherjee A, Gong P, Coetzee J, Raghu S, Diaz R (2021) Climate mismatch between introduced biological control agents and their invasive host plants: improving biological control of tropical weeds in temperate regions. Insects 12(6):549
Heimpel GE, Mills NJ (2017) Biological control: ecology and applications. Cambridge University Press, Cambridge
Henderson L (2020) Invasive alien plants in South Africa (Handbook no. 21). Plant Protection Research Institute, Pretoria
Henderson L, Wilson JR (2017) Changes in the composition and distribution of alien plants in South Africa: an update from the Southern African Plant Invaders Atlas. Bothalia 47(2):a2172
Hill MP, Hulley PE (1995) Host-range extension by native parasitoids to weed biocontrol agents introduced to South Africa. Biol Control 5(2):297–302
Hill MP, Moran VC, Hoffmann JH, Neser S, Zimmermann HG, Simelane DO, Klein H, Zachariades C, Wood AR, Byrne MJ, Paterson ID (2020) More than a century of biological control against invasive alien plants in South Africa: a synoptic view of what has been accomplished. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, pp 549–568
Hughes CE, Ringelberg JJ, Lewis GP, Catalano SA (2022) Disintegration of the genus Prosopis L. (Leguminosae, Caesalpinioideae, mimosoid clade). PhytoKeys 205:147–189
Impson FAC, Moran VC, Hoffmann JH (1999) A review of the effectiveness of seed-feeding bruchid beetles in the biological control of Mesquite, Prosopis species (Fabaceae), in South Africa. Biological control of weeds in South Africa (1990–1998). Afr Entomol Mem 1:81–88
Kleinjan CA, Hoffmann JH, Heystek F, Ivey P, Kistensamy Y (2021) Developments and prospects for biological control of Prosopis (Leguminosae) in South Africa. Afr Entomol 29(3):859–874
Kriticos DJ, Maywald GF, Yonow T, Zurcher EJ, Herrmann NI, Sutherst RW (2015) Dymex simulator (CLIMEX) version 4. CSIRO, Canberra
Mazibuko DM (2012) Phylogenetic relationships of Prosopis in South Africa: an assessment of the extent of hybridization, and the role of genome size and seed size in the invasion dynamics. MSc dissertation, Stellenbosch University
Moran VC, Zachariades C, Hoffmann JH (2021) Implementing a system in South Africa for categorizing the outcomes of weed biological control. Biol Control 153:104431
Muskett PC, Paterson ID, Coetzee JA (2020) Ground-truthing climate-matching predictions in a post-release evaluation. Biol Control 144:104217
Phillips CB, Kean JM, Vink CJ, Berry JA (2018) Utility of the CLIMEX ‘match climates regional’ algorithm for pest risk analysis: an evaluation with non-native ants in New Zealand. Biol Invasions 20(3):777–791
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
Reynolds C, Venter N, Cowie BW, Marlin D, Mayonde S, Tocco C, Byrne MJ (2020) Mapping the socio-ecological impacts of invasive plants in South Africa: are poorer households with high ecosystem service use most at risk? Ecosyst Serv 42:101075
Richardson DM, Foxcroft LC, Latombe G, Le Maitre DC, Rouget M, Wilson JR (2020) The biogeography of South African terrestrial plant invasions. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, pp 549–568
Robertson MP, Kriticos DJ, Zachariades C (2008) Climate matching techniques to narrow the search for biological control agents. Biol Control 46(3):442–452
RStudio Team (2022) RStudio: integrated development environment for R. RStudio (version “Mountain Hydrangea”), PBC, Boston. http://www.rstudio.com/
SAPIA (2018) South African Plant Invaders Atlas (SAPIA) database. Developed by Lesley Henderson and Carin Pretorius and curated by South African National Biodiversity Institute (SANBI)
Schulze RE (1997) South African atlas of agrohydrology and climatology: contribution towards a final report to the Water Research Commission on project 492: modelling impacts of the agricultural environment on water resources: TT82-96. Water Research Commission (WRC), Pretoria
Senaratne KW, Palmer WA, Sutherst RW (2006) Use of CLIMEX modelling to identify prospective areas for exploration to find new biological control agents for prickly acacia. Aust J Entomol 45(4):298–302
Shackleton RT, Le Maitre DC, Richardson DM, van Wilgen BW (2015) The impact of invasive alien Prosopis species (Mesquite) on native plants in different environments in South Africa. S Afr J Bot 97:25–31
Shackleton RT, Le Maitre DC, van Wilgen BW, Richardson DM (2016) Identifying barriers to effective management of widespread invasive alien trees: Prosopis species (Mesquite) in South Africa as a case study. Global Environ Chang 38:183–194
Shackleton RT, Le Maitre DC, van Wilgen BW, Richardson DM (2017) Towards a national strategy to optimise the management of a widespread invasive tree (Prosopis species; Mesquite) in South Africa. Ecosyst Serv 27:242–252
Sudmeyer R (2016) Climate in the Pilbara. Bulletin 4873, Department of Agriculture and Food, Perth
Taylor SJ, Downie DA, Paterson ID (2011) Genetic diversity of introduced populations of the water hyacinth biological control agent Eccritotarsus catarinensis (Hemiptera: Miridae). Biol Control 58(3):330–336
van Klinken RD (2012) Prosopis spp. Mesquite. In: Julien M, McFadyen RE, Cullen J (eds) Biological control of weeds in Australia. CSIRO Publishing, Melbourne, pp 477–485
van Klinken RD (2014) Mesquite (Prosopis species) biological control in Australia. In: Impson FAC, Kleinjan CA, Hoffmann JH (eds) Proceedings of the XIV international symposium on biological control of weeds. Kruger National Park, Skukuza, pp 100–101
van Klinken RD, Burwell C (2005) Evidence from a gelechiid leaf-tier on Mesquite (Mimosaceae: Prosopis) that semi-concealed Lepidopteran biological control agents may not be at risk from parasitism in Australian rangelands. Biol Control 32:121–129
van Klinken RD, Heard TA (2000) Estimating fundamental host range: a host-specificity study of a potential biocontrol agent for Prosopis species (Leguminosae). Biocontrol Sci Technol 10(3):331–342
van Klinken RD, Hoffmann JH, Zimmermann HG, Roberts AP, Muniappan R (2009) Prosopis species (Leguminosae). In: Biological control of tropical weeds using arthropods. Cambridge University Press, Cambridge, UK, pp 353–377
van Wilgen BW, Wilson JR (2018) The status of biological invasions and their management in South Africa 2017. South African National Biodiversity Institute, Kirstenbosch and DST-NRF Centre of Excellence for Invasion Biology, Stellenbosch
van Klinken RD, Fichera G, Cordo H (2003a) Targeting biological control across diverse landscapes: the release, establishment, and early success of two insects on Mesquite (Prosopis spp.) insects in Australian rangelands. Biol Control 26(1):8–20
van Klinken RD, Fichera G, Parr R, McCormick E, Cobon R, Fleck L, March N, McMahon J (2003b) Challenges facing the successful management of widely distributed weeds: biological control of Mesquite (Prosopis species). In: Proceedings of the thirteenth Australian weeds conference, Perth, pp 370–372.
van Wilgen BW, Forsyth GG, Le Maitre DC, Wannenburgh A, Kotzé JD, van den Berg E, Henderson L (2012) An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biol Conserv 148(1):28–38
van Wilgen BW, Wannenburgh A, Wilson JR (2022) A review of two decades of government support for managing alien plant invasions in South Africa. Biol Conserv 274:109741
Ward SM, Gaskin JF, Wilson LM (2008) Ecological genetics of plant invasion: what do we know? Invasive Plant Sci Manag 1(1):98–109
Winston RL, Schwarzländer M, Hinz HL, Day MD, Cock MJ, Julien MH (2014) Biological control of weeds: a world catalogue of agents and their target weeds, 5th edn. USDA Forest Service, Forest Health Technology Enterprise Team, Morgantown
Zachariades C (2021) A catalogue of natural enemies of invasive alien plants in South Africa: classical biological control agents considered, released and established, exotic natural enemies present in the field, and bioherbicides. Afr Entomol 29(3):1077–1142
Zachariades C, Hoffmann JH, Roberts AP (2011) Biological control of Mesquite (Prosopis species) (Fabaceae) in South Africa. Afr Entomol 19(1):402–415
Acknowledgements
Resources made available from the Agricultural Research Council—Plant Health and Protection (ARC-PHP) and the University of the Witwatersrand are also gratefully acknowledged.
Funding
Open access funding provided by University of the Witwatersrand. This work is supported by funding from the South African Working for Water (WfW) programme of the Department of Forestry, Fisheries and the Environment: National Resource Management Programmes (DFFE: NRMP). Funding was also provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation (NRF) of South Africa. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors, and the funders do not accept any liability in this regard.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no potential conflicts of interest relevant to the work presented in this submission.
Informed consent
In addition, no human subjects or animals requiring ethics were used in this study and there are therefore no issues of informed consent.
Additional information
Handling Editor: Michelle Rafter
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cowie, B.W., Heystek, F. & Paterson, I.D. Will climate affect the establishment and efficacy of Agnippe sp. #1 (Lepidoptera: Gelechiidae), a promising biological control agent of Mesquite in South Africa?. BioControl 68, 681–695 (2023). https://doi.org/10.1007/s10526-023-10221-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10221-6