Abstract
Rice bakanae disease, caused by Fusarium fujikoroi, is an economically important disease that poses a threat to rice cultivation. It causes serious yield losses quantitatively and qualitatively. The current study aims to suppress rice bakanae disease by using indigenous antagonistic bacteria. The antagonistic bacteria significantly inhibited the growth of F. fujikoroi RB-01% in vitro. Seven out of twenty antagonistic strains significantly decreased the disease as compared to the negative control. The consortium of two highly antagonistic bacterial strains, viz Bacillus sp. KFP7 and KFP17, effectively reduced the disease as compared to the negative control. They elicited the peroxidase activity and increased the grain yield by 323.08 gm−2 in the bacteria-treated plants. These findings encourage the utilization of these antagonistic bacteria to control the rice bakanae disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza Sativa L.) is one of the major crops as it feeds 60% of the world's population (Katoch et al. 2019). Bakanae disease caused by Fusarium fujikoroi is one of the devastating diseases that severely reduce rice yield (Katoch et al. 2019). It is also known as “foolish seedling disease” because the infected seedlings show different abnormalities i.e. etiolation, elongation of stem/internodes, slenderness, a wider angle of the leaf, and ultimately death (Bashyal et al. 2016; Saito et al. 2021). The infected plants occasionally survive till maturity but do not produce any grains inside panicles (Katoch et al. 2019).
The rice bakanae pathogen can be seed- or soil-borne as it perpetuates from the residues of infected plants from one season to the next (Abbas et al. 2016). However, infected seeds provide the primary inoculum for disease development (Sunani et al. 2020). The pathogen produces ascospores and conidia which are transmitted to other plants by wind and water (Matic et al. 2017). Several approaches such as cultural means, chemicals, and resistant cultivars are being used to manage the bakanae disease (Hossain et al.2015). Chemical control is the most effective one and has been widely used in most of the rice growing areas (Hossain et al.2016). However, the emergence of fungicide-resistant strains and loss of resistance in the inbred rice cultivars have threatened rice production (Chen et al. 2016). Hence, there is an immense need to develop alternative control measures for bakanae disease.
Biological control is a promising approach employed in crop disease management. Antagonistic bacteria are effective biocontrol agents due to their multifaceted properties (Kohl et al. 2019). The indigenous antagonistic bacteria obtained from certain crop rhizosphere have intrinsic mechanisms to cope with the pathogens of that crop by adapting the native rhizosphere environment. These bacteria adopt multiple mechanisms to control the pathogens such as inhibition of their propagation and induction of systemic resistance in the plants (Berendsen et al, 2018). They produce various secondary metabolites to effectively control rice bakanae disease (Sarwar et al. 2018). These metabolites include siderophores, hydrolytic enzymes, and antibiotics (Saraf et al. 2014).
Induction of systemic resistance by the antagonistic bacteria in plants includes triggering various defense responses (Olenska et al. 2020). One such response is the production and accumulation of defense-related enzymes such as peroxidase (POD), phenyl ammonium lyase (PAL), and polyphenol oxidase (PPO) in plants (Wu et al. 2019). These antioxidant enzymes scavenge the reactive oxygen species (ROS) at the site of infection and prevent the proliferation and colonization of the pathogen (Ullah et al. 2020). The antagonistic rhizobacteria also stimulate plant growth by producing phytohormones and solubilizing the nutrients (Emmani et al. 2019).
Application of rhizobacteria under consortium conditions is an emerging trend nowadays. As reported by Dhonge et al. (2021), rhizobacterial consortium enhanced the yield and aroma of rice. Similarly, microbial consortium also reduced the nematode infestation on cucumber (Panpatte et al. 2021) and controlled root rot of tomato (Cai et al. 2021).
Bacillus sp. are ubiquitous biocontrol agents against numerous soil-borne and foliar phytopathogens (Rais et al. 2018; Weerapol et al. 2019). They have been reported in various habitats such as the soil rhizosphere, roots endosphere, and phyllosphere of numerous plants (Srikhong et al. 2018). They can survive for a long time by colonizing the plant roots under diverse environmental conditions (Hasheem et al. 2019). They are categorized as risk factor-1 and a potential source of biopesticide (Rasul et al. 2019). Thus, understanding the biocontrol effects of Bacillus sp. on multiple pathogens of their indigenous host may help in the development of a sustainable crop-specific disease management strategy. In our previous studies, twenty rice associated antagonists (KFP1–KFP20) significantly antagonized the rice blast pathogen (Pyricularia oryzae) in vitro. Out of these antagonists, three strains, viz Bacillus spp. KFP5, Bacillus spp. KFP7 and Bacillus spp. KFP17, suppressed the rice blast by 40–52% and enhanced grain yield by 3.2–3.9 t ha−1 under field conditions (Rais et al. 2018). In current study, we hypothesized that these indigenous Bacillus sp. could have potential to combat the rice bakanae disease. To test this hypothesis, we applied Bacillus sp. to rice crop as individual and consortium under field conditions for two consecutive years. We observed the effect of Bacillus spp. consortium on incidence of bakanae disease, growth parameters, activity of defense related enzymes and grain yield of rice.
Material and methods
Microbes and culture conditions
The bacterial strains KFP1–KFP20 including Bacillus spp. KFP5, Bacillus spp. KFP7, and Bacillus spp KFP17, were previously isolated from the rhizosphere/endosphere of rice (Rais et al. 2016). These strains exhibited antagonistic activity against the rice blast pathogen (P. oryzae) in vitro (Rais et al. 2016). Two strains, viz Bacillus spp. KFP7 and Bacillus spp. KFP17, significantly suppressed the blast disease on rice under net house and field conditions (Rais et al. 2016, 2018). These antagonistic strains were routinely grown on Luria Broth (LB) at 30 ± 2 °C for 24–74 h and stored in 20% glycerol at − 20 °C. The rice bakanae pathogen F. fujikoroi RB-01 (nucleotide accession number KJ719445) was obtained from the Applied Microbiology and Biotechnology Lab, Department of Biosciences, COMSATS University Islamabad, Pakistan and grown on potato dextrose agar (PDA) at 28 ± 2 °C for 5–7 days.
In vitro suppression assay of Fusarium fujikoroi
The twenty bacteria (T1–T20) were tested for their antagonistic potential against F. fujikoroi by dual culture assay. A 5 mm plug of actively growing (seven-day-old) culture of fungus was placed at the center of PDA plates and the bacteria were spotted at equal distances around the fungus. The plates were incubated at 28 ± 2 °C for 72–96 h. The percentage growth inhibition was calculated as reported in earlier studies (Sivan et al. 1987). The treatments were repeated thrice.
Biocontrol efficacy of individual bacteria (field experiment 1)
The antagonistic bacteria showing maximum in vitro antagonistic activity were evaluated for their biocontrol efficacy at the fields of the National Agriculture Research Center (NARC) Islamabad, Pakistan. Two independent experiments were conducted and each experiment was repeated twice during consecutive years (2012–2013 and 2013–2014).
The field experiment 1 was conducted for the consecutive years 2012–2013 on two rice varieties i.e. basmati-385 and basmati-super in field with the following soil characteristics: clay loam, pH 8, saturation 48%, electrical conductivity 1.3 dS m−1, soil organic matter 0.73%, nitrogen 0.04%, phosphorous 1.59 mg kg−1, sodium 4.7 meq l−1, calcium magnesium ion 7.1 meq l−1, exchangeable sodium percentage 2.4%, sodium absorption ratio 2.5 and zinc 0.77 mg kg−1. The treatments included Bacillus sp. KFP 5 and F. fujikoroi (T1), Bacillus sp. KFP 7 and F. fujikoroi (T2), KFP 12 and F. fujikoroi (T3), Bacillus sp. KFP 17, and F. fujikoroi (T4), KFP18 and F. fujikoroi (T5), fungicide thiophanate methyl 2% (w/v) and F. fujikoroi (T6), F. fujikoroi only (T7). The nursery was grown in a separate block of same field using field soil as substrate under natural conditions of June. The nursery was transplanted to the field as described by Rais et al (2018). Briefly, 35–40 days old seedlings were dipped in suspension consisting of bacteria (109 cfuml−1), fungal spores (1.5 × 106ml−1), and 2% carboxymethylcellulose (Biochem) for 4–5 h and transplanted to the field (two-three per hole). The bacteria suspension was prepared by growing them in LB broth, harvested by centrifuging at 8500 rpm for 7–8 min, and re-suspended in 0.85% saline. The fungus was grown on PDA, dissolved in sterile water and filtered through a muslin cloth to obtain spores. The spore concentration was adjusted by using a hemocytometer. A second dose of antagonistic bacteria was applied on the 30th day post-transplantation by soil drenching i.e. 1–2 ml of the cell suspension (109 cfu ml−1), with a sterile syringe next to the roots. The experiment was replicated thrice per treatment in a randomized complete block design. About 15–20 plants were employed in each replication. All agronomic practices were adopted uniformly for all treatments. Each replication block was irrigated separately to avoid the mixing of bio-inoculants. The infected plants were identified based on symptoms development (abnormal elongation, slenderness, and necrosis). Ten plants at the flowering stage were randomly selected from each replication and disease incidence was assessed phenotypically (Zainudin et al. 2008).
Biocontrol efficacy of bacterial consortium (field experiment 2)
Field experiment 2 was conducted for the consecutive years 2013–2014. In this experiment, the antagonistic Bacillus strains (KFP7 and KFP17) exhibiting the best biocontrol efficacy in rice in the first field experiment were evaluated as co-inoculation in rice variety basmati-385. The experiment was conducted as described in field experiment 1 (design, replicates, microbial inoculation and cultural practices). The treatments included F. fujikoroi only (T1), consortium of antagonistic strains (KFP7 and KFP17) and F. fujikoroi (T2), fungicide thiophanate methyl 2% (w/v) and F. fujikoroi (T3), consortium of antagonistic strains (KFP7 and KFP17) (T4). Disease incidence was assessed as described in field experiment 1 but at the time of maturity i.e. approximately 120–125 days after transplantation. The plant height was measured by using a scale while the tillers per plant were counted manually. Grain yield was measured after harvesting the plants and separating the grains by manual threshing.
The activity of defense-related enzymes
The representative treated plants from field experiment 2 were evaluated for their defense-related enzyme activities at the 3rd day post-application of the second bacterial antagonist’s dose (33rd day of post-transplantation). Plant shoots (five plants) were randomly collected from each replication, sealed in plastic bags, dipped in liquid nitrogen, and stored at − 80 °C. One gram of plant shoot was crushed with liquid nitrogen and mixed either with 0.1 M phosphate or respective buffer mentioned in each section. The supernatant was separated by centrifuging at 10,000 rpm for 10 min and used as a crude enzyme extract. The experiment was replicated thrice (biological replications).
Peroxidase (POD) activity
The activity of the peroxidase enzyme was determined by following the standard protocol of Hammerschmidt et al. (1982). The reaction mixture (2.9 ml) was prepared by mixing the pyrogallol (0.25% v/v), sodium phosphate buffer (0.01 M), and H2O2 (0.1 M). The enzyme extract (0.1 ml) was added to initiate the reaction. The POD activity was measured at 470 nm for 3 min with an interval of 1 min. The activity was expressed as a change in the absorbance (∆A min−1 g−1 of fresh weight). In control, the enzyme extract was replaced with phosphate buffer (0.1 M, pH 7).
Polyphenol oxidase (PPO) activity
Polyphenol oxidase (PPO) activity was assayed by following the method described by Mayer et al. (1966) with a slight modification. The reaction consisted of enzyme extract (200 µl), 0.1 M sodium phosphate buffer (1.5 ml, pH 6.5), and 0.01 M catechol (200 µl as a reaction initiator). As a control, enzyme extract was replaced with phosphate buffer (0.1 M, pH7). PPO activity was measured spectrophotometrically at 395 nm for 3 min with an interval of 1 min. The activity was expressed as ∆A min−1 g−1 of fresh weight.
Phenylalanine ammonia-lyase (PAL) activity
The PAL activity was measured as described by Liu et al. (2017). The plant shoot was homogenized in 50 mmol borate buffer (pH 8.8) containing 0.15% polyvinyl pyrrolidone and 15 mM β-mercaptoethanol, 1 mM phenylmethanesulfonyl fluoride and 5 mM EDTA. The homogenate was centrifuged at 11,000 rpm for 20 min and the supernatant was used as the crude enzyme extract. The reaction was prepared by mixing 30 µl enzyme extract, 3 mM L-phenylalanine (prepared in 150 mM Tris HCl buffer), and 0.1 mM borate buffer (pH 8.8). Tris HCl buffer in the control reaction replaced the enzyme extract. The enzyme activity was measured at 290 nm for 3 min with an interval of 1 min. The activity was expressed as ∆A min−1 g−1 of fresh weight.
Statistical analysis
All the percentage data were arcsine-transformed and analysed with ANOVA. Difference between the mean values was compared by the least significant difference (LSD) test at p ≤ 0.05.
Results
In vitro suppression of pathogen by antagonistic bacteria
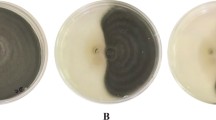
The bakanae pathogen (F. fujikoroi) was significantly inhibited by the rice associated antagonistic rhizobacteria (Fig. 1). Five out of 20 strains, viz KFP5, KFP7, KFP12, KFP17, and KFP18, caused the most prominent inhibition of the F. fujikoroi (≥ 60%) in vitro. The strains KFP12 and KFP17 were the strongest inhibitors (inhibition = 64%) followed by KFP5, KFP7 and KFP18 (inhibition = 60–62%). However, these strains were statistically similar in respect to their inhibitory activity against the pathogen.
In vitro suppression of Fusarium fujikoroi by rice antagonistic bacteria. T stands for isolate name “KFP”. Values are mean of replicates (n = 3) and different letters indicate significant difference at p ≤ 0.05 according to the Fisher’s least significant difference (LSD) test. Bars represent SE. (F19, 40 = 13.3, p < 0.001)
Biocontrol efficacy of rhizobacteria against rice bakanae disease
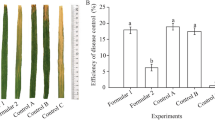
The antagonistic bacteria significantly suppressed the rice bakanae disease under field conditions. In field experiment 1 (individual strain inoculation), the antagonistic Bacillus sp. KFP7 and KFP17 significantly suppressed the bakanae disease (disease incidence ≤ 25% in bacteria treated plants versus disease incidence ≥ 75% in negative control) in both rice varieties (Fig. 2). Their efficacy was equal to that of fungicide (disease incidence ≤ 25% in fungicide treated plants versus disease incidence ≥ 75% in negative control). In field experiment 2, the inoculation of antagonistic Bacillus sp. KFP7 and KFP17 as consortium also significantly suppressed the bakanae disease (disease incidence ≤ 25% in bacteria treated plants versus disease incidence ≥ 95% in negative control), improved the number of tillers (14 tillers in bacteria treated plants versus eight tillers in negative control), plant height (115 cm versus 121.77 cm in negative control), and grain yield (323.08 gm−2 versus 120.5 g/m2 in negative control) as shown in Fig. 3. Once again, the efficacy of the bacterial consortium was equal to that of fungicide. In both experiments, the antagonistic bacteria showed consistent biocontrol efficacy during the consecutive years (Fig. 3). There was no significant effect of the year on disease incidence, number of tillers, plant height, and yield (Tables 1, 2). The rhizobacteria also enhanced the growth parameters and yield (376.45 gm−2 versus 120.5 g/m2 in negative control) in absence of pathogen. These findings suggest their use as potential bioinoculants of rice.
In planta suppression of rice bakanae disease by antagonistic bacteria (Field experiment 1, pooled analysis of two years). T1 = Bacillus sp. KFP5 + F. fujikoroi, T2 = Bacillus sp. KFP7 + F. fujikoroi, T3 = KFP12 + F. fujikoroi, T4 = Bacillus sp. KFP17 + F. fujikoroi, T5 = KFP18 + F. fujikoroi, T6 = fungicide thiophanate methyl 2% (w/v) + F. fujikoroi, T7 = F. fujikoroi (control). Values are mean of replicates (n = 3) and different letters indicte significant difference at p ≤ 0.05 according to the Fisher’s least significant difference (LSD) test. Bars represent SE. (F6,75 = 249.22, p ≤ 0.01)
Effect of Bacillus sp. KFP7 and KFP17 on disease incidence, plant height, no. of tillers, and yield in basmati-385 (Field experiment 2, pooled analysis of two years). T1 = F. fujikoroi, T2 = bacterial consortium (KFP7 and KFP17) with F. fujikoroi, T3 = thiophanate methyl 2% (w/v) with F. fujikoroi, T4 = bacterial consortium (KFP7 and KFP17). Values are mean of replicates (n = 3) and different letters indicate significant difference at p ≤ 0.05 according to the Fisher’s least significant difference (LSD) test. Bars represent SE. a Plant height (F3,16 = 10.84, p < 0.001), b Number of tillers (F3,16 = 8.04, p < 0.01), c Disease incidence (F3,16 = 501.25, p < 0.001), d Yield (F3,16 = 2210.35, p < 0.001)
The activity of defense-related enzymes
The bacterial consortium treatment induced the activity of the POD enzyme (ΔΑ = 0.353) over negative control i.e. treated with fungi only (ΔΑ = 0.124). The POD activity in Fusarium-infected plants treated with the bacterial consortium was significantly higher (ΔΑ = 0.353) than all other treatments. While PAL activity was induced almost equally in both bacterial consortium (ΔΑ = 0.187) and fungicide treated plants (ΔΑ = 0.195). The antagonistic bacteria did not induce the PPO activity (Table 3, Fig. 4).
Effect of Bacillus sp. KFP7 and KFP17 on antioxidant enzymes activity in the rice (pooled analysis of two years). T1 = F. fujikoroi, T2 = bacterial consortium (KFP7 and KFP17) with F. fujikoroi, T3 = thiophanate methyl (2% w/v) with F. fujikoroi, T4 = bacterial consortium (KFP7 and KFP17). Values are mean of replicates (n = 3) and different letters indicate significant difference at p ≤ 0.05 according to the Fisher’s least significant difference (LSD) test. Bars represent SE. a POD (F3,16 = 15.3, p < 0.001), b PPO (F3,16 = 2.52, p = 0.09), c PAL (F3,16 = 2.99, p = 0.06)
Discussion
Antagonistic bacteria have great potential to suppress disease and promote plant growth based on their multifaceted traits. They simultaneously help the plants by protecting them from pathogens and with the acquisition of nutrients to enhance their yield. Previously, certain antagonistic bacterial strains were reported for the suppression of rice blast disease and enhance the grain yield (Rais et al. 2016, 2018). Here, we further investigated their potential to suppress the rice bakanae, which is another economically important disease of rice. In our study, the bakanae pathogen was effectively suppressed by the antagonistic Bacillus bacteria (KFP7 and KFP17). Their antagonism might be due to the production of antibiotics and induction of defense-related enzyme (POD) activity. Antibiosis and induced systemic resistance are the broad mechanisms adopted by Bacillus spp. against numerous pathogens including F. fujikoroi (Sarwar et al. 2018).
These antagonistic bacterial strains, KFP7 and KFP17, have been already reported as effective biocontrol agents of blast disease in the same varieties (Rais et al. 2018). In this study, their consortium exhibited biocontrol efficacy against rice bakanae disease. Their biocontrol efficacy against bakanae disease could be justified by their indigenous nature. The indigenous rhizobacteria can survive in the rhizosphere by adapting the indigenous ecological niche and compete with other microbes (Rasul et al. 2019). The efficacy against bakanae disease further strengthens their use as a potential bio-fungicide for rice.
Bacillus sp. exert diverse mechanisms to control the phytopathogens (Islam et al. 2019). These mechanisms include suppression of fungal proliferation and inducing systemic resistance in plants (Chung et al. 2015). The Bacillus sp. significantly induced the systemic resistance in rice against blast and bakanae disease (Rais et al. 2018; Sarwar et al. 2018). They induced the activity of peroxidase (POD) in basmati- 385 which is a major defense-related enzyme. POD scavenges the reactive oxygen species (ROS) by acting as a high antioxidant. Induction of POD resulted in reduced infection of F. fujikori as reported in numerous studies (Sakhuja et al. 2021; Kalboush et al. 2019; Elamawi et al. 2020; Ji et al. 2018). In contrast, the effect of strains on the activity of other enzymes was not statistically significant. The role of antioxidant enzymes in controlling the disease is well documented in different studies (Chung et al. 2015; Hossain et al. 2016).
The antagonistic Bacillus strains (KFP7 and KFP17) were found almost equally effective against rice bakanae either applied as a single or consortium treatment. Their equal effect depicts their maximum potential against the disease irrespective of the application method. Our findings are consistent with the earlier studies where numerous biocontrol agents are used to control the bakanae disease (Motlagh et al. 2020; Ramesh et al. 2020; Hossain et al. 2016). The consortium of Bacillus sp. enhanced the yield in absence of a pathogen. This effect might be due to their plant growth-promoting traits like nutrient solubilization (Rais et al. 2016). These findings advocate the utilization of examined Bacillus spp as biofertilizer and biofungicide of rice.
There are several reports describing the efficacy of the rhizobacteria applied as a consortium. For instance, the rhizobacterial consortia enhanced the nutrient uptake, biomass, and yield in rice under drought stress (Joshi et al. 2020). Similarly a microbial consortium also suppressed the root rot disease on tomato (Abdeljalil et al. 2021). As reported by Izquierdo-García et al (2021), microbial consortia effectively suppressed the Fusarium wilt disease on cape gooseberry.
The antagonistic bacterial strains showed consistent efficacy against bakanae disease for consecutive years. The findings of the present study suggest that rice-associated antagonistic bacteria screened to control one disease could be effective for other diseases. However, multi-location trials under different ecological conditions are further required to recommend these rhizobacteria as potential bio-inoculants for rice.
References
Abbas Q, Rehman A, Sad M (2016) An overview of bakanae disease of rice. American-Eurasian J Agric Environ Sci 16:270–277
Abdeljalil NO, Vallance J, Gerbore J, Yacoub A, Daami-Remadi M, Rey P (2021) Combining potential oomycete and bacterial biocontrol agents as a tool to fight tomato Rhizoctonia root rot. Biol Control 155:104521
Berendsen RL, Vismans G, Yu K, Song Y, de Jonge R, Burgman WP, Burmølle M, Herschend J, Bakker PA, Pieterse CM (2018) Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J 12:1496–1507
Bashyal BM, Aggarwal R, Sharma S, Gupta S, Singh U (2016) Single and combined effects of three Fusarium species associated with rice seeds on the severity of bakanae disease of rice. J Plant Pathol 98:405–412
Cai X, Zhao H, Liang C, Li M, Liu R (2021) Effects and mechanisms of symbiotic microbial combination agents to control tomato Fusarium crown and root rot disease. Front Microbiol 12:1555
Chen YC, Lai MH, Wu CY, Lin TC, Cheng AH, Yang CC, Wu HY, Chu SC, Kuo CC, Wu FY, Lin GC (2016) The genetic structure, virulence, and fungicide sensitivity of Fusarium fujikuroi in Taiwan. Phytopathology 106:624–635
Chung EJ, Hossain MT, Khan A, Kim KH, Jeon CO, Chung YR (2015) Bacillus oryzicola sp. Nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth-promoting, and systemic resistance inducing activities in rice. Plant Pathol J 31:152–164
Dhondge HV, Pable AA, Barvkar VT, Dastager SG, Nadaf AB (2021) Rhizobacterial consortium mediated aroma and yield enhancement in basmati and non-basmati rice (Oryza sativa L.). J Biotechnol 328:47–58
Elamawi RM, Tahoon AM, Elsharnoby DE, El-Shafey RA (2020) Bio-production of silica nanoparticles from rice husk and their impact on rice bakanae disease and grain yield. Arch Phytopathol Pflanzenschutz 53:459–478
Emmani S, Alikhani HA, Pourbabaei AA, Etesami H, Sarmadian F, Motesssharezadeh B (2019) Effect of rhizopheric and endophytic bacteria with multiple plant growth promoting traits on wheat growth. Environ Sci Pollut 26:19804–19813
Hammerschmidt R, Nuckles E, Kuć J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82
Hashem A, Tabassum B, Abd-Allah EF (2019) Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci 26:1291–1297
Hossain KS, Mia MT, Bashar MA (2015) Management of Bakanae disease of rice. Bangladesh J Bot 44:277–283
Hossain MT, Khan A, Chung EJ, Rashid MH, Chung YR (2016) Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. Plant Pathol J 32:228–241
Izquierdo-García LF, Cotes AM, Moreno-Velandia CA (2021) Screening for effective microbial consortia against Fusarium wilt of cape gooseberry (Physalis peruviana). BioControl 66:713–725
Islam MT, Rahman MM, Pandey P, Boehme MH, Haesaert G (2019) Bacilli and agrobiotechnology: phytostimulation and biocontrol. Springer, Cham
Ji H, Kim TH, Lee GS, Kang HJ, Lee SB, Suh SC, Kim SL, Choi I, Baek J, Kim KH (2018) Mapping of a major quantitative trait locus for bakanae disease resistance in rice by genome resequencing. Mol Genet Genom 293:579–586
Joshi B, Chaudhary A, Singh H, Kumar PA (2020) Prospective evaluation of individual and consortia plant growth promoting rhizobacteria for drought stress amelioration in rice (Oryza sativa L.). Plant Soil 457:225–240
Kalboush ZA, Hassan AA (2019) Antifungal potential and characterization of plant extracts against Fusarium fujikuroi on rice. J Plant Prot Pathol 10:369–376
Katoch P, Katoch A, Paudel M, Upreti S (2019) Bakanae of rice: a serious disease in Punjab. Int J Curr Microbiol App Sci 8:129–136
Köhl J, Kolnaar R, Ravensberg WJ (2019) Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci 10:845
Liu N, Song F, Zhu X, You J, Yang Z, Li X (2017) Salicylic acid alleviates aluminum toxicity in soybean roots through modulation of reactive oxygen species metabolism. Front Chem 5:96
Matic S, Gullino ML, Spadaro D (2017) The puzzle of bakanae disease through interactions between Fusarium fujikuroi and rice. Front Biosci 9:333–344
Mayer A, Harel E, Ben-Shaul R (1966) Assay of catechol oxidase—a critical comparison of methods. Phytochemistry 5:783–789
Motlagh MR, Dashti M (2020) Biological control of Fusarium fujikuroi, the causal agent of bakanae using some antagonistic bacteria in Gilan province. J Plant Prot 34:287–295
Olenska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J (2020) Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ 7:140682
Panpatte DG, Shelat HN, Jhala YK, Vyas RV (2021) Fortified bacterial consortium: a novel approach to control root knot nematode in cucumber (Cucumis sativum). Biol Control 155:104528
Rais A, Shakeel M, Hafeez FY, Hassan MN (2016) Plant growth-promoting rhizobacteria suppress blast disease caused by Pyricularia oryzae and increase grain yield of rice. BioControl 61:769–780
Rais A, Shakeel M, Malik K, Hafeez FY, Yasmin H, Mumtaz S, Hassan MN (2018) Antagonistic Bacillus sp. reduce blast incidence on rice and increase grain yield under field conditions. Microbiol Res 208:54–62
Ramesh N, Naeimi S, Rezaee S, Berdi FK (2020) Biological control of rice Bakanae disease caused by Fusarium fujikuroi using some endophytic fungi. J Appl Entomol 87:281–296
Rasul M, Yasmin S, Zubair M, Mahreen N, Yousaf S, Arif M, Sajid ZI, Mirza MS (2019) Phosphate solubilizers as antagonists for bacterial leaf blight with improved rice growth in phosphorus deficit soil. Biol Control 136:103997
Sakhuja M, Zhawar VK, Pannu PP (2021) Regulation of antioxidant enzymes by abscisic acid and salicylic acid under biotic stress caused by Fusarium fujikuroi in rice. Indian Phytopathol 13:1–8
Saito H, Sasaki M, Nonaka Y, Tanaka J, Tokunaga T, Kato A, Thuy TT, Vang LV, Tuong LM, Kanematsu S, Suzuki T (2021) Spray application of nonpathogenic fusaria onto rice flower controls bakanae disease (caused by Fusarium fujikuroi) in the next plant generation. Appl Environ Microbiol 87:e01959-e2020
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth-promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169:18–29
Sarwar A, Hassan MN, Imran M, Iqbal M, Majeed S, Brader G, Sessitsch A, Hafeez FY (2018) Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol Res 209:1–13
Sivan A, Ucko O, Chet I (1987) Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field conditions. Plant Dis 71:587–592
Sunani SK, Bashyal BM, Kharayat BS, Prakash G, Krishnan SG, Aggarwal R (2020) Identification of rice seed infection routes of Fusarium fujikoroi inciting bakanae disease of rice. J Plant Pathol 102:113–121
Srikhong P, Lertmongkonthum K, Sowanpreecha R, Rerngsamran P (2018) Bacillus sp. strain M10 as a potential biocontrol agent protecting chili pepper and tomato fruits from anthracnose disease caused by Colletotrichum capsici. BioControl 63:833–842
Ullah H, Yasmin H, Mumtaz S, Jabeen Z, Naz R, Nosheen A, Hassan MN (2020) Multitrait Pseudomonas sp. isolated from monocropped wheat (Triticum aestivum) suppress Fusarium root and crown rot. Phytopathology 110:582–592
Weerapol Y, Nimraksa H, Paradornuwat A, Sriamornsak P (2019) Development of ready-to-use products derived from Bacillus subtilis strain CMs026 for plant disease control. BioControl 64:173–183
Wu Z, Huang Y, Li Y, Dong J, Liu X, Li C (2019) Biocontrol of Rhizoctonia solani via induction of the defense mechanism and antimicrobial compounds produced by Bacillus subtilis SL-44 on Pepper (Capsicum annuum L.). Front Microbiol 10:2676
Zainudin NIM, Razak A, Salleh B (2008) Bakanae disease of rice in Malaysia and Indonesia: etiology of the causal agent based on morphological, physiological and pathogenicity characteristics. J Plant Prot Res 48:475–485
Acknowledgements
The authors would like to thank the National Agricultural Research Centre (NARC) for providing field facilities. We would also thank the Pakistan Science Foundation (PSF) for providing funds under Research Grant No. C-141.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflicts of interests.
Ethical approval
The research presented in the article does not involve human participants and/or animals.
Additional information
Handling Editor: Sotiris Tjamos
Rights and permissions
About this article
Cite this article
Nawaz, MEN., Malik, K. & Hassan, M.N. Rice-associated antagonistic bacteria suppress the Fusarium fujikoroi causing rice bakanae disease. BioControl 67, 101–109 (2022). https://doi.org/10.1007/s10526-021-10122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-021-10122-6