Abstract
Pantoea ananatis is a plant pathogenic bacterium that severely impacts rice. In spite of its worldwide prevalence, limited studies have been conducted so far on the control of P. ananatis. Bacitracin A is a non-ribosomal peptide antibiotic with strong antibacterial activity produced by Bacillus licheniformis strain HN-5. We investigated the mechanisms of action underlying the biocontrol and bactericidal efficacy of bacitracin A against P. ananatis. Fluorescence microscopy and bacterial cell viability analyses revealed that the median effective concentration of bacitracin A against P. ananatis was 9.10 μg ml−1. Scanning and transmission electron microscopy showed that bacitracin A damaged the cell wall and membrane of P. ananatis. Quantitative real-time PCR indicated that the transcriptional expression of ftsZ, glmS, and gumD, which are involved in cell division, cell-wall biosynthesis, and extracellular polymeric substance biosynthesis, respectively, was upregulated at 12 h and significantly downregulated at 24 h after bacitracin A treatment in P. ananatis. Bacitracin A caused cell leakage and changes to membrane permeability in P. ananatis, supporting its use as a natural biocontrol agent for P. ananatis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa) is a staple in the diet of more than three billion individuals worldwide, sustaining the nutritional needs of an ever-expanding global population (Skamnioti et al. 2009). Pantoea ananatis is a diverse species that occupies various unusual ecological niches and causes diseases such as sheath rot, grain discoloration, stem necrosis, and leaf blight in rice (Cother et al. 2004; Choi et al. 2012; Shyntum et al. 2015). P. ananatis affects cultivated and wild crops and trees, including rice, corn, onions, honeydew melons, and pineapples, and can cause bacteremia in humans (Coutinho et al. 2009; Kido et al. 2010; Maayer et al. 2014; Shyntum et al. 2015). In 2004, P. ananatis was classified as a rice pathogen in Australia (Cother et al. 2004). South Korea issued the same decree in 2010 (Choi et al. 2012). Furthermore, in 2008, a new variant of leaf blight in rice caused by P. ananatis was reported in India (Mondal et al. 2011). Current methods of controlling P. ananatis primarily involve the application of copper-based pesticides. However, the accumulation of such chemical pesticides in crops results in long-term environmental damage and the development of pesticide-resistant pathogens, thus limiting their applications (Zeriouh et al. 2011; Silva et al. 2012). Therefore, a cost-effective, convenient, and environment-friendly method of P. ananatis eradication is urgently needed. Biological control agents are increasing in popularity owing to their eco-friendliness, versatility, and potential to successfully control disease (Fravel 2005).
The intrinsic attributes of certain microorganisms can be manipulated and used to manage plant diseases (Zeriouh et al. 2011). Bacillus spp. are gram-positive rod-shaped endospore-forming organisms, which may be obligate aerobes or facultative anaerobes. In the traditional taxonomy system, B. subtilis encompasses multiple closely related species such as B. licheniformis, B. subtilis, and B. amyloliquefaciens (Wang et al. 2007; Borshchevskaya et al. 2013). Biopeptides primarily interact with fungal plasma membrane components (Magnet-Dana et al. 1994; Straus et al. 2006; Romero et al. 2007; Jin et al. 2020). Bacitracin is an important non-ribosomal peptide antibiotic produced by certain strains of B. subtilis and B. licheniformis. It is a potent antibiotic against gram-positive and certain gram-negative bacteria. The bacitracin synthetase gene cluster in B. licheniformis is composed of the bacABC operon and bacT, which encode non-ribosomal peptide synthetase and thioesterase, respectively (Konz et al. 1997; Wang et al. 2017). B. licheniformis is used for the industrial production of bacitracin, which is widely used as an additive in livestock feed (Schallmey et al. 2004). The mechanisms underlying the effects of bacitracin in reducing infectious diseases in livestock have been previously investigated (Hancock 2005). However, whether this antimicrobial efficacy of bacitracin A in humans and livestock translates to its potential for the biocontrol of plant diseases has not yet been elucidated. Thus, we conducted this study to evaluate the potential of bacitracin A as a biocontrol agent for P. ananatis and determine the mechanisms underlying its antibacterial activity.
Material and methods
Microorganisms and plants
Pantoea ananatis strain DZ-12 (stored at China General Microbiological Culture Collection Center, ID CGMCC 1.13877) was obtained from professor Gao (Nan Jing Agriculture University, China) and cultured in nutrient broth (NB) medium. B. licheniformis strain HN-5 (Genebank ID No. MK648261.1) was isolated from soil collected from Hainan island on April 18, 2015, and stored at Hainan University, China until use. It was then cultured in Luria-broth (LB) medium. All microorganism strains were stored at −80 °C in an appropriate medium with 40% glycerol. Rice (cultivar IR24) which is grown to the tillering stage, was used in this study. The rice was grown in autoclaved soil in 1 l plastic pots (nine plants per pot) under glasshouse conditions of temperature 26 ± 1 °C, RH 70 ± 5%, and ambient light (L:D 12:12).
Isolation and identification of bacitracin A
Bacillus licheniformis strain HN-5 was cultured in LB medium at 28 °C for 48 h with 180 rpm shaking. The culture (20 l) was reduced in vacuo to approximately 1 l at 60 °C. The metabolites in the concentrated medium were extracted thrice (3 × 1 l) using n-butyl alcohol at 28 °C. The extracts were combined, dried, and concentrated in vacuo. The obtained n-butyl alcohol extract (3.84 g) was separated into ten fractions on a silica gel column (200–300 mesh) using sequential gradient elution, first with 100% chloroform (CHCl3), followed by a mixture of CHCl3/methanol (MeOH) (100:1, 50:1, 25:1, 15:1, 10:1, 5:1, 2:1, 1:1 v/v), and finally 100% MeOH. The resulting fractions were combined according to their thin-layer chromatographic profiles on silica gel GF254 (Marine Chemical Industry Factory, Qingdao, China). Based on a bioassay using the paper disk method, the fractions were incubated at 28 °C for 24 h to test their ability to inhibit P. ananatis (Zuo et al. 2014). Then, 560 mg of Fraction 2 was used for chromatography and separated using Sephadex LH-20 (Merck, Darmstadt, Germany) and a silica gel column with CHCl3/MeOH (2:1 v/v) to yield bacitracin A (234.25 mg). The bacitracin A sample was purified using high-performance liquid chromatography (HPLC), and the composition of the high-grade bacitracin A was analyzed by LC-MS (Pavli et al. 2001). Standard bacitracin A was purchased from Wako (FUJIFILM Wako Pure Chemical Corporation, Chuo-Ku Osaka, Japan).
Antibacterial assay
Pantoea ananatis was cultured for 24 h in NB medium at 28 °C. Then, 10 μl of bacitracin A (5 μg ml−1) filtered through 0.22 μm Millipore membranes was added to a paper disk (6 mm diameter) and placed on an NB agar plate inoculated with P. ananatis. The plates were incubated at 28 °C for 48 h, and the diameters of the zones of inhibition were measured (Zuo et al. 2014). Six NB agar plates inoculated with P. ananatis were treated with either ultrapure water or bacitracin A, and the assays were performed independently and repeated thrice.
LIVE/DEAD BacLight bacterial viability staining
A viability assay was performed using the LIVE/DEAD BacLight bacterial viability staining kit L7012 (Invitrogen, Molecular Probes, USA) and observed using fluorescence microscopy, as previously described (Wu et al. 2015). The kit contains a green-fluorescent SYTO 9 stain and a red fluorescent propidium iodide (PI) stain used for two-color fluorescence staining. This assay was used to test the membrane integrity of P. ananatis upon exposure to bacitracin A. Live bacteria with intact membranes show green fluorescence, whereas bacteria with damaged membranes show red fluorescence. P. ananatis cells (1 ml at a concentration of 107–108 CFU ml–1) were incubated with bacitracin A (final concentration 10, 40, 80, 181.82, and 333.33 μg ml−1) for 24 h, centrifuged at 1000×g for 10 min, and then resuspended in 10 mM sodium phosphate buffer (pH 7.4). Further, 10 μl of the molecular probes, prepared as per the manufacturer’s guidelines, were added to the suspension and incubated for 15 min at 25 °C in the dark. The number of cells was observed using an Olympus BX43 microscope with cellSens standard software (Tokyo, Japan). The bactericidal activity of bacitracin A was calculated using the following equation: percentage survival (%) = (1−treatment/control) × 100, where treatment indicates the cell densities of P. ananatis treated with bacitracin A and the control is the P. ananatis cell density without any treatment (Kim et al. 2007). Six sample suspensions inoculated with P. ananatis were treated with either water or bacitracin A, and the assays were repeated thrice, independently. The MIC50 value of bacitracin A was obtained from the sigmoidal inhibition curves fitted by profit regression analysis (SPSS 19.0).
Microstructural and ultrastructural observations
The effects of bacitracin A on the microstructure and ultrastructure of P. ananatis were observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as previously described (Zeriouh et al. 2011). Briefly, P. ananatis cells were harvested at a concentration of 107–108 CFU ml−1 after preparation for 48 h at 28°C and then incubated with 9.10 μg ml−1 (final concentration) bacitracin A for 12 h. The samples were centrifuged (8000×g for 10 min at 4 °C.) and prefixed with 2.5% glutaraldehyde. The fixed cells were then washed thrice with 100 mM phosphate-buffered saline (PBS) for 10 min and dehydrated using an ethanol gradient. For SEM analysis, the samples were coated with gold and analyzed using Hitachi S-3000N SEM (Hitachi, Japan). For TEM analysis, the cells were post-fixed for 3 h in 1% osmium tetroxide, embedded in Epon 812, sectioned with an ultramicrotome, and examined under a Hitachi H-600 TEM (Jin et al. 2017). Six sample suspensions inoculated with P. ananatis were treated with either water or bacitracin A, and the analyses were repeated thrice, independently.
RNA extraction and qRT-PCR
RNAseq analysis (data not included) and microscopy were used to predict the cell membranes and the sites of cell division that may be targeted by bacitracin A. To this end, we designed and synthesized primers for glucosamine-6-phosphate synthase and cell division as well as the EPS biosynthesis gene primers for P. ananatis. The primers were designed using Primer 5.0 based on the genome of P. ananatis strain DZ-12 (GenBank: NZ_CM012203.1; Table 1). Primer specificity was confirmed by agarose gel electrophoresis and melting curve analysis. The expression of three target genes (gumD, ftsZ, and glmS) of P. ananatis was monitored by qRT-PCR using 16S rRNA as the internal reference (Wu et al. 2015). P. ananatis was exposed to bacitracin A (final concentration of 9.10 μg ml−1) for 12 and 24 h. Untreated P. ananatis culture was used as a control. Total RNA of P. ananatis was extracted using a Bacterial RNA Kit (Omega Biotek, USA) according to the manufacturer’s instructions, and the complementary DNA (cDNA) was synthesized using the Prime Script RT-PCR kit (TaKaRa, Japan). A reaction volume of 20 μl, including SYBR Premix EX Taq (TaKaRa), was used for the qRT-PCR assay, which was performed on an ABI Prism 7500 system (Applied Biosystems Inc, Foster City, CA, USA). The cycling conditions consisted of a hot-start activation step for 14 s at 95 °C, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. Six samples, suspensions in test tubes inoculated with P. ananatis, were treated with water or bacitracin A, and the experiments were performed independently in triplicate. The data were normalized according to the 2−△△CT method (Livak et al. 2001).
Pathogenicity test on rice plants
A pathogenicity assay was conducted in a glasshouse at 25–28 °C as previously described (Qian et al. 2013). In brief, P. ananatis was cultivated in NB medium at 28 °C and mixed at 200 r min−1 for 24 h. One hour before inoculation with the bacterial pathogen, rice (IR24) leaves were sprayed with 9.10 μg ml−1 bacitracin A or water (control). To determine the length of lesions due to P. ananatis, two-month-old rice plants were inoculated with a suspension of 107–108 CFU ml−1 P. ananatis by the spraying method. Disease symptoms were recorded after incubation for 15 days, and the protective rate of bacitracin A was calculated using the following equation: protective rate (%) = (1−treatment/control) × 100, where treatment and control are lesion lengths with and without bacitracin A, respectively (Wu et al. 2015). For each test, 270 plants (rice IR24) were treated with either water or bacitracin A, and the experiment was performed independently in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS (version 19.0; SPSS Inc). Data were analyzed using ANOVA, followed by a Fisher least significant difference test (p < 0.05). MIC50 values were calculated using probit regression. Each treatment had three biological replicates. All data were expressed as means ± SD.
Results
Purification and structure elucidation of bacitracin A
We purified the active substance, designated as compound 1. Compound 1 appeared as a single peak when evaluated by HPLC, confirming the presence of a single compound. The retention time of compound 1 was 24.043 min at 254 nm (Suleiman et al. 2017). Liquid chromatography with tandem mass spectrometry (LC-MS/MS) was performed on a triple quadrupole mass spectrometer using the positive electrospray ionization (ESI) mode. Composition analysis by LC-MS showed that compound 1 and standard bacitracin A had nearly the same compositions. The ESI-MS result for compound 1 matched that for bacitracin-1422 Da (Suleiman et al. 2017; Choi et al. 2017).
Assessment of bactericidal activity of bacitracin A
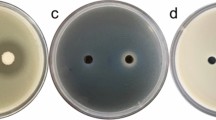
For the agar disk diffusion assays, MeOH, control, n-butyl alcohol extract (5 μg ml−1), and bacitracin A (5 μg ml−1) samples were added to disks inoculated with P. ananatis and achieved 0, 0, 11.930 ± 0.003, and 20.328 ± 0.046 mm zones of inhibition, respectively. To accurately determine the bacteriostatic effect of bacitracin A, we labeled the P. ananatis cells using a LIVE/DEAD BacLight bacterial viability staining kit. This was followed by fluorescence microscopy to determine the bacteriostatic effect of bacitracin A (Table 2). P. ananatis cell staining revealed stable, low levels of red fluorescent cells and high levels of green fluorescent cells, indicating many live intact cells. Following exposure to 10 μg ml−1 (final concentration) bacitracin A for 24 h, the proportion of red fluorescent cells increased to 48.49 ± 0.15 % (p < 0.05). Exposure to 40 μg ml−1 (final concentration) bacitracin A increased the proportion of red fluorescent cells to 75.26 ± 0.15 % owing to the accumulation of the red probe in the damaged P. ananatis membranes (p < 0.05). The cells were intensely stained with the red probe upon exposure to 333.33 μg ml−1 (final concentration) bacitracin A. Severe damage to the cell membranes was more evident in the cells exposed to higher concentrations of bacitracin A. Bacitracin A showed strong antibacterial activity against P. ananatis, with an MIC50 value of 9.10 ± 0.18 μg ml−1 at 24 h (F4,10 = 2654.97; p < 0.01).
Scanning and transmission electron microscopy
SEM and TEM analyses revealed that bacitracin A damaged the cell walls and membranes of P. ananatis, resulting in the leakage of intracellular components and forming an empty space. The untreated cells had a normal, plump, rod-shaped appearance with smooth intact surfaces (Fig. 1a), whereas the cells treated with bacitracin A were loose, distorted, and heavily disrupted (Fig. 1b). TEM indicated that the untreated cells displayed normal prokaryotic morphology with intact cell membranes and a uniformly distributed cytoplasm (Fig. 1c). On the contrary, the cells exposed to bacitracin A displayed distinct damage with the formation of deep pits on the cell surface, condensed cytoplasm, and lysis of organelles and chromosomes (Fig. 1d). Lysed cells and empty vesicles were visible owing to plasmolysis and the efflux of the intracellular components.
Electron micrographs of the microstructure and ultrastructure of the Pantoea ananatis cells upon exposure to 9.10 μgml−1 (final concentration) bacitracin A for 24 h. a Untreated control P. ananatis cells determined by SEM; bars: 1 μm; b P. ananatis cells exposed to 9.10 μg ml−1 (final concentration) bacitracin A for 24 h as determined by SEM; bars: 1 μm; c Untreated control P. ananatis cells determined by TEM; bars: 0.2 μm. d P. ananatis exposed to 9.10 μg ml−1 (final concentration) bacitracin A for 24 h as determined by TEM; bars: 0.2 μm. N: nucleus, CW: cell wall. The arrows point to bacitracin A damaged cell wall and membrane of P. ananatis
Effect of bacitracin A on P. ananatis gene expression
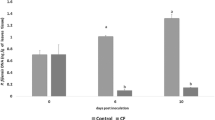
The ftsZ gene is involved in cell division. Glucosamine-6-phosphate synthase is encoded by glmS, which is important in peptidoglycan biosynthesis, and gumD, which is in the gum operon, is responsible for extracellular polysaccharide (EPS) biosynthesis and required for virulence. Therefore, ftsZ, glmS, and gumD were assessed to investigate the effects of bacitracin A on P. ananatis gene expression. The qRT-PCR analysis revealed that ftsZ, glmS, and gumD exhibited higher transcript expression levels (1.15-, 1.75-, and 1.38-fold higher, respectively) than the control upon exposure to bacitracin A at 12 h (Fig. 2). However, compared to the control, ftsZ, glmS, and gumD transcripts were approximately 0.49-, 0.78-, and 0.58-fold less abundant, respectively, at 24 h. After 12 and 24 h, significant differences were observed between the untreated controls and the samples treated with bacitracin A (Fig. 2).
Expression analysis of ftsZ, glmS, and gumD in Pantoea ananatis cells upon exposure to 9.10 μg ml−1 (final concentration) bacitracin A for 12 and 24 h. The values were normalized to the levels of 16S rRNA, which is a housekeeping gene, as an internal reference gene. The Expression of ftsZ, glmS, and gumD gene in treated with bacitracin A compared with untreated control P. ananatis cells are significantly different. The y-axis represents mean expression values ± SD relative to the control. The “*” and “**” means significantly different according to Fisher’s least-significant difference tests at p < 0.05 and p < 0.01, respectively
Biological control of rice diseases caused by P. ananatis
We examined whether bacitracin A protects rice (cultivar IR24) against P. ananatis infection. The virulence of P. ananatis in the infected rice plants treated with bacitracin A was significantly lower than that in the untreated controls, and lesion length and disease severity were remarkably reduced. Lesion length was significantly shorter in the cells treated with bacitracin A than in the untreated controls (12.03 ± 1.12b and 17.86 ± 1.21a cm, respectively (p < 0.05). The protective rates of bacitracin A were 49.94 ± 1.56%.
Discussion
Bacillus spp. protect against plant pathogens through their antimicrobial properties (Ongena et al. 2005; Stein et al. 2005; Romero et al. 2007). Certain strains of B. subtilis produce broad-spectrum antifungals and can control cucurbit powdery mildew (Romero et al. 2007). B. subtilis can generate a wide variety of antibiotics, which are essential for the biocontrol of bacteria and fungi (Moyne et al. 2001; Ongena et al. 2008; Nasir et al. 2012). Bacitracin is a mixture of closely related compounds containing at least ten distinct dodecapeptides that differ by one or two amino acids. The most active and abundant of these peptides is bacitracin A (Morris 1994). Few studies have evaluated whether bacitracin inhibits gram-negative phytopathogens. We found that bacitracin A, isolated from B. licheniformis strain HN-5, was a remarkable growth inhibitor of P. ananatis and has potential as a bactericidal agent for use in the biological control of plant diseases.
Hydrophobic groups on antimicrobial peptides interact with phospholipids and cholesterol in the bacterial cell membranes, allowing them to enter the cell (Carrillo et al. 2003; Makovitzki et al. 2006; Ciesiołka et al. 2014; Oh et al. 2018). Bacitracin A is particularly active against gram-positive bacteria. It inhibits bacterial cell wall biosynthesis and forms a tight ternary complex with C55-isoprenyl pyrophosphate. C55-isoprenyl pyrophosphate acts as a membrane-associated carrier for bacteria during the synthesis of the repeating subunits of peptidoglycan. Blockage of its recycling is fatal to cells (Pollock et al. 1994). Divalent metal cations from the bacitracin A-C55-isoprenyl pyrophosphate complex block the recycling of C55-isoprenyl pyrophosphate. This is fatal to the cell (Storm et al. 1973; Wang et al. 2017). The amino acid building blocks of bacitracin A are L-Ile1, L-Cys2, L-Leu3, D-Glu4, L-Ile5, L-Lys6, D-Orn7, L-Ile8, D-Phe9, L-His10, D-Asp11, and L-Asn12 (Drablos et al. 1999). The amino acid structure of bacitracin A contains an amino-terminal linear pentapeptide moiety with an isoleucine-cysteine thiazoline ring and a C-terminal heptapeptide ring, in which the free α-carboxy group of the C-terminal Asn is bound to the ε-amino group of lysine (Stone et al. 1971). Based on the structure and ultrastructural analysis of bacitracin A, we hypothesized that the isoleucine ring of bacitracin A binds to the fat-soluble phospholipids in the cell wall of the bacteria and penetrates the cell membrane in a fashion comparable to a balloon pierced with a needle. Owing to this mechanism of action along with its amphiphilic nature, there is a low probability of pathogens becoming resistant to bacitracin A. This is proved by the antagonistic effects of bacitracin against the newly identified rice pathogen owing to its amphiphilic nature.
Similar to previous findings by Wu et al. (2015), we found that, in P. ananatis, bacitracin stress regulates the gene expression of ftsZ and glmS, which are involved in cell division and biosynthesis, respectively. Bacitracin may prevent cell membrane repair and induce the production of EPS in P. ananatis cells. The decreased levels of ftsZ and glmS may be attributed to the high levels of dead P. ananatis cells caused by the severe cell wall damage. EPS is an important virulence factor that protects pathogens from host defenses (Torres et al. 2006; Yun et al. 2006; Li and Wang 2011). Downregulation of gumD by bacitracin may have resulted in EPS reduction and the attenuation of P. ananatis virulence. We plan to further evaluate mRNA and protein expression in our future studies.
To the best of our knowledge, this is the first study to perform microscopy and qRT-PCR to clarify the mechanisms underlying the antibacterial effects of bacitracin A against gram-negative phytopathogens. Bacitracin A caused severe ultrastructural damage to P. ananatis cell walls and membranes revealed by the examination of changes in the morphology of P. ananatis. Bacterial inhibition by bacitracin can be partially explained by its ability to cause cell leakage and impede the exchange of materials through the cell membrane owing to changes in membrane permeability.
Bacitracin A, isolated from B. licheniformis strain HN-5, exerted strong bactericidal activity against P. ananatis and showed potential as a natural biological control of this rice pathogen. Our results improved the current understanding of the mechanisms underlying the antibacterial biocontrol elicited by bacitracin A.
References
Borshchevskaya L, Kalinina A, Sineokii S (2013) Design of a PCR test based on the gyrA gene sequence for the identification of closely related species of the Bacillus subtilis group. Appl Biochem Microbiol 49(7):646–655
Carrillo C, Teruel JA, Aranda FJ, Ortiz A (2003) Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta 1611(1–2):91–97
Choi O, Kim H, Lee Y, Kim J, Moon JS, Hwang I (2012) First report of sheath rot of rice caused by Pantoea ananatis in Korea. Plant Pathol J 28(3):331–332
Choi Y, Cho S, Simkhada J, Rahman M, Choi Y, Kim C, Yoo J (2017) A novel multifunctional peptide oligomer of bacitracin with possible bioindustrial and therapeutic applications from a Korean food-source Bacillus strain. PLoS ONE 12(5):e0176971
Ciesiołka J, Jeżowska-Bojczuk M, Wrzesiński J, Stokowa-Sołtys J, Nagaj J, Kasprowicz A, Błaszczyk L, Szczepanik W (2014) Antibiotic bacitracin induces hydrolytic degradation of nucleic acids. BBA-Gen Subj 1840(6):1782–1789
Cother E, Reinke R, Mckenzie C, Lanoiselet V, Noble D (2004) An unusual stem necrosis of rice caused by Pantoea ananas and the first record of this pathogen on rice in Australia. Plant Path 33(4):495–503
Coutinho T, Venter S (2009) Pantoea ananatis: an unconventional plant pathogen. Mol Plant Pathol 10(3):325–335
Drablos F, Nicholson D, Ronning M (1999) EXAFS study of zinc coordination in bacitracin A. BBA-Protern. Struct M 1431(2):433–442
Fravel D (2005) Commercialization and implementation of biocontrol. Annu Rev Phytopathol 43:337–359
Hancock R (2005) Mechanisms of action of newer antibiotics for Grampositive pathogens. Lancet Infect Dis 5(4):209–218
Jin P, Wang H, Liu W, Zhang S, Lin C, Zheng F, Guo M (2017) Bactericidal metabolites from Phellinus noxius HN-1 against Microcystis aeruginosa. Sci Rep 7(1):3132–3141
Jin P, Wang H, Tan Z, Xuan Z, Dahar G, Li Q, Miao W, Liu W (2020) Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic Biochem Phys 163:102–107
Kido K, Hasegawa M, Hioyuki M, Kobayashi M, Yuichi T (2010) Pantoea ananatis strains are differentiated into three groups based on reactions of tobacco and welsh onion and on genetic characteristics. J Gen Plant Pathol 76(3):208–218
Kim J, Kim B, Lee C (2007) Alga-lytic of Pseudomonas fluorescens against the red tide causing marine alga Heterosigma akashiwo (Raphidophyceae). Biol Control 41(3):296–303
Konz D, Klens A, Schorgendorfer K, Marahiel M (1997) The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetases. Chem Biol 4(12):927–937
Li J, Wang N (2011) The wxacO gene of Xanthomonas citri ssp. citri encodes a protein with a role in lipopolysaccharide biosynthesis, biofilm formation, stress tolerance and virulence. Mol Plant Pathol 12(4):381–396
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDC (T) method. Methods 25(4):402–408
Maayer P, Chan W, Rubagotti E, Venter S, Toth I, Birch P, Coutinho T (2014) Analysis of the Pantoea ananatis pan-genome reveals factors underlying its ability to colonize and interact with plant, insect and vertebrate hosts. BMC Genomics 15(1):404–417
Magnet-Dana R, Peypoux F (1994) Iturins, a special class of poreforming lipopeptides: biological and physiological properties. Toxicology 87(1–3):151–174
Makovitzki A, Avrahami D, Shai Y (2006) Ultrashort antibacterial and antifungal lipopeptides. Proc Natl Acad Sci USA 103(43):15997–16002
Mondal K, Mani C, Singh J, Kim J, Mudgett M (2011) A new leaf blight of rice caused by Pantoea ananatis in India. Plant Dis 95(12):1582–1583
Morris M (1994) Primary structural confirmation of components of the bacitracin complex. Biol Mass Spectrom 23(2):61–70
Moyne A, Shelby R, Cleveland T, Tuzun S (2001) Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J Appl Microbiol 90(4):622–629
Nasir M, Besson F (2012) Conformational analyses of bacillomycin D, a natural antimicrobial lipopeptide, alone or in interaction with lipid monolayers at the air-water interface. J Colloid Interf Sci 387(1):187–193
Oh E, Bae J, Kumar A, Choi H, Jeon B (2018) Antioxidant-based synergistic eradication of methicillin-resistant Staphylococcus aureus (MRSA) biofilms with bacitracin. Int J Antimicrob Ag. 52(1):96–99
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125
Ongena M, Jacques P, Touré Y, Destain J, Jabrane A, Thonart P (2005) Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl Microbiol Biot 69(1):29–38
Pavli V, Kmetec V (2001) Optimization of HPLC method for stability testing of bacitracin. J Pharmaceut Biomed 24(5–6):977–982
Pollock T, Thorne L, Yamazaki M, Mikolajczak M, Armentrout R (1994) Mechanism of bacitracin resistance in gram-negative bacteria that synthesize exopolysaccharides. J Bacteriol 176(20):6229–6237
Qian G, Zhou Y, Zhao Y, Song Z, Wang S, Fan J, Hu B, Venturi V, Liu F (2013) Proteomic analysis reveals novel extracellular virulence-associated proteins and functions regulated by the diffusible signal factor (DSF) in Xanthomonas oryzae pv. oryzicola. J Proteome Res 12(7):3327–3341
Romero D, de Vicente A, Olmos J, Dávila J, Pérez-García A (2007) Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaera fusca. J Appl Microbiol 103(4):969–976
Schallmey M, Singh A, Ward O (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50(1):1–17
Shyntum D, Theron J, Venter S, Moleleki L, Toth I, Coutinho T (2015) Pantoea ananatis utilizes a type VI secretion system for pathogenesis and bacterial competition. Mol Plant-Microbe Interact 28(4):420–431
Silva I, Regasini L, Petrônio M, Silva D, Bolzani V, Belasque J, Sacramento L, Ferreira H (2012) Antibacterial activity of alkyl gallates against Xanthomonas citri subsp citri. J Bacteriol 195(1):85–94
Skamnioti P, Gurr S (2009) Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol 27(3):141–150
Stein T (2005) Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol Microbiol 56(4):845–857
Stone K, Strominger J (1971) Mechanism of action of bacitracin: complexation with metalion and C55-isoprenyl pyrophosphate. P Natl Acad Sci 68(12):3223–3227
Storm D, Strominger J (1973) Complex formation between bacitracin peptides and isoprenyl pyrophosphates. The specificity of lipid-peptide interactions. J Biol Chem 248(11):3940–3945
Straus S, Hancock R (2006) Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim Biophys Acta Biomembr 1758(9):1215–1223
Suleiman S, Song F, Su M, Hang T, Song M (2017) Analysis of bacitracin and its related substances by liquid chromatography tandem mass spectrometry. J Pharm Anal 7(1):48–55
Torres M, Jones J, Dangl J (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141(2):373–378
Wang L, Lee F, Tai C, Kasai H (2007) Comparison of gyrB gene sequences, 16SrRNA gene sequences and DNA-DNA hybridization in the Bacillus subtilis group. Int J Syst Evol Microbiol 57(8):1846–1850
Wang Q, Zheng H, Wan X, Huang H, Li J, Christopher T, Wang C, Chen S (2017) Optimization of inexpensive agricultural by-products as raw materials for bacitracin production in Bacillus licheniformis DW2. Appl Biochem Biotechnol 183(4):1146–1157
Wu L, Wu H, Chen L, Yu X, Borriss R, Gao X (2015) Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci Rep 5:12975–12983
Yun M, Torres P, Oirdi M, Rigano L, Gonzalez-Lamothe R, Marano M, Castagnaro A, Dankert M, Bouarab K, Vojnov A (2006) Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol 141(1):178–187
Zeriouh H, Romero D, García-Gutiérrez L, Cazorla F, de Vicente A, Perez-Garcia A (2011) The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol Plant-Microbe Interact 24(12):1540–1552
Zuo W, Jin P, Dong W, Dai H, Mei W (2014) Metabolites from the endophytic fungus HP-1 of Chinese eaglewood. Chin J Nat Med 12(2):151–153
Acknowledgements
We thank Xuewen Gao (College of Plant Protection, Nanjing Agricultural University, Key Laboratory of Integrated Management of Crop Diseases and Pests, Ministry of Education, Nanjing, China) for a kind support. The authors declare no competing financial interests.
Funding
The funding was provided by the National Natural Science Foundation of China (Grant No. 31960552), the Scientific Research Foundation for Advanced Talents (Grant No. KYQD(ZR)1842) and Hainan Provincial Science and Technology Foundation Youth Talent Innovation Program (Grant No. QCXM201903).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest in this work.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling Editor: Jane Debode.
Rights and permissions
About this article
Cite this article
Jin, P., Tan, Z., Wang, H. et al. Antimicrobial effect of Bacillus licheniformis HN-5 bacitracin A on rice pathogen Pantoea ananatis. BioControl 66, 249–257 (2021). https://doi.org/10.1007/s10526-020-10052-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-020-10052-9