Abstract
This study aimed to evaluate Metarhizium anisopliae (Metschn.) Sorokin (Hypocreales: Clavicipitaceae) persistence in the soil and its impact on Rhipicephalus microplus Canestrini (Acari: Ixodidae) larval recovery in a semifield trial after the treatment of female ticks. Nine strains from the genus Metarhizium Sorokin were isolated from the soil in Brazil and taxonomically classified using the ef1-α gene. The thermotolerance of the strains and their in vitro virulence to tick larvae were tested. One M. anisopliae strain was selected and formulated for the semifield test. The presence of M. anisopliae in the soil ranged from 0.4 × 105 to 1.4 × 105 colony forming units per gram of soil after the treatment during the five months of the survey. The fungus-treated grass pots had significantly fewer larvae than did the control pots. Evidence was gathered about the soil persistence of a native M. anisopliae strain and its efficacy in the biological control of ticks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhipicephalus microplus Canestrini (Acari: Ixodidae) ticks, popularly known as cattle ticks, cause direct damage to cattle through blood feeding, leading to anemia and ultimately death. This parasite is the vector of serious pathogenic agents to cattle and may cause leather damage and a propensity to myiasis (Ybañes et al. 2018). Its economic impact is estimated to be more than three billion dollars per year in Brazil alone (Grisi et al. 2014). The life cycle of this tick has two main phases: a parasitic phase (feeding larvae, nymphs and adults) and a non-parasitic phase (completely engorged females, eggs, and unfed larvae), the life stages of which are found on the ground. Studies have demonstrated that unfed larvae are more susceptible than adults to entomopathogens (Kaaya and Hassan 2000; Camargo et al. 2012). Thus, successful and more complete management for tick control must consider not only the parasitic stages (on the cattle) but also the non-parasitic stages. Chemical acaricides are the most commonly used method to control this tick. When inappropriately applied, these chemicals result in the selection for resistant tick populations (Klafke et al. 2017; Rodrigues-Vivas et al. 2017) in addition to environmental, meat, and milk contamination (Banumathi et al. 2017; Goméz-Perez et al. 2014). The use of entomopathogenic fungi against ticks has become a more sustainable and promising alternative (Bernardo et al. 2018; Camargo et al. 2014, 2016; De Paulo et al. 2016; Fernández-Sálas et al. 2017; Murigu et al. 2016). Field tests to control ticks in the non-parasitic stages demonstrated the efficiency of oil-based Metarhizium formulations applied directly to animals (Camargo et al. 2014, 2016; Kaaya et al. 2011) or the soil, e.g., to control fully engorged R. annulatus larvae/females, as reported by Samish et al. (2014), and R. microplus larvae, as reported by Bittencourt et al. (2003) and Ojeda-Chi et al. (2010).
The successful use of entomopathogenic fungi to control arthropods depends not only on virulence levels but also on the interactions between the fungus and its target host and their environment. The environmental factors that can negatively influence entomopathogenic fungal action include high solar irradiance, high temperatures, low RH, and soil conditions (e.g., texture, moisture content, agricultural inputs, soil microbes, and the chemical characteristics of the rhizosphere) (Jaronski 2007). Although ticks are naturally much less susceptible than insects to entomopathogenic fungi (Ment et al. 2012), the hypothesis here was that using a soil-borne entomopathogenic fungus isolated from localities where the R. microplus tick population is maintained could benefit fungal action, since native isolates tend to be naturally more adapted to the environment of the tick, as they are exposed to the same abiotic conditions. In addition, when applied to soil, the microclimate and microbiota can be a challenge for exotic isolates before they contact an arthropod host (Bilgo et al. 2018). It is also known that exposure to high temperatures may reduce the viability and/or delay the conidial germination of entomopathogenic fungi (Keyser et al. 2014; Rangel et al. 2005), and the optimal temperature for these fungi is between 25 and 35 °C (Cooney and Emerson 1964; Roberts and Campbell 1977). For that reason, entomopathogenic fungal conidia are rarely applied in the field as unformulated propagules, as adjuvants can protect them against detrimental abiotic factors, extend their field persistence, and increase their infectivity (Paixão et al. 2017).
Here, the novel aspects involved an analysis of the entomopathogen persistence in a semifield trial and its efficacy on an R. microplus larval outbreak initiated by the insertion of completely engorged female ticks in a pasture. Accordingly, the female ticks were placed in grass pots previously treated with a fungus-based oil-in-water emulsion, simulating tick detachment from the host. The fungal strain used in the semifield test was isolated from soil samples and selected based on its thermotolerance and in vitro virulence to unfed larvae. The soil persistence of the Metarhizium anisopliae (Metschn.) Sorokin (Hypocreales: Clavicipitaceae) soil-borne native isolate used in the assay was also assessed.
Materials and methods
Selection of a Metarhizium isolate for the semifield test using virulence and thermotolerance tests
Native Metarhizium strains (Supplementary Table S1) were isolated from soil using a selective artificial medium (Fernandes et al. 2010) based on their morphological characteristics according to Humber (2012). Then, these isolates underwent molecular identification. The ef1-α (eukaryotic translation elongation factor 1-α) gene was used for identification. The sequencing primers were the same as those used for the PCR. The sequences obtained were compared with 14 selected ef1-α sequences of different Metarhizium species from the GenBank database (Supplementary Table S2). The sequences were aligned by the Clustal W progressive alignment method, and a dendrogram was constructed using an algorithm based on the “Neighbor-joining” method (the protocols for the strains’ isolation and classification are described in detail in the supplementary material). These native isolates were tested for virulence against unfed R. microplus larvae. To obtain the tick larvae, engorged R. microplus females were collected from the floors of cattle pens holding artificially infected calves (CEUA/IV/UFRRJ protocol #037/2014) at the W. O. Neitz Parasitological Research Station/Department of Animal Parasitology, Veterinary Institute, Rio de Janeiro Federal Rural University (UFRRJ), Brazil. The test of virulence to the tick larvae was conducted according to Quinelato et al. (2012): ten test tubes were used per group, with approximately 1000 larvae (50 mg of R. microplus eggs) in each tube. The eggs were previously incubated at 27 °C ± 1 °C with a RH ≥ 80% for 15 days until larval hatching. The tubes with less than 98% hatchability were discarded. Nine Metarhizium spp. isolates (Supplementary Table S1) were tested in four different concentrations of aqueous conidial suspensions (i.e., 1.0 × 105, 1.0 × 106, 1.0 × 107, and 1.0 × 108 conidia ml−1). The control group was treated with polyoxyethylene sorbitan monoleate (Tween 80, Vetec Fine Chemicals Ltda, Rio de Janeiro, RJ, Brazil) solution at 0.01% (v/v). Conidial viability was determined by plating an aliquot (~ 50 μl) of the 105 conidia ml−1 suspension on potato dextrose agar (PDA) medium, followed by incubation at 25 ± 1 °C and ≥ 80% RH for 24 h. Conidial germination was observed by microscopy (×400) after 24 h. Tick larval mortality was assessed five days after the treatment.
The effect of heat on the relative germination of the nine Metarhizium spp. isolates was assessed according to Fernandes et al. (2008). The fungal suspensions were exposed to 40 °C ± 0.1 or 42 °C ± 0.1 for 4 h. The control conidial suspensions were subjected to the same protocol but without heat exposure (the tubes were incubated at 27 °C ± 0.1 for 4 h), and Metarhizium robertsii Bisch., Rehner & Humber (Hypocreales: Clavicipitaceae) ARSEF 2575 was used as a standard isolate to validate the assays, as its thermotolerance is already known (Rangel et al. 2005). Plates from all the groups were incubated for 24 h in the dark at 28 ± 1 °C. Germination of at least 300 conidia per plate was assessed after 24 h, and relative percentages were calculated by comparing the germination of the heated with unheated conidia (Braga et al. 2001a, b). The experiments were repeated on three different days with a new batch of conidia for each isolate on each day.
Biological assay using Rhipicephalus microplus females under semifield conditions
Based on the R. microplus larval bioassay results and thermotolerance tests, M. anisopliae isolate LCM S04 was selected as the best isolate for the semifield test using female ticks. LCM S04 conidia were suspended in 0.01% Tween 80 aqueous solution at 1.0 × 108 conidia ml−1. An aliquot of 10 ml of fungal suspension was inoculated into spawn bags with 1 kg of rice and 300 ml of 0.5% pre-autoclaved peptone solution (Santi et al. 2011). The rice bags were stored at 25 ± 1 °C and RH ≥ 80% for 21 days. The fully colonized substrates were then washed with 1% Tween 80 sterile distilled water solution, sieved and quantified. Mineral oil (Proquímios Comércio e Indústria Ltda, Rio de Janeiro, RJ, Brazil) was added to the aqueous fungal suspension, resulting in a 10% oil-in-water emulsion at 1.0 × 108 conidia ml−1.
Thirty plastic pots (21 cm height, 24 cm width, and 24 cm length) were filled with 1/3 sand and stone for water drainage and 2/3 non-sterile soil conditioner (Natussolos do Brasil Ltda, Taubaté, SP, Brazil). The pots were previously cultivated with Brachiaria brizantha (Hochst. Ex A. Rich.) Stapf (Poales: Poaceae) cv. Marandu (forage grass) (Wolf seeds do Brasil, Ribeirão Preto, SP, Brazil) for three months (from October to December 2017) in an open field (22° 45′ 54.9″ south, 43° 41′ 57.2″ west). In each pot, the B. brizantha leaf height was standardized at 35 cm from the soil surface to the top of the leaves. Three groups with eight pots each were formed: (1) the negative control (untreated grass pots), (2) the oil control (mineral oil-in-water emulsion with no fungus added; each pot was treated with 86 ml of 10% mineral oil-in-water emulsion), and (3) the fungus-treated group. Each grass pot from the fungus-treated group was treated with 86 ml of M. anisopliae LCM S04 oil-in-water emulsion at 1.0 × 108 conidia ml−1 (equivalent to 1.5 × 107 conidia cm−2). The treatment was carried out with sprayers to be homogeneous over the entire surface (soil and grass stem) of the pots. On the same day, after the fungal treatment, a group of five completely engorged females ticks of the same weight were placed on each of the 30 grass pots. The pots were surveyed for female oviposition and larval hatching. The larvae were collected from the tops of the B. brizantha leaves and counted starting 60 days after the fungal treatment. The grass pots were then surveyed every day until no more larvae were observed. The collected larvae were frozen and counted.
Metarhizium persistence in the treated soil
Every 15 days, 30 days after the fungus treatment, soil samples were collected from each pot (treated or control groups) and inoculated on CTC (PDA plus chloramphenicol, thiabendazole and cycloheximide) artificial culture medium (Fernandes et al. 2010). Soil from three different sites of each pot was collected using a spatula, homogenized in one sample, and then weighed. The soil (0.35 g) was transferred to a 1.5 ml microtube with 1 ml of 0.01% Tween 80 sterile distilled water solution. Fifty microliters of this homogenized suspension was plated on CTC artificial medium using a Drigalski handle (one plate per pot per collection) according to Fernandes et al. (2010). The number of Metarhizium colony forming units (CFUs) from each soil collection was calculated per gram of fungus-treated soil based on the number of Metarhizium colonies observed in each Petri dish, considering the analysis of the ten pots.
The Metarhizium colonies re-isolated from each collection were transferred to PDA and identified based on their colony aspect, color, background, and conidial shape (Humber 2012). Morphologically similar colonies from the same group and collection day were considered the same Metarhizium colony type. Molecular identifications were performed for every Metarhizium morphological colony type re-isolated from each of the ten soil collections from the fungus-treated group. The Metarhizium colonies isolated from the control groups (the untreated pots and pots treated with mineral oil) were also analyzed. The colonies underwent molecular analysis to confirm their identities as Metarhizium LCM S04 using the ef1-α gene, following the protocol described in the supplementary material.
A fragment of every ef1-α consensus sequence from each re-isolated colony was aligned and compared with the LCM S04 (i.e., the isolate previously sprayed on the grass pots) sequence, five other M. anisopliae sensu stricto sequences, and one M. acridum sequence (Driver & Milner) Bisch., Rehner & Humber (Hypocreales: Clavicipitaceae). Information about the Metarhizium isolates and their respective collections and GenBank codes are presented in Supplementary Table S2.
Climatological data
From January 26 to June 27 2018, temperature and RH were monitored in the open field close to the pots (22° 45′ 54.9″ south and 43° 41′ 57.2″ west) every 3 h using a HOBO H8 data logger (Onset Computer Corporation, Bourne, MA, USA). The period of study included the summer, autumn, and winter seasons in the Southern Hemisphere.
Statistical analysis
The data were checked for normality using a Shapiro–Wilk test. R. microplus in vitro larval mortality (in vitro virulence test) and R. microplus larval recovery under semifield conditions (semifield trial) had non-normal distributions. The conidial relative germination (thermotolerance test) and the number of CFUs per gram of soil (M. anisopliae soil persistence analysis) had normal distributions. M. anisopliae CFUs data were statistically analyzed using one-way ANOVA followed by a t test (LSD) using BioEstat 5.3® (Mamirauá Institute, Belem, Pará, Brazil). Two-way ANOVA followed by a Bonferroni test were used to analyze the data from the thermotolerance test (which examined the effect of two independent factors: the fungal isolate and the temperature) and from the in vitro virulence test (which examined the effect of two independent factors: the fungal isolate and the conidial concentration), using GraphPad Prism 5.00 (San Diego California, USA). The data from the in vitro virulence test were arcsine square root transformed prior to the analysis to better meet the assumptions of normality and homogeneity of variance. The data from the semifield trial were analyzed using a Kruskal–Wallis test followed by a Dunn’s test using GraphPad Prism 5.00. The correlation between the environmental temperature and the number of CFUs g−1 recovered from the soil was determined by Pearson’s correlation using BioEstat 5.3®. Eventual missing observations from the biological assays due to lost samples were not considered in the statistical analyzes. P-values ≤ 0.05 were considered significant.
Results
Selection of a Metarhizium isolate for the semifield test
The identifications based on the ef1-α fragment of the nine strains are shown in Supplementary Fig. S1. Significant differences in the mortality of the R. microplus larvae were detected among the treatments with the nine Metarhizium isolates for each of the different fungal concentrations (fungal isolate: F9,304 = 347.6 and P < 0.0001; conidial concentration: F3,304 = 1167 and P < 0.0001) (Table 1). The interaction between the conidial concentration and the fungal isolate had a significant effect on larval mortality (F27,304 = 86.7 and P < 0.0001). LCM S04 yielded the highest mortality when the larvae were treated with 106 conidia ml−1.
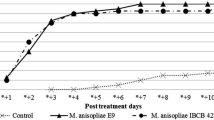
The thermotolerance profiles indicated that 40 °C was easily bearable by these isolates, with some of them exhibiting close to 100% relative germination (Table 2). When submitted to 42 °C, the conidial relative germination showed significant differences (fungal isolate: F9,34 = 6.6 and P < 0.0001; temperature: F1,34 = 466.1 and P < 0.0001). LCM S04 exhibited higher average relative germination rates than LCM S03, LCM S05, LCM S06, LCM S07, LCM S08, and LCM S09 at 42 °C (Table 2). The interaction between the temperature and the fungal isolate had a significant effect on conidial germination (F9,34 = 5.5 and P = 0.0001). The experiments were validated by the relative germination of M. robertsii ARSEF 2575 conidia, which ranged from 55.3 to 73.9% when exposed to 42 °C, as expected.
Biological assay using Rhipicephalus microplus females under semifield conditions
The larvae recovered in the fungus-treated group were significantly different in comparison to the other groups (χ2 = 20.0; df = 2; P < 0.0001). The untreated group exhibited the highest average number of recovered larvae (2596 ± 390.1), which was not significantly different from that of the oil control group (740 ± 164.7) (P > 0.05), while in the fungus-treated group, only 11 larvae were recovered from the pots (average 1.4 ± 1.37) (P < 0.001) (Fig. 1). The means and SE from each group were assessed based on the analysis of eight pots.
Average and SE of Rhipicephalus microplus larval recovery from Brachiaria brizantha pots two months after the fungal treatment. Untreated: untreated pots; Oil control: pots treated with a mineral oil-in-water emulsion with no fungus; LCM S04: pots treated with an oil-in-water fungal emulsion. Each group had eight pots, which received five fully engorged females of a homogenous weight. The different letters indicate significant differences (P ≤ 0.05) among the treatments. The data were analyzed by a Kruskal–Wallis test followed by a Dunn’s test
Metarhizium persistence in the treated soil
Metarhizium colonies were abundant in every soil sample collected from the fungus-treated group, with a reasonable fluctuation in the number of CFUs per gram of soil among the ten collections (F9,69 = 5.0; P < 0.0001) (Fig. 2). There was a propensity for higher CFU rates in the last soil collections (i.e., collections 1 2, 3, and 4 exhibited significantly fewer CFUs than those in collections 6, 8 and 9) (Fig. 2). The CFUs means and SE were assessed based on the analysis of eight pots.
Average and SE of the Metarhizium colony forming units (CFUs) per gram of soil. The colonies were recovered from the fungus-treated group over the ten soil collections (corresponding to the numbers 1–10 on the x-axis), every 15 days and 30 days after the treatment. The average number of CFUs per gram of soil is a result of the eight replicates (eight pots). The different letters indicate significant differences in the Metarhizium density (P ≤ 0.05). The statistical analyses consisted of a one-way ANOVA followed by a t test
Usually, only one widespread colony type (morphologically similar to the others) was recovered from the soil samples of the Metarhizium-treated group in each collection. When a morphologically different Metarhizium colony type was detected in the samples of a given collection, it was labeled as a different colony type. Accordingly, 13 colony types from the fungus-treated group were analyzed (one widespread colony detected in each of the ten soil collections and three additional colonies detected in collection numbers 1, 9, and 10).
Only six Metarhizium colony types were detected in the control groups during the study: one colony in collection 9 from the untreated grass pots and five other colonies collected from the oil control pots (one in collection 6, one in collection 7, and three in collection 9). The ef1-α fragments (449 bp) of the 19 colony types were aligned with the LCM S04 fragment. All the colonies that were re-isolated from the fungus-treated grass pots as well as the Metarhizium colonies re-isolated in soil collections 6, 7, and 9.1 from the oil control group and in collection 9 from the untreated pots shared 100% sequence identity with LCM S04. Two colonies from collection 9 (9.2 and 9.3) of the oil control group exhibited 96.7% and 68.5% similarity to LCM S04 and were considered new Metarhizium isolates (Metarhizium colony 9.2) or unresolved placement (Metarhizium colony 9.3).
Climatological data analysis
The temperature and humidity averages during the study were 25.8 °C and 77.9%, respectively. Nevertheless, the maximum temperature recorded was 49 °C, and the minimum temperature was 11 °C. The maximum RH recorded was 100%, and the minimum was 26%. There was a weak negative correlation between the temperatures observed and the CFU data (r = − 0.6; P = 0.03).
Discussion
The present study addressed, for the first time, the soil persistence of a native, soil-borne entomopathogenic isolate of M. anisopliae and its efficacy against R. microplus larval outbreaks under semifield conditions. These findings demonstrated the fungal action against different non-parasitic phases of ticks (fully engorged females, eggs and unfed larvae). In the present study, one Metarhizium isolate was selected (from the nine screened native soil-borne isolates) for the semifield test based on its tolerance to heat and in vitro virulence against R. microplus larvae. In tropical areas, temperatures above 40 °C are easily reached in the field, which makes the selection of highly thermotolerant isolates crucial for the success of field applications. Isolates that naturally show good thermotolerance are expected to have a better performance after the incorporation of adjuvants. Here, significant variability in conidial thermotolerance was found among the tested isolates, even though they share the same origin (city) (Table 2 and Supplementary Table S1). Rangel et al. (2005) did not find a correlation between conidial heat resistance and latitude of origin. These authors also highlighted the importance of thermotolerance for isolate choice, since it is a feature that may interfere with biological control potential in the field. In the present study, the isolates had relatively low wet-heat tolerance when exposed to 42 °C for 4 h (Table 2). Only LCM S01 and LCM S04 had satisfactory results at the highest temperature, exhibiting similar tolerance to that of M. robertsii ARSEF 2575.
In vitro biological assays should be one of the first steps (before field tests) to select new entomopathogens against arthropod pests. In the present study, the R. microplus in vitro bioassay provided information about the virulence profile of the new Metarhizium isolates against larvae, which is considered the most susceptible phase of the tick lifecycle. Three isolates, including LCM S04, resulted in more than 90% larval tick mortality only five days after the fungal treatment using the highest conidial concentration (Table 1). Excellent in vitro results were obtained for the LCM S04 isolate a short time after the treatment and with a very low conidial concentration for ticks (i.e., 1.0 × 106 conidia ml−1). Therefore, this isolate was chosen for the field test of R. microplus control.
One of the main hypotheses of the present study was confirmed: M. anisopliae conidia can negatively impact R. microplus larval outbreaks in the field even when fungal formulations are not applied directly to the tick larvae. Although Bittencourt et al. (2003) performed field tests, these authors reported larval control through direct larval treatment. Here, M. anisopliae isolate LCM S04 significantly reduced the number of R. microplus larvae recovered in the fungus-treated grass pots approximately 60 days after the treatment, supporting the results obtained for larval control under in vitro conditions. It is important to consider that the fungus could negatively affect non-parasitic phases of the tick other than larvae (viz., completely engorged females and eggs). Accordingly, the reduced number of recovered larvae from the treated group observed here may reflect not only the larval mortality but also a negative interference in the reproductive capacity of R. microplus engorged females as well as a low larval hatching rate.
In the present study, the success of the native isolate LCM S04 against R. microplus under semifield conditions may be attributed, among other reasons, to the fact that the isolate was possibly adapted to the soil environment (based on the positive results in the soil persistence analyses and presence of a rich fungal microbiota in the control plates). Here, the density of the CFUs in some soil collections exhibited considerable variation (Fig. 2). In fact, the grass coverage, once homogeneous among the pots, became more heterogeneous over the months, which is suggested to explain the variation in the CFUs recovered from the different pots of the treated group. The lower the coverage, the more exposed to heat and sunlight the fungi on the soil surface. It is also interesting that the highest rates of Metarhizium recovery from the soil occurred when temperatures tended to be milder (i.e., in the autumn and winter). In contrast, lower recovery rates were observed during the summer (Fig. 2). This could be explained by the negative correlation observed between the environmental temperature and the recovered CFUs. Even though this correlation was weak, in the field, other variables, such as humidity and soil microbiota, can also contribute to the variation in the fungal recovery rates.
The identities of the Metarhizium strains re-isolated from the soil samples were assessed based on a comparison of the ef1-α fragment sequences from the recovered colonies and the LCM S04 isolate previously used to treat the pots. All the strains recovered from the fungus-treated group exhibited 100% similarity with M. anisopliae LCM S04. Some collections from the control group also exhibited Metarhizium sp. colonies, and the LCM S04 identity of the colonies suggested contamination by horizontal dispersion. Interestingly, the LCM S04 Metarhizium colonies were not detected in the control pots at the beginning of the experiment but when the fungal concentration in the treated pots was higher (i.e., in collections 6, 8, and 9) (Fig. 2), suggesting that this finding could be a result of contamination by wind dispersal, the activity of soil arthropods and/or rain, as reported by other authors (Castro et al. 2016; Pilz et al. 2011). Two colonies isolated from samples of the oil control group did not exhibit 100% similarity with LCM S04, suggesting that they are indigenous Metarhizium isolates.
The environmental persistence of an entomopathogenic fungus, i.e., the period in which the fungus is biologically active against an arthropod pest in the environment, has the potential to be inversely proportional to the number of fungal applications in the field (Bruck 2010). Accordingly, once a fungus is applied, it may control future generations of soil arthropod pests. In the present study, the use of a native soil-borne M. anisopliae isolate to control R. microplus is suggested to increase the chances of optimal tick control. Entomopathogenic fungi persistence in the soil represents a key feature for the success of the biological control of the arthropod pests present in this environment, such as the non-parasitic life stages of R. microplus (i.e., eggs, unfed larvae, and completely engorged females). The M. anisopliae CFU density in the soil reported here provides crucial information about the amount of fungus required in the soil to control non-parasitic phases of R. microplus. These data will assist future tick management strategies applied to the non-parasitic phase of R. microplus.
References
Banumathi B, Vaseeharan B, Rajasekar P, Prabhu NM, Ramasamy P, Murugan K, Canaçe A, Benelli G (2017) Exploitation of chemical, herbal and nanoformulated acaricides to control the cattle tick Rhipicephalus (Boophilus) microplus—a review. Vet Parasitol 244:102–110
Bernardo CC, Barreto LP, Silva CRS, Luz C, Arruda W, Fernandes ÉKK (2018) Conidia and blastospores of Metarhizium spp. and Beauveria bassiana s.l.: their development during the infection process and virulence against the tick Rhipicephalus microplus. Ticks Tick-borne Dis 9:1334–1342
Bilgo E, Lovett B, Leger RJST, Sanon A, Dabiré RK, Diabaté A (2018) Native entomopathogenic Metarhizium spp. from Burkina Faso and their virulence against the malaria vector Anopheles coluzzii and non-target insects. Parasit Vectors 11:209–215
Bittencourt VREP, Bahiense TC, Fernandes EK, Souza EJ (2003) Avaliação da ação in vivo de Metarhizium anisopliae (Metschnikoff. 1879) Sorokin. 1883 aplicado sobre Brachiaria decumbens infestada com larvas de Boophilus microplus (Canestrini. 1887) (Acari: Ixodidae). Rev Bras Parasitol Vet 12:38–42
Braga GU, Flint SD, Messias CL, Anderson AJ, Roberts DW (2001a) Effect of UV-B on conidia and germlings of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycol Res 105:874–882
Braga GU, Flint SD, Miller CD, Anderson AJ, Roberts DW (2001b) Variability in response to UV-B among species and strains of Metarhizium isolated from sites at latitudes from 61 N to 54 S. J Invertebr Pathol 78:98–108
Bruck DJ (2010) Fungal entomopathogens in the rhizosphere. BioControl 55:103–112
Camargo MG, Golo PS, Angelo IC, Perinotto WM, Sá FA, Quinelato S, Bittencourt VREP (2012) Effect of oil-based formulations of acaripathogenic fungi to control Rhipicephalus microplus ticks under laboratory conditions. Vet Parasitol 188:140–147
Camargo MG, Marciano AF, Sá FA, Perinotto WMS, Quinelato S, Gôlo PS, Angelo IC, Prata MCA, Bittencourt VREP (2014) Commercial formulation of Metarhizium anisopliae for the control of Rhipicephalus microplus in a pen study. Vet Parasitol 205:271–276
Camargo MG, Nogueira MRS, Marciano AF, Perinotto WMS, Coutinho-Rodrigues CJB, Scott FB, Angelo IC, Prata MCA, Bittencourt VREP (2016) Metarhizium anisopliae for controlling Rhipicephalus microplus ticks under field conditions. Vet Parasitol 223:38–42
Castro T, Mayerhofe J, Enkerli J, Eilenberg J, Meyling NV, Moral RA, Demétrio CGB, Delalibera I Jr (2016) Persistence of Brazilian isolates of the entomopathogenic fungi Metarhizium anisopliae and M. robertsii in strawberry crop soil after soil drench application. Agric Ecosyst Environ 233:361–369
Cooney DG, Emerson R (1964) Thermophilic fungi: an account of their biology, activities, and classification. WH Freeman, San Francisco
De Paulo JF, Ferreira JRT, Marciano AF, Freitas MC, Coutinho-Rodrigues CJB, Camargo MG, Ângelo IC, Bittencourt VREP, Gôlo PS (2016) Association between Metarhizium anisopliae sensu lato and cypermethrin to control Rhipicephalus microplus. Bras J Vet Med 38:66–74
Fernandes ÉKK, Keyser CA, Rangel DEN, Foster RN, Roberts DW (2010) CTC medium: a novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol Control 54:197–205
Fernandes ÉKK, Rangel DE, Moraes ÁM, Bittencourt VREP, Roberts DW (2008) Cold activity of Beauveria and Metarhizium, and thermotolerance of Beauveria. J Invertebr Pathol 98:69–78
Fernández-Salas A, Alonso-Díaz MA, Alonso-Morales RA, Lezama-Gutiérrez R, Rodríguez-Rodríguez JC, Cervantes-Chávez JA (2017) Acaricidal activity of Metarhizium anisopliae isolated from paddocks in the Mexican tropics against two populations of the cattle tick Rhipicephalus microplus. Med Vet Entomol 31:36–43
Goméz-Pérez ML, Romero-González R, Plaza-Bolaños P, Génin E, Martínez Vidal JL, Garrido FA (2014) Wide-scope analysis of pesticide and veterinary drug residues in meat matrices by high resolution MS: detection and identification using Exactive-Orbitrap. J Mass Spectrom 49:27–36
Grisi L, Leite RC, Martins JRS, Barros ATM, Andreotti R, Cançado PD, Leon AAP, Pereira JB, Villela HS (2014) Reassessment of the potencial economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet 23:150–156
Humber RA (2012) Identification of entomopathogenic fungi. In: Lacey LA (ed) Manual of techniques in invertebrate pathology, 2nd edn. Academic Press, Washington, pp 151–187
Jaronski ST (2007) Soil ecology of the entomopathogenic ascomycetes: a critical examination of what we (think) we know. In: Ekesi S, Maniania NK (eds) Use of entomopathogenic fungi in biological pest management. Research Signpost, Kerala, pp 91–143
Kaaya GP, Hassan S (2000) Entomogenous fungi as promising biopesticides for tick control. Exp Appl Acarol 24:913–926
Kaaya GP, Samish M, Hedimbi M, Gindin G, Glazer I (2011) Control of tick populations by spraying Metarhizium anisopliae conidia on cattle under field conditions. Exp Appl Acarol 55:273–281
Keyser CA, Fernandes ÉKK, Rangel DE, Roberts DW (2014) Heat-induced post-stress growth delay: a biological trait of many Metarhizium isolates reducing biocontrol efficacy? J Invertebr Pathol 120:67–73
Klafke G, Webster A, Agnol BD, Prade E, Silva J, de La Canal LH, Becker M, Osório MF, Mansson M, Barreto R, Scheffer R, Souza UA, Corassini VB, Santos J, Reck J, Martin JR (2017) Multiple resistance to acaricides in field populations of Rhipicephalus microplus from Rio Grande do Sul state, Southern Brazil. Ticks and Tick-Borne Dis 8:73–80
Ment D, Churchill AC, Gindin G, Belausov E, Glazer I, Rehner SA, Rot A, Donzelli BGG, Samish M (2012) Resistant ticks inhibit Metarhizium infection prior to haemocoel invasion by reducing fungal viability on the cuticle surface. Environ Microbiol 14:1570–1583
Murigu MM, Nana P, Waruiru RM, Nga’nga CJ, Ekesi S, Maniania NK (2016) Laboratory and field evaluation of entomopathogenic fungi for the control of amitraz-resistant and susceptible strains of Rhipicephalus decoloratus. Vet Parasitol 225:12–18
Ojeda-Chi MM, Rodriguez-Vivas RI, Galindo-Velasco E, Lezama-Gutiérrrez R (2010) Laboratory and field evaluation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for the control of Rhipicephalus microplus (Acari: Ixodidae) in the Mexican tropics. Vet Parasitol 170:348–354
Paixão FRS, Muniz ER, Barreto LP, Bernardo CC, Mascarin GM, Luz C, Fernandes ÉKK (2017) Increased heat tolerance afforded by oil-based conidial formulations of Metarhizium anisopliae and Metarhizium robertsii. Biocontrol Sci Technol 27:324–337
Pilz C, Enkerl J, Wegensteiner R, Keller S (2011) Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J Appl Entol 135:393–403
Quinelato S, Golo PS, Perinotto WM, Sá FA, Camargo MG, Angelo IC, Moraes AML, Bittencourt VREP (2012) Virulence potential of Metarhizium anisopliae sl isolates on Rhipicephalus (Boophilus) microplus larvae. Vet Parasitol 190:556–565
Rangel DEN, Braga GUL, Anderson AJ, Roberts DW (2005) Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. J Invertebr Pathol 88:116–125
Roberts DW, Campbell AS (1977) Stability of entomopathogenic fungi. In: Ignoffo CM, Hostetter DL (eds) Environmental stability of microbial insecticides. Ann Entomol Soc Am, Lanham, pp 19–76
Rodriguez-Vivas RI, Jonsson NN, Bhushan C (2017) Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res 117:3–29
Samish M, Rot A, Ment D, Barel S, Glazer I, Gindin G (2014) Efficacy of the entomopathogenic fungus Metarhizium brunneum in controlling the tick Rhipicephalus annulatus under field conditions. Vet Parasitol 206:258–266
Santi L, Silva LAD, Silva WOB, Corrêa APF, Rangel DEN, Carlini CR, Schrank A (2011) Virulence of the entomopathogenic fungus Metarhizium anisopliae using soybean oil formulation for control of the cotton stainer bug Dysdercus peruvianus. World J Microbiol Biotechnol 27:2297–2303
Ybañez AP, Mingala CN, Ybañez RHD (2018) Historical review and insights on the livestock tick-borne disease research of a developing country: the Philippine scenario. Parasitol Int 62:262–266
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, providing MSc scholarship for A.R.C. Corval, E.S. Mesquita, and A.F. Marciano, and National Council for Scientific and Technological Development (CNPq) of Brazil for providing undergraduate scholarship for T.A. Correa and a PhD scholarship for J. Fiorotti. This research was supported by grants of CNPq (Project #40910220164) and Carlos Chagas Filho Foundation for Research of the State of Rio de Janeiro (FAPERJ). We are grateful to Caroline F. Pereira and Danilo M. Akiau (formerly FAPERJ and CNPq undergraduate fellowships) for helping with the heat assay and with the in vitro tests with ticks. We appreciate the advices of the statisticians Dr. Wagner Tassinari, Dr. Celso G. Barbosa, and Dr. Marcus Sandes Pires. V.R.E.P. Bittencourt is a CNPq researcher.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that there are no conflict of interest to declare.
Ethical approval
The ticks used in the present study were obtained from artificially colonized calves after approval by the Federal Rural University of Rio de Janeiro Ethics Committee on Animal Use (CEUA/UFRRJ number 037/2014). As the present study accessed Brazilian genetic heritage, the research was registered at the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under the code AA47CB6.
Additional information
Handling Editor: Nicolai Meyling
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mesquita, E., Marciano, A.F., Corval, A.R.C. et al. Efficacy of a native isolate of the entomopathogenic fungus Metarhizium anisopliae against larval tick outbreaks under semifield conditions. BioControl 65, 353–362 (2020). https://doi.org/10.1007/s10526-020-10006-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-020-10006-1