Abstract
The compatibility of microsclerotial granules of the entomopathogenic fungus Metarhizium brunneum (Petch) strain F52 with fungicides (chlorothalonil, iprodione, propiconazole, pyraclostrobin+metconazole, and propiconazole+trifloxystrobin) was determined. In vitro, chlorothalonil was not detrimental to conidial production and viability at all concentrations (1–1000 mg a.i. (active ingredient) l−1). Iprodione reduced conidial production at ≥ 100 mg a.i. l−1 but had no effect on viability. Propiconazole reduced conidial production at ≥ 10 mg a.i. l−1 and reduced viability at 1000 mg a.i. l−1. A pyraclostrobin+metconazole combination reduced conidial production at ≥ 10 mg a.i. l−1 and viability at ≥ 100 mg a.i. l−1. A propiconazole+trifloxystrobin combination reduced conidial production at all concentrations and viability at ≥ 100 mg a.i. l−1. In the greenhouse, propiconazole suppressed colony forming unit (CFU) counts at both low and high rates. Chlorothalonil and iprodione had no inhibitory effect on fungal growth. The number of CFUs recovered did not differ significantly ten, 20 or 30 days after treatment. This study provides baseline information for the potential commercial use of the microsclerotia in pest control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The entomopathogenic fungus Metarhizium brunneum (Petch) strain F52 (formerly M. anisopliae F52) (Hypocreales: Clavicipitaceae) has been widely used in mycoinsecticides for the control of many insect pests since it is safe to vertebrates and other non-target organisms (Zimmermann 2007). To date, the commercialized products of this fungus, regardless of formulation, have infective conidia as the active ingredient. However, proper placement of liquid-based conidial sprays and their homogenous distribution through the soil matrix is difficult to achieve with these formulations (Jaronski and Jackson 2008). This may compromise product efficacy given that the target insects need to have adequate physical contact with a number of viable conidia to become infected. As an alternative to liquid sprays, granular formulations consisting of conidia bound to inert or nutritive carriers may be used. The practical use of granular formulations, however, is often impinged by high production costs, poor shelf life and physical barriers (Jaronski and Jackson 2008).
Formulations based on microsclerotia may offer an alternative to conidia-based formulations of entomopathogenic fungi. Microsclerotia are melanized hyphal aggregated survival structures and are known as overwintering structures produced by plant pathogenic fungi (Coley-Smith and Cooke 1971; Cooke 1983). The microsclerotia of some plant fungi, such as Colletotrichum truncatum (Schwein.) Andrus & W.D. Moore (Glomerellales: Glomerellaceae) and Mycoleptodiscus terrestris (Gerd.) Ostaz. (Magnaporthales: Magnaporthaceae) have been considered for commercialization as bioherbicides against weedy plants (Jackson and Schisler 1995; Shearer and Jackson 2006). The production of microsclerotia has also been reported in several entomopathogenic fungi, such as Cordyceps Fr. (Hypocreales: Cordycipitaceae) (Evans and Samson 1982), Hirsutella Pat. (Hypocreales: Ophiocordycipitaceae) (Speare 1920), Metarhizium (= Nomuraea) rileyi (Farl.) Kepler, Rehner & Humber (Hypocreales: Clavicipitaceae) (Sprenkel and Brooks 1977), and Akanthomyces (= Lecanicillium) lecanii (Zimm.) Spatafora et al. (Hypocreales: Cordycipitaceae) (Wang et al. 2013). In Metarhizium spp. (Hypocreales: Clavicipitaceae), microsclerotia have been reported to be produced successfully in M. anisopliae (Metchnikoff) Sorokin (strains TM109, MA1200), M. acridum (Driver & Milner) J.F. Bisch., Rehner & Humber, and M. robertsii J.F. Bisch., S.A. Rehner & Humber (Jackson and Jaronski 2009; Mascarin et al. 2014). Studies have shown that M. brunneum is able to form microsclerotia that are capable of surviving unfavorable conditions, especially desiccation, and these microsclerotia can be dehydrated and resume conidiation upon rehydration (Jaronski and Jackson 2008; Jackson and Jaronski 2009).
Microsclerotia-based formulations have been considered as a viable option for the use of M. brunneum against soil-dwelling insect pests and have provided control of the sugar beet root maggot, Tetanops myopaeformis (Röder) (Diptera: Ulidiidae) (Jackson and Jaronski 2009) and Japanese beetle, Popillia japonica Newman (Coleoptera: Scarabaeidae), larvae in turfgrass (Behle et al. 2015). Compared with liquid or granular conidial formulations, microsclerotia possess the advantage of multiplying the rate of fungus applied by their type of reproduction. Upon sufficient rehydration, M. brunneum microsclerotia are capable of producing up to 1010 infective conidia per gram of dry microsclerotia under ideal laboratory conditions (Behle et al. 2013). In addition, microsclerotia formulated onto clay-based granules had more rapid and profuse conidial production and were able to germinate at lower water activity levels compared to granules with conidia bound to nutritive carriers (corn grit granules), causing higher larval mortality of T. myopaeformis (Jaronski and Jackson 2008). Valuable attributes of the microsclerotia, including ease-of-use, lower cost of production in liquid culture, desiccation tolerance and profuse conidial germination, make microsclerotia-based formulations a promising alternative for M. brunneum commercialization, especially in the management of soil inhabiting insect pests (Jaronski and Jackson 2008; Jackson and Jaronski 2009; Behle and Jackson 2014).
Conidia-based commercial products of M. brunneum are compatible with many pesticides including some fungicides (e.g., iprodione, etc.) in foliar and soil applications, but other fungicides (e.g., chlorothalonil, pyraclostrobin, trifloxystrobin, etc.) may have compatibility issues with the fungus (Bruck 2009; Moorhouse et al. 1992). Rachappa et al. (2007) reported the compatibility of M. anisopliae with many insecticides and herbicides but moderate to strong inhibitory effects of fungicides on fungal growth. For example, propiconazole, chlorothalonil and two other fungicides were highly detrimental to fungal growth and sporulation in vitro. However, to the best of our knowledge, there have been no reported studies on the effect of fungicides on microsclerotia from entomopathogenic fungi. The compatibility of M. brunneum F52 microsclerotia with fungicides is presently unknown.
To develop baseline data on the compatibility of microsclerotia-based M. brunneum formulations, the current study first evaluated under in vitro conditions the effect of five fungicide products, which have been reported to be compatible or incompatible with other conidia-based M. brunneum formulations, on microsclerotia sporulation and germination. Our ultimate goal was to explore the use of microsclerotia-based formulations for the management of insect pests in turfgrass including on golf courses. However, golf course fairways and especially putting greens are regularly treated with fungicides for the management of a multitude of turf diseases (Kerns and Tredway 2013). Given that environmental conditions affecting potential interactions between entomopathogenic fungi and fungicides may differ between different crops and commodities, we further explored the effect of three of the most commonly used golf turf fungicides on M. brunneum microsclerotia in turfgrass grown in pots in the greenhouse.
Materials and methods
Fungus and fungicides
The granular formulation of M. brunneum F52 microsclerotia used in this study was prepared at the United States Department of Agriculture, Agricultural Research Service, Crop Bioprotection Research Unit in Peoria, IL, USA as described in Jackson and Jaronski (2009, 2012) and Behle and Jackson (2014) except that diatomaceous earth rather than clay was used as the carrier material. The majority of granules (~ 85%) were 0.25–1.0 mm in size giving about 2500 granules per gram of formulation. The fungicides used in the laboratory and greenhouse studies are listed in Table 1.
Compatibility of microsclerotia with fungicides in vitro
The effect that fungicides had on the number of conidia produced from the microsclerotia and the viability of the produced conidia was tested in vitro in the laboratory. Each of five fungicides (Table 1) was tested at six concentrations (1, 10, 100, 250, 500, 1000 mg a.i. (active ingredient) l−1 of agar medium) in addition to an untreated control (water agar). In each experimental trial, each fungicide concentration was tested in two replicates. The tests on propiconazole, iprodione and chlorothalonil were repeated in four experimental trials. The pyraclostrobin+metconazole and propiconazole+trifloxystrobin treatments were repeated in three trials.
The fungicides were incorporated into 1.2% water agar when it had reached 60 °C to prevent possible fungicide degradation at higher temperatures, and the mixtures were poured into 60 mm (diam) Petri dishes to solidify. After agar plates had cooled, 0.1 g of M. brunneum microsclerotia granules was evenly spread across the agar surface of each Petri dish. The dishes were then incubated (26 °C, L:D 12:12) for nine days to allow the microsclerotia to grow and produce conidia. After incubation, for each dish the fungal materials (conidia+mycelia) were scraped carefully from the agar surface and placed into 5 ml of 0.05% Tween 80 solution in deionized water in sterilized 50 ml centrifuge tubes. To each tube, 3 mm glass beads were added to promote agitation and to break up the conidial clumps during mixing. The suspension was vortex-mixed for 1 min and then diluted to approximately 1 × 107 conidia ml−1. The number of conidia was counted under × 450 magnification using a hemocytometer loaded with 10 µl of solution. The average of four subsamples for each tube was used to assess the number of conidia produced per gram of microsclerotia in each treatment. The half maximal inhibitory concentration (IC50) inhibiting conidial production in relation to the untreated control was determined for each fungicide.
The viability of conidia (measured as germination rate) produced on fungicide treated plates was determined on half strength Sabouraud dextrose agar plus yeast (SDAY) selective media (32.5 g SDA, 7.5 g agar and 5 g yeast extract in 1 l deionized water). Antibiotics selective for entomopathogenic fungi were added to prevent microbial contamination as follows: gentamicin: 1 ml l−1, chloramphenicol: 0.25 g l−1, ampicillin: 0.3 g l−1, streptomycin sulphate: 0.3 g l−1, and cycloheximide: 0.03 g l−1. The conidial suspension was serially diluted to approximately 1 × 106 conidia ml−1, and 100 µl of diluted suspension was spread evenly over the agar medium in 60 mm Petri dishes. The dishes were wrapped with parafilm and incubated at 26 °C and L:D 12:12 for 16–20 h. Then the germination rate was determined under × 450 magnification. In each sample, the germinated and non-germinated proportion in the first 50–200 conidia were determined under two cover slips (50 conidia were used for treatments with low conidial production; 200 were used for others). Two drops (20 µl) of lactophenol cotton blue (Sigma-Aldrich, St. Louis, MO, USA) were added under each cover slip to stain the fungus for better observation of germ tubes. Conidia were considered as germinated when the germ tubes were at least twice as long as the conidium.
Compatibility of microsclerotia with fungicides under greenhouse conditions
For greenhouse testing, 540 ml plastic containers (Fabri-Kal®) with small drainage holes, filled with soil (surface area 86.6 cm2) were seeded with creeping bentgrass (Agrostis stolonifera L.) seeds (0.05 g per pot). The grass was allowed to grow for two months before treatment. The soil was a mixture of pasteurized (70 °C for 3 h) sandy loam (61% sand, 27% silt, 12% clay, 2.3% organic matter, pH 5.9) and sand (3:1 ratio). The greenhouse was set at 14 h light at 25 °C: 10 h dark at 19º C, and natural light was supplemented with 400 watt high pressure sodium lamps when intensity fell below 600 mmol m−2. Plants were fertilized weekly (20-20-20 NPK, The Scotts Miracle Gro Co., Marysville, OH, USA), watered as necessary and clipped twice a week (13 mm height, typical golf course fairway mowing height).
Each pot received 0.02 g (23 kg ha−1, producing 0.5–2 × 1014 viable conidia ha−1 under favorable conditions) microsclerotia granules, which were evenly applied to the soil surface. The pots had been watered well with tap water one day before granule application and, immediately after granule application, received light irrigation (3 mm = 26 ml per pot) to wash the granules below the turf canopy onto the substrate surface where many of the potential target insects in turfgrass are active. Fungicides were applied 24 h later using a Generation III Research sprayer (748 l ha−1 spray volume). The three fungicides propiconazole, iprodione and chlorothalonil (Table 1) labeled for turfgrass were applied at two rates representing the low and high end of the recommended rate range for turfgrass applications (propiconazole: 0.5 and 1.8 kg a.i. ha−1, iprodione: 1.5 and 6 kg a.i. ha−1, chlorothalonil: 2.2 and 8.6 kg a.i. ha−1). Thereafter, the pots were irrigated with 7 mm (= 60 ml per pot) water every other day to provide moisture for microsclerotia and grass growth. The average greenhouse temperature during the experiment was 19.6 °C (range 15.2 to 27.6 °C).
Results were evaluated in different pots at ten, 20 and 30 days after application of the microsclerotial granules by determining the number of CFUs recovered from each pot. The top 2.5 cm of soil and the grass from each sampled pot were collected into a bag and brought to the laboratory to determine the conidial concentration. The contents of each bag were mixed thoroughly before two subsamples (approximately 5 g each) were taken from it. Each subsample was diluted with 50 ml sterile 0.05% Tween 20 and shaken vigorously by hand for 5 min to ensure a homogenous suspension. Following 5 s for sedimentation, 1 ml of the soil suspension was transferred to 9 ml 0.05% Tween 20 to make a 10% dilution. The diluted suspension was vortexed for 1 min with 3 mm glass beads, and a 0.1 ml aliquot was removed from the surface layer and spread onto each of two potato dextrose agar plus yeast extract (PDAY) plates with 0.015% dodine (Syllit 65 WP, Agriphar S.A., Ougrée, Belgium) supplemented with 0.15 g l−1 chloramphenicol (Rangel et al. 2010). Plates were incubated (26 ± 1 °C, L:D 12:12) for seven days before the number of colonies appearing on the plates was counted and the CFUs per pot were determined. The moisture content of each sample (i.e., the top 2.5 cm of soil and grass of each pot) was determined by drying a pre-weighed quantity of collected soil in an oven for three days at 72 °C and then air-drying for another three days. The grass pots before treatment were tested to be free of M. brunneum with PDAY dodine plates. Each treatment was replicated in three grass pots in each of three experimental trials.
Data analysis
Generalized linear model (PROC GLIMMIX, SAS 9.4) (SAS Institute 2016) was used to determine the effect of fungicide product type and concentration on the number of conidia produced and conidial viability (germination rate) in vitro, and to determine the effect of fungicide treatment and evaluation time (ten, 20 and 30 days post-microsclerotial application) on the number of CFUs produced in soil under greenhouse conditions. In vitro conidial production and germination rate were first analyzed using a one-way method to evaluate the treatment effects as compared to the control. Then data were converted to percent conidial reduction and relative germination and analyzed with two-way factorial methods. Major factors (fungicide and concentration in vitro, fungicide and evaluation time in greenhouse) were analyzed as fixed effects with type III error. Counts of conidia and CFUs were analyzed with negative binomial distribution and a log link function, and proportional data (germination rate and percent conidial reduction) were analyzed with beta distribution and a logit link function. Means were separated using Tukey’s HSD test (α = 0.05). The IC50 of each fungicide in vitro was calculated using a generalized linear model with binomial distribution and a probit link function. Pearson correlation (PROC CORR) was used to examine the data for any correlation between soil moisture and CFU concentration in the greenhouse experiment.
Results
Compatibility of microsclerotia with fungicides in vitro
The compatibility of the microsclerotial granules of M. brunneum F52 with five fungicides was tested in vitro at fungicide concentrations ranging from 1 to 1000 mg a.i. l−1. Compared to the untreated control plates (4.7 × 109), the number of conidia produced per gram microsclerotia granules was significantly reduced (F30, 193 = 43.47, P < 0.001) by iprodione at ≥ 100 mg a.i. l−1 (82.8–94.0%), by propiconazole (95.4–99.7%) and pyraclostrobin+metconazole (95.6–99.4%) at ≥ 10 mg a.i. l−1, and by propiconazole+trifloxystrobin (93.8–99.4%) at all concentrations (Table 2). Chlorothalonil did not significantly reduce conidia production at any rate compared to the untreated control. After conversion of the data to percent conidial reduction, percent reduction in number of conidia produced was significantly affected by fungicide type (F4, 186 = 121.22, P < 0.0001) and concentration (F5, 186 = 50.88, P < 0.0001). The two factors interacted significantly (F20, 186 = 5.45, P < 0.001). Percent reduction in conidial numbers was the lowest for iprodione at 1 mg a.i. l−1 although not significantly lower than for chlorothalonil at 1–100 mg a.i. l−1 (Table 2). Percent reduction was the greatest for propiconazole and pyraclostrobin+metconazole at 100–1000 mg a.i. l−1, and propiconazole+trifloxystrobin at 10–1000 mg a.i. l−1, but was not significantly higher than for propiconazole and pyraclostrobin+metconazole at 10 mg a.i. l−1, iprodione at 100–1000 mg a.i. l−1, and propiconazole+trifloxystrobin at 1 mg a.i. l−1. Generally, chlorothalonil had the least effect on conidiogenesis (IC50 > 1000 mg a.i. l−1), followed by iprodione (IC50 of 15.6 mg a.i. l−1), and propiconazole (IC50 of 1.4 mg a.i. l−1). Pyraclostrobin+metconazole and propiconazole+trifloxystrobin had the strongest inhibition on conidia production (IC50 < 1 mg a.i. l−1) (Table 2).
The viability of conidia (i.e., percent germination) from the above fungicide treatments compared to the untreated control plates (99.2% germination) was significantly affected (F30, 193 = 15.80, P < 0.0001) by propiconazole at 1000 mg a.i. l−1 (reduced by 24.1%) and by the two combination products at ≥ 100 mg a.i. l−1 (reduced by 28.4–45.9% for pyraclostrobin+metconazole; 21.1–45.1% for propiconazole+trifloxystrobin) (Table 3). Chlorothalonil and iprodione did not suppress conidial viability significantly at any concentration. After conversion of the data to percent germination, percent germination was significantly affected by fungicide type (F4, 186 = 53.19, P < 0.0001) and concentration (F5, 186 = 24.67, P < 0.001). Fungicide type and concentration interacted significantly (F20, 186 = 2.61, P = 0.0004). Percent germination was similarly high for chlorothalonil and iprodione at all rates, propiconazole at ≤ 500 mg a.i. l−1, and both combination products at ≤ 10 mg a.i. l−1 (Table 3). Germination was the lowest for pyraclostrobin+metconazole at 500–1000 mg a.i. l−1 and propiconazole+trifloxystrobin at 1000 mg a.i. l−1 but was not significantly lower than any the remaining combination product rates at ≥ 100 mg a.i. l−1.
Compatibility of microsclerotia with fungicides under greenhouse conditions
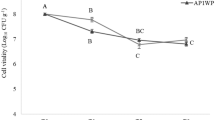
Under greenhouse conditions, the compatibility of the microsclerotia with fungicides was tested with three commonly used turf fungicides, chlorothalonil, iprodione and propiconazole applied at two rates representing the low and a high end of labelled field rates for turf. Because rates differed among fungicide types, rate was not included as a separate factor. Recovered CFU counts were significantly affected by fungicide treatment (fungicide type-rate combination) (F(6, 168) = 6.74, P < 0.0001) but not by evaluation time (ten, 20 or 30 days after treatment (DAT)) (P = 0.211), with no significant interaction between the factors (P = 0.083). For data combined across DAT, only propiconazole (at both low and high rates) significantly reduced the CFU counts compared to the untreated control. Further, the low rate of chlorothalonil had significantly higher CFU counts than the low and high rate of propiconazole. Other treatments (except for propiconazole-low rate) had significantly more CFUs than the high rate of propiconazole (Fig. 1). In none of the fungicides did the CFU counts differ significantly between the low and higher rate.
Number (mean + SE) of CFU of Metarhizium brunneum F52 recovered from pots with grass that were left untreated (untreated control = UTC) or treated with a low (L) and a high (H) rate of the fungicides propiconazole (Prop), iprodione (Ipro) and chlorothalonil (Chlor). Data are combined for samples taken at ten, 20 and 30 DAT, because DAT had no significant effect and did not interact with treatment (P > 0.05). Means with the same letter are not significantly different (Tukey’s test, α > 0.05)
The soil moisture determined at the time of evaluation averaged 14.1 (± 0.3) %. Combining all evaluation times, a weak correlation between soil moisture and CFU counts was detected for the low rates of propiconazole (r = 0.436, P = 0.023) and chlorothalonil (r = 0.400, P = 0.039). No significant correlation was observed for any other treatments (P > 0.05).
Discussion
This study showed varying effects of different fungicides on the sporulation and germination of conidia produced from M. brunneum F52 microsclerotia under in vitro conditions and on fungal survival and subsequent growth when applied to grass grown in pots in the greenhouse. To our knowledge, no studies on compatibility of microsclerotia-based formulations of entomopathogenic fungi with fungicides have been conducted previously. The degree of compatibility of these fungal propagules with the fungicides in vitro ranged from no significant suppression of any parameters by chlorothalonil, to limited suppression only at higher concentrations by iprodione, to strong suppression of sporulation and germination, even at low rates by a combination of propiconazole and trifloxystrobin. Under greenhouse conditions, chlorothalonil and iprodione did not suppress fungal growth at low or high field recommended rates and propiconazole showed inhibitory effects at both low and high rates.
Fungicides, especially chlorothalonil and iprodione, seem to have a more limited impact on the ability of microsclerotia of M. brunneum to grow out and produce viable conidia, compared to their impact on germination of conidia and growth of mycelium in some other studies. In particular, chlorothalonil was found to be compatible with the microsclerotia in conidiation and viability in vitro here, whereas several other studies reported inhibitory effects of chlorothalonil on mycelial growth, conidia production, or germination of M. anisopliae isolates (Moorhouse et al. 1992; Chandler and Davidson 2005; Rachappa et al. 2007; Yáñez and France 2010). In addition, in this study iprodione inhibited conidial production starting at 100 mg a.i. l−1 and had no effect on germination at any rates (up to 1000 mg a.i. l−1). This is similar to the findings of Moorhouse et al. (1992) and Bruck (2009) that iprodione at 1000 mg a.i. l−1 was not detrimental to conidia germination but inhibit growth of M. anisopliae (including strain F52). However, Yáñez and France (2010) found inhibition of iprodione on M. anisopliae growth at lower concentrations (beginning with ≤ 10 mg a.i. l−1). Also, Shah et al. (2009) found iprodione significantly suppressed germination of M. anisopliae V275 conidia starting at 0.1 × of recommended rate and inhibited mycelial growth at the recommended rate. Differences in methodology, i.e. viability of conidia harvested from fungicide-infused plates in our test versus conidia directly exposed to fungicide-infused plates in other studies, might explain the less profound effect of fungicides on spore germination. However, variation in fungal strains and exposure type, microsclerotia versus conidia-based, might also account for the difference in susceptibility to fungicides. Similar to our findings, adverse effects of propiconazole, trifloxystrobin, and pyraclostrobin were reported on germination, growth, and conidiation of M. anisopliae F52 and other strains (Rachappa et al. 2007; Bruck 2009; Silva et al. 2013). If microsclerotia are indeed more tolerant of fungicides than conidia, this can likely be ascribed to their structure and physiology, i.e. the melanized, compact hyphal aggregates (Jaronski and Jackson 2008; Jackson and Jaronski 2009), which are designed to overcome stress that might typically kill conidia or mycelium. The difference between microsclerotia and conidia-based formulations of entomopathogenic fungi in their susceptibility to fungicides merits further studies.
The impact of fungicides, especially iprodione, which was tested both in vitro and in the greenhouse, on fungal growth and conidiation was less profound in soil versus in vitro, despite the fungicide concentrations being much higher in the greenhouse study. Our observations are consistent with the findings of Bruck (2009), who reported fungistatic effects in vitro and compatibility in the rhizosphere for iprodione, pyraclostrobin, trifloxystrobin and several other fungicides with M. anisopliae F52. Similarly, Moorhouse et al. (1992) found no effect of chlorothalonil on the number of colonies recovered from potting mix with peat compost. Also, Chandler and Davidson (2005) found that the growth of M. anisopliae 389.93 was suppressed by iprodione in vitro, but the efficacy against the larval and pupal stages of cabbage root fly Delia radicum (L.) was unaffected in the greenhouse. Declining concentration of fungicides due to rapid degradation might have played a significant role in enhancing compatibility. Most fungicides have relatively short persistence after application (Griffin 1994). For example, iprodione was reported to have a half-life of less than seven days in soil at pH 7 (Tomlin 1997). In addition, fungicides may also be subject to rapid decline following rainfall or irrigation (Shah et al. 2009). In contrast, fungi are continuously exposed to very stable fungicide concentrations in direct contact in vitro, and thus the inhibitory effects are more profound. In the greenhouse test, the fungicides were applied 24 h after the microsclerotia, which may have reduced the suppressive effect of the fungicide somewhat compared to a simultaneous application which the in vitro conditions essentially represent. However, propiconazole was still detrimental to conidiation and growth in the greenhouse. This may affect fungal infectivity and control success in pest management. For fungicide treatments incompatible with the microsclerotia, like propiconazole, increasing time intervals between fungicide and fungus applications may alleviate adverse effects of fungicides. Although applications of incompatible agents in pest management are commonly separated by time and space, the theory of delayed applications, especially the sequence and optimal time intervals between fungicide and microsclerotia applications in turfgrass, may warrant future studies.
Goble et al. (2016a) found that conidia production in hydromulch applications increased over time and reached maximum density at 20–30 day under forest conditions. Here, in the grass pot studies in the greenhouse, the number of CFUs did not vary significantly between evaluations conducted at ten, 20 and 30 DAT. Also, the number of CFUs per gram of microsclerotia produced in the untreated control was lower in the greenhouse (0.72–0.97 × 109) than in the laboratory at constant 26 °C (1.45 × 1010). According to Goble et al. (2016a, b, 2017), successful production of conidia was strongly associated with temperature, RH and rainfall/irrigation. During the experimental period, the greenhouse temperature ranged from 15.2 to 27.6 °C averaging 19.6 °C. The variable and occasionally low temperature in the greenhouse might have interfered with conidiation and fungal growth. In addition, the CFU numbers might have been lowered due to overhead irrigation washing some conidia downwards along the pot edges. In this study, soil moisture averaged 14% (v/w) (approx. 50% field capacity) at evaluation, well above the 15% field capacity sufficient for fungal outgrowth and sporulation of M. brunneum microsclerotia reported by Jaronski and Jackson (2008). Thus, it is unlikely the reason for reduced conidiation. Here, soil moisture was not strongly correlated to CFU production, probably because watering on every other day provided sufficient moisture to maintain maximum conidia production during the experiment.
In conclusion, M. brunneum F52 microsclerotia sensitivity varied with fungicides and concentration, and the suppressive effects were less profound in soil than in vitro. Chlorothalonil and iprodione should be fully compatible with microsclerotia-based formulations of M. brunneum F52 in turfgrass. Propiconazole, on the other hand, may need to be applied some time before or after the application of the entomopathogenic fungus to minimize any suppressive effects. However, the benefit of separating the applications in time needs to be investigated in future studies. Differences between our findings with microsclerotia-based formulations and those of other studies with conidial formulations suggest potential differences between formulations in susceptibility to fungicides. Our study provides baseline reference for the potential commercial use of the microsclerotia in pest control with regard to fungicide compatibility especially in turfgrass, which may require frequent fungicide applications. Future research may be directed to testing the compatibility of the microsclerotia granules with other fungicide groups, e.g., the succinate dehydrogenase inhibitors (SDHI), comparison between microsclerotial and conidial formulations in susceptibility to fungicides, and time intervals between fungus and fungicide applications to maximize the fungal activities in pest control.
References
Behle RW, Jackson MA (2014) Effect of fermentation media on the production, efficacy, and storage stability of Metarhizium brunneum microsclerotia formulated as a prototype granule. J Econ Entomol 107:582–590
Behle RW, Jackson MA, Flor-Weiler LB (2013) Efficacy of a granular formulation containing Metarhizium brunneum F52 (Hypocreales: Clavicipitaceae) microsclerotia against nymphs of Ixodes scapularis (Acari: Ixoididae). J Econ Entomol 106:57–63
Behle RW, Richmond DS, Jackson MA, Dunlap CA (2015) Evaluation of Metarhizium brunneum F52 (Hypocreales: Clavicipitaceae) for control of Japanese beetle larvae in turfgrass. J Econ Entomol 108:1587–1595
Bruck DJ (2009) Impact of fungicides on Metarhizium anisopliae in the rhizosphere, bulk soil and in vitro. BioControl 54:597–606
Chandler D, Davidson G (2005) Evaluation of entomopathogenic fungus Metarhizium anisopliae against soil-dwelling stages of cabbage maggot (Diptera: Anthomyiidae) in glasshouse and field experiments and effect of fungicides on fungal activity. J Econ Entomol 98:1856–1862
Coley-Smith JR, Cooke RC (1971) Survival and germination of fungal sclerotia. Ann Rev Phytopathol 9:65–92
Cooke R (1983) Morphogenesis of sclerotia. In: Smith JE (ed) Fungal differentiation: a contemporary synthesis. Marcel Dekker Inc., New York, pp 397–418
Evans HC, Samson RA (1982) Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. I. The Cephalotes (Myrmicinae) complex. Trans Br Mycol Soc 79:431–453
Goble TA, Gardescu S, Fisher JJ, Jackson MA, Hajek AE (2016a) Conidial production, persistence and pathogenicity of hydromulch formulations of Metarhizium brunneum F52 microsclerotia under forest conditions. Biol Control 95:83–93
Goble TA, Gardescu S, Jackson MA, Hajek AE (2016b) Evaluating different carriers of Metarhizium brunneum F52 microsclerotia for control of adult Asian longhorned beetles (Coleoptera: Cerambycidae). Biocontrol Sci Technol 26:1212–1229
Goble TA, Gardescu S, Jackson MA, Hajek AE (2017) Evaluating Metarhizium brunneum F52 microsclerotia in hydromulch formulations using different tackifiers under forest and orchard conditions. BioControl 62:769–778
Griffin DH (1994) Fungal physiology. Wiley-Liss, New York
Jackson MA, Jaronski ST (2009) Production of microsclerotia of the fungal entomopathogen Metarhizium anisopliae and their potential for use as a biocontrol agent for soil inhabiting insects. Mycol Res 113:842–850
Jackson MA, Jaronski ST (2012) Development of pilot-scale fermentation and stabilization processes for the production of microsclerotia of the entomopathogenic fungus Metarhizium brunneum strain F52. Biocontrol Sci Technol 22:915–930
Jackson MA, Schisler DA (1995) Liquid culture production of microsclerotia of Colletotrichum truncatum for use as bioherbicidal propagules. Mycol Res 99:879–884
Jaronski ST, Jackson MA (2008) Efficacy of Metarhizium anisopliae microsclerotial granules. Biocontrol Sci Technol 18:849–863
Kerns JP, Tredway LP (2013) Advances in turfgrass pathology. In: Stier JC, Horgan BP, Bonos SA (eds) Turfgrass: biology, use, and management. Agronomy Monograph 56. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, pp 733–776
Mascarin GM, Kobori NN, de Jesus Vital RC, Jackson MA, Quintela ED (2014) Production of microsclerotia by Brazilian strains of Metarhizium spp. Using submerged liquid culture fermentation. World J Microbiol Biotechnol 30:1583–1590
Moorhouse ER, Gillespie AT, Sellers EK, Charnley AK (1992) Influence of fungicides and insecticides on the entomogenous fungus Metarhizium anisopliae, a pathogen of the vine weevil, Otiorhynchus sulcatus. Biocontrol Sci Technol 2:49–58
Rachappa V, Lingappa S, Patil RK (2007) Effect of agrochemicals on growth and sporulation of Metarhizium anisopliae (Metschnikoff) Sorokin. Karnataka J Agric Sci 20:410–413
Rangel DEN, Dettenmaier SJ, Fernandes ÉKK, Roberts DW (2010) Susceptibility of Metarhizium spp. and other entomopathogenic fungi to dodine-based selective media. Biocontrol Sci Technol 20:375–389
SAS Institute (2016) SAS® 9.4 procedures guide. SAS Institute Inc., Cary
Shah FA, Ansari MA, Watkins J, Phelps Z, Cross J, Butt TM (2009) Influence of commercial fungicides on the germination, growth and virulence of four species of entomopathogenic fungi. Biocontrol Sci Technol 19:743–753
Shearer JF, Jackson MA (2006) Liquid culturing of microsclerotia of Mycoleptodiscus terrestris, a potential biological control agent for the management of Hydrilla. Biol Control 38:298–306
Silva RAD, Quintela ED, Mascarin GM, Barrigossi JAF, Lião LM (2013) Compatibility of conventional agrochemicals used in rice crops with the entomopathogenic fungus Metarhizium anisopliae. Sci Agric 70:152–160
Speare AT (1920) On certain entomogenous fungi. Mycologia 12:62–76
Sprenkel RK, Brooks WM (1977) Winter survival of the entomogenous fungus Nomuraea rileyi in North Carolina. J Invertebr Pathol 29:262–266
Tomlin CDS (1997) A world compendium: the pesticide manual. British Crop Protection Council, Surrey
Wang H, Lei Z, Reitz S, Li Y, Xu X (2013) Production of microsclerotia of the fungal entomopathogen Lecanicillium lecanii (Hypocreales: Cordycipitaceae) as a biological control agent against soil-dwelling stages of Frankliniella occidentalis (Thysanoptera: Thripidae). Biocontrol Sci Technol 23:234–238
Yáñez M, France A (2010) Effects of fungicides on the development of the entomopathogenic fungus Metarhizium anisopliae var. anisopliae. Chil J Agric Res 70:390–398
Zimmermann G (2007) Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Technol 17:879–920
Acknowledgements
We thank Claire Moreland-Ochoa, Nadege Aoki and Eva Morgan for their assistance with some of the germination studies and thank Francoise Vermeylen (statistician from Cornell University) for assisting with data analysis. Thanks to Dr. Bruce Clarke (Department of Plant Biology, Rutgers University) for providing fungicides for the tests. The project was funded by United States Golf Association, Rutgers Center for Turfgrass Science, the Tri State Research Foundation, the New York State Turfgrass Association, and the USDA National Institute of Food and Agriculture Hatch Multistate projects 0206130 and 1014679 through the New Jersey Agricultural Experiment Station, Hatch Multistate projects NJ08295 and NJ08235, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that the manuscript and the authors are in full compliance with the outlined ethical standards.
Additional information
Handling Editor: Nicolai Meyling
Rights and permissions
About this article
Cite this article
Wu, S., Kostromytska, O.S., Goble, T. et al. Compatibility of a microsclerotial granular formulation of the entomopathogenic fungus Metarhizium brunneum with fungicides. BioControl 65, 113–123 (2020). https://doi.org/10.1007/s10526-019-09983-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09983-9