Abstract
Strains IMI 330189 of Metarhizium acridum (Driver & Milner) J.F. Bisch., Rehner & Humber (Hypocreales: Clavicipitaceae) and EABb 90/2-Dm of Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordycipitaceae) are a promising biocontrol tool of Dociostaurus maroccanus (Thunberg) (Orthoptera: Acrididae), although the effects of thermoregulation and Moroccan locust-fever on the infection process of these fungi remain unknown. In vitro experiments, measuring conidial germination and hyphal growth either at constant or at fluctuating temperatures simulating thermoregulatory conditions, indicated that strain IMI 330189 had greater fitness at temperatures above 27 °C and the strain EABb 90/2-Dm was better adapted to the temperature range of 10–25 °C. These effects were mirrored in vivo, where locust thermoregulation caused a marked reduction in the virulence of EABb 90/2-Dm in comparison to no thermoregulation conditions (average survival time: 6.10 vs. 15.83 days; mortality: 100% vs. 73.7%) but only a moderate reduction in the virulence of IMI 330189 (average survival time: 4.57 vs. 8.26 days; mortality: 100% vs. 100%). Thermal gradient experiments revealed that the strain IMI 330189 induced behavioral fever in D. maroccanus (preferred temperatures approximately 4 °C above the uninfected control), although it only led to a slight reduction in virulence. Strain EABb 90/2-Dm did not induce such a clear behavioral response. Under the temperature conditions of the main breeding areas of the Moroccan locust, strain IMI 330189 is likely a better candidate for use in biocontrol, although strain EABb 90/2-Dm could be also a good alternative in more temperate environments either as a stand-alone one or in mixed combinations of the two fungal strains, potentially providing more effective control over a broader range of temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of temperature, determined by environmental conditions and insect thermoregulation, is probably the main factor affecting the performance of bioinsecticides based on fungal entomopathogens for locust control, potentially reducing and/or delaying insect mortality (Arthurs and Thomas 2001; Blanford and Thomas 2000; Blanford et al. 1998; Klass et al. 2007; Ouedraogo et al. 2004; Vega and Kaya 2012). Temperature can affect the virulence of entomopathogenic fungi at different phases of the infection process, with inter- and intra-specific variations in thermal requirements mainly related to habitat, geographical origin, and even insect host (Inglis et al. 2001). To this end, some studies have shown that Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordycipitaceae) is a fungal species more psychrophilic than Metarhizium anisopliae (Metschn.) Sorokin (Hypocreales: Clavicipitaceae) in soils from temperate and near-northern habitats from Otario, Canada (Bidochka et al. 1998). Indeed, it has been reported that certain genetic groups within these two species dominate soils of Artic and forested habitats (those with ability for cold active growth, i.e. 8 °C) and other genetic groups dominate soils of agricultural habitats (those with ability for growth at high temperatures, i.e. 37 °C) (Bidochka et al. 2000, 2002).
In vitro studies indicate that conidial germination of Beauveria spp. and Metarhizium spp. isolates is usually delayed or decreased at temperatures at the range of 10–15 °C and 35–40 °C (Fargues et al. 1997b; Inglis et al. 1996; Yeo et al. 2003). Hyphal growth, in general, is optimal at the range of 20–30 °C, and it is reduced at temperatures below 5 °C and above 37 °C (Fargues et al. 1997a; Quesada-Moraga et al. 2006; Vega and Kaya 2012). In vivo experiments also show that virulence of fungal entomopathogens is decreased when insects are exposed to daily time periods at high temperatures (> 30 °C) or allowed to thermoregulate (Anderson et al. 2013b; Elliot et al. 2002; Fisher and Hajek 2014; Inglis et al. 1996, 1999; Keyser et al. 2014; Ouedraogo et al. 2004; Springate and Thomas 2005; Thomas and Jenkins 1997).
Temperature fluctuates to a considerable extent in some habitats reaching suboptimal levels for pathogen growth. Moreover, locusts and grasshoppers demonstrate behavioral thermoregulation (Chappell and Whitman 1990), using basking and/or selection of thermal microenvironment to maintain their body temperatures several degrees above ambient (preferred or “set point” body temperature) by absorbing heat directly from the sun as well as from warm substrates (Carruthers et al. 1992; Jaronski 2010). This thermoregulatory behavior can be more pronounced upon infection with a pathogen, a phenomenon termed “behavioral fever” which is an adaptive defensive response resulting in suppression of pathogen growth and enhanced performance of the host’s immune system (Blanford et al. 1998; Elliot et al. 2002; Hunt et al. 2011; Inglis et al. 1996; Ouedraogo et al. 2004).
Dociostaurus maroccanus (Thunberg) (Orthoptera: Acrididae) is an important plague locust of the Mediterranean region (Latchininsky and Launois-Luong 1992; Quesada-Moraga and Santiago-Álvarez 2001). Two fungal strains, Beauveria bassiana (EABb 90/2-Dm) and Metarhizium acridum (IMI 330189), have been evaluated as promising candidates to control this pest. Strain EABb 90/2-Dm was isolated from D. maroccanus in the permanent breeding area of La Serena (Badajoz) in southern Spain (Hernandez-Crespo and Santiago-Álvarez 1997) and strain IMI 330189 is the standard isolate used in locust biocontrol in Africa (LUBILOSA project) (Lomer et al. 2001). Both strains have proven acceptable efficacy against D. maroccanus in laboratory bioassays and field applications (Collar-Urquijo et al. 2002; Jiménez-Medina et al. 1998; Klass et al. 2007; Quesada-Moraga 1998). Whilst the effect of constant temperatures on hyphal growth in vitro of both strains has been evaluated (Ouedraogo et al. 1997; Quesada-Moraga et al. 2006; Thomas and Jenkins 1997), their hyphal growth and conidial germination under constant and fluctuating temperature regimes, and virulence against D. maroccanus when locusts can thermoregulate remain unknown. Field temperatures in La Serena, an important breeding area for D. maroccanus in Spain, tend to show large diurnal variation, with daily maximum temperatures exceeding 40 °C and minimum temperatures consistently falling below 20 °C (Klass et al. 2007). In this setting, D. maroccanus has been shown to be an active behavioral thermoregulator, maintaining body temperatures in the range 38–40 °C for 8–10 h a day (Klass et al. 2007). Thus, the effect of temperature and thermoregulation is crucial in the evaluation of both strains to be developed for D. maroccanus control.
The present research is a comparative study of the effect of thermoregulation on the virulence of strains B. bassiana (EABb90/2-Dm) and M. acridum (IMI 330189) to D. maroccanus. For that, the thermal requirements of both strains were investigated in vitro under constant and fluctuating temperatures, simulating locust thermoregulation. Then, in vivo experiments compared the survival of D. maroccanus following infection with each strain, using conditions in which locusts were able to thermoregulate versus those in which thermoregulation was constrained. The potential existence of behavioral fever in D. maroccanus, as a response to the infection with these strains, was also evaluated.
Materials and methods
Fungal culture and inoculum preparation
The strain M. acridum (IMI 330189) was obtained from the mycological culture collection held by CABI Bioscience (UK). This isolate has been researched extensively for use in locust and grasshopper control in Africa in the LUBILOSA project (Lomer et al. 2001). The strain B. bassiana (EABb 90/2-Dm) belongs to the collection of the Agricultural Entomology Research group at University of Cordoba (Spain) and was isolated from a nymph of D. maroccanus in the breeding area of La Serena in Badajoz (Spain) in 1990 (Hernandez-Crespo and Santiago-Álvarez 1997). For the different bioassays, subcultures of these strains were made in Sabouraud Dextrose Agar (SDA) in Petri dishes and incubated at 27 °C for 14 days to ensure proper sporulation. Conidial suspensions were prepared by scraping conidia from Petri plates into an aqueous solution of 0.05% Tween 80. The concentration of conidia was enumerated using a Malassez hemocytometer and suspensions were stored at 4 °C until their use.
Insects
The locusts for the bioassays were obtained from a laboratory population maintained at the Agricultural Entomology Research Group (University of Cordoba, Spain) derived from adults collected in the permanent breeding area of La Serena (Badajoz, Spain) (38°55′N, 5°29′W). The locusts were reared in an insectary regulated at 27 °C, 60% RH, under a 13:11 L:D photoperiod. Rearing cages were made of wooden frames and metallic mesh (50 cm × 50 cm × 50 cm for nymphs and 30 cm × 30 cm × 30 cm for adults). To enable insect thermoregulation, a heat gradient was provided within the cages by incandescent light bulbs (60 W) that were mounted in the middle of the upper wall of the cages. A metallic mesh was placed under the bulb to permit the vertical movement of the locusts.
Conidial germination
For the evaluation of the effect of constant temperatures on conidial germination in vitro, petri dishes (Ø 90 mm) of SDA were inoculated with each isolate at the center of the plate with 2 μl of 5 × 106 spores ml−1 (1 × 104 spores) of the conidial suspension and then spread over the whole surface of the plate. Dishes were sealed with Parafilm and placed in different incubators at 5, 10, 15, 20, 25, 30, 35, 40 and 45 °C. Conidial germination was evaluated at nine time intervals during the 72 h period following inoculation (2, 4, 6, 8, 10, 12, 24, 48 and 72 h). Each experimental treatment (combination temperature × incubation time) had three replicates (dishes) for a total of 243 dishes per isolate. After each incubation period, conidia were fixed with cotton blue in lactophenol (100 g l−1). A total of 100 conidia per dish, selected in random fields of view, were assessed microscopically for germination. A conidium was considered germinated if it had a germ tube at least as long as the smallest diameter of the conidium (Yeo et al. 2003).
For the evaluation of the effect of daily temperature increase on conidial germination in vitro, under conditions simulating insect thermoregulation, conidia were exposed to two different temperature regimes (Fig. 2a). For each isolate, one group of dishes (thermoregulation simulated) was placed in the insectary within locust bioassay cages at conditions of room temperature plus additional heat gradient provided by a bulb mounted in the middle of the upper wall of the cages (60 W) (temperature > 35 °C for approx. 10 h day−1, 26.5–28.5 °C rest of the day). The other group of dishes (no thermoregulation simulated, 26.5–28.5 °C 24 h day−1) was placed in the same bioassay cages but with no additional heat source. Temperatures of both thermal regimes were measured in the middle point of the cage with Gemini dataloggers, one for cages with no thermoregulation simulated and one for thermoregulation simulated. These dataloggers were randomly placed in different cages during the experiment. The experimental design was similar to the one described above for constant temperatures, with three replicates (dishes) per isolate, incubation period (2, 4, 6, 8, 10, 12, 24, 48 and 72 h) and thermal regimes (thermoregulation simulated, no thermoregulation) for a total of 54 dishes per isolate.
Hyphal growth
For the evaluation of the effect of constant temperatures on hyphal growth in vitro, SDA plates (Ø 90 mm) were marked with two perpendicular lines on the base of the plate and spot inoculated on the central cross with 2 μl of 5 × 106 spores ml−1 suspension (1 × 104 spores) using a micropipette. Three replicate plates for each isolate were incubated at 5, 10, 15, 20, 25, 30, 35 and 40 °C. Colony diameters along the two marked lines were measured daily from day 2 until day 20, and the mean of the two readings was recorded for each plate. For the evaluation of the effect of daily temperature increase on hyphal growth in vitro, the two isolates were exposed to the two different temperature regimes described previously (Fig. 2a, thermoregulation simulated, no thermoregulation). The dishes were placed in the insectary within the locust bioassay cages and followed the same experimental procedure.
Fungal virulence
The in vivo experiment was conducted simultaneously and under the same temperature conditions as the in vitro experiments at fluctuating temperatures previously explained (Fig. 2a). Six different treatments were evaluated: Control with thermoregulation (Control*), control with no thermoregulation (Control), M. acridum (IMI 330189) with thermoregulation (Ma*), M. acridum (IMI 330189) with no thermoregulation (Ma), B. bassiana (EABb 90/2-Dm) with thermoregulation (Bb*) and B. bassiana (EABb 90/2-Dm) with no thermoregulation (Bb). For each treatment, 80 adult insects were inoculated (four replicates of 20 insects in a completely randomized design). Each replicate comprised ten male and ten female locusts housed in a wood framed cage (30 cm × 30 cm × 30 cm) with metallic mesh sides and roof. The treatments with thermoregulation were provided with heat gradient by a bulb mounted in the in the middle of the upper wall of the cages (60 W), with a metallic mesh under the bulb as climbing frame to enable basking behavior, thus providing identical thermal conditions as those of the in vitro experiments explained above (temperature measured at the middle point of the cage >35 °C for approx. 10 h day−1, 26.5–28.5 °C rest of the day). For the treatments under no thermoregulation conditions, same type of cages were used, with the metallic mesh as climbing frame but with no bulb as heat source (26.5–28.5 °C 24 h day−1).
Conidial suspensions were formulated in water with tween 80 (0.05%). Each locust received 2 μl of 5 × 106 spores ml−1 suspension (1 × 104 spores per insect) applied with a micropipette beneath the dorsal pronotal shield. This dose (approx. LD50), was selected based on previous studies of pathogenicity of the isolate EABb 90/2-Dm to D. maroccanus (Jiménez-Medina et al. 1998) and IMI 330189 to Zonocerus variegatus L. (Thomas and Jenkins 1997). Controls received blank formulation (water with tween 80). Time to death for each insect was recorded daily.
Behavioral fever
Thermal preference of locusts infected by the fungal strains was evaluated using similar methodology to the one first described by Inglis et al. (1996). Fifteen insects, randomly selected from the following treatments of the in vivo experiment explained above: Control with thermoregulation (Control*), M. acridum (IMI 330189) with thermoregulation (Ma*) and B. bassiana (EABb 90/2-Dm) with thermoregulation (Bb*), were placed in a temperature gradient at four and five days after infection.
The thermal gradient was provided by an aluminum tray (100 cm × 20 cm × 10 cm) with one end of the tray resting on a hot plate (Thermix, Model 310T, Fisher Scientific, Ottawa, ON, Canada). As an indicator of the thermal gradient, 11 surface temperatures of the tray (25 °C to 45 °C) were marked in 2 °C increments. The tray was covered with a Plexiglas top to prevent locusts from escaping. Groups of five insects (from each of the three replicates or cages, total 15 insects with sexes balanced) were introduced into the middle of the gradient and were left for 30 min to acclimate. Preferred temperatures were then recorded every 15 min at three different times (30 min, 45 min and 60 min). Locust positions were marked on the tray, and the actual temperatures of those positions in the gradient were recorded with a thermometer (Hanna, ± 0.1 °C). This process was performed at four and five days after the infection for a total of 45 temperature readings per treatment and day.
Statistical analysis
The effect of constant temperatures and time of exposure (hours) on the proportion of conidial germination was evaluated with a response surface model, with observations binomially distributed and the probit link function, using the equation:
where \(Temperature_{i}\) is the incubation temperature, \(Time_{j}\) is the incubation time (hours) and k denotes replicate. In the experiment of fluctuating temperatures with thermoregulation simulated conditions (temperature >35 °C for approx. 10 h day−1, 26.5–28.5 °C rest of the day) the effect of daily temperature increase on conidial germination was evaluated with the two parameter, nonlinear exponential model (response binomially distributed):
where \(Time_{i}\) is the incubation time (hours) and j denotes replicate. In the case of no thermoregulation simulated (26.5–28.5 °C, 24 h day−1), the probit model was used (binomial distribution and probit link function):
where \(Time_{i}\) is the incubation time (hours) and j denotes replicate . The estimation method for models 1, 2, and 3 (binomial response models) was maximum likelihood, and model effects were evaluated by likelihood ratio χ2 tests (α = 0.05). Statistical comparison of the times for the germination of the 50% of conidia (GT50), between isolates at each temperature, was performed by calculating the 95% confidence interval of the ratio of those parameters (GT50Bb/GT50Ma) according to Fieller’s theorem (Robertson et al. 2007). If the confidence interval of the ratio does not include 1, then the two parameters are considered to be significantly different.
In vitro hyphal growth rate (\(v\), mm day−1) for each isolate and temperature regime was calculated as the slope of the regression line:
where \(Colony\;\emptyset _{ij}\) is the average of the two colony diameters at each day i per replicate j. The effect of the temperature on the growth rate was then modeled with the beta function (Quesada-Moraga et al. 2006):
where \(v\left( {Temperature} \right)_{ij}\) is the hyphal growth rate at each temperature i and replicate j; \(Temperature_{i}\) is the temperature of incubation; \(T_{min}\), \(T_{max}\) and \(T_{opt}\) are the lowest, highest and optimal temperature for the fungal growth respectively; \(v_{Topt}\) is the fungal growth rate at the optimal temperature (\(T_{opt}\)); and \(T_{b}\) is a shape parameter of the beta curve. According to experimental data, \(T_{min}\) was fixed at 5 °C for both isolates, enabling improvement of the model fit and precision of the parameter estimates (Quesada-Moraga et al. 2006). Nonlinear models with beta function, were estimated by least squares with Newton optimization method for response normally distributed. Statistical comparisons of the regression parameters, between isolates, were performed with t-tests (α = 0.05).
Average survival time (AST, average of the survival times of the different insects) and median survival time (MST, time for mortality of 50% of insects) were estimated with the Kaplan–Meier procedure. ASTs were compared between treatments and between sexes within treatment with log-rank tests (α = 0.05) with Bonferroni correction for multiple testing. Differences in thermal preference for insects infected by M. acridum and B. bassiana and exposed to the temperature gradient were analyzed with a linear mixed model for repeated measures to evaluate main effects and interactions of the factors treatment, day, and time of temperature recording:
where \(Thermal preference_{ijkl}\) is the temperature in the gradient for each insect position (l); \(treatment_{i}\) = Control, B. bassiana and M. acridum under thermoregulation regime (Control*, Bb*, Ma*); \(day_{j}\) = four and five days after inoculation; \(time_{k}\) = temperature recording at 30, 45 and 60 min. Correlation between repeated measures was modeled with the compound symmetry covariance matrix. Each replicate, a group of five insects randomly selected (three replicates per treatment, total 15 insects), was the experimental unit measured over time. Data was square root transformed to achieve linear model assumptions. The linear mixed model was estimated with the restricted maximum likelihood (REML) method, and means were compared with Tukey’s tests (α = 0.05) (Stroup 2012). Statistical analyses were performed with JMP Pro 12.2.0 (SAS 2015).
Results
Conidial germination
The temperature profile of conidial germination of B. bassiana (EABb 90/2-Dm) showed significant effect of temperature with the probit model (model test χ2 = 16178.14; P < 0.0001; df = 4). All model effects: Temperature, log(Time), Temperature × log(Time) and Temperature2 were statistically significant (χ2 test P < 0.0001; df = 1). After 72 h of incubation, the optimum temperature for germination was 21.09 °C (95% CI 19.07–21.36). There was no conidial germination at 5 °C, 40 °C and 45 °C. At 35 °C, the percentage of conidial germination predicted by the model was only 21.4% of germination. Temperatures in the range 13–30 °C produced >95% conidial germination. (Table 1, Fig. 1a).
In vitro experiments at constant temperatures. a and b Effect of temperature on conidial germination of B. bassiana (EABb 90/2-Dm) and M. acridum (IMI 330189). Graphs represent surface response model (Eq. 1) with the following parameter estimates (± SE): B. bassiana, \(\beta_{0} = - 13.62 \pm 0.31\), \(\beta_{1} = 0.42 \pm 0.01\), \(\beta_{2} = 1.85 \pm 0.03\), \(\beta_{3} = - 0.11 \pm 0.003\), \(\beta_{4} = - 0.02 \pm 0.0004\); M. acridum, \(\beta_{0} = - 9.91 \pm 0.21\), \(\beta_{1} = 0.32 \pm 0.007\), \(\beta_{2} = 1.31 \pm 0.02\), \(\beta_{3} = - 0.02 \pm 0.001\), \(\beta_{4} = - 0.02 \pm 0.0003\). c and d Effect of temperature on hyphal growth rate of B. bassiana (EABb 90/2-Dm) and M. acridum (IMI 330189). Graphs represent beta function (Eq. 5) with the following parameter estimates (± SE): B. bassiana,\(v_{Topt} = 4.17 \pm 0.16\), \(T_{opt} = 23.87 \pm 0.49\), \(T_{max} = 35.10 \pm 3.83\), \(T_{b} = 3.29 \pm 0.24\), \(T_{min} = 5.0\) fixed; M. acridum, \(v_{Topt} = 3.34 \pm 0.04\), \(T_{opt} = 28.96 \pm 0.21\), \(T_{max} = 37.10 \pm 0.52\), \(T_{b} = 1.42 \pm 0.24\), \(T_{min} = 5.0\) fixed. Error bars in c and d represent the SE
For M. acridum (IMI 330189), the probit model also showed significant effect of temperature on conidial germination (model test χ2 = 17493.53; P < 0.0001; df = 4). All model effects: Temperature, log(Time), Temperature × log(Time) and Temperature2 were statistically significant (χ2 test P < 0.0001; df = 1). After 72 h of incubation, the optimum temperature for conidial germination was 27.59 °C (95% CI 24.96–27.76). There was no germination at 5 °C, 10 °C or 45 °C. At 40 °C the percentage of conidial germination predicted by the model was only 42.4% of germination. Temperatures in the range 19–36 °C produced >95% of conidial germination (Table 1, Fig. 1b). Time for germination of 50% of conidia (GT50), showed significantly faster germination of B. bassiana versus M. acridum at 15 °C and at 20 °C. Conidial germination was faster for M. acridum than for B. bassiana at 25 °C and at 30 °C (Table 1).
When the conidial germination was evaluated with the fluctuating temperature regime that simulated thermoregulation conditions (Fig. 2b), it was shown that the daily increase of temperature largely reduced the conidial germination both for M. acridum and B. bassiana in comparison to the environment at insectary temperature with no thermoregulation (26.5–28.5 °C). Probit and exponential models of conidial germination over time were statistically significant (Fig. 2b; B. bassiana no thermoregulation: χ2 = 2025.68, P < 0.0001, df = 1; M. acridum no thermoregulation: χ2 = 1934.97, P < 0.0001, df = 1; B. bassiana thermoregulation simulated: χ2 = 60.20, P < 0.0001, df = 1; M. acridum thermoregulation simulated: χ2 =147.10, P < 0.0001, df = 1). At 72 h, in the case of fluctuating temperature, there were significant differences between fungal strains for the proportion of conidial germination, with only 36.9% (M. acridum) and 13.1% (B. bassiana) of conidia germinated (t48 = 11.28; P < 0.0001). In the environment with insectary temperature with no thermoregulation conditions (26.5–28.5 °C), there were significant differences between GT50s, 4.49 h (95% CI 4.23–4.74) for M. acridum and 8.76 h (95% CI 8.35–9.18) for B. bassiana with 95% CI ratio = (0.476–0.551), but there were no significant differences in the proportion of conidial germination at 72 h, higher than 99% for both M. acridum and B. bassiana.
Experiments at fluctuating temperatures. a Temperature regimes for experiments in figures b, c, and d: No thermoregulation (dashed line) = insectary conditions with no additional heat gradient provided by bulbs (26.5–28.5 °C); Thermoregulation (solid line) = insectary conditions with additional heat gradient provided by bulbs in rearing cages (26.5–42.5 °C). b Conidial germination in vitro, comparing simulated thermoregulation (solid symbols) versus no thermoregulation (hollow symbols). X-axis (Time-hours) is on logarithmic scale. The model for thermoregulation conditions (Eq. 2) has the following parameter estimates (± SE): B. bassiana, \(\beta_{0} = 0.13 \pm 0.01\), \(\beta_{1} = 0.12 \pm 0.02\); M. acridum, \(\beta_{0} = 0.36 \pm 0.01\), \(\beta_{1} = 0.18 \pm 0.02\) The model for no thermoregulation conditions (Eq. 3) has the following parameter estimates (± SE): B. bassiana, \(\beta_{0} = - 2.79 \pm 0.10\), \(\beta_{1} = 1.28 \pm 0.04\); M. acridum, \(\beta_{0} = - 1.87 \pm 0.08\), \(\beta_{1} = 1.24 \pm 0.05\). c Hyphal growth in vitro, comparing simulated thermoregulation (solid symbols) versus no thermoregulation (hollow symbols). The model for both conditions (Eq. 4) has the following parameter estimates (± SE): B. bassiana no thermoregulation, \(v = 2.40 \pm 0.03\); B. bassiana thermoregulation, \(v = 0.66 \pm 0.01\); M. acridum no thermoregulation, \(v = 3.39 \pm 0.04\); M. acridum thermoregulation, \(v = 2.03 \pm 0.02\). d Insect cumulative survival, using Kaplan-Meier analysis. For figures b, c and d: open circle: M. acridum (IMI 330189) with no thermoregulation, filled circle: M. acridum (IMI 330189) with thermoregulation, open triangle: B. bassiana (EABb 90/2-Dm) with no thermoregulation, filled triangle: B. bassiana (EABb 90/2-Dm) with thermoregulation, open square: control with no thermoregulation, filled square: control with thermoregulation. Error bars in b, c and d represent the SE
Hyphal growth
Temperature profiles of hyphal growth in vitro (Fig. 1c and d) indicated that the optimum temperature for B. bassiana was 23.87 ± 0.49 °C (mean ± SE), which was significantly lower than the optimum temperature for M. acridum, 28.96 ± 0.21 °C (t34 = 9.51, P = 0.0001). The growth rates at optimum temperatures were also significantly different: B. bassiana = 4.17 ± 0.16 mm day−1; M. acridum = 3.34 ± 0.04 mm day−1; (t34 = 2.765, P = 0.0001). B. bassiana did not show hyphal growth at 5 °C, and at 35 °C the growth was very slow = 0.08 ± 0.02 mm day−1. M. acridum did not show hyphal growth at 5 °C or 10 °C, but the growth at 35 °C was 1.25 ± 0.04 mm day−1. Neither of the strains grew at 40 °C, and there were not significant differences between maximum temperatures estimated by the model, B. bassiana = 35.10 ± 3.83 °C, M. acridum = 37.10 ± 0.52 °C (t34 = 0.517; P = 0.608).
When the fungal strains were exposed to a gradual increase in temperature that simulated thermoregulation conditions, growth rate decreased significantly for both isolates in comparison to their growth rate at insectary temperature with no additional heat source provided (26.5–28.5 °C, Fig. 2a). In the case of B. bassiana, the growth rate decreased from 2.40 ± 0.03 to 0.66 ± 0.01 mm day−1 (t56 = 51.761; P < 0.0001). In the case of M. acridum, the growth rate decreased from 3.39 ± 0.04 to 2.03 ± 0.02 mm day−1 (t56 = 30.641; P < 0.0001) (Fig. 2c). M. acridum growth rate was significantly higher than B. bassiana growth rate at both temperature regimes (thermoregulation simulated, t56 = 64.01; P < 0.0001; thermoregulation not simulated t56 = 19.26; P < 0.0001).
Fungal virulence and behavioral fever
M. acridum was more virulent on D. maroccanus locusts than B. bassiana in both temperature regimes (Table 2, Fig. 2d). When locust were allowed to thermoregulate, virulence decreased both for B. bassiana and M. acridum. The survival analysis did not show significant differences in average survival times between males and females within each treatment (log rank test χ2; P values ranged from 0.205 to 0.815; df=1), and results are presented combining both sexes. In the case of M. acridum, the increase in average survival time was 1.8 times (from 4.57 to 8.26 days; log rank test χ2 = 102.06; P < 0.0001; df = 1), although at the end of the 20 days of experiment the mortality was 100% for both temperature regimes. In the case of B. bassiana, the increase in average survival time was 2.6 times (from 6.10 to 15.83 days; log rank test χ2 = 129.55; P < 0.0001; df = 1). The insect mortality decreased from 100% to 73.7%. The percentage of cadavers with external fungal growth was 86.2, 63.7, 60.0 and 42.5% for Ma, Bb, Ma* and Bb* respectively.
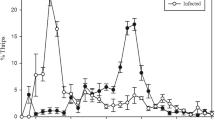
The analysis of the thermal preference of the locusts placed in the temperature gradient showed significant effect of treatment (F2,36 = 3.88; P = 0.0298), but no significant effect of day of assessment (F1,36 = 0.1360; P = 0.1360), time of temperature recording (F2,36 = 2.43; P = 0.1022), or any other of the second and third level interactions of the linear mixed model for repeated measures (Eq. 6). The mean preferred temperature for Ma* (40.6 ± 0.94 °C) was significantly higher than the preferred temperature for the control (Control*) (36.7 ± 0.95 °C), (t36 = 2.86; P = 0.0226). Bb* showed higher preferred temperature than the control (38.8 ± 0.94 °C), but the difference was not statistically significant (t36 = 1.50; P = 0.3032) (Fig. 3).
Thermal gradient preference for insects infected by B. bassiana (EABb 90/2-Dm) and M. acridum (IMI 330189) and maintained in thermoregulation conditions. Each dot represents an individual measurement at four and five days post-inoculation and at 30, 45 and 60 min after the insects being placed into the temperature gradient. Box and whisker plots show data distribution: the two ends of the box represent the 25th and 75th quantiles (1st and 3rd quartile respectively); the horizontal line within the box represents the median; the whiskers extend from each end of the box to the outmost data point that falls within the distance of 1.5 × interquartile range
Discussion
Thermal requirements for hyphal growth and conidial germination have been common criteria for selection of isolates with potential to control locusts and grasshoppers, and other insect pests (Darbro et al. 2011; Jaronski 2010; Ouedraogo et al. 1997; Quesada-Moraga et al. 2006; Yeo et al. 2003). Optimum temperature for growth of B. bassiana (EABb 90/2-Dm) in our work (23.87 °C) was the same as the one reported previously by Quesada-Moraga et al. (2006). Only optimum temperature for growth of M. acridum (IMI 330189) showed a minor difference with that reported previously by Thomas and Jenkins (1997), 28.96 versus 27 °C, probably due to the different nonlinear regression model or experimental variability. However, our in vitro experiments at constant temperatures suggests better adaptation of M. acridum (IMI 330189) at temperatures above 27 °C, and better adaptation of B. bassiana (EABb 90/2-Dm) at temperatures ranges of 10–25 °C, especially for hyphal growth. In the previous work on thermal requirements of IMI 330189 strain at constant temperatures (Thomas and Jenkins 1997), conidial germination, which did not occur in the range of 5 to 15°C, was measured after 17 h of incubation. In our work, IMI 330189 strain conidial germination at 15 °C was observed after 24 h of incubation and onwards, with a maximum rate of 57.8% after 72 h of incubation. Similarly, due to shorter incubation time, Thomas and Jenkins (1997) reported low germination rates (ca. 1%) at 40 °C, which reached 42.4% after 72 h of incubation in the present work (although < 5% after 24 h of incubation).
When both fungal strains were compared under conditions of daily temperature fluctuation simulating thermoregulation, the reduction in conidial germination and hyphal growth relative to the values observed at the more stable insectary temperature (26.5–28.5 °C) was higher for B. bassiana (EABb 90/2-Dm) than for M. acridum (IMI 330189). This can be explained by looking at the thermal performance curves: temperatures above 35 °C are limiting for both strains, but the range of temperatures 26.5–35 °C is clearly more favorable to M. acridum than to B. bassiana. Negative effects of transient exposure to high temperatures has been previously described for B. bassiana (Inglis et al. 1996), for M. anisopliae and B. bassiana (Liu et al. 2003), for B. bassiana and Metarhizium flavoviride (Inglis et al. 1999) and for Metarhizium brunneum Petch (Hypocreales: Clavicipitaceae) (Fisher and Hajek 2014).
In addition to the direct impact of the delay/lack of germination and growth during periods of unfavorable temperatures, the ability to recover normal metabolic activity after exposure to high temperatures could also affect the strains’ response e.g. Klass et al. (2007), Rangel et al. (2010). Keyser et al. (2014) studied the ability of Metarhizium spp. isolates to recover normal metabolic activity after exposure to high temperature for several hours daily (fungal colonies were exposed to 40 °C for 4 h or 8 h followed by 20 h or 16 h at 28 °C respectively). Evidence suggests that certain isolates require a temporary repair or “retooling” period on return to favorable temperatures. Similar results have been shown with B. bassiana in studies on houseflies (Anderson et al. 2013a).
Fungal virulence also was affected by thermoregulation. The average survival time and total mortality of the insects infected with both fungal strains were equivalent when insects were maintained under standard insectary conditions and unable to thermoregulate behaviorally. However, when insects were allowed to thermoregulate, the virulence of B. bassiana (EABb 90/2-Dm) was considerably reduced, increasing the average survival time and the number of surviving insects after 20 days of experiment. The reduction of the virulence of M. acridum (IMI 330189) to D. maroccanus was less marked. Although AST increased 1.8 times, all the locusts died during the experiment (20 days). The reduction in virulence of Metarhizium spp. and Beauveria spp. due to thermoregulation has been described in grasshoppers and locusts in different studies (see references in the Introduction section) and also in other insect species (Anderson et al. 2013b; Hunt and Charnley 2011; Thomas and Blanford 2003). Whilst it has been reported that the possible effect of sex on fungal virulence varies across species of Orthoptera (Milner et al. 1996; Wang et al. 2013), our study has shown that no differences in fungal virulence were found due to the host’s sex in either thermoregulation or no-thermoregulation conditions.
M. acridum (IMI 330189) has been shown to induce behavioral fever in several species of locusts and grasshoppers, including Oedaleus senegalensis (Krauss) (Blanford et al. 1998), Schistocerca gregaria (Forskål) (Elliot et al. 2002, 2003; Thomas et al. 2003), and Locusta migratoria migratorioides (Reiche, L.J. & Fairmaire) (Ouedraogo et al. 2002, 2004). B. bassiana isolates have also been shown to induce behavioral fever on grasshoppers, Melanoplus sanguinipes (Fabricius) (Inglis et al. 1996), and an isolate from the breeding area of La Serena (Spain) induced behavioral fever in S. gregaria (Thomas et al. 2003). Our results suggest that M. acridum (IMI 330189) induced behavioral fever in D. maroccanus. Locusts infected with this strain selected temperatures approximately 4 °C above the temperatures preferred by the uninfected control. Nonetheless, this strain was still virulent under these experimental conditions. Locusts infected with B. bassiana (EABb 90/2-Dm) also selected higher (approximately 2 °C) than control insects, although the differences in preferred temperatures were not statistically significant in our experiment.
Overall, our study suggests that M. acridum (IMI 330189) is more heat tolerant than B. bassiana (EABb 90/2-Dm). Under the temperature conditions of La Serena, the main breeding area for D. maroccanus in Spain, with daily maximum temperatures exceeding 40 °C and minimum temperatures consistently falling below 20 °C, (Klass et al. 2007), M. acridum (IMI 330189) is likely a better candidate for use in biocontrol. It has to be noted that maximum temperature for growth of IMI 330189 strain is slightly above 37 °C (i.e. 37.1 °C), whereas the actual growth rate at this temperature is minimal or null, limiting the potential concern of selecting isolates with growth potential above human body temperature. Moreover, the very few cases of human infection by entomopathogenic fungi have been reported for B. bassiana in immunocompromised individuals even with fungal strains unable to grow at 37 °C (Henke et al. 2002; Tucker et al. 2004).
B. bassiana EABb 90/2-Dm strain has shown higher hyphal growth rate than M. acridum (IMI 330189) at temperatures below 27 °C, suggesting that in more temperate environmental conditions, the former strain would be a good candidate, either as a stand-alone one or in mixed combinations of the two fungal strains potentially providing more effective control over a broader range of conditions. Nonetheless, such a strain combination strategy might potentially alter population dynamics of a particular insect–pathogen interaction, especially in sub- and pre-lethal conditions (Klinger et al. 2015; Thomas et al. 2003), which remains to be elucidated in our D. maroccanus-IMI 330189 - EABb 90/2-Dm system.
References
Anderson RD, Blanford S, Jenkins NE, Thomas MB (2013a) Discriminating fever behavior in house flies. PLoS ONE 8(4):e62269. https://doi.org/10.1371/journal.pone.0062269
Anderson RD, Blanford S, Thomas MB (2013b) House flies delay fungal infection by fevering: at a cost. Ecol Entomol 38:1–10
Arthurs S, Thomas MB (2001) Effects of temperature and relative humidity on sporulation of Metarhizium anisopliae var. acridum in mycosed cadavers of Schistocerca gregaria. J Invertebr Pathol 78:59–65
Bidochka MJ, Kasperski JE, Wild GAM (1998) Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Can J Bot-Rev Can Bot 76:1198–1204
Bidochka MJ, Melzer MJ, Lavender TM, Kamp AM (2000) Genetically related isolates of the entomopathogenic fungus Metarhizium anisopliae harbour homologous dsRNA viruses. Mycol Res 104:1094–1097
Bidochka MJ, Menzies FV, Kamp AM (2002) Genetic groups of the insect-pathogenic fungus Beauveria bassiana are associated with habitat and thermal growth preferences. Arch Microbiol 178:531–537
Blanford S, Thomas MB (2000) Thermal behavior of two acridid species: effects of habitat and season on body temperature and the potential impact on biocontrol with pathogens. Environ Entomol 29:1060–1069
Blanford S, Thomas MB, Langewald J (1998) Behavioral fever in the Senegalese grasshopper, Oedaleus senegalensis, and its implications for biological control using pathogens. Ecol Entomol 23:9–14
Carruthers RI, Larkin TS, Firstencel H, Feng ZD (1992) Influence of thermal ecology on the mycosis of a rangeland grasshopper. Ecology 73:190–204
Chappell MA, Whitman DW (1990) Grasshopper thermoregulation. In: Chapman RF (ed) Biology of grasshoppers. Wiley Interscience, New York, pp 143–172
Collar-Urquijo JL, Celma-Calamita J, Blandford S, Thomas MB (2002) Control de Dociostaurus maroccanus y Calliptamus italicus (Orthoptera: Acrididae) mediante aplicaciones en campo de Metarhizium anisopliae var acridum. Bol San Veg Plagas 28:185–192
Darbro JM, Graham RI, Kay BH, Ryan PA, Thomas MB (2011) Evaluation of entomopathogenic fungi as potential biological control agents of the dengue mosquito, Aedes aegypti (Diptera: Culicidae). Bicontrol Sci Technol 21:1027–1047
Elliot SL, Blanford S, Thomas MB (2002) Host-pathogen interactions in a varying environment: temperature, behavioural fever and fitness. Proc R Soc Lond Ser B-Biol Sci 269:1599–1607
Elliot SL, Blanford S, Horton CM, Thomas MB (2003) Fever and phenotype: transgenerational effect of disease on desert locust phase state. Ecol Lett 6:830–836
Fargues J, Goettel MS, Smits N, Ouedraogo A, Rougier M (1997a) Effect of temperature on vegetative growth of Beauveria bassiana isolates from different origins. Mycologia 89:383–392
Fargues J, Ouedraogo A, Goettel MS, Lomer CJ (1997b) Effects of temperature, humidity and inoculation method on susceptibility of Schistocerca gregaria to Metarhizium flavoviride. Biocontrol Sci Technol 7:345–356
Fisher JJ, Hajek AE (2014) Thermoregulatory behavior and fungal infection of Anoplophora glabripennis (Coleoptera: Cerambycidae). Environ Entomol 43:384–392
Henke MO, de Hoog GS, Gross U, Zimmerman G, Kraemer D, Weig M (2002) Human deep tissue infection with an entomopathogenic Beauveria species. J Clin Microbiol 40:2698–2702
Hernandez-Crespo P, Santiago-Álvarez C (1997) Entomopathogenic fungi associated with natural populations of the Moroccan locust Dociostaurus maroccanus (Orthoptera: Gomphocerinae) and other Acridoidea in Spain. Biocontrol Sci Technol 7:357–363
Hunt VL, Charnley AK (2011) The inhibitory effect of the fungal toxin, destruxin A, on behavioural fever in the desert locust. J Insect Physiol 57:1341–1346
Hunt VL, Lock GD, Pickering SG, Charnley AK (2011) Application of infrared thermography to the study of behavioural fever in the desert locust. J Therm Biol 36:443–451
Inglis GD, Johnson DL, Goettel MS (1996) Effects of temperature and thermoregulation on mycosis by Beauveria bassiana in grasshoppers. Biol Control 7:131–139
Inglis GD, Duke GM, Kawchuk LM, Goettel MS (1999) Influence of oscillating temperatures on the competitive infection and colonization of the migratory grasshopper by Beauveria bassiana and Metarhizium flavoviride. Biol Control 14:111–120
Inglis GD, Goettel MS, Butt TM, Strasser H (2001) Use of hyphomicetous fungi for managing insect pests. In: Butt TM, Jackson CW, Magan N (eds) Fungi as biocontrol agents: progress, problems and potential. CABI International, Wallingford, pp 23–69
Jaronski ST (2010) Ecological factors in the inundative use of fungal entomopathogens. BioControl 55:159–185
Jiménez-Medina J, Aldebis HK, Santiago-Alvarez C (1998) Valoración insecticida de diversos aislados de hongos hifomicetos para el control de la langosta mediterránea, Dociostaurus maroccanus (Thunberg). Bol San Veg Plagas 24:867–872
Keyser CA, Fernandes EKK, Rangel DEN, Roberts DW (2014) Heat-induced post-stress growth delay: a biological trait of many Metarhizium isolates reducing biocontrol efficacy? J Invertebr Pathol 120:67–73
Klass JI, Blanford S, Thomas MB (2007) Development of a model for evaluating the effects of environmental temperature and thermal behaviour on biological control of locusts and grasshoppers using pathogens. Agric For Entomol 9:189–199
Klinger EG, Vojvodic S, DeGrandi-Hoffman G, Welker DL, James RR (2015) Mixed infections reveal virulence differences between host-specific bee pathogens. J Invertebr Pathol 129:28–35
Latchininsky AV, Launois-Luong MH (1992) Le criquet marocain, Dociostaurus maroccanus (Thunberg, 1815) dans la partie orientale de son aire de distribution. Etude monographique à l’ex-URSS et aux pays proches. Montpellier/VIZR, Saint-Petersbourg
Liu HP, Skinner M, Brownbridge M, Parker BL (2003) Characterization of Beauveria bassiana and Metarhizium anisopliae isolates for management of tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae). J Invertebr Pathol 82:139–147
Lomer CJ, Bateman RP, Johnson DL, Langewald J, Thomas M (2001) Biological control of locusts and grasshoppers. Annu Rev Entomol 46:667–702
Milner RJ, Staples JA, Prior C (1996) Laboratory susceptibility of Locusta migratoria (L), Austracris guttulosa (Walker) and Valanga irregularis (Walker) (Orthoptera: Acrididae) to an oil formulation of Metarhizium flavoviride Gams and Rozsypal (Deuteromycotina: Hyphomycetes). Aust J Entomol 35:355–360
Ouedraogo A, Fargues J, Goettel MS, Lomer CJ (1997) Effect of temperature on vegetative growth among isolates of Metarhizium anisopliae and M. flavoviride. Mycopathologia 137:37–43
Ouedraogo RM, Kamp A, Goettel MS, Brodeur J, Bidochka MJ (2002) Attenuation of fungal infection in thermoregulating Locusta migratoria is accompanied by changes in hemolymph proteins. J Invertebr Pathol 81:19–24
Ouedraogo RM, Goettel MS, Brodeur J (2004) Behavioral thermoregulation in the migratory locust: a therapy to overcome fungal infection. Oecologia 138:312–319
Quesada-Moraga E (1998) Biología y ecología de la reproducción y desarrollo embrionario de la langosta mediterránea Dociostaurus maroccanus (Thunberg) y su posible interferencia como estrategia de lucha. PhD Thesis, University of Cordoba. Spain
Quesada-Moraga E, Santiago-Álvarez C (2001) Rearing and breeding of the Moroccan locust Dociostaurus maroccanus (Thunberg) (Orthop., Acrididae) under laboratory conditions. J Appl Entomol-Z Angew Entomol 125:121–124
Quesada-Moraga E, Maranhao EAA, Valverde-Garcia P, Santiago-Alvarez C (2006) Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements, and toxicogenic activity. Biol Control 36:274–287
Rangel DEN, Fernandes EKK, Dettenmaier SJ, Roberts DW (2010) Thermotolerance of germlings and mycelium of the insect-pathogenic fungus Metarhizium spp. and mycelial recovery after heat stress. J Basic Microbiol 50:344–350
Robertson JL, Russell RM, Preisler HK, Savin NE (2007) Bioassays with arthropods, 2nd edn. CRC Press, Boca Raton
SAS (2015) JMP pro 12.2.0 SAS Institute Inc., Cary, NC
Springate S, Thomas MB (2005) Thermal biology of the meadow grasshopper, Chorthippus parallelus, and the implications for resistance to disease. Ecol Entomol 30:724–732
Stroup WW (2012) Generalized linear mixed models: modern concepts, methods and applications. CRC Press, Boca Raton
Thomas MB, Blanford S (2003) Thermal biology in insect-parasite interactions. Trends Ecol Evol 18:344–350
Thomas MB, Jenkins NE (1997) Effects of temperature on growth of Metarhizium flavoviride and virulence to the variegated grasshopper, Zonocerus variegatus. Mycol Res 101:1469–1474
Thomas MB, Watson EL, Valverde-Garcia P (2003) Mixed infections and insect-pathogen interactions. Ecol Lett 6:183–188
Tucker DL, Beresford CH, Sigler L, Rogers K (2004) Disseminated Beauveria bassiana infection in a patient with acute lymphoblastic leukemia. J Clin Microbiol 42:5412–5414
Vega FE, Kaya HK (2012) Insect pathology, 2nd edn. Academic Press, San Diego
Wang Y, Yang P, Cui F, Kang L (2013) Altered immunity in crowded locust reduced fungal (Metarhizium anisopliae) pathogenesis. PLoS Pathog 9(1):e1003102. https://doi.org/10.1371/journal.ppat.1003102
Yeo H, Pell JK, Alderson PG, Clark SJ, Pye BJ (2003) Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Manag Sci 59:156–165
Acknowledgements
We thank Dr. Elizabeth Maranhão and Dr. Karl D. Schnelle for their scientific collaboration and review of the document. This research was supported by the project AGL2016-80483-R from the Spanish Ministry of Science, Innovation and Universities. Dr. Inmaculada Garrido-Jurado thanks the Ministry of Economy and Competitiveness of the Spanish Government for a Juan de la Cierva post-doctoral Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Helen Roy.
Rights and permissions
About this article
Cite this article
Valverde-Garcia, P., Santiago-Alvarez, C., Thomas, M.B. et al. Comparative effects of temperature and thermoregulation on candidate strains of entomopathogenic fungi for Moroccan locust Dociostaurus maroccanus control. BioControl 63, 819–831 (2018). https://doi.org/10.1007/s10526-018-9904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-018-9904-6