Abstract
The silver fly Leucopis glyphinivora Tanasijtshuk (Diptera: Chamaemyiidae) is an aphidophagous predator during its larval stage. Few studies have examined the predation habits of this species for biological control. Larval voracity of L. glyphinivora was measured under laboratory and controlled greenhouse conditions and compared with a commercially available biocontrol agent, Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae). Laboratory tests were conducted in Petri dishes using Myzus persicae Sulzer (Hemiptera: Aphididae) on potato leaves. In greenhouse tests, predator voracity was evaluated with various plant-aphid treatments. In the laboratory, silver fly larvae consumed 39% more aphids than A. aphidimyza throughout their larval development. In the greenhouse, L. glyphinivora consumed more aphids than A. aphidimyza regardless of treatment. The highest voracities were obtained on tomato and bell pepper infested with M. persicae. No antagonistic predatory effects were observed when predators were used together. This study provides useful insight on L. glyphinoivora as an efficient aphid predator but more research is needed to establish its potential for biological control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aphids are major crop pests in many greenhouses (Blackman and Eastop 2007; Sorensen 2009). The use of chemical pesticides has led to pest resistance and environmental problems by having adverse effects on non-target species. Biological pest control is used as an alternative to traditional chemical pesticides to avoid these risks while still maintaining pest populations low (van Lenteren and Woets 1988; Blackman and Eastop 2007; Hoddle and van Driesche 2009; Sorensen 2009). While the market for biological control is growing and many species have been tested, only a fraction of the potential present in nature has been studied for biocontrol evaluations (Sloggett 2005; Begum et al. 2017; van Lenteren et al. 2018). It is therefore likely that many interesting species have been overlooked for biocontrol programs in greenhouses. Various criteria are used to determine if a predatory insect has the potential to become an effective biocontrol agent. Specificity to the target pest, ability to suppress pest populations, numerical response, searching ability, reproductive behaviour, generation time and ability to be mass-reared for commercial production are all important factors that need to be well understood before a biocontrol program can be established (Coppel and Mertins 1977; Stiling and Cornelissen 2005).
The silver fly Leucopis glyphinivora Tanasijtshuk (Diptera: Chamaemyiidae) is an aphidophagous predator in its larval stage (Rad et al. 2003; Satar et al. 2015; Mehrparvar et al. 2016). Very little information is currently available about the biology and ecology of this species, specifically on its efficiency as a biological control agent. L. glyphinivora has a Holarctic distribution, being found throughout much of Europe and North America (Carroll and Hoyt 1984; Brewer and Noma 2010; Rakhshani et al. 2010; Natshuk and Bagachanova 2013; Kahanpää 2014; Satar et al. 2015). Larvae of this silver fly feed on a wide variety of aphid species, many of which are agricultural pests such as the black bean aphid Aphis fabae Scopoli (Rad et al. 2003; Mustaţă et al. 2010), the soybean aphid Aphis glycines Matsumura (Kaiser et al. 2007) and the green apple aphid Aphis pomi DeGeer (Carroll and Hoyt 1984). Other species in the Leucopis Meigen genus have been studied as potential biological control agents in both agriculture and forestry. L. gaimarii Tanasijtshuk and L. ninae Tanasijtshuk were used against the Russian wheat aphid Diuraphis noxia Mordvilko (Hemiptera: Aphididae) (Gaimari and Turner 1996a, 1997; Mohamed et al. 2000; Brewer and Elliott 2004; Noma et al. 2005). In vineyards, L. simplex Loew was observed to be an important natural enemy of Daktulosphaira vitifoliae Fitch (Hemiptera: Phylloxeridae) (Stevenson 1967). In forestry, L. hennigrata McAlpine was used against the balsam woolly adelgid Adelges picea Ratzeburg (Hemiptera: Adelgidae) (McAlpine 1978; Humble 1994) while L. argenticollis Zetterstedt and L. piniperda Malloch were used to control hemlock woolly adelgid A. tsugae Annand (Hemiptera: Adelgidae) (Wallace and Hain 2000; Preisser et al. 2014; Kohler et al. 2016).
We have selected the predatory gall midge Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae) as a reference species to evaluate the potential of L. glyphinivora as a biological control agent. The midge is extensively used in greenhouses around the world to control aphid outbreaks (Malais and Ravensberg 2006; Völkl et al. 2007; Alotaibi 2008). This species was chosen for its resemblance with L. glyphinivora due to taxonomic proximity (dipteran), size similarity, presence of a predatory larval stage and dietary preference for aphids. Aphidoletes aphidimyza larvae use a so-called furtive predation strategy. Such a strategy enables A. aphidimyza larvae to both live and feed within the aphid colony without causing significant disturbance (Lucas and Brodeur 2001). This furtive strategy is a rare trait which allows A. aphidimyza to not only reduce the risk of food shortage by limiting aphid dispersal (Fréchette et al. 2008), but also to hide from intraguild predators through both a dilution effect generated by aphids (Lucas and Brodeur 2001) and a selfish herd effect by selecting a central position within the colony (Dumont et al. 2015). Commercial use of A. aphidimyza for control of crop pests in greenhouses began in the early 1980s and has remained a popular alternative to chemical pesticides (van Lenteren and Woets 1988; Malais and Ravensberg 2006; Powell and Pell 2007). Aphidoletes aphidimyza does, however, present certain undesirable characteristics. To avoid drying out, larvae pupate in the soil if RH is low, thus making them vulnerable to ground dwelling predators (van Schelt and Mulder 2000; Yukawa et al. 2008; Le Goff et al. 2016). There is also a high mortality rate amongst A. aphidimyza pupae and emerging adults are often unable to reach the soil surface (Yukawa et al. 2008). Aphidoletes aphidimyza larvae are also unable to disperse more than 63 mm before dying from starvation (Wilbert 1973) meaning once eggs are laid, biological control happens on a very local scale on the host plant. It is therefore important to study other potential biological control agents that might lack such negative traits while still sharing the same ecological niche.
The aim of this study is to evaluate the potential of L. glyphinivora as a new biological control agent by establishing its predation ability against aphid populations. This trait is then compared to that of a similar commercially available biological control agent, A. aphidimyza. While voracity is just one aspect of what makes an effective biocontrol agent, this information will provide a first estimation of its overall efficacy against aphid pests in a greenhouse environment. First, daily and life time voracity of larvae of the two predators were evaluated and compared under laboratory conditions. Second, we observed the in situ impact of larvae against aphids in a greenhouse using different aphid-host plant combinations. Finally, the combined use of the two predators together was evaluated to determine the possibility of synergistic or antagonistic effects. The main hypothesis is that, due to the slower development time of L. glyphinivora, this species is more voracious than A. aphidimyza (Canale et al. 2002; Rad et al. 2003; Barriault et al. 2018). Moreover, L. glyphinivora is known to be a rather polyphagous species inhabiting a wide variety of host plants (Tanasijtshuk 1986; Satar et al. 2015). We can then expect that the specific crop plants or pest aphids will not significantly alter its predation efficacy. This behaviour is also observed throughout the Leucopis Meigen genus (Brewer and Noma 2010; Zhou et al. 2014; Colares et al. 2015; Satar et al. 2015; Kohler et al. 2016).
Materials and methods
Laboratory experiment
Insect material
Wild L. glyphinivora specimens were collected from greater burdock (Arctium lappa L.) infested with the black bean aphid Aphis fabae Scopoli (Hemiptera: Aphididae) found on campus grounds at the Université du Québec à Montréal (Montréal, Canada) (43°30′34″N; 73°34′08″O) throughout July 2016. Specimens were mostly collected as larvae and pupae. Aphidoletes aphidimyza were commercially supplied as pupae from Plant-Products Québec (Laval, Quebec, Canada). Both predators were reared in the same growth chamber under identical conditions, and for multiple generations using the Gaimari and Turner (1996b) method. Mass rearing cages consisted of a cubic polyvinyl chloride (PVC) frame (35 × 35 × 35 cm3) covered with a sheet of white muslin. Potato plants, Solanum tuberosum (var. Norland), infested with the green peach aphid M. persicae, were introduced into rearing cages and served as the main host plant and prey aphid for both predators. A saturated solution of water and table sugar (sucrose) was supplied as an additional food source for adults. A dry mixture of table sugar and brewer’s yeast (Saccharomyces cerevisiae) (1:1 ratio) was also added into the L. glyphinivora cages as another food source for adults. Every week, a single non-infested potato plant was introduced into each rearing cage until adult emergence began, after which adults were transferred into new rearing cages. Old cages were kept for seven days before discarding plant material to ensure all adults had emerged. Rearing cages were kept in a growth chamber at 24 °C, RH 75%, and L:D 16:8.
Laboratory tests
Laboratory tests were conducted in small Petri dishes (diameter = 5 cm) with agar gel and a potato leaf lining the bottom of the dish. A single egg (L. glyphinivora or A. aphidimyza) was collected from a rearing cage and introduced into the Petri dish. A colony of 20 first stage (N1) M. persicae was also introduced into the Petri dish, allowing larvae to feed as soon as they hatched. Once larvae hatched, larval voracity was noted every 24 h until pupation occurred. Observations continued until each larva had pupated. Additional aphids were added daily into the Petri dishes to re-establish the colony at 20 aphids.
A predator free control treatment was conducted under identical conditions as those with a predator. The control treatment was used to consider natural aphid mortality. The following model was used to calculate voracity while taking natural prey mortality into account (Soares et al. 2003):
where V0 is the number of prey eaten by a given predator, A is the number of prey available at the start of the test, a is the number of prey remaining after a certain time frame (24 h) and ra is the ratio of prey alive in the control treatment after the same time frame.
Greenhouse experiment
Insect material
Leucopis glyphinivora specimens came from a pre-existing rearing colony held at the biological control laboratory at the Université du Québec à Montréal. Wild specimens were collected on campus grounds (Montréal, Canada) from apple trees (Malus pumilla Miller) infested with the green citrus aphid Aphis spiraecola van Der Goot (Hemiptera: Aphididae) throughout July 2009. Wild L. glyphinivora were regularly collected and added to the experimental rearing colony. As with the laboratory tests, A. aphidimyza specimens came from Plant-Products Québec as pupae. Both predators were reared in cubic PVC cages (35 × 35 × 35 cm3) covered with a sheet of white muslin. Insects used for the greenhouse tests were reared with the same procedure used for the laboratory test rearing. Rearing cages for both predators were kept in a growth chamber at 24 °C, RH 75%, and L:D 16:8.
Greenhouse tests
Two types of tests were carried out: single predator and combined predator. All greenhouse tests were held at the Fermes Lufa Inc. © greenhouse in Montréal, Canada. Greenhouse conditions were 23 °C, RH 73%, and L:D 16:8. Single predator greenhouse tests were conducted from April to August 2013. The crop plants used were eggplant Solanum melongena L. (var. Jaylo), tomato S. lycopersicum L. (var. Rebelski), bell pepper Capsicum annuum L. (var. Red Knight) and cucumber Cucumis sativus L. (var. Camaro). All crop plants used were at the fruit bearing stage and had between 15 and 20 leaves. Three pest aphid species were used in these tests as well: the potato aphid Macrosiphum euphorbiae Thomas (Hemiptera: Aphididae), the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae) and the peach-potato aphid M. persicae. Various crop plant-pest aphid combinations were used: eggplant with M. euphorbiae, eggplant with M. persicae, tomato with M. euphorbiae, tomato with M. persicae, bell pepper with M. persicae and cucumber with A. gossypii.
In the M. euphorbiae and M. persicae treatments, aphid colonies were established at 20 aphids. For the A. gossypii treatment, aphid colonies were set to 30 aphids due to the smaller size of this species. All colonies were made up of N1 stage aphid nymphs. Aphid colonies were placed on a crop plant leaf and confined to a clip cage (diameter = 5 cm) (MacGillivray and Anderson 1957). A single predator larva having hatched less than 24 h prior was released into each clip cage at the start of tests. A predator free control treatment was also established. Aphid mortality was recorded seven days after predator releases.
Combined predator greenhouse tests took place throughout July and August 2013. Cucumber plants infested with A. gossypii were used for these tests. As with the single predator tests, an aphid colony was established in a clip cage set on the crop plant. Colonies were comprised of 60 N1 stage aphid nymphs. Four treatments were set: a single L. glyphinivora larva, a single A. aphidimyza larva, one L. glyphinivora larva with one A. aphidimyza larva combined and a predator free control treatment. The combined treatment had a single larva of each predator simultaneously. All predators were introduced in the clip cage less than 24 h after hatching. Clip cages were observed three days after predator introduction to establish aphid mortality. In order to assess the effect of combining both predators, expected combined voracity was calculated using the following model (Soluk 1993):
where C is the expected combined consumption, Np is initial prey density and P1 and P2 are the proportion of prey consumed by each predator respectively when alone. As with the laboratory tests, control treatments established in the greenhouse tests were used to account for natural aphid mortality using Eq. 1.
Statistical analysis
Mean consumption was compared between predator species for each treatment. A Shapiro–Wilk test was conducted on the data to test for normality before further analysis. Appropriate statistical tests were selected accordingly. With the laboratory test data, mean daily aphid voracity was compared between predators with a Wilcoxon test since data were shown to not follow a normal distribution. Total larval voracity was compared between predators using Welch’s two sample t test. A two-way ANOVA was used to compare mean daily predator voracity between species throughout larval development. Mean proportion of aphids consumed in the single predator greenhouse tests were compared between predators and treatments with a two-way ANOVA. A treatment consisted of a specific aphid pest-crop plant assemblage, which means that six treatments were observed in total. The proportion of aphids consumed was used since not all treatments had the same number of aphids at the start of each test. A Tukey HSD post-hoc test was conducted to identify significant differences between treatments. A one-way ANOVA was used for the combined predator test to compare mean 3-day voracity between L. glyphinivora alone, A. aphidimyza alone, both predators together and expected voracity of both predators together. The R statistical software version 3.3.1 (R Development Core Team 2016) was used to conduct statistical analyses with each data set.
Results
Laboratory experiments
There was no significant difference in the mean daily voracity between L. glyphinivora and A. aphidimyza with M. persicae on potato leaves (Wilcoxon test: U = 9101; P = 0.567). Mean daily voracity was 4.26 ± 0.26 (mean ± SE) aphids per day for L. glyphinivora and 4.43 ± 0.39 aphids per day for A. aphidimyza. The mean total amount of aphids consumed throughout larval development was significantly different with L. glyphinivora consuming significantly more aphids than A. aphidimyza (Welch t test: df = 31.621; t = − 4.759; P < 0.001). On average, a single L. glyphinivora larva killed a total of 38.13 ± 0.60 aphids while a single A. aphidimyza larva killed a total of 23.22 ± 1.29 aphids. Total larval consumption was 39% higher for L. glyphinivora.
Silver fly reached peak voracity on the 7th day of larval development, with an average of 7.23 aphids consumed (Fig. 1a). On the 3rd day of larval development, A. aphidimyza larvae reached a peak consumption of 9.34 aphids (Fig. 1a). The pattern of mean daily larval voracity varied significantly with larval development (Fig. 1a) (two-way ANOVA: F5,262 = 20.345; P < 0.001). Mean aphid consumption was not significantly different between L. glyphinivora and A. aphidimyza in the first 48 h after hatching (Fig. 1a). Mean daily voracity was significantly higher with A. aphidimyza on the 3rd and 4th days of larval development (Fig. 1a). Leucopis glyphinivora had a mean daily voracity significantly higher than A. aphidimyza on the 5th and 6th days after hatching (Fig. 1a). Leucopis glyphinivora larvae began to pupate on the 8th day of development. All larvae had reached pupation by the 12th day. For A. aphidimyza pupation happened between the 4th and 6th days of development.
Predator voracity dynamics for L. glyphinivora (n = 20) and A. aphidimyza (n = 19) under laboratory conditions shown as a mean daily voracity (± SE) and b cumulative population consumption throughout complete larval development. Larvae having reached pupation were progressively removed from the experimental population. Significant differences (P < 0.05) between species for each time since hatching are indicated by an asterisk (*). Day 1 = hatch day
The cumulative aphid consumption for 20 L. glyphinivora reached 763 N1 stage M. persicae over a span of ten days (Fig. 1b). A population of 19 A. aphidimyza consumed a cumulative total of 469 M. persicae over a period of five days (Fig. 1b). Cumulative prey consumption by L. glyphinivora surpassed what was observed with A. aphidimyza on the 6th day after hatching (Fig. 1b). This coincided with the end of larval development in A. aphidimyza and with peak larval consumption in L. glyphinivora (Fig. 1a). Both predator species started attacking aphids less than 24 h after hatching.
Greenhouse experiments
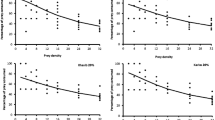
Leucopis glyphinivora consumed significantly more aphids than A. aphidimyza, regardless of treatment (two-way ANOVA: F1,286 = 18.310; P < 0.001). There was a significant difference in predator consumption between the various prey aphid-host plant combination treatments used (two-way ANOVA: F5,286 = 7.413; P < 0.001) with M. persicae on tomato and bell pepper having the greatest effect (Fig. 2). A higher proportion of aphids was consumed in the aforementioned treatments by both predators since the interaction between predator species and treatment was not significant (two-way ANOVA: F5,286 = 0.994; P = 0.422), (Fig. 2).
There was no significant difference in the 3-day larval voracity between L. glyphinivora and A. aphidimyza when they were released individually with a colony of 60 A. gossypii (Fig. 3). Voracity was significantly higher when both predators were released together and was not significantly different from the expected value (Fig. 3) (ANOVA: F3,105 = 23.38; P < 0.001).
Mean number of A. gossypii consumed (± SE) on cucumber by L. glyphinivora larvae (n = 26), A. aphidimyza larvae (n = 31) and both predators together (n = 26) in controlled greenhouse conditions. The dotted line indicates expected combined consumption. Different letters indicate significant differences between treatments (P < 0.05)
Discussion
Larval voracity for L. glyphinivora was observed in both laboratory and controlled greenhouse conditions. The ability of a predator to control its target prey is an essential quality for a biological control agent and the most basic measure of its efficiency (Hoddle and van Driesche 2009). This chamaemyiid species was compared with a similar commercial biological control agent, A. aphidimyza, in order to estimate its efficacy in suppressing pest aphids. The mean daily number of aphids killed does not differ among predator species. Leucopis glyphinivora does, however, consume a larger amount of aphids throughout its entire larval development, thus supporting our first hypothesis. This might be caused by its longer larval development (Canale et al. 2002; Rad et al. 2003; Barriault et al. 2018). Although this was not the case in our study, Latham and Mills (2010) observed an unidentified species of Leucopis spp. that had an increased daily consumption rate and spent more time feeding compared to A. aphidimyza when preying on Hyalopterus pruni Geoffroy (Hemiptera: Aphididae) in California plum orchards. Wild populations of L. americana in Florida, USA were reported to have a consumption rate of 17 A. spiraecola per day (Miller 1928), almost four times as high as what we observed, although aphid size and environmental conditions were not specified in this study. This still suggests that there is much variation in the predatory habits of different Leucopis spp. species.
Observation of a predator’s voracity dynamics (mean daily aphid voracity for the entire larval stage) reveals the predation pattern for both predators. Aphid consumption with L. glyphinivora increases steadily in the early stages of larval development to reach a sort of plateau, followed by a steady decline of predation before pupation occurs. With A. aphidimyza, consumption increases rapidly, peaking two days after hatching, and sharply declines afterwards. Differences in the rates of development for these two predators should lead to a different biological control application. The slow nature of L. glyphinivora is best suited for more mid-term biological control while the immediate effect of A. aphidimyza seems more optimized for very short term use. Biological control programs using both predators can therefore be tailored to the nature of pest outbreaks. Application of L. glyphinivora would be for a moderate and longer sustained pest control due to a longer larval development. Aphidoletes aphidimyza would be more suitable for immediate suppression of an intense aphid outbreak. Something else to consider is the timing of peak aphid consumption in both predators and how this would work with aphid population growth dynamics. More research is required in order to understand how these findings can be implemented into a biological control program.
Greenhouse experiments comparing aphid voracity between L. glyphinivora and A. aphidimyza demonstrated that L. glyphinivora always consumes a higher proportion of aphids, regardless of prey aphid species or host plant used. This means that L. glyphinivora is a rather polyphagous predator with the potential for being used on a wide variety of crops. Indeed, L. glyphinivora has been reported to attack nearly 80 different species of aphids and is found on over 70 different host plants (Tanasijtshuk 1986; Raspi and Ebejer 2008; Bokina 2009; Brewer and Noma 2010; Mustaţă et al. 2010; Rakhshani et al. 2010; Satar et al. 2015; Mehrparvar et al. 2016). Interestingly, the best results were observed with M. persicae on different crops. These were done on tomato and bell pepper, two structurally different plants. Tomato leaves are rather pubescent, presenting a high density of trichomes. These structures provide an important microhabitat component for a furtive predator such as A. aphidimyza by reducing the potential for intraguild predation by some active searching predators such as coccinelids (Lucas and Brodeur 1999; Griffin and Yeargan 2002). A slow moving vermiform larva like L. glyphinivora may also benefit from such protection. Unlike with tomato, bell pepper leaves are glabrous. One would not expect such a crop to be an optimal host plant choice. Surprisingly, the least effective treatments used eggplant as a target crop, one treatment involving M. persicae. Similarly to tomatoes, eggplants have many trichomes and would therefore be expected to make a suitable habitat for L. glyphinivora and A. aphidimyza. Also, M. persicae provided both the best and least effective results. More information concerning preferential host plants and prey aphids is necessary to make an effective biological control program involving L. glyphinivora. In our experiment, L. glyphinivora was confined to a controlled aphid colony within a clip cage. If L. glyphinivora were to be released in a greenhouse for a biological control program, larvae would be free to move around the host plant and even onto other nearby plants. Aphid colony density and age structure varies between colonies on nearby host plants. Rad et al. (2003) observed that L. glyphinivora larvae tend to leave small colonies composed of large aphids in search of a new colony, preferring a higher density of small aphids. Further research is needed to define the exact predatory habits of free roaming L. glyphinivora, as one would see in an actual biological control setting.
In the combined predator greenhouse test, the observed predatory effect of L. glyphinivora and A. aphidimyza together was not significantly different from the expected effect. This means there is no antagonistic effect, such as interference, and a biological control program involving the combined use of both predators should generate additive effects on the focal prey. Both L. glyphinivora and A. aphidimyza larvae were found alive at the end of the test period in 25 of the 26 replicates when used together. Only one replicate contained a single L. glyphinivora and no A. aphidimyza at the end of the 3-day trials indicating a low potential for intraguild predation. Since A. aphidimyza uses a furtive predation strategy and both predators are rather passive slow moving vermiform larvae, this may help them avoid intraguild predation with each other (Lucas et al. 1998; Fréchette et al. 2008). Aphidoletes aphidimyza is a known furtive predator (Lucas et al. 1998; Lucas and Brodeur 2001) and this strategy may also occur in L. glyphinivora. In fact, L. glyphinivora larvae are slow moving and do not seem to actively forage for aphids as one would typically see in an active searching predator. Aphids also did not seem to modify their behaviour when coming into contact with L. glyphinivora. Absence of significant defensive behaviour by prey when in the presence of a predator is a key characteristic of furtive predation (Lucas and Brodeur 2001). Wild L. glyphinivora sampled for this study were found within aphid colonies tended by ants, which are known to attack active searching aphid predators (Katayama and Suzuki 2003; Stewart-Jones et al. 2008). While collecting our field samples, ants were never seen attacking L. glyphinivora larvae. This is in line with furtive predation behaviour (McLean 1992; Sentis et al. 2012). Furtive predation has also been observed with another close species of silver fly, Leucopis annulipes (Fréchette et al. 2008). If intraguild predation between L. glyphinivora and A. aphidimyza is low, a biological control program using both predators simultaneously could be applied. This could also be extended to other, more active searching aphidophagous predators. Active searching predators, such as ladybirds (Coleoptera: Coccinellidae), can be seen as more efficient predators in that they consume a larger amount of aphids more rapidly (Dixon 1959; Marks 1977; Soares et al. 2001; Cabral et al. 2009). A drawback of these predators is that they are susceptible to engage in intraguild predation (Rosenheim et al. 1993; Hindayana et al. 2001; Völkl et al. 2007) and cannibalism (Osawa 1992; Burgio et al. 2002), which limits their potential at high predator densities. While also susceptible to intraguild predation, furtive predators living within the prey aphid colony benefit from a dilution effect (Lucas and Brodeur 2001) and a selfish herd effect (Dumont et al. 2015) which reduce the negative impact of intraguild predation. At low aphid densities, however, intraguild predation tends to occur more frequently (Polis et al. 1989; Lucas 2005). Predatory mites such as the intraguild predator Amblyseius swirskii Athias-Henriot and the hyperpredator Neoseiulus cucumeris Oudemans have also been shown to have negative effects on A. aphidimyza despite the midge’s furtive behaviour (Messelink et al. 2011, 2013). While unable to control large aphid populations, certain zoophytophagous mirids can prevent aphid outbreaks by establishing a population prior to aphid invasion through the use of supplementary food sources (Messelink et al. 2015). Even though these generalist predators engage in intraguild predation, their combined use with A. aphidimyza still results in a greater aphid control than A. aphidimyza alone (Messelink et al. 2013). Further research on the predatory behaviour of L. glyphinivora and on the interactions in a multi-predator environment is still necessary. This may lead to more efficient biological control programs involving the use of L. glyphinivora in conjunction with other beneficial insects against aphids.
Our study shows promising results of L. glyphinivora as an effective predator against aphid pests in greenhouses. Further research is, however, necessary before establishing its efficacy as a biological control agent. Searching ability and dispersal capacity are all important traits a good biological control agent would need for use against aphid pests in a greenhouse setting. Fertility, fecundity, oviposition preference and general reproductive behaviour are all key aspects of L. glyphinivora biology that remain to be explored and will provide essential information pertaining to mass-rearing conditions and multi-generation population dynamics in this species. All tests in our study were done under very controlled conditions. Actual predator release trials will provide useful information on the behaviour of a free roaming L. glyphinivora population in a greenhouse setting. These types of studies, combined with our current study and previous work on the life cycle and survival of L. glyphinivora (Barriault et al. 2018) will paint a global picture on how to effectively use L. glyphinivora to its full potential.
References
Alotaibi S (2008) Mass production and utilization of the predatory midge, Aphidoletes aphidimyza Rondani for controlling aphids. Global J Biotech Biochem 3:1–7
Barriault S, Soares AO, Gaimari SD, Lucas E (2018) Leucopis glyphinivora Tanasijtshuk (Diptera: Chamaemyiidae), a new aphidophagous biocontrol agent; development, survival and comparison with Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae). Bull Entomol Res. https://doi.org/10.1017/S0007485318000767
Begum M, Lees E, Ampt P, Mansfield S (2017) Development of Australian commercial producers of invertebrate biological control agents from 1971 to 2014. BioControl 62:525–533
Blackman RL, Eastop VF (2007) Taxonomic issues. In: van Emden HF, Harrington R (eds) Aphids as crop pests. CAB International, London, pp 1–29
Bokina IG (2009) The influence of vegetation on the abundance of cereal aphid entomophages in the forest-steppe of western Siberia. Entomol Rev 89:757–769
Brewer MJ, Elliott NC (2004) Biological control of cereal aphids in North America and mediating effects of host plant and habitat manipulations. Annu Rev Entomol 49:219–242
Brewer MJ, Noma T (2010) Habitat affinity of resident natural enemies of the invasive Aphis glycines (Hemiptera: Aphididae), on soybean, with comments on biological control. J Econ Entomol 103:583–596
Burgio G, Santi F, Maini S (2002) On intra-guild predation and cannibalism in Harmonia axyridis (Pallas) and Adalia bipunctata L. (Coleoptera: Coccinellidae). Biol Control 24:110–116
Cabral S, Soares AO, Garcia P (2009) Predation by Coccinella undecimpunctata L. (Coleoptera: Coccinellidae) on Myzus persicae Sulzer (Homoptera: Aphididae): effect of prey density. Biol Control 50:25–29
Canale A, Canova R, Raspi A (2002) Leucopis glyphinivora Tanasijtshuk (Diptera Chamaemyiidae): allevamento di laboratorio e prove preliminari dell’influenza di temperature costanti sulla durata dello sviluppo preimmaginale. Atti XIX Congresso nazionale italiano di Entomologia pp 529–533
Carroll DP, Hoyt SC (1984) Natural enemies and their effects on apple aphid, Aphis pomi DeGeer (Homoptera: Aphididae), colonies on young apple trees in central Washington. Environ Entomol 13:469–481
Colares F, Michaud JP, Bain CL, Torres JB (2015) Recruitment of aphidophagous arthropods to sorghum plants infested with Melanaphis sacchari and Schizaphis graminum (Hemiptera: Aphididae). Biol Control 90:16–24
Coppel HC, Mertins JW (1977) Biological insect pest suppression. Springer, New York, pp 234–255
Dixon AFG (1959) An experimental study of the searching behaviour of the predatory coccinellid beetle. J Anim Ecol 28:259–281
Dumont F, Lucas E, Brodeur J (2015) Do furtive predators benefit from a selfish herd effect by living within their prey colony? Behav Ecol Sociobiol 69:971–976
Fréchette B, Larouche F, Lucas É (2008) Leucopis annulipes larvae (Diptera: Chamaemyiidae) use a furtive predation strategy within aphid colonies. Eur J Entomol 105:399–403
Gaimari SD, Turner WJ (1996a) Larval feeding and development of Leucopis ninae Tanasijtshuk and two populations of Leucopis gaimarii Tanasijtshuk (Diptera: Chamaemyiidae) on Russian wheat aphid, Diuraphis noxia (Mordvilko) (Homoptera: Aphididae), in Washington. Proc Entomol Soc Wash 98:667–676
Gaimari SD, Turner WJ (1996b) Methods for rearing Aphidophagous Leucopis spp. (Diptera: Chamaemyiidae). J Kansas Entomol Soc 69:363–369
Gaimari SD, Turner WJ (1997) Behavioral observations on the adults and larvae of Leucopis ninai and L. gaimarii (Diptera: Chamaemyiidae), predators of Russian wheat aphid, Diuraphis noxia (Homoptera: Aphididae). J Kansas Entomol Soc 70:153–159
Griffin ML, Yeargan V (2002) Factors potentially affecting oviposition site selection by the lady beetle Coleomegilla maculata (Coleoptera: Coccinellidae). Environ Entomol 31:112–119
Hindayana D, Meyhofer R, Scholz D, Poehling HM (2001) Intraguild predation among the hoverfly Episyrphus balteatus de Geer (Diptera: Syrphidae) and other aphidophagous predators. Biol Control 20:236–246
Hoddle MS, van Driesche RG (2009) Biological control of insect pests. In: Resh VH, Cardé RT (eds) Encyclopedia of insects, 2nd edn. Elsevier, Burlington, pp 91–101
Humble LM (1994) Recovery of additional exotic predators of balsam wooly adelgid, Adelges picea (Ratzeburg) (Homoptera: Adelgidae), in British Columbia. Can Entomol 126:1101–1103
Kahanpää J (2014) Checklist of the fly families Chamaemyiidae and Lauxaniidae of Finland (Insecta, Diptera). ZooKeys 441:277–283
Kaiser ME, Noma T, Brewer MJ, Pike KS, Vockeroth JR, Gaimari SD (2007) Hymenopteran parasitoids and dipteran predators found using soybean aphid after its Midwestern United States invasion. Ann Entomol Soc Am 100:196–205
Katayama N, Suzuki N (2003) Bodyguard effects for aphids of Aphis craccivora Koch (Homoptera: Aphididae) as related to the activity of two ant species, Tetramorium caespitum Linnaeus (Hymenoptera: Formicidae) and Lasius niger L. (Hymenoptera: Formicidae). Appl Entol Zool 38:427–433
Kohler GR, Wallin KF, Ross DW (2016) Seasonal phenology and abundance of Leucopis argenticollis, Leucopis piniperda (Diptera: Chamaemyiidae), Laricobius nigrinus (Coleoptera: Deridontidae) and Adelges tsugae (Hemiptera: Adelgidae) in the Pacific Northwest USA. B Entomol Res 106:546–550
Latham DR, Mills NJ (2010) Quantifying aphid predation: the mealy plum aphid Hyalopterus pruni in California as a case study. J Appl Ecol 47:200–208
Le Goff GJ, Nicolas A, Al Mohamad R, Hance T (2016) Impact of humidity on the biological development of Aphidoletes aphidimyza (Diptera: Cecidomyiidae). J Econ Entomol 109:1482–1486
Lucas É (2005) Intraguild predation among aphidophagous predators. Eur J Entomol 102:351–364
Lucas É, Brodeur J (1999) Oviposition site selection by the predatory midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae). Environ Entomol 28:622–627
Lucas É, Brodeur J (2001) A Fox in sheep’s clothing: furtive predators benefit from the communal defense of their prey. Ecology 82:3246–3250
Lucas É, Coderre D, Brodeur J (1998) Intraguild predation among aphid predators: characterization and influence of extraguild prey density. Ecology 79:1084–1092
MacGillivray ME, Anderson GB (1957) Three useful insect cages. Can Entomol 89:43–46
Malais MH, Ravensberg WJ (2006) Les pucerons et leurs ennemis naturels. Connaître et reconnaître : la biologie des ravageurs des serres et de leurs ennemis naturels. Koppert B.V, Berkel en Rodenrijs, pp 131–172
Marks RJ (1977) Laboratory studies of plant searching behaviour by Coccinella septempunctata L. larvae. Bull Entomol Res 67:235–241
McAlpine JF (1978) A remarkable new species of Leucopis from western Canada (Diptera: Chamaemyiidae). Proc Entomol Soc Wash 79:14–18
McLean IFG (1992) Behaviour of larval and adult Leucopis (Diptera: Chamaemyiidae). Br J Ent Nat Hist 5:35–36
Mehrparvar M, Mansouri SM, Hatami B (2016) Some bioecological aspects of the rose aphid, Macrosiphum rosae (Hemiptera: Aphididae) and its natural enemies. Acta Univ Sap Agr Environ 8:74–88
Messelink GJ, Bloemhard CMJ, Cortes JA, Sabelis MW, Janssen A (2011) Hyperpredation by generalist predatory mites disrupts biological control of aphids by the aphidophagous gall midge Aphidoletes aphidimyza. Biol Control 57:246–252
Messelink GJ, Bloemhard CMJ, Sabelis MW, Janssen A (2013) Biological control of aphids in the presence of thrips and their enemies. BioControl 58:45–55
Messelink GJ, Bloemhard CMJ, Hoogerbrugge H, van Schelt J, Ingegno BL, Tavella L (2015) Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J Appl Entomol 139:333–341
Miller RL (1928) Biology and natural control of the green citrus aphid Aphis spiraecola Patch. Fla Entomol 12:49–56
Mohamed AH, Lester PJ, Holtzer TO (2000) Abundance and effects of predators and parasitoids on the Russian wheat aphid (Homoptera: Aphididae) under organic farming conditions in Colorado. Environ Entomol 29:360–368
Mustaţă G, Mustaţă M, Andriev S-O, Prelipcean C (2010) The complex of predators controlling the colonies of Aphis fabae Scop. (Homoptera, Aphididae) in eastern Romania. Analele Ştiinţifice ale Universităţii”Al. I. Cuza” Iaşi, s. Biologie animală 56:97–100
Natshuk EP, Bagachanova AK (2013) Silver-flies (Diptera, Chamaemyiidae) of Yakutia, Russia. Eur Entomol J 12:70–78
Noma T, Brewer MJ, Pike KS, Gaimari SD (2005) Hymenopteran parasitoids and dipteran predators of Diuraphis noxia in the west-central Great Plains of North-America: species records and geographic range. BioControl 50:11–97
Osawa N (1992) A life table of the ladybird beetle Harmonia axyridis Pallas (Coleoptera, Coccinellidae) in relation to the aphid abundance. Jpn J Ent 60:575–579
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Powell W, Pell JK (2007) Biological control. In: van Emden HF, Harrington R (eds) Aphids as crop pests. CAB International, Wallingford, pp 469–513
Preisser EL, Oten KLF, Hain FP (2014) Hemlock woolly adelgid in the eastern United States: what have we learned? Southeast Nat 13:1–15
R Core Team (2016) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. URL https://www.r-project.org/
Rad SG, Hatami B, Asadi G (2003) Biology of Leucopis glyphinivora Tanas. (Dip.: Chamaemyiidae) and its efficiency in biological control of Aphis fabae Scop. JWSS 6:195–207
Rakhshani H, Ebadi R, Hatami B, Rakhshani E, Gharali B (2010) A survey of alfalfa aphids and their natural enemies in Isfahan, Iran, and the effect of alfalfa strip-harvesting on their populations. J Entomol Soc Iran 30:13–28
Raspi A, Ebejer MJ (2008) New records of Diptera Chamaemyiidae from the Mediterranean and Oman with a description of a new species: Parochthiphila (Euestelia) argentiseta from Turkey and a redescription of Parochthiphila (Parochthiphila) inconstans (Becker). Entomol Fennica 19:55–64
Rosenheim JA, Wilhoit LR, Armer CA (1993) Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 96:439–449
Satar S, Raspi A, Özdemir I, Tusun A, Karacaoğlu M, Beneli G (2015) Seasonal habits of predation and prey range in aphidophagous silver flies (Diptera chamaemyiidae), an overlooked family of biological control agents. Bull Insectol 68:173–180
Sentis A, Lucas É, Vickery WL (2012) Prey abundance, intraguild predators, ants and the optimal egg-laying strategy of a furtive predator. J Insect Behav 25:529–542
Sloggett JJ (2005) Are we studying too few taxa? Insights from aphidophagous ladybird beetles (Coleoptera: Coccinellidae). Eur J Entomol 102:391–398
Soares AO, Coderre D, Schanderl H (2001) Fitness of two phenotypes of Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 98:287–293
Soares AO, Coderre D, Schanderl H (2003) Effect of temperature and intraspecific allometry on predation by two phenotypes of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Environ Entomol 35:939–944
Soluk DA (1993) Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology 74:219–225
Sorensen JT (2009) Aphids. In: Resh VH, Cardé RT (eds) Encyclopedia of insects, 2nd edn. Elsevier, Burlington, pp 27–31
Stevenson AB (1967) Leucopis simplex (Diptera: Chamaemyiidae) and other species occurring in galls of Phylloxera vitifoliae (Homoptera: Phylloxeridae) in Ontario. Can Entomol 99:815–820
Stewart-Jones A, Pope TW, Fitzgerald JD, Poppy GM (2008) The effect of ant attendance on the success of rosy apple aphid populations, natural enemy abundance and apple damage in orchards. Agr Forest Entomol 10:37–43
Stiling P, Cornelissen T (2005) What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol Control 34:236–246
Tanasijtshuk VN (1986) Leucopis (Leucopis) glyphinivora. Fauna of the USSR. Nauka Publishers, St. Petersburg, pp 289–295
van Lenteren JC, Woets J (1988) Biological and integrated pest control in greenhouses. Ann Rev Entomol 33:239–269
van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl 63:39–59
van Schelt J, Mulder S (2000) Improved methods of testing and release of Aphidoletes aphidimyza (Diptera: Cecidomyiidae) for aphid control in glasshouses. Eur J Entomol 97:511–515
Völkl W, Mackauer M, Pell JK, Brodeur J (2007) Predators, parasitoids and pathogens. In: van Emden HF, Harrington R (eds) Aphids as crop pests. CAB International, London, pp 187–233
Wallace MS, Hain FP (2000) Field surveys and evaluation of native and established predators of the hemlock woolly adelgid (Homoptera: Adelgidae) in the southeastern United States. Environ Entomol 29:638–644
Wilbert H (1973) Zur Suchfähigkeit der Eilarven von Aphidoletes aphidimyza (Diptera: Cecidomyiidae). Entomol Exp Appl 16:514–524
Yukawa J, Abe J, Mizota K (2008) Improvement in the practical use of an aphidophagous gall midge, Aphidoletes aphidimyza (Diptera: Cecidomyiidae), in greenhouses. In: Mason PG, Gillespie DR, Vincent C (eds) Proceedings of the third international symposium on biological control of aphids. Christchurch, pp 77–87
Zhou H, Yu Y, Tan X, Chen A, Feng J (2014) Biological control of insect pests in apple orchards in China. Biol Control 68:47–56
Acknowledgements
Our deepest thanks go out to Stephen D. Gaimari, California Department of Food and Agriculture, for identification and rearing advice with Leucopis glyphinivora. We would like to extend our gratitude to Jill Vandermeerschen for her help with statistical analysis, Simon Chaussé and Tina Lévesque for technical assistance and the team at LUFA, particularly Javier Campos and Lauren Rathmell. We would also like to thank the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Ministère de l’Agriculture des Pêcheries et de l’Alimentation du Québec (MAPAQ), through their Programme de Soutien à l’Innovation Horticole (PSIH), for funding this research project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
Rights and permissions
About this article
Cite this article
Barriault, S., Fournier, M., Soares, A.O. et al. Leucopis glyphinivora, a potential aphidophagous biocontrol agent? Predation and comparison with the commercial agent Aphidoletes aphidimyza. BioControl 64, 21–31 (2019). https://doi.org/10.1007/s10526-018-09909-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-018-09909-x