Abstract
A persistent problem in weed biocontrol is how to reliably predict whether a plant that supports development in laboratory host-specificity testing will be utilized in field conditions, and this is undoubtedly preventing releases of safe and effective agents. Moreover, the potential for unanticipated undesirable indirect effects of weed biocontrol on ecological networks has raised concerns by policy-makers and the general public. The key to minimizing risks of non-target impacts is prioritizing candidate agents that are both host-specific and effective, such that the number of agents required to bring the weed under control is minimized. As a consequence both the weed and its biocontrol agents become minor components of the local biota. Here we review recent attempts in New Zealand to improve the predictive ability of host-range testing, to avoid potentially safe and effective agents being rejected. Research in New Zealand aimed at predicting whether an agent is likely to experience enemy-release (i.e. reduced parasitism and predation) could assist agent prioritization, potentially making biocontrol both environmentally safer and more effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environmental safety of weed biocontrol has been debated for several decades (e.g. Louda et al. 1997; Strong 1997; Suckling and Sforza 2014). A problem that troubles biocontrol practitioners to this day is reliably predicting whether a plant that supports development in laboratory host-specificity testing is likely to be utilized in field conditions (Louda et al. 2003). A risk-averse approach to regulating the introduction of new organisms would be to reject all candidate biocontrol agents that can complete development on any valued non-target plants. Inflexibly applying this approach, however, is likely to result in environmentally safe and potentially successful agents being rejected, because the ‘field’ or ‘ecological’ host-range of an agent is usually only a subset of its fundamental host-range. This is because, when given no-choice in a laboratory test, candidate agents may feed on plants that they would never attack in field conditions (van Klinken 2000). Authorities have become more risk-averse and gaining approval for the introduction of a biocontrol agent into at least some jurisdictions has been subject to delays, or is becoming increasingly difficult (Becker et al. 2013; Hill et al. 2013; Hinz et al. 2014; Klein et al. 2011; Sheppard et al. 2003). It has also been noted that a number of safe and effective biocontrol agents that were released in the past would not be approved for release under current regulatory regimes (Groenteman et al. 2011; Hinz et al. 2014). Examples such as the denial of a release permit for Ceratapion basicorne (Illiger) (Coleoptera: Brentidae) in the USA, despite open field specificity tests which demonstrated that C. basicorne is highly specific to the target weed [Centaurea solstitialis, Asteraceae: Cardueae; (Cristofaro et al. 2013)], indicates that risk averse regulatory authorities are preventing the release of safe and potentially effective agents in some jurisdictions.
Concerns have also been raised regarding the potential indirect impacts of weed biocontrol, for example via interactions in food webs (e.g. Carvalheiro et al. 2008; Pearson and Callaway 2005; Willis and Memmott 2005). Although rarely demonstrated experimentally, there are examples where natural enemies of introduced biocontrol agents have interfered with biocontrol efficacy and many other cases where biotic interference has been suspected or blamed for biocontrol failure (Goeden and Louda 1976). Selecting agents that are likely to experience enemy-release should improve the prospective success of a biocontrol program while also minimizing the potential for indirect impacts (e.g. Paynter et al. 2010). Improved predictive ability regarding direct and indirect non-target impacts has the potential to enhance both the safety and the success of weed biocontrol. Here we review selected biological control literature with a focus on New Zealand (henceforth NZ) research to highlight developments aimed at improving host-range testing protocols and the ability to predict biotic interference in weed biocontrol programs.
Direct impacts on non-target hosts
A recent systematic global review of non-target attack in weed biocontrol programs indicated that significant negative impacts on non-target species occur rarely and the few cases documented are the result of historic releases that would not be permitted under current regulatory regimes (Suckling and Sforza 2014). This indicates that current host-range testing protocols are assessing risk adequately. Although it could still be argued that the number of detected cases may be a fraction of those that have occurred (Simberloff and Stiling 1996) because systematically collected national survey data from countries that practice weed biocontrol is largely lacking. For example, Suckling and Sforza’s (2014) review indicated that, world-wide only 43 of 512 (8%) arthropod agents released attack non-target plants. In NZ, where systematic surveys have been conducted, eight out of 33 (24%) arthropod agents surveyed are known to attack non-target plants (Paynter et al. 2004) (Table 1). This difference is likely due to greater sampling effort, rather than the NZ flora being uniquely susceptible to non-target attack. According to Suckling and Sforza’s (2014) damage scale, none of the NZ examples have affected crops and the five (15%) agents that attack native plant species only cause minimal or minor damage.
Although it is possible that Suckling and Sforza (2014) may have underestimated the number of cases of non-target attack, it seems unlikely that there could be any examples of hitherto undetected attack causing major impacts on non-target plants, given the large amount of research on non-target attack in the last two decades. Moreover, non-target survey work in NZ indicated several ways by which the environmental safety of weed biocontrol could be improved. For example, unexpected non-target attack from the gorse pod moth Cydia succedana (Denis and Schiffermüller) (Lepidoptera: Tortricidae) indicated that (1) while choice tests generally represent a more natural scenario than no-choice tests, there are situations in which choice tests can be unreliable, and (2) only a tested population of an agent should be released. Cydia succedana has two generations in southern England coinciding with the main flowering periods of two gorse species: Ulex europaeus L. (Fabales: Fabaceae) in spring and Ulex minor Roth (Fabales: Fabaceae) in summer. In NZ, U. minor is an uncommon weed and it was hoped that emergence of the second generation of C. succedana would coincide with the autumn flowering period of U. europaeus (Paynter et al. 2008a). Instead, C. succedana emergence has remained poorly synchronized with autumnal flowering of U. europaeus, and C. succedana attacks other non-native summer flowering Fabaceae in NZ, such as Lotus corniculatus L. (Fabales: Fabaceae) (Paynter et al. 2008a). Both choice and no-choice tests were done prior to the release of C. succedana in NZ and no-choice starvation tests indicated that C. succedana sourced from England should be highly host-specific. However, moths collected in Portugal were also released in NZ. Retrospective testing indicated that C. succedana sourced from Portugal have a broader host-range than English moths, explaining the non-target attack. This study also indicated that choice tests underestimated the risk of non-target attack because Portuguese moths exhibited a very strong preference for U. europaeus over L. corniculatus in choice oviposition tests, but there was no significant difference in the number of eggs laid on U. europaeus and L. corniculatus in no-choice tests. In this case no-choice tests were a better predictor of the field host-range of C. succedana due to asynchrony between the agent and the target weed in the field (Paynter et al. 2008a).
Predicting the risk of non-target attack from host-specificity tests
Paynter et al. (2015b) noted that the fundamental host-range for development can usually be reliably determined by no-choice laboratory tests, but uncertainties remain regarding the ability to predict the field (also termed realized or ecological) host-range of an agent. As the field host-range is commonly narrower than the fundamental host-range, it follows that no-choice tests may overestimate risks (van Klinken 2000). Developing means to reliably predict the field host-range may, therefore, enable practitioners to differentiate between safe and risky species and avoid rejecting safe agents.
Open field tests conducted in the native range of the candidate agent are generally considered the most natural form of host-range testing (e.g. Marohasy 1998). But they are logistically difficult and interpretation can be problematic because the presence of the primary host may cause females to be unresponsive to lower-ranked potential hosts (Paynter et al. 2015b).
Paynter et al. (2015b) tested the hypothesis that quantifying the relative performance of candidate weed biocontrol agents on test and target plants during laboratory host-range testing can predict the probability of test plants being attacked in the field. They concluded that this approach can help predict risk of non-target attack because there was a clear threshold score above which non-target attack became likely and below which non-target attack did not occur. This ‘threshold score’ approach has been adopted by NZ regulatory authorities. The release of a leaf beetle Chrysolina abchasica (Weise) (Coleoptera: Chrysomelidae) for the biological control of tutsan, Hypericum androsaemum L. (Malpighiales: Hypericaceae), was recently approved despite native NZ Hypericum species being within its fundamental host-range, on the basis that the combined risk scores for native Hypericum species were well below the predicted threshold for non-target attack. It was not possible to import and grow native NZ Hypericum species for use in field tests in Georgia, where C. abchasica is native (Hugh Gourlay, pers. comm.), so approval to release C. abchasica in NZ would not have been possible without the development of the Paynter et al. (2015b) approach to laboratory host-range testing. Chrysolina abchasica will be closely monitored to test the prediction that native Hypericum species will not become permanent field hosts.
Host-range testing could potentially be further refined by developing a more rigorous basis for determining the amount of replication required to assess risk. Withers et al. (2013) used a range of statistical tools to investigate the risk that the buddleia leaf weevil Cleopus japonicus (Wingelmüller) (Coleoptera: Curculionidae) might attack native Hebe speciosa (A. Cunn.) Andersen (Lamiales: Plantaginaceae) plants. The power analyses performed by Withers et al. (2013) focused on trying to detect relatively rare events. For example, they calculated that ca. 300 replicates would be required to have a 80% chance of detecting survival if only 2% of C. japonicus larvae could develop to pupation on H. speciosa. This is about an order of magnitude higher than the amount of replication per test plant species currently used in NZ. However, the analysis by Paynter et al. (2015b) indicated that the lowest relative survival rate above which non-target attack was reported was 0.34 (i.e. percentage survival on the non-target plant was 34% of the survival level on the target weed; Fig. 1a Paynter et al. 2015b). The level of replication required to detect a risky candidate agent is therefore likely to be significantly lower than suggested by Withers et al. (2013), but will vary depending on the survival rate on the target plant. For example, assuming a threshold relative survival rate of 0.34, it will be necessary to detect a survival rate of ca. 27% on a test plant if the survival rate on the target weed is 80%. Intuitively it seems likely that current levels of replication are adequate, given that examples of unexpected non-target attack are rare (Suckling and Sforza 2014). However, greater replication may be desirable to ensure reliable estimates of relative performance are generated when predicting whether a fundamental host plant is likely to be utilized in the field.
As noted by Louda et al. (2003), ecological studies can also refine risk, although there are relatively few examples worldwide of potentially risky agents being approved for release on the basis of ecological studies. The most common cases are where climate matching has indicated potential non-target hosts occur in regions with climates that are unsuitable for the candidate agent (e.g. Hasan and Delfosse 1995).
There is a risk that biocontrol agents could rapidly evolve altered use of fundamental hosts through quantitative genetic changes, although evidence is lacking (van Klinken and Edwards 2002). This hypothetical risk has resulted in at least one candidate agent being rejected. Manrique et al. (2014) demonstrated that rearing Paectes longiformis Pogue (Lepidoptera: Noctuidae), a candidate agent for Schinus terebinthifolia Raddi (Sapindales: Anacardiaceae) in Florida, exclusively on the native Rhus aromatica Aiton (Sapindales: Anacardiaceae) for multiple generations resulted in enhanced performance on the potential non-target plant. It is hard to predict whether there would be a selection pressure for P. longiformis to perform better on Rhus aromatica had P. longiformis been released. For example, S. terebinthifolia is an abundant weed and it may be unlikely that populations of P. longiformis could persist exclusively on Rhus aromatica for multiple generations. Another advantage of using quantitative host-range testing data is that evidence for rapid evolution could be obtained retrospectively by comparing the relative performance of agents collected from the non-target hosts with the original host-testing data or with agents collected from the original source population (Paynter et al. 2015b).
Parasitoids, predators and diseases
Parasitism
In NZ, ten out of 28 (35%) arthropod weed biocontrol agents surveyed were found to be hosts to 19 parasitoid species (Paynter et al. 2010). The majority of parasitoid species belong to the Hymenoptera, which are poorly described in NZ (Berry 2007), making predictions regarding the risk of future agents being hosts to parasitoids problematic (Paynter et al. 2010). Nevertheless, a method for predicting the likelihood of parasitism was developed by Paynter et al. (2010), who found that parasitoid species richness on weed biocontrol agents in NZ was positively correlated to richness in the area of origin. However, only agents with native ‘ecological analogues’ (i.e. a native NZ insect that is taxonomically related to the agent, and has a similar lifestyle niche and feeds on the target weed) contributed significantly to this pattern. This ‘native analogue’ approach has subsequently been used to prioritize candidate biocontrol agents in NZ. For example, a potentially host-specific moth, Lobesia coccophaga Falkovitch (Lepidoptera: Tortricidae), was recently given low priority for biocontrol of Japanese honeysuckle Lonicera japonica (Thunb.) (Dipsacales: Caprifoliaceae), due to presence of native analogues (native tortricid moths) feeding on L. japonica in NZ that are likely to be hosts of parasitoids that are capable of attacking and potentially reducing the impact of L. coccophaga (Paynter et al. 2017).
Not all parasitized agents listed by Paynter et al. (2010) possess a native analogue. For example, some agents are parasitized by exotic parasitoids that have no known native hosts. Nevertheless, the analogue approach could be expanded as there is evidence that the presence of a parasitized ‘introduced analogue’ (defined here as an introduced herbivore that is taxonomically related to the subsequent agent, and has a similar lifestyle niche and feeds on the target weed or a congeneric plant) indicates the risk of any subsequent introductions being parasitized. For example Procecidochares alani introduced into NZ in 2001 to control Ageratina riparia (Regel) R.M. King and H. Rob (Asterales: Asteraceae), is parasitized by Megastigmus sp. (Hymenoptera: Torymidae), which was already known to attack a congeneric agent P. utilis, which was introduced into NZ in 1958 to control A. adenophora (Spreng.) R.M. King and H. Rob. (Asterales: Asteraceae) (Paynter et al. 2010).
Preliminary investigation reveals that releasing agents that are analogues of already-released parasitized agents appears to be a relatively frequent occurrence. McFadyen and Jacob (2004) listed 54 international records of parasitism of weed biocontrol agents which contains eight (~15%) examples of parasitized agents which were released after a parasitized ‘introduced analogue’.
Predation
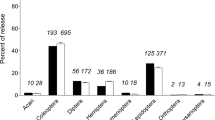
Predation is much harder to detect than parasitism as parasitoids can be reared, while predators generally have to be ‘caught in the act’ or their prey items detected using molecular techniques (e.g. Chen et al. 2000). Therefore, predation is likely to be under-recorded. In NZ, there are mostly anecdotal observations of predation on only 17 out of 39 (~43%) arthropod agents confirmed to have established by December 2014 (Supplementary information, Table S1).
The native analogue approach appears less useful for predicting predation, perhaps because most predators are generalists (Petráková et al. 2015). Nevertheless, there are two examples in NZ where introduced biocontrol agents are attacked by oligophagous predators that prey on analogous native hosts: the gorse spider mite T. lintearius is attacked by a native coccinellid beetle Stethorus bifidus (Kapur) (Coleoptera: Coccinellidae), which naturally attacks native Tetranychus species, feeding on native Carmichaelia species (Fabales: Fabaceae); the Scotch broom gall mite Aceria genistae Nalepa (Trobidiformes: Eriophyidae) is attacked by predatory mites that also occur in the galls of Aceria carmichaeliae Lamb (Trobidiformes: Eriophyidae) found on native brooms (Carmichaelia species), with the main predators being Typhlodromus caudiglan Schuster (Mesostigmata: Phytoseiidae) and Zetzellia maori González-Rodríguez (Trobidiformes: Stigmaeidae) (Mala 2013). Furthermore, the predators associated with T. lintearius and A. genisteae in NZ are closely-related to predators which feed on these species in their native ranges indicating that native range surveys of natural enemies could help predict the food webs associated with species that possess analogous native species: in England, the main predator of T. lintearius was reported to be Stethorus punctilium Weise (Coleoptera: Coccinellidae) (Kirby 2006) and, in France, a predatory phytoseiid mite Typhlodromus pyri Scheuten (Mesostigmata: Phytoseiidae) was commonly found preying on A. genistae (Q. Paynter, pers. obs.). Therefore, with hindsight, predation of T. lintearius and A. genistae in NZ was predictable and it could be argued that the potential impact of these predators was predictable as well: predation is considered the main reason why outbreaks of T. lintearius fail to persist in NZ (Peterson et al. 2000) yet extremely damaging outbreaks of A. genistae have endured in NZ since it was introduced in 2008, perhaps because A. genistae mites are smaller than their phytoseiid predators so that there is a refuge from predation within the highly convoluted gall-like leaf curls (Z-Q Zhang, pers. comm.). In the native range, the status of both agents is similar to the situation in NZ: Tetranychus lintearius is considered to be uncommon in Europe (Kirby 2006; van Eyndhoven 1967), whereas A. genistae is common in the Cévennes region of France, where it appears to be one of the few natural enemies capable of killing Cytisus scoparius (L.) Link (Fabales: Fabaceae) plants (Hosking 1990).
Larvae of endophytic feeders appear to be less susceptible to predation than exposed feeders. Arthropod weed biocontrol agents (listed in Supplementary information, Table S1) were classified as having endophytic (e.g. seed, or stem-borers, gall-formers, leaf-miners) or externally-feeding juvenile stages (including partially concealed leaf-tiers). Predation has been reported for only 5/21 (~24%) of endophytic agents released in NZ. By contrast, 12/18 (~67%) of externally-feeding agents have been reported to be subject to predation and the proportion of agents subject to predation varied significantly according to concealment (Supplementary information, Table S1; P = 0.011, Fischer’s exact test). Moreover, exposed feeders can be more prone to predation when honeydew-producing Hemiptera are present and attract predatory ants onto host plants (Paynter et al. 2012).
Impact of predation and parasitism
Parasitism was associated with biocontrol failure in NZ, with 8 of 15 (53%) unsuccessful agents being parasitized, while only one of nine successful agents was parasitized (Paynter et al. 2010). Five agent species were found to be heavily parasitized to an extent that is likely to significantly influence their efficacy: the old man’s beard leafminer, Phytomyza vitalbae Kaltenbach (Diptera: Agromyzidae), where combined parasitism and predation (attributed to eulophid parasitoids, which eat more prey than they parasitize) rates averaged at least 58%; Tyria jacobaeae L. (Lepidoptera: Erebidae) (up to 78% parasitism), Zeuxidiplosis giardi (Kieffer) (Diptera: Cecidomyiidae) (41% parasitism); Procecidochares alani Steyskaland (Diptera: Tephritidae) (68% parasitism); and P. utilis Stone (Diptera: Tephritidae) (up to 100% parasitism) (Paynter et al. 2010).
Predation is considered to be reducing the efficacy of four agents: Urophora solstitialis L. (Diptera: Tephritidae), Tortrix s.l. sp. chrysanthemoides, Urophora cardui (L.) (Diptera: Tephritidae) and T. lintearius (Supplementary information, Table S1). Overall, at least nine agent species are therefore currently impacted by parasitism and/or predation to an extent where it is believed to significantly reduce their impacts on the target weed. This is a significant proportion of agents established in NZ and a major cause of agent failure: of the 39 arthropod weed biocontrol agent species confirmed to be established in NZ by 2014, 16 species are considered to contribute to complete or partial control of their target weeds. The impacts of four species are uncertain and the remaining 19 species are considered to be unsuccessful (Supplementary information, Table S1). Moreover, future surveys may demonstrate that predation has reduced the efficacy of other unsuccessful agents in NZ, such as Monophadnus spinolae (Klug) (Hymenoptera: Tenthredinidae), where the cause of failure is currently unknown (Supplementary information, Table S1). Therefore, prioritizing the release of agents that are likely to find enemy-free space (e.g. Fowler et al. 2010) could have a tangible effect on the success rate of weed biocontrol introductions, as well as reducing the risk of unwanted indirect impacts on native food webs.
Disease
Disease is a factor that has affected rearing programs in NZ (Paynter et al. 2015a) although there is little evidence to suggest that it negatively affects weed biocontrol agents in the field in NZ. However, there is increasing awareness that the effects of some diseases may be more subtle compared to the epizootics discussed by Goeden and Louda (1976). For example, a gregarine (sporozoan protozoan) gut parasite of the leaf beetle, Neolema ogloblini (Monrós) (Coleoptera: Chrysomelidae), discovered while still in containment, was not lethal but appeared to reduce beetle fecundity, longevity and general vigor, potentially compromising its biological control efficacy (Smith et al. 2013). This pathogen was eliminated from the culture prior to the release of N. ogloblini. Since 1984, permission to release agents from containment in NZ has been made conditional on testing agent cultures for freedom of pathogenic organisms using light microscopy techniques (Paynter et al. 2016). Surveys are currently underway to investigate whether disease is present and impacting on weed biocontrol agent populations in the field.
Discussion
Host-specificity tests and predicting the risk of non-target attack
Paynter et al.’s (2015b) quantitative relative risk approach could provide regulatory authorities with a defendable decision-making mechanism and help ensure that correct decisions are made. Further analyses using datasets from other countries are desirable. For example, by 2010, 49 weed biocontrol agent species had been released in NZ (Fowler et al. 2010) of which only 23 species met the criteria for inclusion in Paynter et al.’s (2015b) analyses (i.e. during host-range testing they developed to adult on potential non-target plant species present in NZ and directed surveys had been conducted to investigate whether these fundamental host plants are also attacked in the field in NZ). By comparison, 94, 242 and 270 weed biocontrol agent species had been released in mainland USA, Australia and South Africa by 2004, 2012 and 2011 respectively (Coombs et al. 2004; Julien et al. 2012; Klein 2011). Much larger data sets from these countries could potentially be generated to (1) rigorously test the reliability of Paynter et al.’s (2015b) approach and (2) refine parameter estimates of the approach, thereby improving confidence when using the approach to predict the risk of non-target attack. Directed surveys to formally assess non-target attack in these countries would be required, before such analyses should proceed, due to the possibility that non-target attack has been under-reported in these countries.
The potential to expand this approach to predict the field host-range of plant pathogen weed biocontrol agents could also be explored. Results of plant pathogen specificity testing are commonly presented as categorical/qualitative damage scores, similar to those first proposed by Mortensen (1984). Globally, pathogens released for the biological control of weeds have apparently only ever caused damage to six non-target species outdoors, yet those same agents damaged 107 non-target species in pre-release tests conducted indoors (Barton 2012). We therefore hypothesise that a relative risk method for determining the degree of resistance/susceptibility of test plants more quantitatively (for example, by quantifying the number of telia/teliospores on test and host plants) could help predict the field host-range of pathogen agents more reliably. As noted by Barton (2012), directed surveys for non-target damage from pathogens released as classical biological control agents are rare, reiterating the need for directed surveys as non-target attack may be underestimated.
Parasitoids, predators and diseases
Agents at the greatest risk of being attacked by parasitoids can be identified by the presence of analogues. Moreover, although specialist predators are rare, the risk they pose to candidate biocontrol agents is predictable, although the definition of what constitutes a native analogue may need to be broadened from that outlined by Paynter et al. (2010). Native analogues of the gorse spider mite and broom gall mite feed on plants in the same family as the target weeds, rather than the target weed itself.
Few studies have investigated food webs associated with weed biocontrol agents in both native and exotic habitats (Veldtman et al. 2011). The potential utility of using the analogue approach to predict predation might become more apparent if more food web association studies were conducted.
The analogue approach does not predict the level of parasitism or predation that an agent might be subjected to. Applying the approach uncritically might, therefore, result in a potentially effective agent being given an inappropriately low priority for introduction. An excellent suggestion for further refining this methodology is to determine the impact of parasitism on the analogue species, assuming that the candidate agent is likely to suffer a similar level of parasitism should it be released (Greg Wheeler, pers. comm.). Similarly, it may prove useful to determine the level or impact of parasitism or predation in the native range, for example using the techniques described by van Driesche et al. (1991) and Luck et al. (1988), as a potential predictor of the impact of natural enemies in the introduced range for species which possess an analogue.
It may also be informative if the utility of the analogue approach is investigated in other countries. Due to its isolated island status, some taxonomic groups are absent or of low diversity in NZ (Wallis and Trewick 2009). We hypothesize that introduced weed biocontrol agents introduced into continental areas, such as Australia, South Africa and the USA, may be more likely to encounter an analogous native species than agents released in NZ. Comparing agent performance in regions where analogues are present and regions where analogues are absent may be informative.
Finally, more detailed case studies and surveys are needed to investigate whether disease is an unrecognized cause of biocontrol failure and cause of indirect impacts. Not all jurisdictions make pathogen screening a condition for approval to release an agent from containment, so it may be informative to compare and contrast the incidence of disease in weed biocontrol agents in NZ and other countries where pathogen screening is not routinely performed (e.g. Australia; A.W. Sheppard, pers. comm.).
References
Barton J (2012) Predictability of pathogen host range in classical biological control of weeds: an update. BioControl 57:289–305
Becker RL, Katovich EJS, Hinz HL, Gerber E, Ragsdale DW, Venette RC, McDougall DN, Reardon R, Riper LCv, Skinner LC, Landis DA (2013) The garlic mustard (Alliaria petiolata) case, what makes a good biological control target: the intersection of science, perspectives, policy and regulation. In: Proceedings of the XIII international symposium on biological control of weeds, Waikoloa, Hawaii, USA, 11-16 September, 2011, 2013, pp 332–339
Berry JA (2007) Alysiinae (Insecta: Hymenoptera: Braconidae). Fauna N Z 58:95
Carvalheiro LG, Buckley YM, Ventim R, Fowler SV, Memmott J (2008) Apparent competition can compromise the safety of highly specific biocontrol agents. Ecol Lett 11:690–700
Chen Y, Giles K, Payton M, Greenstone M (2000) Identifying key cereal aphid predators by molecular gut analysis. Mol Ecol 9:1887–1898
Coombs EM, Clark JK, Piper GL, Cofrancesco AF Jr (eds) (2004) Biological control of invasive plants in the United States. Oregon State University Press, Corvallis
Cristofaro M, De Biase A, Smith L (2013) Field release of a prospective biological control agent of weeds, Ceratapion basicorne, to evaluate potential risk to a nontarget crop. Biol Control 64:305–314
ERMA (2007) Environmental risk management authority decision NOR06005. http://www.epa.govt.nz/search-databases/HSNO%20Application%20Register%20Documents/NOR06005.doc. Accessed 11 May 2017
Fowler SV, Gourlay AH, Hill RH, Withers T (2004) Safety in New Zealand weed biocontrol: a retrospective analysis of host-specificity testing and the predictability of impacts on non-target plants. In: Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK (eds) Proceedings of the XI international symposium on biological control of weeds. CSIRO Entomology, Canberra, Australia, pp 265–270
Fowler SV, Paynter Q, Hayes L, Dodd S, Groenteman R (2010) Biocontrol of weeds in New Zealand: an overview of nearly 85 years. In: 17th Australasian weeds conference. New frontiers in New Zealand: together we can beat the weeds. Christchurch, New Zealand, 26–30 September, 2010, pp 211–214
Goeden RD, Louda SM (1976) Biotic interference with insects imported for weed control. Annu Rev Entomol 21:325–342
Groenteman R, Fowler SV, Sullivan JJ (2011) St. John’s wort beetles would not have been introduced to New Zealand now: a retrospective host range test of New Zealand’s most successful weed biocontrol agents. Biol Control 57:50–58
Hasan S, Delfosse ES (1995) Susceptibility of the Australian native, Heliotropium crispatum, to the rust fungus Uromyces heliotropii introduced to control common heliotrope, Heliotropium europaeum. Biocontrol Sci Technol 5:165–174
Heenan PB, de Lange PJ (2004) Alternanthera denticulata (amaranthaceae) in New Zealand: a new addition to the indigenous or naturalised flora? N Z J Bot 42:739–745
Hill R, Campbell D, Hayes L, Corin S, Fowler S (2013) Why the New Zealand regulatory system for introducing new biological control agents works. In: Wu Y, Johnson T, Sing S, Raghu S, Wheeler G, Pratt P, Warner K, Center T, Goolsby J, Reardon R (eds) Proceedings of the XIII international symposium on biological control of weeds. Morgantown, West Virginia, USA: USDA Forest Service Forest Health Technology Enterprise Team, pp 75–83
Hinz HL, Gassmann A, Bourchier RS, Schwarzlander M (2014) Successes we may not have had: a retrospective comparison of predicted versus realized host range of weed biological control agents in North America. Invasive Plant Sci Manag 7:564–579
Hosking J (1990) The feasibility of biological control of Cytisus scoparius (L.) Link. Report on overseas study tour, June-September 1990. Unpublished report, New South Wales Department of Agriculture and Fisheries, Tamworth, Australia
Julien MH, McFadyen R, Cullen J (2012) Biological control of weeds in Australia. CSIRO Publishing, Melbourne
Kirby M (2006) Population fluctuations in gorse mites. Trans Suffolk Nat Soc 42:55–61
Klein H (2011) A catalogue of the insects, mites and pathogens that have been used or rejected, or are under consideration, for the biological control of invasive alien plants in South Africa. Afr Entomol 19:515–549
Klein H, Hill M, Zachariades C, Zimmermann H (2011) Regulation and risk assessment for importations and releases of biological control agents against invasive alien plants in South Africa. Afr Entomol 19:488–497
Louda SM, Kendall D, Connor J, Simberloff D (1997) Ecological effects of an insect introduced for the biological control of weeds. Science 277:1088–1090
Louda SM, Pemberton RW, Johnson MT, Follett PA (2003) Nontarget effects - the Achilles’ heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annu Rev Entomol 48:365–396
Luck RF, Shepard BM, Kenmore P (1988) Experimental methods for evaluating arthropod natural enemies. Annu Rev Entomol 33:367–389
Mala VN (2013) The ecology and impact of the broom gall mite (Aceria genistae) on its host plant Scotch broom (Cytisus scoparius). M.Sc. Thesis. The University of Auckland
Manrique V, Diaz R, Condon T, Overholt W (2014) Host range tests reveal Paectes longiformis is not a suitable biological control agent for the invasive plant Schinus terebinthifolia. BioControl 59:761–770
Marohasy J (1998) The design and interpretation of host-specificity tests for weed biological control with particular reference to insect behaviour. Biocontrol News Inf 19:13N–20N
McFadyen R, Jacob HS (2004) Insects for the biocontrol of weeds: predicting parasitism levels in the new country. In: Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK (eds) Proceedings of the XI international symposium on biological control of weeds, 2004. CSIRO Entomology, Canberra, Australia, pp 135–140
Mortensen K (1984) A proposal for standardized scale of attack and its application to biocontrol agents of weeds in laboratory screening tests. In: Delfosse ES (ed) Proceedings of the VI international symposium on biological control of weeds. Agriculture Canada, pp 643–650
Paynter QE, Fowler SV, Gourlay AH, Haines ML, Harman HM, Hona SR, Peterson PG, Smith LA, Wilson-Davey JA, Winks CJ, Withers TM (2004) Safety in New Zealand weed biocontrol: a nationwide survey for impacts on non-target plants. N Z Plant Protect 57:102–107
Paynter Q, Gourlay AH, Oboyski PT, Fowler SV, Hill RL, Withers TM, Parish H, Hona S (2008a) Why did specificity testing fail to predict the field host-range of the gorse pod moth in New Zealand? Biol Control 46:453–462
Paynter Q, Martin N, Berry J, Hona S, Peterson P, Gourlay AH, Wilson-Davey J, Smith L, Winks C, Fowler SV (2008b) Non-target impacts of Phytomyza vitalbae a biological control agent of the European weed Clematis vitalba in New Zealand. Biol Control 44:248–258
Paynter Q, Fowler SV, Gourlay AH, Groenteman R, Peterson P, Smith L, Winks CJ (2010) Predicting parasitoid accumulation on biological control agents of weeds. J Appl Ecol 47:575–582
Paynter Q, Forgie SA, Winks CJ, Peterson PG, Ward DF, Nicholson L, van Zoelen R (2012) Biotic resistance: facilitation between invasive Homoptera and invasive ants limits the establishment of an introduced weed biocontrol agent in New Zealand. Biol Control 63:188–194
Paynter Q, Fowler SV, Hayes L, Hill RL (2015a) Factors affecting the cost of weed biocontrol programs in New Zealand. Biol Control 80:119–127
Paynter Q, Fowler SV, Hugh Gourlay A, Peterson PG, Smith LA, Winks CJ (2015b) Relative performance on test and target plants in laboratory tests predicts the risk of non-target attack in the field for arthropod weed biocontrol agents. Biol Control 80:133–142
Paynter Q, Fowler SV, Gourlay AH, Peterson PG, Smith LA, Winks CJ (2016) The influence of agent rearing success and release size on weed biocontrol programs in New Zealand. Biol Control 101:87–93
Paynter Q, Konuma A, Dodd SL, Hill RL, Field L, Gourlay AH, Winks CJ (2017) Prospects for biological control of Lonicera japonica (Caprifoliaceae) in New Zealand. Biol Control 105:56–65
Pearson DE, Callaway RM (2005) Indirect nontarget effects of host-specific biological control agents: implications for biological control. Biol Control 35:288–298
Peterson PG, McGregor PG, Springett BP (2000) Density dependent prey-feeding time of Stethorus bifidus (Coleoptera: Coccinellidae) on Tetranychus lintearius (Acari: Tetranychidae). N Z J Zool 27:41–44
Petráková L, Líznarová E, Pekár S, Haddad CR, Sentenská L, Symondson WOC (2015) Discovery of a monophagous true predator, a specialist termite-eating spider (Araneae: Ammoxenidae). Sci Rep 5:14013
Sheppard AW, Hill R, DeClerck-Floate RA, McClay A, Olckers T, Quimby PC, Zimmermann HG (2003) A global review of risk–benefit–cost analysis for the introduction of classical biological control agents against weeds: a crisis in the making? Biocontrol News Inf 24:77N–94N
Sheppard A, Haines M, Thomann T (2006) Native-range research assists risk analysis for non-targets in weed biological control: the cautionary tale of the broom seed beetle. Aust J Entomol 45:292–297
Simberloff D, Stiling P (1996) How risky is biological control? Ecology 77:1965–1974
Smith LA, Fowler SV, Paynter Q, Pedrosa-Macedo JH, Wigley P (2013) Successfully eliminating parasitic gregarines from Neolema ogloblini (Coleoptera: Chrysomelidae)—a biological control agent for Tradescantia fluminensis (Commelinaceae). In: Wu Y, Johnson T, Sing S, Raghu S, Wheeler G, Pratt P, Warner K, Center T, Goolsby J, Reardon R (eds) XIII international symposium on biological control of weeds. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team, 2012–2007, p 36
Strong DR (1997) Fear no weevil? Science 277:1058–1059
Suckling DM, Sforza RFH (2014) What magnitude are observed non-target impacts from weed biocontrol? PLoS ONE 9(1):e84847
van Driesche R, Bellows T, Elkinton J, Gould J, Ferro D (1991) The meaning of percentage parasitism revisited: solutions to the problem of accurately estimating total losses from parasitism. Environ Entomol 20:1–7
van Eyndhoven G (1967) Tetranychus lintearius Dufour, 1932, is a valid species (Acar.) Notalae ad Tetranychidas II. Entomol Ber 27:90–100
van Klinken RD (2000) Host specificity testing: why do we do it and how we can do it better. In: van Driesche R, Heard T, McClay A, Reardon R (eds) Host-specificity testing of exotic arthropod biological control agents: the biological basis for improvement in safety, USDA Forest Service Bulletin, Morgantown, West Virginia, USA, pp 54–68
van Klinken RD, Edwards OR (2002) Is host-specificity of weed biological control agents likely to evolve rapidly following establishment? Ecol Lett 5:590–596
Veldtman R, Lado TF, Botes A, Procheş Ş, Timm AE, Geertsema H, Chown SL (2011) Creating novel food webs on introduced Australian acacias: indirect effects of galling biological control agents. Divers Distrib 17:958–967
Wallis GP, Trewick SA (2009) New Zealand phylogeography: evolution on a small continent. Mol Ecol 18:3548–3580
Webb C, Sykes W, Garnock-Jones P (1988) Flora of New Zealand, vol IV. DSIR Botany Division, Christchurch
Willis AJ, Memmott J (2005) The potential for indirect effects between a weed, one of its biocontrol agents and native herbivores: a food web approach. Biol Control 35:299–306
Withers TM, Carlson CA, Gresham BA (2013) Statistical tools to interpret risks that arise from rare events in host specificity testing. Biol Control 64:177–185
Acknowledgements
This work was was supported by core funding to Landcare Research from the Ministry of Business, Innovation and Employment. QP thanks Mark Schwartzlaender for the opportunity to attend the International Congress of Entomology. We thank Cliff Moran, Mark Schwarzländer and S. Raghu and two anonymous reviewers for helpful comments on an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: S. Raghu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paynter, Q., Fowler, S.V. & Groenteman, R. Making weed biological control predictable, safer and more effective: perspectives from New Zealand. BioControl 63, 427–436 (2018). https://doi.org/10.1007/s10526-017-9837-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9837-5