Abstract
Advancing age is characterized by functional and phenotypic alterations in the distribution of circulating T-cell subsets, some of which are exacerbated by a latent infection with the persistent herpesvirus, cytomegalovirus (CMV). The influence of age, sex and CMV-infection on T-cell subpopulations in the peripheral blood remains incompletely understood. Here, T cells from 157 participants of the Berlin Aging Study II (BASE-II) were characterized at 21–34 (n = 59) and 62–85 (n = 98) years of age. We found that the frequency of naïve CD8+ T cells was significantly lower in the older group than in the young, and was different in men and women. Elderly men had a significantly lower proportion of naïve CD8+ T cells than younger men, regardless of their CMV-status, but in older women, this was seen only in the CMV-seropositive group. Reciprocally, older men had a higher proportion of late-differentiated, potentially “senescent” CD57+ T cells. Thus, T-cell senescence may be more pronounced in older men than women. Within the CD4+ population, in the elderly of both sexes there was a significantly higher proportion of late-differentiated TEMRA cells (T effector memory cells re-expressing CD45RA), but these were present exclusively in CMV-positive subjects. Finally, for the first time, we examined the so-called TSCM cell (T-stem cell-like memory) subpopulations in both CD4+ and CD8+ subsets and found that neither CMV-seropositivity nor age or sex affected their frequencies. This study confirms significant cross-sectional age-associated differences of T-cell subset distribution in a representative German urban population and emphasizes the impact of both sex and CMV-infection on T-cell naïve and memory phenotypes, but unaffected frequencies of T-stem cell-like memory cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in the human immune system accompanying aging are generally referred to as indicating “immunosenescence”. Many factors and mechanisms are attributed to immunosenescence including defects in hematopoiesis, thymus involution and defects in formation, maturation, migration and homeostasis of peripheral lymphocytes (Müller et al. 2013). Age-related modulation of the immune system can be assessed as differences in the distribution of peripheral T cells at different stages of differentiation. The frequencies of memory T cells depend on lifetime exposure of the individual to pathogens, above all to cytomegalovirus (CMV). As many studies now confirm, infection with this herpesvirus drives specific T cells to a late stage of differentiation, which may be confused with the process of aging itself if not properly controlled for (Chidrawar et al. 2009; Derhovanessian et al. 2010; Fülöp et al. 2013; Looney et al. 1999; Pawelec 2014a, b; Smithey et al. 2012). These late-stage differentiated CMV-specific CD8+ T cells have a reduced or absent proliferative capacity, increased ability for activation of senescence pathways and a significantly increased resistance to apoptosis in vitro (Akbar and Fletcher 2005). In aged people, oligoclonally expanded T cells show increased expression of “late-stage” differentiation markers. The accumulation of these highly differentiated T-cell pools in combination with a reduced frequency of naïve T cells could possibly contribute to causing age-related mortality (Almanzar et al. 2005; Chidrawar et al. 2009; Pawelec 2014b; Qi et al. 2014a, b; Wertheimer et al. 2014). Nonetheless, some recent findings indicate that this late-differentiated T-cell constellation is not universally to be viewed as detrimental to survival, but depends on the circumstances. Thus, in an extremely elderly Dutch population, lower frequencies of naïve CD8+ T cells and higher frequencies of late-differentiated CD8+ T cells were associated with a survival benefit on 7 year follow-up (Derhovanessian et al. 2012). Thus, the immunological remodeling of the memory cell pool, which takes place with advancing age, is likely to represent an adaptation of the aged immune system conferring survival advantages (Pawelec 2012). Hence, it remains important to establish the impact of age and CMV-infection in different human populations experiencing different current and earlier exposures and environments, and to establish whether this is different in men and women. For this reason, the Berlin BASE II study was established to examine the impact of multiple health, socioeconomic, psychological and other parameters on healthy ageing, enabling a large-scale study of the contribution of immune ageing to this process. Here we present a subset analysis of the cross-sectional base-line of this study, for which longitudinal follow-up will be available later.
We have used polychromatic flow cytometry to simultaneously measure multiple surface marker phenotypes, defining the following subpopulations: (N) naïve (CD45RA+CCR7+CD27+CD28+); (CM) central memory (CD45RA−CCR7+CD27+CD28+); (EM3) effector memory (CD45RA−CCR7−CD27−CD28−); (E) terminally-differentiated T-effector memory cells as an extended TEMRA phenotype (CD45RA+CCR7−CD27−CD28−); “exhausted” T cells [PD-1+ (CD279+)]; potentially “senescent” T cells (CD57+) and T stem cell-like memory T cells (TSCM, defined as CD45RA+CCR7+CD27+CD28+CD95+). We have analyzed the frequency of these subpopulations from the viewpoint of age, influence of sex and effect of a latent CMV-infection. For this reason we first examined the effect of age on the differentiation status of the T cells in both CMV-positive and CMV-negative men and women. The influence of sex in different age groups with different CMV-status and the influence of the CMV-status in men and women in different age groups have been explored. In addition, to the best of our knowledge for the first time, the frequency of the TSCM phenotype (Lugli et al. 2013) and of PD-1+ T-cells has been included, and the effects of age, CMV-serostatus and sex on their frequency have been examined.
Materials and methods
Subjects
A subgroup of 157 participants of the BASE-II study selected on the basis of a distribution of age, sex and CMV-infection similar to the whole cohort has been analyzed here (Table 1). BASE-II is a multidisciplinary and multi-institutional project that ascertains a large number of ageing-related variables from a wide range of different functional domains (Bertram et al. 2014). Phenotypic assessments include factors related to geriatrics and internal medicine, immunology, genetics, psychology, sociology and economics. Baseline recruitment of the BASE-II cohort was recently completed and has led to the sampling of 1600 older adults (age range 60–85 years), as well as 600 younger adults (20–35 years) serving as the basic population for in-depth analyses. The study was approved by the local ethics committees and written informed consent was obtained from all participants.
Samples
Venous blood was taken from the subjects of the BASE-II study during medical examinations by the Geriatric Research Group at the Charité in Berlin and sent to Tübingen in three EDTA tubes (7 ml) packed in iso-containers, to minimize temperature variations. The PBMC were further isolated under sterile conditions and frozen at −196 °C in the gas phase of liquid nitrogen until further processing.
Flow cytometry
All staining steps were performed in PFEA buffer (PBS, 2 % FCS, 2 mM EDTA, and 0.01 % azide). After thawing, PBMCs were treated with human Ig, GAMUNEX (Bayer, Leverkusen, Germany), and ethidium monoazide (EMA) bromide (MoBiTec GmbH, Göttingen, Germany) for 10 min on ice to block surface FcRs and label nonviable cells. Cells were first stained with unconjugated primary Ab CCR7 (R&D System) for 20 min at 4 °C, followed by staining with Pacific Orange-conjugated F(ab´) fragment of goat anti-mouse IgG (Invitrogen) for another 20 min on ice. Mouse serum (Millipore, Temecula California, USA) was added for 15 min to block nonspecific binding to anti-mouse secondary Ab, followed by addition of directly conjugated mAbs, CD3-Alexa Fluor700, CD4-PerCP, CD8-allophycocyanin-H7 (all from BD Biosciences, Heidelberg, Germany), CD27-allophycocyanin (BioLegend, San Diego, CA), CD45RA-V450 (BD Horizon, Heidelberg, Germany) CD28-PE (BD Pharmingen, Germany), PD1-PerCP-Cy5.5 (BioLegend, San Diego, CA), CD95-PE-Cy7 (eBioscience, San Diego, CA), and CD57-FITC (Immunotools, Freiburg, Germany). After 20 min incubation on ice, cells were washed and analyzed immediately on a LSR II cytometer (BD, Heidelberg) with FACSDiva software (BD Biosciences). The spectral overlap between all channels was calculated automatically by the BD FACSDiva software, after measuring negative and single-color controls. Data were analyzed using FlowJo 7.6.5 software (Tree Star, Portland, USA). T-cell subsets were characterized according to previously published models (Derhovanessian et al. 2010; Romero et al. 2007; Sallusto et al. 1999). The gating strategy is shown in Supplemental Material, Fig. S1, (a, b). In brief, the lymphocyte population was gated in FSC versus SSC dot plots. After exclusion of EMA+ dead cells, viable lymphocytes were gated within CD3+ gate and then selected for either CD8+ (Fig. S1a) or CD4+ (Fig. S1b) T-cell subsets, which have further been subdivided into main T-cell subsets (N, CM, EM and TEMRA) using CD45RA and CCR7. These subsets were also stained for CD27 and CD28 expression to better characterize their differentiation status: N (CD45RA+CCR7+CD27+CD28+); CM (CD45RA−CCR7+CD27+CD28+); EM3 (CD45RA−CCR7−CD27−CD28−); E (an extended TEMRA phenotype defined as CD45RA+CCR7−CD27−CD28−). Additionally, N cells have been gated for CD95 expression to identify TSCM (CD45RA+CCR7+CD27+ CD28+CD95+) cells and PD-1 (CD279) was determined within the CD3+ population. Flow cytometry staining and data analysis were performed on blinded samples.
Screening of CMV-serostatus
Anti-CMV IgG titers were measured in plasma of BASE-II participants using a CMV IgG kit (Omega Diagnostic Group, Scotland, UK) based on enzyme immunoassay technology.
Statistics
Statistical analysis used GraphPad V6 (GraphPad Software, Inc., La Jolla, USA). For comparisons between two independent groups the Mann–Whitney U Test was used. For all analyses, the significance level (p-values) has been set to 0.05, adjusted for multiple comparisons using the Bonferroni correction.
Results
Frequency of CD4+ and CD8+ T cells within the CD3+ T-cell population
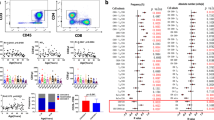
Age-related differences in the proportion of CD4+ T cells within the CD3+ pool are apparent in Fig. 1a and Table S1 (Supplementary Material). Older CMV-seronegative women have significantly higher frequencies of peripheral CD4+ T cells than younger subjects (p = 0.0004). However, a latent infection with CMV is associated with a lower percentage of CD4+ T cells, regardless of sex and age of the subjects (p = 0.0109 for young and p = 0.0007 for old women; p = 0.0026 for young and p = 0.0067 for old men).
Frequency of CD4+ (a), CD8+ (b) T cells within the CD3+ population; frequency of naïve (N) T cells (c, d) and central memory (CM) T cells (e, f) within CD4+ and CD8+ T cell populations for every subject of the eight different groups. The horizontal bars represent the median values for each group. N T cells are defined as CD45RA+CCR7+CD27+CD28+; CM cells are defined as CD45RA−CCR7+CD27+CD28+; y young, o old. Significance levels: *p < 0.05 and **p < 0.01 Bonferroni-corrected
The percentage of CD8+ T cells is significantly lower in elderly subjects of either sex (p = 0.0021 for women; p = 0.0024 for men), but only in CMV-negative people (Fig. 1b; Table S1, in Suppl). However, no significant differences were observed between sexes. An effect of CMV infection on this parameter was only seen in the elderly where both older women (p = 0.0017), and older men (p = 0.0024) had a higher percentage of CD8+ T cells than CMV-seronegatives.
No significant differences in the percentage of CD3+ T cells (Fig. 3f; Table S1) were observed between different subgroups, except in the group of old females, where CMV-positive individuals had a significantly higher frequency of CD3+ T cells than CMV-negative (p = 0.0053).
Frequency of early-differentiated T cells within the CD4+ and CD8+ T-cell subsets
We found a significant difference between old and young subjects in the frequency of naïve T cells (defined as CD45RA+CCR7+CD27+CD28+) within the CD4+ T-cell population. Elderly women had a lower percentage (p = 0.008) of naïve CD4+ T cells compared to younger women, but only in the group of CMV-negative individuals (Fig. 1c). In contrast, although there were also lower frequencies of CD4+ naïve T cells in older men, in this case this was only seen in the CMV-seropositive group (p = 0.0063). These results imply that at least some of the observed sex differences in naïve T-cell distribution in the elderly are not only dependent on the different frequency of CMV-infection in men and women, but reflect a difference in response to CMV after infection.
CD8+ T cells are generally reported to show greater age-associated differences than CD4+ T cells. Consistent with this, Fig. 1d shows a greater impact of age on the frequencies of CD8+ naïve T cells than CD4+ naïve T cells, in both CMV+ and CMV− subjects. Elderly men had a significantly lower proportion of these naïve T cells than younger men, regardless of their CMV-status (p = 0.0012 for CMV−; p < 0.0001 for CMV+). However, in women, this was only the case in the CMV-positive group (p = 0.0004) (Fig. 1d; Table S1, in Suppl). There is an additional sex difference, in that older men had a lower proportion of naïve CD8+ T cells (p = 0.0081) than elderly women, only in the CMV-positive group (Fig. 1d).
Similarly, age-related differences can be seen in the central memory (CM) CD4+ T-cell population (CD45RA−CCR7+CD27+CD28+), particularly evident in the CMV-seronegative female group. Thus, elderly women had a significantly higher frequency (p = 0.0027) of CM T cells than younger women (Fig. 1e).
In the CD8+ T-cell population, a significant influence of age on the distribution of CM T cells was seen only in CMV-seronegative men (Fig. 1f; Table S1, in Suppl). The percentage of CD8+ CM T cells in elderly subjects was significantly higher (p = 0.0023) in comparison to younger people. In addition, CMV-seropositive older men have a significantly lower percentage (p = 0.0016) of CM cells than seronegative elderly men.
Frequency of late-differentiated T cells within the CD4+ and CD8+ T-cell subsets
Age-related differences in the frequencies of effector memory (EM3) T cells (CD45RA−CCR7−CD27−CD28−) can be clearly seen in CMV-positive women (Fig. 2a). Thus, the proportion of CD4+ EM3 T cells in older women is significantly higher than in the younger women (p = 0.01). A strong effect of CMV-infection is also apparent in that CMV-negative subjects exhibit a significantly lower frequency of CD4+ EM3 T cells than CMV-positive subjects, regardless of sex and age (p = 0.0003 for young and p = 0.0002 for old women; p = 0.0115 for young and 0.0014 for old men). Thus, sex seems to make no difference to the distribution of EM3 T cells.
Frequency of effector memory (EM3) (a, b) and TEMRA effector (E) (c, d) T cells within CD4+ (a, c) and CD8+ (b, d) T-cell populations for every subject of the eight different groups. The horizontal bars represent the median values for each group. EM3 T cells are defined as CD45RA−CCR7−CD27−CD28−; E cells are defined as CD45RA+CCR7−CD27−CD28−; y young, o old. Significance levels: *p < 0.05 and **p < 0.01 Bonferroni-corrected
In contrast to the CD4+ T-cell population, no effect of age, sex or CMV-status was noted for the CD8+ population of EM3 T cells (Fig. 2b; Table S1, in Suppl).
Finally, we assessed the frequencies of the potentially “terminally-differentiated” T-effector memory T cells characterized by their re-expression of CD45RA (so-called TEMRA cells). Although the presence of cells with this phenotype in the CD8+ T-cell subset is well-accepted, it has remained controversial whether they exist in the CD4+ T-cell subset. Therefore we analyzed an extended phenotype of TEMRA subset, designated effector (E) T cells and characterized by negativity for the two costimulatory receptors CD27 and CD28 (CD45RA+CCR7−CD27−CD28−). Within the CD4+ population of CMV-seronegative people, it was indeed the case that this subset of TEMRA cells was essentially absent (Fig. 2c). However, in CMV-infected older subjects, some individuals did possess CD4+ T cells with this phenotype (Fig. 2c). There was a significantly higher proportion of these cells in CMV-positive people (p = 0.0044 for young and p = 0.0002 for old women; and p = 0.0116 for old men) (Fig. 2c).
Concerning the frequency of CD8+ E cells, we found a significant influence of age on this subset but again, only in subjects with latent CMV-infection (p = 0.0021 for women; p = 0.0072 for men). Furthermore, the frequency of these cells in CMV-positive elderly individuals was significantly higher (p = 0.0062 for women; p = 0.0028 for men) in comparison to CMV-negative old people, regardless of their sex. In the younger subjects no significant differences could be detected (Fig. 2d; Table S1, in Suppl).
Frequency of CD57+ T cells within the CD4+ and CD8+ T-cell subsets
With the objective to corroborate the results described above, the expression of CD57 (as an independent marker of late-differentiated, potentially “senescent” T cells) has been investigated and compared within the CD4+ and CD8+ populations. CD57 is often referred to in the literature as a marker of T-cell “senescence” reflecting its presence on cells thought to be beyond the terminally differentiated state and possibly indicating true replicative senescence, at least in the CD8+ subset. (Tarazona et al. 2000). We found CD57 expression also on CD4+ T-cells in the elderly, relative to younger subjects, especially those who were CMV-infected (p = 0.0039 for women; p = 0.0007 for men). With respect to the influence of CMV-status, the frequency of CD57+ T cells was significantly higher in CMV-positive than CMV-negative subjects, regardless of both age and sex (p = 0.0002 for young and p < 0.0001 for old women; p = 0.0005 for young and p = 0.0002 for old men).
Also in the CD8+ T-cell subset (Fig. 3b; Table S1, in Suppl), we found that the percentage of CD57+ T cells in elderly CMV-positive subjects was significantly higher than in younger subjects, regardless of sex (p = 0.0098 for women; p = 0.0002 for men).
Frequency of CD57+ T cells within CD4+ (a) and CD8+ (b) T cell populations as well as percentage of PD-1 (CD279+) T cells within the CD8+ T cell population (c) for every subject of the eight different groups. Frequency of TSCM cells within CD4+ (d) and CD8+ (e) T cell populations for every subject of the four different groups. The horizontal bars represent the median values for each group. TSCM cells are defined as CD45RA+CCR7+CD27+ CD28+CD95+; y young, o old. Significance levels: *p < 0.05 and **p < 0.01 Bonferroni-corrected
As in the CD4+ T-cell population, a clear influence of CMV-status can be seen on the expression of CD57 by CD8+ T cells. The percentage of CD57+ T cells in CMV-positive older women (p = 0.0083) and in CMV-positive older men (p = 0.0017) was significantly higher compared to CMV-negative subjects (Fig. 3b).
Frequency of PD-1+ (CD279+) T cells within the CD8+ subset
We also investigated the expression of PD-1 (programmed cell death protein 1), which is mostly expressed on CD8+ T cells and is known as an inhibitory immunoregulator, potentially marking “exhausted” T cells (Barber et al. 2006). We found that elderly men had a significantly higher percentage of CD279+ T cells than younger subjects, but only in the CMV-negative group (p = 0.0098) (Fig. 3d; Table S1, in Suppl).
Considering the influence of CMV-status on the expression of PD-1, a significant difference was found only for CMV-negative-versus-positive men who had a higher proportion of PD-1+ T cells (p = 0.0095). No sex-related differences were observed.
Frequency of T-stem cell-like memory (TSCM)-cells within the CD4+ and CD8+ T-cell subsets
A novel T-cell subpopulation with stem cell-like properties has been described (Gattinoni et al. 2011), characterized by the phenotype CD45RA+CCR7+CD27+ CD28+CD95+, and designated TSCM. This long-lived memory T-cell population has an increased capacity for self-renewal and for the generation of multipotent CM-, EM-, and E-cells (Gattinoni et al. 2011). However, this rare population has not been studied in terms of the influence of age or CMV-serostatus. Thus, we examined the distribution of TSCM cells within the CD4+ and CD8+ populations, and summarize the results in Fig. 3d and e. It is apparent that neither CMV-status nor age has any significant associations with the frequency of TSCM-cells. We also found no effect of sex (data not shown). Hence, this potentially important source of effector and memory T cells appears to be well-conserved in both sexes regardless of either age or CMV-infection, all of which are factors markedly affecting the distribution of other T-cell differentiation phenotypes in humans.
Discussion
Influence of age on differentiation status in CMV-positive and CMV-negative men and women
The results of our study have confirmed a general tendency for reduced frequencies of naïve T cells in the peripheral blood of subjects at advanced age, regardless of CMV-infection. This is more pronounced in the CD8+ subset but is also apparent in CD4+ T cells. Interestingly, this tendency is particularly pronounced in women who are CMV-negative women but in men who are CMV-positive, suggesting that there is a sex difference in the immunological impact of CMV. A recent study by another group yielded slightly different results, where aging in the absence of CMV was associated with decreased naïve CD8+ but not CD4+ T cells (Wertheimer et al. 2014). However, that study did not examine men and women separately. This may explain the difference, because we found that the age-associated lower frequency of naïve T cells in CMV-seronegative individuals was sex-sensitive.
We have observed a higher frequency of CM CD4+ T cells exclusively in the CMV-negative elderly, while CMV-positive individuals had a more advanced differentiation phenotype of EM3 and E CD4 T cells. These results are consistent with CMV predominantly driving the accumulation of late-stage CD4+ as well as CD8+ memory T cells in both sexes. Thus, as in most previous studies, more marked than for CD4+ T cells, we have observed lower frequencies of naïve CD8+ T cells at older age for all groups. Both CMV-positive and CMV-negative old subjects of both sexes showed a lower percentage of naïve CD8+ T cells. Similar to the CD4+ T cells, also here a corresponding increase in the frequency of CM cells in CMV-seronegative elderly people was observed. The same trend was observed for the “late-differentiated” effector population in CMV-seropositive subjects.
Consistent with this interpretation, higher frequencies of T cells expressing CD57 in both the CD4+ and CD8+ subsets in the elderly were observed exclusively in CMV-seropositive subjects. As CD57 is considered to be a potential “senescence marker” for late differentiated T cells (Koch et al. 2008; Pera et al. 2014; Strioga et al. 2011; Tarazona et al. 2000), this finding was expected. Again, there were marked differences between the CD4+ and CD8+ subsets, especially in the young. Interestingly, and perhaps counter-intuitively, the frequencies of “exhausted” PD-1+ CD8+ T cells were not affected by age or CMV infection; the same was true for TSCM cells in these subjects.
Age-related thymic involution is associated with a decreased efficiency of T-cell development and with a reduced migration of naïve T cells into the periphery (Lynch et al. 2009; Qi et al. 2014b). The consequences of thymic involution in old people contribute to a reduction of naïve T cells in periphery, regardless of CMV-infection. However, the higher frequency of memory cells present exclusively in CMV-positive subjects could be explained by the coexistence of latent CMV and duration of immune system stimulation by the virus, requiring permanent immunosurveillance. According to Stowe et al. CMV-reactivation may occur more often in older people and this could provide an explanation for an age-related increase in the memory T-cell pool in this age group (Stowe et al. 2007).
Influence of sex in different age groups with different CMV-status
It is known that the impact of aging on immunity in men and women is different (Caruso et al. 2013) but details on immune status are sparse. Although the total number of lymphocytes in the peripheral blood of both sexes is similar, men have a lower percentage of T cells within their lymphocyte population (Bouman et al. 2004; Hirokawa et al. 2013). The difference in incidence of infectious diseases and the different prevalence of autoimmune diseases between men and women could also be attributed to sex-related differences in the immune system (McCombe et al. 2009; Qi et al. 2014a). However, little is known about the effects of sex differences on the aging immune system (Nunn et al. 2009). There are few publications so far on sex differences in the age-related distribution of T-cell subpopulations. Yan et al. studied men and women of different age groups, but without taking their CMV-status into account. They reported significantly higher frequencies of EM cells in older men, but not in older women (Yan et al. 2010), but in a study of the Cuban population (with a higher prevalence of CMV-infection at all ages) the frequency of highly differentiated T cells was higher in women (Garcia Verdecia et al. 2013). Thus, the impact of sex and CMV-persistence on distinct T-cell subpopulations in the peripheral blood remains incompletely quantified and understood. In our experiments reported here, we have observed multiple sex-related differences in the effects of age and CMV-infection on the differentiation status of both CD4+ and CD8+ T cells. Together, the data suggest that the CMV-associated “senescence of T cells” in older men may be more pronounced than in elderly women.
Although sex-specific differences in sex hormone secretion patterns and their changes over the lifespan are clearly candidates intimately involved in controlling ageing trajectories, their impact on immunity is not well-established (Nussinovitch and Shoenfeld 2012; Tower and Arbeitman 2009). It is known that estrogens enhance humoral immunity, while androgens and progesterone tend to suppress it (Gameiro and Romao 2010; Sakiani et al. 2013). Women in general seem to have stronger humoral and cell-mediated immune responses to immune stimulation compared to men. In addition, they generally have higher antibody levels and increased levels of circulating IL-1, IL-4 and IFN-γ (Ansar Ahmed et al. 1985; Yan et al. 2010). It has been reported that men have more pronounced thymic involution, a process that could be due to higher androgens levels in men (Ongradi and Kovesdi 2010).
Although the functionality of the immune system in men and women seems to be different, these differences can only partly explain our results. Such differences for example, seem to play no crucial role in the subpopulation of CMV-negative individuals on the distribution of T-cell subpopulations. Therefore, we can only assume that the sex differences in the differentiation status of the T cells could first emerge under the immunomodulating effect of the stress of long-term immunosurveillance to control CMV-infection. It might be assumed that CMV reactivation in men and women manifests differently. However, this has not been investigated yet, and currently available methods of serological detection neither allow the determination of the duration of virus persistence nor the number of reactivations occuring over the life span.
Influence of CMV-status in men and women in different age groups
As discussed above, repetitive reactivation of CMV or re-infection could lead to exhaustion and dysfunction of CMV-specific T cells, so that larger amounts of immune cells are needed to control the CMV-infection. In our experiments we found that the CMV-serostatus had a decisive influence on the distribution of different subpopulations of both CD4+ and CD8+ T cells. In CD4+ T cells, an accumulation of “late-differentiated” subpopulations was directly associated with the CMV-status, being seen only in infected individuals. This finding on the subgroup of BASE-II participants is in line with our earlier results in other cohorts (Derhovanessian et al. 2012; Derhovanessian and Pawelec 2012; Koch et al. 2008) and by Lachmann et al. (2012). These data are consistent with the hypothesis that persistent CMV-infection accelerates age-related increases in the proportion of memory T cells. This is likely to be influenced by many other factors, such as general health and genetic background, which still have not been taken into account at this stage of the study. Because BASE-II is collecting a large data set on health parameters, cognitive and psychosocial data, as well as genetic data, these variables will also be taken into account as the study progresses.
The importance of this type of analysis, and including the hitherto “innocuous” CMV as an important parameter is emphasized by earlier findings that the CMV-induced accumulation of late-differentiated T cells with advancing age may correlate with increased mortality. The Swedish OCTO and NONA longitudinal studies defined an immune risk profile (IRP) predicting 2-, 4- and 6-year survival. All subjects in the IRP group were CMV-positive, as opposed to 85 % of those not in the IRP group (Pawelec et al. 2009; Wikby et al. 2006).
Frequency of the TSCM-cells among CD4+ and CD8+ T-cell populations
To the best of our knowledge, in the present study we have investigated for the first time the impact of age and CMV-status on the frequency of the relatively rare long-lived TSCM subpopulation with stem-cell-like characteristics. Interestingly, neither age, sex or CMV infection affected the frequency of TSCM cells within the CD4+ and CD8+ subsets. Retention of these potentially important cells through life could be a crucial aspect of the maintenance of immune system functionality. As there is age-related impairment of progenitor cell production from the bone marrow (Warren and Rossi 2009), as well the age-dependent decreased thymic function discussed above, the TSCM population might represent an important additional source of T-cell regeneration in the periphery. It remains to be determined whether the maintained levels of TSCM cells in the elderly are also functionally intact.
Study limitations
Some limitations of the present study need to be acknowledged. First, the study included a small subgroup of the BASE-II study containing 157 participants. Although selected on the basis of a distribution similar to the whole cohort, the sample size is small, especially after further subdivision into subgroups, based on the differences in age, sex and CMV-positivity. Also the differences in the number of CMV-seronegative young participants compared to CMV-seropositive donors may affect our results and limit the power to detect some significant associations. Further, there are several outliers mainly in the older groups that can affect results of our statistical analysis, although we used nonparametric tests that are recommended for the data being subject to outliers or extreme values. Therefore we are planning further analyses on the whole BASE-II cohort of 2200 participants to confirm and to generalize these results and to include social and other data.
Conclusions
Data obtained from a subgroup of participants of the BASE-II study have demonstrated that age, sex and CMV-status all influence the differentiation phenotypes of peripheral blood T lymphocytes. The multidisciplinary nature of BASE-II will allow the correlation of immunologic parameters with health and socio-economic status of the subjects, their genetic background and psychological characteristics. This will allow us to dissect out the influences of these parameters on immune function, and vice versa, at baseline of the planned longitudinal follow-up of BASE-II.
References
Akbar AN, Fletcher JM (2005) Memory T cell homeostasis and senescence during aging. Curr Opin Immunol 17:480–485. doi:10.1016/j.coi.2005.07.019
Almanzar G et al (2005) Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol 79:3675–3683. doi:10.1128/JVI.79.6.3675-3683.2005
Ansar Ahmed S, Penhale WJ, Talal N (1985) Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol 121:531–551
Barber DL et al (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. doi:10.1038/nature04444
Bertram L et al (2014) Cohort profile: the Berlin Aging Study II (BASE-II). Int J Epidemiol 43:703–712. doi:10.1093/ije/dyt018
Bouman A, Schipper M, Heineman MJ, Faas MM (2004) Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol 52:19–26. doi:10.1111/j.1600-0897.2004.00177.x
Caruso C, Accardi G, Virruso C, Candore G (2013) Sex, gender and immunosenescence: a key to understand the different lifespan between men and women? Immun Ageing 10:20. doi:10.1186/1742-4933-10-20
Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P (2009) Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol 155:423–432. doi:10.1111/j.1365-2249.2008.03785.x
Derhovanessian E, Pawelec G (2012) Vaccination in the elderly. Microb Biotechnol 5:226–232. doi:10.1111/j.1751-7915.2011.00283.x
Derhovanessian E et al (2010) Hallmark features of immunosenescence are absent in familial longevity. J Immunol 185:4618–4624. doi:10.4049/jimmunol.1001629
Derhovanessian E et al (2012) Lower proportion of naive peripheral CD8+ T cells and an unopposed pro-inflammatory response to human Cytomegalovirus proteins in vitro are associated with longer survival in very elderly people. Age (Dordr) 35:1387–1399. doi:10.1007/s11357-012-9425-7
Fülöp T, Larbi A, Pawelec G (2013) Human T cell aging and the impact of persistent viral infections. Front immunol 4:271. doi:10.3389/fimmu.2013.00271
Gameiro C, Romao F (2010) Changes in the immune system during menopause and aging. Front Biosci (Elite Ed) 2:1299–1303
Garcia Verdecia B et al (2013) Immunosenescence and gender: a study in healthy Cubans. Immun Ageing 10:16. doi:10.1186/1742-4933-10-16
Gattinoni L et al (2011) A human memory T cell subset with stem cell-like properties. Nat Med 17:1290–1297. doi:10.1038/nm.2446
Hirokawa K, Utsuyama M, Hayashi Y, Kitagawa M, Makinodan T, Fulop T (2013) Slower immune system aging in women versus men in the Japanese population. Immun Ageing 10:19. doi:10.1186/1742-4933-10-19
Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G (2008) Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing 5:6. doi:10.1186/1742-4933-5-6
Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, Kern F (2012) Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J Virol 86(2)1001–1009. doi:10.1128/JVI.00873-11
Looney RJ et al (1999) Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol 90:213–219. doi:10.1006/clim.1998.4638
Lugli E et al (2013) Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 123:594–599. doi:10.1172/JCI66327
Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD (2009) Thymic involution and immune reconstitution. Trends Immunol 30:366–373. doi:10.1016/j.it.2009.04.003
McCombe PA, Greer JM, Mackay IR (2009) Sexual dimorphism in autoimmune disease. Curr Mol Med 9:1058–1079
Müller L, Fülöp T, Pawelec G (2013) Immunosenescence in vertebrates and invertebrates. Immun Ageing 10:12. doi:10.1186/1742-4933-10-12
Nunn CL, Lindenfors P, Pursall ER, Rolff J (2009) On sexual dimorphism in immune function. Philos Trans R Soc London B 364:61–69. doi:10.1098/rstb.2008.0148
Nussinovitch U, Shoenfeld Y (2012) The role of gender and organ specific autoimmunity. Autoimmun Rev 11:A377–A385. doi:10.1016/j.autrev.2011.11.001
Ongradi J, Kovesdi V (2010) Factors that may impact on immunosenescence: an appraisal. Immun Ageing 7:7. doi:10.1186/1742-4933-7-7
Pawelec G (2012) Hallmarks of human “immunosenescence”: adaptation or dysregulation? Immun Ageing 9:15. doi:10.1186/1742-4933-9-15
Pawelec G (2014a) Immunosenenescence: role of cytomegalovirus. Exp Gerontol 54:1–5. doi:10.1016/j.exger.2013.11.010
Pawelec G (2014b) T-cell immunity in the aging human. Haematologica 99:795–797. doi:10.3324/haematol.2013.094383
Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A (2009) Cytomegalovirus and human immunosenescence. Rev Med Virol 19:47–56. doi:10.1002/rmv.598
Pera A, Campos C, Corona A, Sanchez-Correa B, Tarazona R, Larbi A, Solana R (2014) CMV latent infection improves CD8+ T response to SEB due to expansion of polyfunctional CD57+ cells in young individuals. PLoS One 9:e88538. doi:10.1371/journal.pone.0088538
Qi Q et al (2014a) Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci USA 111:13139–13144. doi:10.1073/pnas.1409155111
Qi Q, Zhang DW, Weyand CM, Goronzy JJ (2014b) Mechanisms shaping the naive T cell repertoire in the elderly—thymic involution or peripheral homeostatic proliferation? Exp Gerontol 54:71–74. doi:10.1016/j.exger.2014.01.005
Romero P et al (2007) Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol 178:4112–4119
Sakiani S, Olsen NJ, Kovacs WJ (2013) Gonadal steroids and humoral immunity. Nat Rev Endocrinol 9:56–62. doi:10.1038/nrendo.2012.206
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. doi:10.1038/44385
Smithey MJ, Li G, Venturi V, Davenport MP, Nikolich-Zugich J (2012) Lifelong persistent viral infection alters the naive T cell pool, impairing CD8 T cell immunity in late life. J Immunol 189:5356–5366. doi:10.4049/jimmunol.1201867
Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R (2007) Chronic herpesvirus reactivation occurs in aging. Exp Gerontol 42:563–570. doi:10.1016/j.exger.2007.01.005
Strioga M, Pasukoniene V, Characiejus D (2011) CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology 134:17–32. doi:10.1111/j.1365-2567.2011.03470.x
Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R (2000) Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev 121:77–88
Tower J, Arbeitman M (2009) The genetics of gender and life span. J Biol 8:38. doi:10.1186/jbiol141
Warren LA, Rossi DJ (2009) Stem cells and aging in the hematopoietic system. Mech Ageing Dev 130:46–53. doi:10.1016/j.mad.2008.03.010
Wertheimer AM et al (2014) Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol 192:2143–2155. doi:10.4049/jimmunol.1301721
Wikby A et al (2006) The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev 127:695–704. doi:10.1016/j.mad.2006.04.003
Yan J, Greer JM, Hull R, O’Sullivan JD, Henderson RD, Read SJ, McCombe PA (2010) The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing 7:4. doi:10.1186/1742-4933-7-4
Acknowledgments
The authors thank participants and colleagues of the interdisciplinary group of the BASE-II study. We would like to thank Karin Hähnel and Lilly Öttinger for their excellent organizational and technical assistance and Nicole Janssen for performing CMV-ELISAs. We thank all colleagues of the TATI-Group and especially Kilian Wistuba-Hamprecht, whose skills and commitment made this study possible. We gratefully acknowledge the support from the German Ministry for Education and Research Grant Nos. 16SV5536K and FKZ 01EI1401 and from the European Commission Grant FP7 259679, as well as from the Max Planck Institute for Human Development, Berlin.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10522_2015_9563_MOESM1_ESM.ppt
Supplementary Figure S1: Gating strategy to identify distinct subsets of CD4+ (a) and CD8+ (b) T cells. Viable lymphocytes were gated within the CD3+ gate and selected for CD8+ or CD4+ T cell subsets, which were subdivided into four (N, CM, EM and TEMRA-E) using CD45RA and CCR7. CD27 and CD28 staining was used to characterize their differentiation status: N (CD45RA+CCR7+CD27+CD28+); CM (CD45RA-CCR7+CD27+CD28+); EM3 (CD45RA−CCR7−CD27−CD28−); E (CD45RA+CCR7−CD27−CD28−). N cells have been gated for CD95 to identify TSCM (CD45RA+CCR7+CD27+ CD28+CD95+) cells. Supplementary material 1 (PPT 345 kb)
Rights and permissions
About this article
Cite this article
Di Benedetto, S., Derhovanessian, E., Steinhagen-Thiessen, E. et al. Impact of age, sex and CMV-infection on peripheral T cell phenotypes: results from the Berlin BASE-II Study. Biogerontology 16, 631–643 (2015). https://doi.org/10.1007/s10522-015-9563-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-015-9563-2