Abstract
Cortical surface area (CSA) has particular relevance for understanding development, behavior, and the connection between brain structure and function. Little is known about genetic and environmental determinants of CSA during development. We utilized bivariate twin methods to identify global and regionally specific genetic factors which influence CSA in a preliminary sample of typically-developing adolescents, with hypotheses based on findings in middle-aged adults. Similar to previous findings, we observed high heritability for total CSA. There was also significant evidence for genetic influences on regional CSA, particularly when these were not adjusted for total CSA, with highest heritability in frontal cortex and relatively fewer genetic contributions to medial temporal cortical structures. Adjustment for total CSA reduced regional CSA heritability dramatically, but a moderate influence of genetic factors remained in some regions. Both global and regionally-specific genetic factors influence regional CSA during adolescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although long neglected by neuroscience, the human adolescent brain is a fruitful target of investigation that provides insights into maturational processes and the links between brain and behavior. With the increased use of non-invasive neuroimaging techniques such as magnetic resonance imaging (MRI), the uniqueness of the adolescent brain is gradually being revealed (van Soelen et al. 2012a). Cortical size, as measured by features such as thickness and cortical surface area (CSA), differs widely among individuals. Recent studies have emphasized that, during the period of adolescent development, the trajectories of cortical thickness and CSA may be very different from one another (Raznahan et al. 2011).
Although there have been several studies of the heritability of cortical thickness and its changes in childhood (Lenroot et al. 2009; van Soelen et al. 2012a, b; Yoon et al. 2010), to date, only one study has used a genetically-informative sample of young adolescent twins to explore the relative role of genes and environment on CSA. van Soelen et al. (2012a, b) found high heritability of total CSA for 12 year olds (h = 0.91 [0.89–0.95]), but did not present regional data.
Better understanding of CSA in adolescence and the role played by genetic and environmental factors could likely advance knowledge about the development of psychiatric disorders, many of which become clinically-apparent during the adolescent period (Kessler et al. 2007; Paus et al. 2008) and often have considerable genetic determinants (Faraone et al. 2005; Muhle et al. 2004). Studying CSA in typically-developing adolescents and how it is influenced by genetic and environmental factors is a first step in understanding how brain development may go awry in psychiatric conditions (Luna and Sweeney 2001). Previous studies in adults have emphasized that there are substantial genetic contributors to normal variation between individuals in the size of the cortex (Kremen et al. 2010; Winkler et al. 2010). One important finding was that cortical thickness and surface area seem to have largely non-overlapping genetic determinants (Panizzon et al. 2009; Winkler et al. 2010). Thus, it cannot be assumed that findings from adolescent samples regarding genetic and environmental determinants of cortical thickness will apply to measures of CSA. Another finding from previous studies is the importance of examining global size measures and how accounting for these measures may influence apparent heritability of regional size (Eyler et al. 2011). The relationship among global and regional size measures may be different during development compared to later in life because overall brain growth may still be occurring.
The current study utilized a twin design to study genetic and environmental influences on total and regional CSA in a preliminary sample of typically-developing adolescents. We based our hypotheses on previous findings in middle-aged adults (Eyler et al. 2011). Specifically, we expected that total and regional CSA would be highly heritable, but that regional heritabilities would be reduced after controlling for global CSA. We also predicted that there would be greater heritability of frontal CSA and lower heritability of medial temporal CSA, as was observed in that study, and as might be theorized based on the sensitivity of medial temporal regions to excitotoxic damage and/or the importance of genetic control over development of the prefrontal cortex, which is the most evolutionarily-expanded region of the human brain. A final aim of the study was to compare heritabilities between the current adolescent sample and the previous middle aged adult sample to discover potential areas where genetic or environmental factors might be playing a relatively larger role in adolescence.
Methods
Participants
Male and female adolescent twins in Chongqing were recruited based on demographic data from the school enrollment system of Chongqing education committee. Inclusion criteria: student aged 12–18 who studies in general school. Exclusion criteria: children with mental retardation or studying in a special school, without normal reading and writing ability or who are illiterate or refused to take part in this study, IQ <90 and left-handers. After an informed consent form was signed by the legal guardian (always parents of subjects), an MRI scan was completed (see below) and a blood sample was collected. Data from 57 twin pairs (114 adolescents) was successfully collected with permission from Ethics Committee of Chongqing Medical University (see Table 1 for age and gender information).
Intelligence testing was also performed at our hospital using the China-Wechsler Intelligence Scale for Children (C-WISC), revised by Yaoxian and Taisheng (1993). The mean IQ of the group was 124.39 (SD = 13.48). One pair was excluded because one of the twins had low IQ (<80). All participants were confirmed to be right-handed by the Edinburgh Handedness Inventory. This exclusion criterion was imposed because of other planned analyses for the study which would examine the relationship of brain measures to behavior and might be influenced by handedness.
Zygosity identification
Zygosity (monozygotic or dizygotic) was determined by DNA from a blood sample (Instrument: Genetic Analyzer 3100AvantTM, Kit: AmpFLSTR® Identifiler® PCR Amplification).We tested 15 short tandem repeat (STR) and Amelogenin to determine zygosity as follows: If 15 STR and Amelogenin were the same in a twin pair, this pair was classified as monozygotic (MZ); if the twins had different gender or one gene loci different, this pair was classified as dizygotic (DZ). MZ reliability can be achieved at the level of 0.999 through such a genetic analysis (Yang et al. 2006).
Image acquisition
Images were acquired on a GE 3.0 T scanner (The First Affiliated Hospital of ChongQing Medical University, China); Sagittal T1-weighted magnetization prepared rapid gradient echo sequences were employed with a time to inversion = 450 ms, time echo = 2.8 ms, time repetition = 6.2 ms, flip angle = 25°, slice thickness = 1.2 mm, and voxel size 0.47 × 0.47 × 1 mm. A total of 166 axial slices were collected. These data were reviewed for quality, registered, and corrected for magnetic field intensity to improve signal to noise. Of the 114 scans available at the time of these analyses, quality control measures excluded 0.6 % (4 cases) due to scanner artifact and 3 % (16 cases) due to inadequate image processing results, thus the MRI analyses are based on 114 cases (30 pairs MZ, 27 pairs DZ).
Image processing
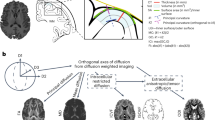
The Freesurfer software package was used to reconstruct the cortical surface (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999, 2004). Image intensities were first normalized, and then extra cerebral tissues were removed. The brain was segmented into gray and white matter using a connected components algorithm and the gray–white boundary was estimated. Based on this, a deformable surface algorithm was used to estimate the pial surface. A Gaussian smoothing kernel with a full width at half maximum of 10 mm was applied to the surface.
The Desikan_Killiany atlas (Desikan et al. 2006) was used to divide the cortical surface into 66 regions of interest (ROIs; 33 per hemisphere; Fischl et al. 2004). Regional CSA was calculated in each subject’s native space, as the sum of the areas of each triangle falling within a given ROI. We also divided the surface into 12 cortical regions of interest at the lobar level (bilateral frontal, parietal, lateral temporal, medial temporal and cingulate cortex; see Table 3 for which Freesurfer parcellations are contained in each lobe). Finally, we calculated total CSA as the sum of the areas of all ROIs.
Statistical analysis
We first examined whether there were any phenotypic correlations of the CSA measures and age. We then examined within-twin-pair correlations separately for MZ and DZ pairs. These correlations were calculated on regional CSA measures as well as regional measures that were adjusted for total CSA. Adjustment for total CSA for these calculations was made by running a linear regression: Regional CSA = b*total CSA + error, and saving the residuals for use in the correlations.
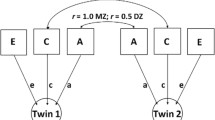
We then estimated the proportion of variance in the CSA measures contributed by additive genetic, shared environmental, and unique environmental factors using an ACE twin model. Additive genetic variance (A) refers to the additive genetic effects of alleles at every contributing locus and is estimated by modeling differences between MZ and DZ twins (who share 1.0 vs. 0.5 [on average] additive genetic effects). Environmental influences that make twins similar to one another are estimated by examining shared environmental variance (C). Unshared, individual-specific environmental influences are captured in the unique environmental variance (E; Eaves et al. 1978; Neale et al. 1992). We used the OpenMx package (Boker et al. 2011) to construct a saturated model and its submodels to first test basic assumptions (equal means and variances for twin 1/twin 2 and MZ/DZ pairs) for the data of total CSA, ‘unadjusted’ and ‘adjusted’ (residuals from the regression with whole brain CSA) regional CSA measures, and to examine twin sample homogeneity to the population.
To estimate A, C, and E contributors to variance, while also allowing us to adjust for total CSA in the context of the twin model, bivariate models (using both regional and total CSA measures) were then tested using the OpenMx package. In our data, we saw very little contribution of shared environment to measures of global CSA, so our final bivariate models included A and E parameters related to total CSA, and A, C and E parameters for each specific ROI. We also tested bivariate models with only A and E influences on both total CSA and regional CSA. We were underpowered to test for dominance effects (i.e., an ADE model), so this was not examined. The bivariate models allowed us to estimate genetic and environmental effects on a ROI that include genetic and environmental contributions shared with total CSA (‘unadjusted’). It also permitted examination of ‘adjusted’ genetic and environmental contributions to regional CSA, that is, the genetic and environmental effects specific to the ROI only (Eyler et al. 2011). Details of the calculation of the adjusted and unadjusted variance components and path of bivariate model can be found in Eyler et al. (2011).
Optimization of model fit to these data used maximum likelihood (Dominicus et al. 2006; Eaves et al. 1978; Neale et al. 1992) by calculating twice the negative log-likelihood of the raw data for each twin pair and summing across all pairs. The use of maximum likelihood in measuring model fit allows for hypothesis testing between an original model (A, C, E) and its nested models (A and C, A and E, and E only). Because the variance component estimates are zero-bounded, the difference between an original model and its respective submodels follows a 50:50 mixture of zero and a χ2 distribution with degrees of freedom equal to the difference in model parameters (df = 1). Model fit indices are presented in Supplemental Tables S1–S4.
Results
There was generally no evidence of strong correlations of total or regional CSA with age in this adolescent sample. Out of 66 regions, 14 lobes, and 1 measure of total CSA examined, we observed only 3 correlations that were significant at p < 0.05. This is approximately the number expected by chance (81*0.05 = 4.05), suggesting no true age relationships.
In univariate twin analysis, we found no significant differences between a saturated model and its submodels where means/variances/covariances are equal by zygosity and between twins for total CSA or for ‘unadjusted’ and ‘adjusted’ regional CSA. So our twin sample was homogeneous with respect to the population.
Total CSA was highly heritable, and unique environmental effects played almost no role (MZ correlation = 0.90, DZ correlation = 0.33; A = 0.92 [95 % CI 0.84; 0.95] and E = 0.08 [95 % CI 0.05; 0.16]). Significance of the additive genetic component was further shown by a significant reduction in model fit if A was dropped from the AE model (p < 0.0001).
Unadjusted genetic and environmental effects on regional CSA
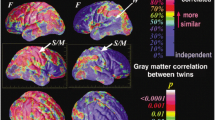
Genetic factors contributed greatly to unadjusted regional measures of CSA. Results summarized by lobes are presented in Table 2; results for all 66 ROIs are presented in Table 3. MZ correlations ranged from −0.09 to 0.81 and DZ correlations ranged from −0.16 to 0.62. The average heritability (under an AE model for regional CSA) for unadjusted CSA across all 66 ROIs was 0.52 in this adolescent sample. For 61 out of 66 ROIs and for all lobar summary measures, additive genetic effects were significant (‘no A’ model resulted in poorer fit at p < 0.05 and a 95 % confidence interval not containing zero). Heritability under an AE model was not significant in bilateral frontal pole, left fusiform gyrus, right temporal pole and left caudal anterior cingulate cortex. At the lobar level, bilateral medial temporal cortex had large (though non-significant) shared environmental contributions, and additive effects were not significant under the ACE model. Heritabilities were numerically largest for frontal lobes and smallest for medial temporal lobes, primarily due to low heritability of two component ROIs: bilateral temporal pole and fusiform gyrus. Left and right heritabilities at the lobar and ROI level were generally comparable in magnitude.
Genetic and environmental contributions to adjusted regional CSA
Heritability estimates for the influences unique to regional CSA and not shared with total CSA were smaller than those seen when total CSA was not in the model. Adjusted lobar results are shown in Table 4 and ROI results are shown in Table 5. When AE models were used for both total and regional CSA, average adjusted heritability across the 66 ROIs was 0.23. Thus, common genetic factors shared between the regional and global CSA accounted for much more of the variance than unique, regional genetic factors.
At the lobar level, only left occipital and right lateral temporal lobes were significantly heritable under a model with ACE influences on each lobe. There were large common environmental effects found for some regions, including right occipital lobe, left lateral temporal lobe, and bilateral medial temporal lobe. The C estimate was significant for the left lateral temporal lobe. For regions without high C estimates, additive genetic estimates under an AE model were significant in bilateral frontal, left occipital, right lateral temporal and right cingulate, indicating that some genetic influences on CSA remained for these lobes even after adjusting for total CSA.
Discussion
As we expected, genetic variation is an important determinant of individual differences in total cortical CSA in adolescents. Individual differences in regional CSA, not adjusted for individual differences in total CSA, were also quite heritable; the magnitude of genetic effects on regional CSA were somewhat lower than for the global measure, especially for small regions which may be more affected by measurement error.
At the lobar level, we observed that the magnitude of genetic influences on regional CSA in adolescents seemed to follow an A–P (anterior–posterior) gradient (with the highest heritabilities in frontal cortex), with bilateral symmetry. As is shown in Tables 1 and 2, heritabilities of bilateral frontal lobes were greater than several other lobes both with and without adjustment for total CSA. It may be that development-relevant genes are having strong regional influence during adolescence. Compared to other lobes, higher common and unique environmental influences were seen in the medial aspect of the temporal lobe, even relative to smaller lobar divisions, such as the cingulate region. This pattern is consistent with the effects seen in middle aged men (Eyler et al. 2011), so may suggest greater environmental influences on CSA of this region at multiple developmental stages, although the possibility of greater measurement error in this region cannot be ruled out. This phenomenon may be related to environmental factors acting to expand or contract the numbers of neurons of the medial temporal lobe in brain development during adolescence. Globally, it is generally acknowledged that gray matter (GM) volume decreases during adolescence (Giedd et al. 2008), and this seems to be due to modest decreases in cortical surface area accompanied by dramatic decreases in cortical thickness between the ages of 8 and 30 years (Ostby et al. 2009). What types of environmental factors might be uniquely acting on medial temporal CSA during this period deserves further study, as this may point to ways to maximize function of these structures which are so important for learning and memory.
It is also of note that for many of our lobar and regional measures of CSA, the MZ correlation was more than twice the DZ correlation. Although we were not powered to examine dominance effects (or, D), the pattern of the results suggests a possible role for these types of influences on surface area of some regions in adolescents which would need to be verified in a larger sample.
One difference between our findings in adolescents and those in middle aged men was a greater apparent influence of shared environment on some regional measures of CSA. This may be due to the fact that the adolescent twins were still living together in the same family and receiving education in the same school. The contributions of shared environment might be lessened in the middle aged twins due to a longer period of time living and working separately from each other. We cannot rule out, however, that our larger C estimates stem from the smaller sample size of the current study, which may have resulted in less stable cross-twin correlations.
Similar to findings in middle-aged men, the genetic factors which affect global and regional CSA seem to overlap considerable, as the portion of genetic variance unique to each region was small. Still, many regions were still significantly heritable under an AE model after accounting for genetic variance related to global CSA. These findings suggest that many of the genes responsible for variation in the area of each individual region are expected to be the same as those responsible for variation in CSA as a whole.
Our sample is characterized by a narrow age range from 12 to 18 years and follow-up MRIs are being performed to detect particular regions in which genetic influences may increase or decrease. Our findings were broadly consistent with those seen in middle aged men, in terms of the magnitudes of heritabilities, the effects of adjustment for global CSA, and apparent regional differences in the relative influences of genes versus environment.
There are several limitations to our study, which guide future work. First, the small size of our sample limited our ability to detect small effects and was generally not powerful enough to separately and reliably estimate the contribution of shared environmental (C) effects, investigate dominant genetic effects (D), or test for sex differences in our genetic models. Second, our sample also had an above average mean IQ, which limits the generalizability of the findings. Third, there are potential limitations of using traditional cortical parcellation schemes, since the regional boundaries of the Desikan-Killiany atlas (Desikan et al. 2006) are not genetically based. Fourth, we did not examine whether the genes that influence CSA are the same between regions. Distinct patterns of shared and non-shared genetic influences between regional CSA measures have been shown in adults and an important future direction will be to see if similar patterns are seen during adolescence.
References
Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J (2011) OpenMx: an open source extended structural equation modeling framework. Psychometrika 76(2):306–317
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9(2):179–194
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980
Dominicus A, Skrondal A, Gjessing HK, Pedersen NL, Palmgren J (2006) Likelihood ratio tests in behavioral genetics: problems and solutions. Behav Genet 36(2):331–340
Eaves LJ, Last KA, Young PA, Martin NG (1978) Model-fitting approaches to the analysis of human behaviour. Heredity (Edinb) 41(3):249–320
Eyler LT, Prom-Wormley E, Panizzon MS, Kaup AR, Fennema-Notestine C, Neale MC, Jernigan TL, Fischl B, Franz CE, Lyons MJ, Grant M, Stevens A, Pacheco J, Perry ME, Schmitt JE, Seidman LJ, Thermenos HW, Tsuang MT, Chen CH, Thompson WK, Jak A, Dale AM, Kremen WS (2011) Genetic and environmental contributions to regional cortical surface area in humans: a magnetic resonance imaging twin study. Cereb Cortex 21(10):2313–2321
Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P (2005) Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57(11):1313–1323
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97(20):11050–11055
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9(2):195–207
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14(1):11–22
Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, Tossell J, Addington A, Gogtay N (2008) Trajectories of anatomic brain development as a phenotype. Novartis Found Symp 289:101–112; discussion 112–108, 193-105
Kessler RC, Angermeyer M, Anthony JC, Demyttenaere RDEG, Demyttenaere K, Gasquet I, Gluzman GDEG, Gurej S, Haro O, Kawakami JM, Karam N, Levinson A, Medina Mora D, Oakley ME, Browne MA, Posada-Villa J, Stein J, Adley DJ, Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustun TB (2007) Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 6(3):168–176
Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, Franz CE, Lyons MJ, Pacheco J, Perry ME, Stevens A, Schmitt JE, Grant MD, Seidman LJ, Thermenos HW, Tsuang MT, Eisen SA, Dale AM, Fennema-Notestine C (2010) Genetic and environmental influences on the size of specific brain regions in midlife: the VETSA MRI study. Neuroimage 49(2):1213–1223
Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN (2009) Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp 30(1):163–174
Luna B, Sweeney JA (2001) Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull 27(3):443–455
Muhle R, Trentacoste SV, Rapin I (2004) The genetics of autism. Pediatrics 113(5):e472–e486
Neale MC, Cardon LR, Organization North Atlantic Treaty, Division Scientific Affairs (1992) Methodology for genetic studies of twins and families. Kluwer Academic Publishers, Boston
Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB (2009) Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 29(38):11772–11782
Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS (2009) Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19(11):2728–2735
Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9(12):947–957
Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN (2011) How does your cortex grow? J Neurosci 31(19):7174–7177
Rubenstein JL, Rakic P (1999) Genetic control of cortical development. Cereb Cortex 9(6):521–523
van Soelen IL, Brouwer RM, Peper JS, van Leeuwen M, Koenis MM, van Beijsterveldt TC, Swagerman SC, Kahn RS, Hulshoff Pol HE, Boomsma DI (2012a) Brain SCALE: brain structure and cognition: an adolescent longitudinal twin study into the genetic etiology of individual differences. Twin Res Hum Genet 15(3):453–467
van Soelen IL, Brouwer RM, van Baal GC, Schnack HG, Peper JS, Collins DL, Evans AC, Kahn RS, Boomsma DI, Hulshoff Pol HE (2012b) Genetic influences on thinning of the cerebral cortex during development. Neuroimage 59(4):3871–3880
Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC (2010) Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53(3):1135–1146
Yaoxian G, Taisheng C (1993) The China-Wechsler Intelligence Scale for Children (C-WISC). Hunan Map Publishing Company, Changhsa
Yang MJ, Tzeng CH, Tseng JY, Huang CY (2006) Determination of twin zygosity using a commercially available STR analysis of 15 unlinked loci and the gender-determining marker amelogenin—a preliminary report. Hum Reprod 21(8):2175–2179
Yoon U, Fahim C, Perusse D, Evans AC (2010) Lateralized genetic and environmental influences on human brain morphology of 8-year-old twins. Neuroimage 53(3):1117–1125
Acknowledgments
Supported by the National Nature Science Foundation of China (81101025), Dr. Eyler’s contributions were supported by the National Institutes of Health National Institutes on Aging (R01 AG22381) and the Desert-Pacific Mental Illness Research, Education, and Clinical Center. Thanks to Kan, Kees-Jan (Department of Biological Psychology, VU University Amsterdam) as well as Neeltje E.M. van Haren (Department of Psychiatry, University Medical Center Utrecht) for assistance with twin methods.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This study was ethically approved by the Ethics Committee, The First Affiliated Hospital of Chongqing Medical University, and performed in accordance with the criteria defined by the rules of the committee.
Additional information
Edited by Kristen Jacobson.
Xingshun Ma and Lisa T. Eyler are Joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, X., Eyler, L.T., Hu, X. et al. Regional Cortical Surface Area in Adolescents: A Preliminary MRI Twin Study of Genetic and Environmental Contributions. Behav Genet 46, 205–216 (2016). https://doi.org/10.1007/s10519-015-9755-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-015-9755-1