Abstract
Unprotected heterosexual intercourse is the leading cause of HIV acquisition in women. Due to the complex nature of correct and consistent condom use by both men and women, developing alternative female-controlled HIV prevention options is a global health priority. Vaginal films containing antiretroviral drugs are a potential delivery system for the prevention of HIV acquisition through sexual contact. In this study, we explored women’s preferences regarding physical characteristics of microbicide vaginal films through questionnaires and focus groups. Eighty-four sexually active, ethnically diverse women 18–30 years of age from Pittsburgh, Pennsylvania, participated in the study. Women visually and manually examined a variety of vaginal films, as well as three other vaginal products undergoing evaluation for HIV prevention: tablet, ring, and gel. Means and standard deviations or frequencies and 95 % confidence intervals were calculated for questionnaire data. Focus groups were audio-recorded, transcribed verbatim, and coded for content analysis. Women most frequently preferred vaginal films to be smooth and thin (63 %), translucent (48 %), and 2″ × 2″ square size (36 %). Driving these preferences were five major themes: ease and accuracy of use, desire for efficacy, discretion, intravaginal comfort and minimal impact, and minimizing disruption of sexual mood/activities. Women’s preferences for various microbicide vaginal film physical attributes represented a balance of multiple values. In general, women desired a comfortable, efficacious, easy to use, and minimally intrusive product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, heterosexual transmission is a leading cause of HIV infection in women (Prejean, Song, An, & Hall, 2008; UNAIDS, 2011). Greater biological susceptibility and gendered power dynamics are known to make women more vulnerable to HIV acquisition through sexual contact than men (Higgins, Hoffman, & Dworkin, 2010). One mechanism to combat this disadvantage is the use of female-controlled HIV prevention products. One potential female-controlled approach is topical pre-exposure prophylaxis or topical microbicides. These are products in development that when applied vaginally or rectally can prevent the acquisition of HIV. Both coitally dependent and independent vaginal microbicides have been evaluated in large clinical trials (Abdool Karim et al., 2010; Baeten et al., 2016; Marrazzo et al., 2015). Adherence issues have arisen with vaginal microbicide gel.
Of interest is the development of a vaginal film containing antiretroviral(s) as an alternative vaginal microbicide dosage form. Hypothetical advantages of microbicide vaginal films include low cost, easy storage, portability, and minimal waste. Characteristics of vaginal films that women have liked include female control; covert use; ease of use; comfort; less mess compared to vaginal gel or suppository; fast dissolving time; less leakage; perception of a tight, dry vagina; and not being felt inside the vagina (Coggins et al., 1998; Green et al., 2001; Nel, Mitchnick, Risha, Muungo, & Norick, 2011; Rustomjee, Abdool Karim, Abdool Karim, Laga, & Stein, 1999; Steiner et al., 1995; Visness, Ulin, Pfannenschmidt, & Zekeng, 1998). The primary problems described with vaginal films have included difficulty with insertion, uncertainty regarding adequacy of film placement using the fingers, and perceived interruption of sex (Bunge et al., 2016; Coggins et al., 1998; Mason et al., 2003; Mauck, Baker, Barr, Abercrombie, & Archer, 1997; Raymond et al., 1999, 2005; Steiner et al., 1995), with some studies identifying film as the least preferred vaginal dosage form (Green et al., 2001; Hardy, Jimenez, de Padua, & Zaneveld, 1998; Pool et al., 2000). First-in-human phase 1 trials of a dapivirine vaginal film and tenofovir vaginal film, both reverse transcriptase inhibitor-containing films, have been conducted. Bunge et al. showed that film users, when compared to gel users, were less likely to report product leakage and to describe the product as uncomfortable, but more likely to define the product as difficult to vaginally insert. For those women who had poor product placement with films, prior tampon and lubricant use were not as prevalent as in those with good film placement (Bunge et al., 2016).
In this study, the primary objective was to describe preferred physical characteristics of a vaginal film for HIV prevention in sexually active women, in order to optimize the design of this dosage form for this indication. The secondary objective was to describe attitudes toward the use of vaginal films for HIV prevention. This study is the first report on women’s preferences for and attitudes about the vaginal film physical characteristics of texture/thickness, size/shape, and appearance.
Method
Participants and Procedure
We employed a blend of questionnaires and focus group discussion to capture a descriptively rich understanding of women’s preferences for vaginal film characteristics and attitudes, beliefs, and motivations that influence these preferences (Giacomini, Cook, & Evidence-Based Medicine Working Group, 2000a). Qualitative approaches such as focus groups allow subjects to describe their thoughts and preferences within an open discussion without the limitations of predetermined items or assumptions that can be encountered with quantitative approaches like surveys and questionnaires. While quantitative tools limit subjects to a selection of responses presumed by investigators to represent their feelings, experiences, and beliefs, qualitative methods provide an open approach allowing subjects to share their perspectives using their own words (Giacomini et al., 2000a; Patton, 2015). Questionnaires were used both as a springboard for group discussion as well as a means to capture key elements of group discussions in a corroborating fashion (Creswell, 2009). The University of Pittsburgh Institutional Review Board (IRB) approved the study.
Participants were eligible if they were (1) female, (2) aged 18–30 years, and (3) able and willing to provide written informed consent. Women who reported no episodes of vaginal intercourse in the prior year or who were pregnant were not eligible for participation. Participants were recruited using IRB-approved advertisements placed in Pittsburgh area neighborhoods, colleges, research registries, craigslist.com, the Magee-Womens Hospital of UPMC Ambulatory Care Clinics, and the Allegheny County Health Department Sexually Transmitted Disease Clinic. Participants in two other studies of reproductive tract infections at Magee-Womens Hospital of UPMC, who gave permission to be contacted for future studies, were also invited to participate.
We employed purposive sampling in terms of socioeconomic and racial/ethnic background through neighborhood recruitment guided by 2000 US Census Bureau Pittsburgh area demographic data (Patton, 2015). Specifically, we sought to include likely users of vaginal microbicides by including women living in communities disproportionately affected by HIV, including African American women (CDC, 2016). Sample size was determined by thematic saturation related to vaginal film physical characteristic preferences; we continued to recruit and conduct focus groups until we noted redundancy in discussions (Giacomini et al., 2000a; Giacomini, Cook, & Evidence-Based Medicine Working Group, 2000b; Patton, 2015). Participants took part in focus groups on a rolling basis depending on scheduling availability, with all focus groups having participants of varying socioeconomic status and racial/ethnic background. All participants received light refreshments and $30 compensation for time and travel.
Measures
After confirming eligibility criteria and providing written informed consent, participants completed an initial questionnaire collecting sociodemographic and sexual history information. Participants then took part in a moderated group discussion lasting approximately 75 min. One trained moderator (MF) led all 12 focus groups. The introduction, format, and primary discussion questions were consistent across all groups. The moderator worked to ensure groups’ discussions were as open and dynamic as possible in order to foster a variety of opinions and perspectives.
During the introduction, the moderator provided a brief overview of vaginal film, tablet, gel, and ring, including a summary of different dosing and application methods as used for contraception. The primary group discussion questions included: (1) thoughts about the idea of a vaginal film; (2) thoughts about hypothetical vaginal film use; (3) characteristics of an ideal vaginal film; (4) preferred film characteristics; and (5) preferred vaginal product type among film, tablet, ring, and gel.
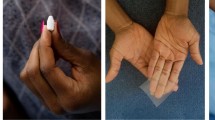
As part of the group discussion, women had the opportunity to view, hold, and touch a variety of placebo and nonoxynol-9 vaginal films immobilized on Plexiglas sheets (vaginal contraceptive film, VCF, by Apothecus) (Fig. 1a–c). Immediately after examining these demonstration films, participants individually ranked their preferences regarding film texture/thickness, size/shape, and appearance using a 3-item questionnaire. Women then shared their rankings with the group and discussed the reasons for their preferences. Again, all vaginal film characteristic preferences and attitudes indicated by the participants are based on hypothetical, not actual product use.
Demonstration vaginal products. a Films of varying texture and thickness. From left to right, textured/thin, textured/thick, smooth/thin. b Films of varying size and shape. From left to right, 2″ × 2″, 1″ × 1″, 1″ × 2″, 1″ × 3″. c Films of varying appearance. From left to right, clear, translucent, and opaque. d From left to right, placebo ring, nonoxynol-9 vaginal tablet, and nonoxynol-9 vaginal gel
Women next had the opportunity to view, hold, and touch a placebo vaginal ring (International Partnership for Microbicides) and nonoxynol-9-containing vaginal tablet and gel (Encare vaginal contraceptive insert by Blairex and vaginal contraceptive gel by Ortho Options) (Fig. 1d). The focus group discussion was followed by a final questionnaire collecting theoretical preferences regarding vaginal products for HIV prevention in general and also specific to vaginal film. Each study session lasted 2 h, including informed consent.
Data Analysis
Questionnaires
Frequencies and 95 % confidence intervals were calculated for categorical data. Means and SDs were calculated for continuous data.
Focus Groups
Focus group discussions were audio-recorded and transcribed verbatim. We performed qualitative content analyses using a modified grounded theory approach (Crabtree & Miller, 1999; Daveson, O’Callaghan, & Grocke, 2008; Sandelowski, 2000; Strauss & Corbin, 1990). Specifically, we relied on the processes of grounded theory, including constant comparison among focus group transcripts and open coding to label and categorize emergent data, but modified this approach with the inclusion of existing research questions to generate the full analysis (Glaser, 2002). Two investigators independently read and coded the transcripts and met to compare coding and create a codebook in an iterative and collaborative manner. Agreement on discrepant codes was achieved through a discussion process between coders. The codebook and coding processes were stored and organized using Atlas.ti qualitative software and were periodically reviewed by a third investigator to further prevent bias. The final codebook was systematically applied to all focus group transcripts by the same two investigators independently to minimize interpretive bias (Patton, 1999). Had there been any differences in interpretation, a third investigator would have arbitrated this difference; no interpretative differences were noted. Patterns and relationships among codes were identified to develop themes and conceptual ordering (Strauss & Corbin, 1990). The final step in data analysis was the process of corroboration, or triangulation, a method used in qualitative analysis to assess analytic credibility, ensure consistency, and guard against bias (Giacomini et al., 2000a; Mays & Pope, 2000; Patton, 1999). In addition to investigator corroboration by using two independent coders, other methods of corroboration included review of study findings among the larger study group, work-in-progress presentations, and review of study findings with HIV microbicide researchers external to the study (Patton, 1999, 2015). All reviewers endorsed good corroboration of our themes.
Results
Study Population
Participant characteristics are shown in Table 1. These characteristics reflect information recorded by the women on one of the self-administered written study questionnaires. Eighty-four women participated in the study. Previous use of vaginal products was common, although only three women reported having ever used a vaginal film. The most commonly used intravaginal product reported was tampons (88 %), while 17 % of women reported past spermicide use, 14 % had used a vaginal ring, and 6 % reported use of vaginal tablets. Participants were predominantly young (mean age 23.4 years) and unmarried. Higher-risk sexual activity was common, with 57 % reporting having more than one sexual partner in the past year, 48 % reporting past reproductive tract infection, 40 % reporting ever having anal sex, and 11 % reporting trading sex for money or other goods. Only 14 % of women reported always using condoms with vaginal sex in the past year.
Attitudes Regarding Vaginal Films
All 84 women completed the written questionnaires. Table 2 presents the participants’ perceptions about vaginal films after visual and manual manipulation. Participants reported their beliefs that films were likely to be very comfortable, very easy to insert, and result in no change in sexual pleasure either personally or for a sexual partner (Table 2). Participants most frequently preferred films to be smooth/thin (63 %), 2″ × 2″ square size (36 %), and translucent (48 %) (Table 3). Participants strongly preferred vaginal films to be odorless (84 %) and flavorless (84 %). For film insertion, 49 % of women desired an applicator, 37 % preferred to use their fingers only, and 14 % were neutral with regard to using an applicator or their fingers. Results presented in Tables 2 and 3 were gathered from the information recorded by the women on written study questionnaires.
Qualitative Analysis
We held a total of 12 focus groups, each with five to ten participants. In discussing preferred physical characteristics of vaginal films, five major themes emerged: ease and accuracy of use, desire for efficacy, discretion, comfort and minimal impact within the vagina, and minimizing disruption of sex. All of these explanatory themes were emergent. Table 4 contains participant quotations that illustrate these five themes.
Ease and Accuracy of Use
Participants justified hypothetical preference for film characteristics based on beliefs that certain characteristics could impact correct versus incorrect use. For example, one woman described how the larger film increased her confidence that she would receive adequate protection. Many women felt that a 1″ × 1″ film was likely to be “lost” during insertion due to its small size. Several others felt that a small vaginal film would simplify insertion. For film appearance, most participants found a minimum level of visual discernibility reassuring and preferred a translucent or opaque film over a clear film.
Women were more divided about the optimal film texture and thickness that would promote easy and accurate insertion. Smooth, thin film was preferred by one woman specifically because of perceived ease of insertion. However, several other participants expressed concerns regarding ease and accuracy of insertion with smooth/thin film, including excessive flimsiness, slipping through the fingers and being lost, being unable to physically sense adequate insertion, and dissolving too rapidly.
Desire for Efficacy
Also prominent in preferred vaginal film characteristics was the desire for efficacy in preventing HIV infection. Film characteristics that many women associated with greater efficacy included larger size, square shape, texture, and translucent or opaque rather than clear appearance. In particular, several women felt that a larger film would offer greater efficacy by dissolving and distributing over the vaginal surface to a greater extent than a smaller film. Only a minority of women felt vaginal film size would not matter with regard to efficacy in HIV prevention since medication type or content would be most important. Square shape was another characteristic that several women associated with better vaginal mucosal coverage.
With regard to film appearance, many women equated a higher degree of film opacity with greater medication content and efficacy. Similarly, several women associated more texture with more medication. Participants were divided regarding whether more or less vaginal film thickness might provide greater efficacy. Many equated thick films to more medication or longer-lasting efficacy, while others envisioned greater medication release with rapid disintegration of thin films.
For several participants, the desire for efficacy in the context of alcohol consumption was of special concern: “I don’t want to end up finding out that it’s not, that it doesn’t work if you’ve been drinking…” Another participant stated that she needed a product that was easy to use effectively, even when inebriated. Women felt efficacy in the context of alcohol consumption was important given their experiences of higher-risk sexual encounters while drinking alcohol. As a result, extended efficacy was viewed as favorable, with suggestions for monthly, weekly, or daily dosing.
Discretion
Many participants desired discreet use of vaginal films. The importance of discreet use varied, however, from unobtrusiveness during sex to permitting concealment. Women perceived concealment in using a vaginal film for HIV prevention to be helpful during suspected infidelity within a relationship: “Say I suspected he was cheating on me. If you could disguise it as a lubricant, for me that would be an effective way to use it.” Several other women wanted vaginal films to be unobtrusive during sex in order to prevent any potential partner reaction to HIV-preventative vaginal film use: “I just want it to be so that the guy doesn’t even know that it’s there. Cause…I don’t know…they kind of trip out. And I don’t want them to even know that they’re being protected, or I’m being protected.”
Translucent and clear films were perceived to be less visually noticeable than opaque film, both during insertion in front of a partner and also in terms of any potential vaginal discharge. For film texture and thickness, participants felt that a smooth/thin film would be most discreet.
Several participants wanted discretion external to their sexual partnerships and expressed the desire for circumspect use around family and strangers:
It would be great if it came in the Listerene pack size. Something cute that you wouldn’t have to…Where you don’t have to worry about somebody going through your purse and being like, “What the hell is this?” Like, I don’t want my mom knowing about it.
Some women considered circumspect use important because of a sense of stigma with HIV or with being at risk for HIV infection:
I think a lot of younger girls would be hesitant to ask their parents for something that just protects them against HIV. Because I know I would. Cause my parents would be like, “Well, what are you doing that you’re going to get HIV?” Whereas if it was paired with birth control or maybe some less severe STDs, I wouldn’t be as embarrassed or ashamed to be like, “I need something that’s going to prevent just HIV.” Because I would be doing things that are putting me at risk.
Minimal Impact
Offering minimal impact on the vaginal environment was a strongly held notion pervasive throughout the focus group sessions that included both physical comfort and nominal impact on the natural state of the vagina. Not being felt in the vagina was highly desirable and perceived to coincide with greater comfort. The vaginal film characteristics most frequently associated with comfort were being smooth and thin. Several women were particularly concerned about textured films possibly being uncomfortable without sufficient vaginal lubrication. Only a small minority of women believed that feeling a vaginal film within the vagina would be preferable, in order to physically sense when a vaginal film is correctly inserted. Several women perceived larger size film as more likely to be felt within the vagina.
To many women, maintaining the natural state of the vagina and preserving the natural color of vaginal fluid meant good vaginal health. The appearance of opaque film, therefore, caused concern for some: “I think with something like C [opaque film], I guess even B [translucent film] and I did pick it, but I think that if something was leaking and it was that color you could mistake it as an infection or something.” Participants felt a change in vaginal fluid color might be worrisome both personally as well as with respect to a partner’s potential reaction.
Minimizing Disruption of Sexual Mood and Activity
Another product characteristic desired by participants was that film use would allow them to carry out sexual activities, from maintaining sexual excitement to not hindering the flow of a sexual episode. Several women felt that if the physical characteristics of a vaginal film presented too much of a challenge in terms of use, the mood for sex might be lost. For example, women expressed concern that the larger film size would be more challenging to insert and thus hamper their sexual interest.
Participants perceived even packaging as potentially affecting the mood: “I hope they just make a funner way to open it [VCF] up. It just does not look like, oh, we’re going to get romantic and into the bed. That look like you got something.” Appearing medicinal was generally regarded as undesirable. Another potential hindrance to the flow of sexual activity was disintegration time, which was perceived to be potentially longer for larger films, greater thickness and texture, and higher opacity.
As seen in Table 1, women in the study engaged in a variety of sexual activity types, including oral and anal sex. As a result, many liked film characteristics conducive to types of sexual activity other than vaginal intercourse. For example, one woman said: “I liked B [1″ × 1″ film] the best because if I wanted it the way I was telling you for my mouth, my anal, plus my vagina. B [1″ × 1″ film] looks like the perfect size just to toss it in my mouth, or you know, just toss it.” Several women also pointed out the importance of inoffensive taste for a dissolved vaginal film in permitting oral sex. Many women described sex occurring outside the home and desired the portability afforded by a smaller film: “I put B [1″ × 1″ film] first just cause I think I’d like the smaller and you’d be able to just have them in your purse or whatever, and they wouldn’t take up as much room.”
Discussion
The results of this study of demonstration vaginal films and hypothetical product use suggest that women most frequently prefer vaginal films to be smooth/thin, 2″ × 2″ square, translucent, odorless, and flavorless. Multiple, sometimes competing reasons drove women’s preferences regarding film characteristics, including ease and accuracy of use, desire for efficacy, discretion, comfort and minimal impact within the vagina, and minimizing disruption of sexual activity. The leading emergent themes for preferring smooth, thin films included comfort within the vagina and discretion, while leading emergent themes for preferring 2″ × 2″ square films included ease and accuracy of use and desire for efficacy. Preference for translucent films appeared most related to desire for efficacy, discretion, and minimal impact within the vagina.
Women were generally receptive to the use of a vaginal film for HIV prevention, despite lack of previous experience with vaginal films. The majority of women expressed the belief that vaginal films were likely to be “very comfortable” and “very easy” to insert. Reasons for liking vaginal films included minimal perception of use by user or her partner, likelihood of physical comfort, potential for less embarrassment compared to condoms, skin-to-skin genital contact, and female control of use. However, concern about inadequate film disintegration was a major factor to some women preferring a vaginal dosage formulation other than film. Further, compatibility of a film with anal and/or oral sex may be a desirable characteristic given the demographics and comments of study participants.
Prior studies of vaginal film acceptability have utilized the VCF, which is a 2″ × 2″ square, smooth, thin, and translucent film (Coggins et al., 1998; Green et al., 2001; Hardy et al., 1998; Mauck et al., 1997; Pool et al., 2000; Raymond et al., 1999, 2005; Steiner et al., 1995). In one recent study of microbicide acceptability among 526 sexually active women in three African countries, participants used a vaginal film, tablet, and soft gel capsule for seven days each. The film used was a translucent, off-white placebo vaginal film (Nel et al., 2011). In this study, both the film and the soft gel capsule were preferred over the tablet, primarily because of faster dissolution and ease of insertion. These data are consistent with the data from the present study conducted among at-risk young women in Pittsburgh. Taken together, these data suggest that film products may have high acceptability among potential consumers.
A tension between perceived convenience and product discretion existed for many of our participants. Women liked the idea of a vaginal film not being noticed personally or by a partner but were concerned that manual insertion could be difficult or prone to error. Interestingly, this concern was also an opinion noted during a recent Phase 1 microbicide film trial (Bunge et al., 2016).
The primary limitation of this study is that it relies on visual and manual product examination (hypothetical use) rather than actual use. Although product preference and acceptability are best determined by actual use, the strategy utilized in our study may predict a woman’s willingness to adopt a new method or product. Another limitation is the inclusion of a fixed range of demonstration products. In particular, only one type of ring, tablet, or gel was shown. Due to the practicality of scheduling participants on a rolling basis, focus groups were not homogenous sets of women. As a result, some participants that may have felt dissimilar to others in the group may not have shared their thoughts as openly. However, the focus group moderator did encourage all group participants to voice opinions during each session. Further, the women in this study were largely young, urban, white or black, never married, and with high school or GED completion. As a result, the findings are likely to be most generalizable to similar populations. Additionally, we did not link specific focus group statements to participant characteristics like partnership status, race/ethnicity, or socioeconomic status in order to identify trends in responses. Addressing these limitations in future studies could enrich and expand upon this current research and prove useful to the development of HIV prevention products.
The proposed preferences shared by women in this study represent a balance of priorities. A majority of women preferred a smooth, thin film over a textured, thin or thick film. Preferences were less distinct in regard to film size, shape, and appearance. Driving women’s choices is a blend of individual, interpersonal, environmental, community, and societal factors—all of which warrant further investigation into broader factors that may influence product preferences and acceptability. However, the complexity of women’s sexual decision-making within the context of this study appears to necessitate as wide a variety of HIV-preventative products as is feasible in terms of resource and developmental allocation. These study findings add to the breadth of vaginal film literature, support the acceptability of vaginal films as a formulation for prevention of HIV transmission, and highlight the importance of multiple attitudes in selecting vaginal film physical characteristics.
References
Abdool Karim, Q., Abdool Karim, S. S., Frohlich, J. A., Grobler, A. C., Baxter, C., Mansoor, L. E., … Gengiah, T. N. (2010). Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science, 329, 1168–1174. doi:10.1126/science.1193748.
Baeten, J. M., Palanee-Phillips, T., Brown, E. R., Schwartz, K., Soto-Torres, L. E., Govender, V., … Siva, S. (2016). Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New England Journal of Medicine. doi:10.1056/NEJMoa1506110.
Bunge, K. E., Dezzutti, C. S., Rohan, L. C., Hendrix, C. W., Marzinke, M. A., Richardson-Harman, N., … Hillier, S. L. (2016). A Phase 1 trial to assess the safety, acceptability, pharmacokinetics and pharmacodynamics of a novel dapivirine vaginal film. Journal of Acquired Immune Deficiency Syndromes, 71, 498–505. doi:10.1097/QAI.0000000000000897.
CDC. (2016, February 4). HIV among African Americans. Retrieved from http://www.cdc.gov/hiv/group/racialethnic/africanamericans/.
Coggins, C., Elias, C. J., Atisook, R., Bassett, M. T., Ettiegnene-Traore, V., Ghys, P. D., … VanDevanter, N. L. (1998). Women’s preferences regarding the formulation of over-the-counter vaginal spermicides. AIDS, 12, 1389–1391.
Crabtree, B. F., & Miller, W. L. (1999). Doing qualitative research (2nd ed.). Thousand Oaks, CA: Sage Publications.
Creswell, J. W. (2009). Research design: Qualitative, quantitative, and mixed methods approaches (3rd ed.). Thousand Oaks, CA: Sage Publications.
Daveson, B., O’Callaghan, C., & Grocke, D. (2008). Indigenous music therapy theory building through grounded theory research: the developing indigenous theory framework. Arts in Psychotherapy, 35, 280–286. doi:10.1016/j.aip.2008.06.005.
Giacomini, M. K., Cook, D. J., & Evidence-Based Medicine Working Group. (2000a). Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Journal of the American Medical Association, 284, 357–362.
Giacomini, M. K., Cook, D. J., & Evidence-Based Medicine Working Group. (2000b). Users’ guides to the medical literature: XXIII. Qualitative research in health care B. What are the results and how do they help me care for my patients? Journal of the American Medical Association, 284, 478–482.
Glaser, B. G. (2002). Conceptualization: On theory and theorizing using grounded theory. International Journal of Qualitative Methods, 1, 23–38.
Green, G., Pool, R., Harrison, S., Hart, G. J., Wilkinson, J., Nyanzi, S., & Whitworth, J. A. (2001). Female control of sexuality: illusion or reality? Use of vaginal products in south west Uganda. Social Science and Medicine, 52, 585–598.
Hardy, E., Jimenez, A. L., de Padua, K. S., & Zaneveld, L. J. (1998). Women’s preferences for vaginal antimicrobial contraceptives. III. Choice of a formulation, applicator, and packaging. Contraception, 58, 245–249.
Higgins, J. A., Hoffman, S., & Dworkin, S. L. (2010). Rethinking gender, heterosexual men, and women’s vulnerability to HIV/AIDS. American Journal of Public Health, 100, 435–445. doi:10.2105/AJPH.2009.159723.
Marrazzo, J. M., Ramjee, G., Richardson, B. A., Gomez, K., Mgodi, N., Nair, G., … Hendrix, C. W. (2015). Tenofovir-based preexposure prophylaxis for HIV infection among African women. New England Journal of Medicine, 372, 509–518. doi:10.1056/NEJMoa1402269.
Mason, T. H., Foster, S. E., Finlinson, H. A., Morrow, K. M., Rosen, R., Vining, S., … Seage, G. R. (2003). Perspectives related to the potential use of vaginal microbicides among drug-involved women: Focus groups in three cities in the United States and Puerto Rico. AIDS and Behavior, 7, 339–351.
Mauck, C. K., Baker, J. M., Barr, S. P., Abercrombie, T. J., & Archer, D. F. (1997). A phase I comparative study of contraceptive vaginal films containing benzalkonium chloride and nonoxynol-9. Postcoital testing and colposcopy. Contraception, 56, 89–96.
Mays, N., & Pope, C. (2000). Qualitative research in health care. Assessing quality in qualitative research. British Medical Journal, 320, 50–52.
Nel, A. M., Mitchnick, L. B., Risha, P., Muungo, L. T., & Norick, P. M. (2011). Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. Journal of Women’s Health, 20, 1207–1214. doi:10.1089/jwh.2010.2476.
Patton, M. Q. (1999). Enhancing the quality and credibility of qualitative analysis. Health Services Research, 34, 1189–1208.
Patton, M. Q. (2015). Qualitative research and evaluation methods (4th ed.). Thousand Oaks, CA: Sage Publications.
Pool, R., Whitworth, J. A., Green, G., Mbonye, A. K., Harrison, S., Wilkinson, J., & Hart, G. J. (2000). An acceptability study of female-controlled methods of protection against HIV and STDs in south-western Uganda. International Journal of STD and AIDS, 11, 162–167.
Prejean, J., Song, R., An, Q., & Hall, H. (2008). Subpopulation estimates from the HIV incidence surveillance system—United States, 2006. Morbidity and Mortality Weekly Report, 57, 985–989.
Raymond, E., Alvarado, G., Ledesma, L., Diaz, S., Bassol, S., Morales, E., … Carlos, G. (1999). Acceptability of two spermicides in five countries. Contraception, 60, 45–50.
Raymond, E. G., Chen, P. L., Condon, S., Luoto, J., Barnhart, K. T., Creinin, M. D., … Blackwell, R. (2005). Acceptability of five nonoxynol-9 spermicides. Contraception, 71, 438–442. doi:10.1016/j.contraception.2004.12.023.
Rustomjee, R., Abdool Karim, Q., Abdool Karim, S. S., Laga, M., & Stein, Z. (1999). Phase 1 trial of nonoxynol-9 film among sex workers in South Africa. AIDS, 13, 1511–1515.
Sandelowski, M. (2000). Whatever happened to qualitative description? Research in Nursing & Health, 23, 334–340.
Steiner, M., Spruyt, A., Joanis, C., Glover, L., Cordero, M., Alvarado, G., & Onoka, C. (1995). Acceptability of spermicidal film and foaming tablets among women in 3 countries. International Family Planning Perspectives, 21, 104–107.
Strauss, A. L., & Corbin, J. M. (1990). Basics of qualitative research: Grounded theory procedures and techniques. Newbury Park, CA: Sage Publications.
UNAIDS. (2011). Global HIV/AIDS response. Retrieved from http://www.who.int/hiv/pub/progress_report2011/hiv_full_report_2011.pdf.
Visness, C. M., Ulin, P., Pfannenschmidt, S., & Zekeng, L. (1998). Views of Cameroonian sex workers on a woman-controlled method of contraception and disease protection. International Journal of STD and AIDS, 9, 695–699.
Acknowledgments
This study was funded by a grant from the Doris Duke Charitable Foundation to the University of Pittsburgh School of Medicine to support Clinical Research Fellow Maria Fan and by Grant Number NIH U19AI082639. The authors wish to thank the study participants, the International Partnership for Microbicides for supplying placebo vaginal rings, and the many researchers who contributed their assistance and advice.
Funding
This study was funded by a grant from the Doris Duke Charitable Foundation to the University of Pittsburgh School of Medicine to support Clinical Research Fellow Maria Fan and by Grant Number NIH U19AI082639.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a real or perceived conflict of interest in any of the material that is presented in the manuscript.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fan, M.D., Kramzer, L.F., Hillier, S.L. et al. Preferred Physical Characteristics of Vaginal Film Microbicides for HIV Prevention in Pittsburgh Women. Arch Sex Behav 46, 1111–1119 (2017). https://doi.org/10.1007/s10508-016-0816-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-016-0816-1