Abstract
A newly isolated rhabdovirus-like agent, named SHRV-In, obtained from snakehead fish in India was further characterized in this study. The virus demonstrated growth in various cell lines including those derived from snakehead and seabass species. It exhibited sensitivity to acidic conditions (pH 3.0) and exposure to 56 °C. Five structural proteins of 20 to 68 kDa were found in the purified virus. Experimental infection trials conducted on catfish (Pangasius pangasius) resulted in mortality 72 h post-infection, whereas no mortality was observed in rohu (Labeo rohita) and common carp (Cyprinus carpio) fingerlings. Molecular characterization of the virus involved sequencing of the N, P, M, and G gene fragments after PCR amplification using specific primers. Sequence analysis of all four viral genes showed high similarity with the previously isolated snakehead rhabdovirus (SHRV) from Thailand. Phylogenetic analysis and gene sequence alignment placed the SHRV-In isolate within the Novirhabdovirus group, which includes viruses like VHSV (viral hemorrhagic septicemia virus), IHNV (infectious hematopoietic necrosis virus), and SHRV. However, our repeated trials failed to amplify the NV gene in this isolate SHRV-In.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
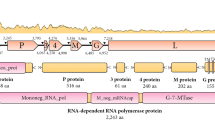
Rhabdoviruses, which infect a vast assortment of host species within mammals, birds, reptiles, amphibians, fish, crustaceans, and insects, are classified within the family Rhabdoviridae. This family falls under the order Mononegavirales, comprising three subfamilies, 46 genera, and a total of 318 species (Walker et al. 2022). The Rhabdoviridae family encompasses RNA genomes that are single-stranded and negative-sense, typically spanning 10 to 16 kb in length. Virions typically exhibit an enveloped morphology, commonly appearing as bullet-shaped or bacilliform, although filamentous non-enveloped forms have also been documented. These genomes typically comprise single RNA molecules with termini that are partially complementary, although variations exist. While most rhabdoviruses possess five genes encoding structural proteins (N, P, M, G, and L), some may feature additional genes or alternative open reading frames (ORFs) within the structural protein genes, enabling the encoding of supplementary proteins (Dietzgen et al. 2017).
The species that infect fish comes under the genera Perhabdovirus, Siniperhavirus, Scophrhavirus, and Sprivivirus are assigned to the subfamily Alpharhabdovirinae (Walker et al. 2022). Genus Novirhabdovirus under the subfamily Gammarhabdovirinae includes the classical rhabdoviruses that infect fish such as viral hemorrhagic septicemia virus (VHSV) and infectious hematopoietic necrosis virus (IHNV). Novirhabdoviruses induce severe hemorrhagic disease in fish and are distinguished phylogenetically from other genera by the presence of an extra gene, NV, located between G and L genes (Kurath and Leong 1985; Schuetze et al. 1996; Kurath et al. 1997).
The rhabdoviruses such as viral hemorrhagic septicemia virus (VHSV), infectious hematopoietic necrosis virus (IHNV), spring viremia of carp virus (SVCV), and which affect commercially important farming fishes such as carps, salmon, and trout are listed under OIE (WOAH) as notifiable diseases. All three diseases affect coldwater species, occurring optimally within a temperature range of 15–25 °C, and are characterized by severe hemorrhagic septicemia (Dietzgen et al. 2017). However, temperature range for SVCV is 4 ± 31 °C, with optimum replication at 20 ± 22 °C, while IHNV, HIRRV, and VHSV all have colder temperature ranges of 4 ± 20 °C, with replication optima of approximately 10 ± 15 °C (Wolf 1988). Since the first identification of a fish rhabdovirus in 1938 from European rainbow trout (Oncorhynchus mykiss) by Schaperclaus, subsequent efforts have led to the isolation, cultivation in cell cultures, and genomic sequencing of several fish rhabdoviruses. These viruses tend to replicate extensively in organs like the kidney, spleen, and brain, with lesser replication occurring in the liver, heart, and gills (Lovy et al. 2012).
The rhabdovirus originating from snakehead fish was initially documented in the early 1980s, having been identified in diseased snakeheads from Thailand, Myanmar, and the Philippines (John and George 2012). Subsequently, it was also associated with cases of epizootic ulcerative syndrome (EUS) (Frerichs et al. 1986; Ahne et al. 1988; John and George 2012). The full genome sequence of one such snakehead rhabdovirus (SHRV) from Southeast Asia is now accessible (AF147498) (Kasornchandra et al. 1992). Additionally, a rhabdovirus, belonging to the genus Vesiculovirus (SHVV), was identified in hybrid snakehead fish in China (Zeng et al. 2014). Notably, unlike other fish rhabdoviruses, these isolates from snakehead fish exhibit optimal multiplication at a temperature of 27 °C. As of now, there is only one documented instance of a rhabdovirus detection and isolation from snakehead fish in India (John et al. 2021), which is further characterized in the present study. The virulence of the isolated virus strain was also evaluated through experimental infection studies conducted on significant tropical cultivable fish species.
Materials and methods
Virus and cell lines
A rhabdovirus-like agent (SNLKD1116, SHRV-In) isolated from the infected snakehead (John et al. 2021) was characterized in the present study. The virus was isolated from infected snakeheads that were suffering from mortalities in the extensive type aquaculture tanks in South Tamil Nadu, India. Clinically, the fish showed surface ulcerations and had hemorrhagic areas in the flank and snout. Internally the fish showed serosanguinous fluid in the peritoneal cavity (John et al. 2021). Blotched snakehead virus (BSNV) (John and Richards 1999) and similar damsel virus (Sivasankar et al. 2017) were used for comparative biophysical studies. The virus isolates were propagated in SSN1 cell lines (Frerichs et al. 1991) and SNKD2a cells (John and George 2006) at 27 °C, and infectivity values were calculated. An L15 medium supplemented with a 1 × antibiotic antimycotic solution and 10% fetal bovine serum (Gibco, USA) was used for culturing the cells. Virus propagation was achieved by infecting cultures reaching 75–80% confluence, with extensive cytopathic effect (CPE) observed after 3 days of incubation. Upon reaching 85–90% CPE, the culture was harvested and centrifuged at 3000 g for 15 min at 4 °C. The viral titer (TCID50 ml−1) of the clarified supernatant was determined using an endpoint dilution assay in 96-well plates and calculated using the method outlined by Spearman and Kärber (1931).
Biophysical and biochemical characterization
The heat stability and resilience to pH (3, 7, and 11) treatment of SNLKD1116 were investigated following the method outlined in a previous study (George et al. 2015). Chloroform sensitivity of the viral isolate was assessed according to the protocol provided by Feldman and Wang (1961). The nature and type of nucleic acid of the isolate were analyzed utilizing 5-iodo-2-deoxyuridine (IUDR) and staining with acridine orange, following the procedure detailed by Rovozzo and Burke (1973). Transmission electron micrographs of the virus were also carried out as reported in our previous study (John et al. 2021).
Purification of virus
Ultracentrifugation was employed to concentrate the rhabdovirus-like agent. Initially, the virus was inoculated into a 175-cm2 flask having a confluent monolayer of SSN1 cells After observing the extensive cytopathic effect (CPE) 3 days post-inoculation, the virus was purified as described earlier (George et al. 2015) with the following variations. The initial clarification was done at 3000 g for 15 min, and the pelleting of the virus by ultracentrifugation was done at 138,000 g for 1 h at 4 ℃. The pooled virus pellet was layered on to a 20% sucrose cushion for purification and spun again at 138,000 g for 1 h at 4 ℃. Purified virus was stored in TNE buffer at −80 ℃ until used.
Analysis of structural proteins
The purified rhabdovirus-like agent’s structural proteins were anlayzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) employing a vertical gel electrophoresis unit (GeNei TM, India), following previously established protocols (George et al. 2015).
Primer designing and sequencing of PCR products
Primer3 plus software was used for designing primers using the snakehead rhabdovirus (SHRV) genome (GenBank Accession No. AF147498) as template. The primer sequences along with their expected fragment sizes are provided in Table 1. Sequencing of the resulting PCR products were done through outsourcing to MWG, Eurofins Genomics India Pvt Ltd, Bangalore, India.
RNA extraction and cDNA synthesis
Total RNA was isolated from each flask of SSN1 cells inoculated with the virus, as well as from the corresponding control flask, separately. The cells were harvested by aspiration, followed by clarification through centrifugation at 2000 g for 5 min. The harvested cells were lysed with 1 ml of Trizol reagent and then incubated at room temperature for 5 min. Subsequently, the mixture was vigorously shaken by hand for 15 s after adding 0.2 ml of chloroform. After further incubation for 2–3 min at room temperature, the lysate was clarified by centrifugation at 12,000 g for 15 min at 4 °C. The aqueous supernatant was collected and combined with 0.5 ml of isopropyl alcohol. Following centrifugation at 12,000 g for 10 min, the supernatant was discarded, and the RNA pellet was washed twice with 75% ethanol by centrifugation at 7500 g for 5 min at 4 °C. The ethanol supernatant was removed, and the RNA pellets were briefly air-dried before being dissolved in RNase-free water. The RNA solution was then incubated at 55–60 °C for 10 min and stored at −70 °C.
For cDNA synthesis, RNA samples were subjected to reverse transcription using a cDNA synthesis kit (Thermo Fisher, UK) and random primers according to the manufacturer’s instructions. Specifically, 10 µl of extracted RNA was mixed with 10 µl of cDNA synthesis mixture containing 2 µl of reverse gene-specific primer, 2 µl of 10 × RT buffer, 0.8 µl of 25 × dNTP, 1 µl of MultiScribe Reverse Transcriptase, 1 µl of RNase inhibitor, and 3.2 µl of nuclease-free water. The reaction mixture was then incubated at 25 °C for 10 min, followed by 37 °C for 160 min, and finally 85 °C for 5 min. The resulting cDNA was subsequently utilized for PCR analysis to further characterize the gene of interest.
PCR amplification
PCR was performed using aliquots of cDNA templates prepared from concentrated virus stock of the rhabdovirus-like agent–infected SSN1 cells, with cDNA extracted from uninfected SSN1 cells serving as a control. The PCR reaction mixture, totaling 25 µl, comprised 2 µl of DNA template, 2 µl each of forward and reverse primers, 12.5 µl of master mix (Smart prime − 2 × PCR master mix), and 6.5 µl of deionized water. Amplification products were visualized in 1.2% agarose gels (SeaKem LE Agarose, Lonza, USA) alongside 100-bp DNA ladder H3 RTU (GeneDireX, Inc.) molecular markers, post-staining with ethidium bromide. A gel documentation system (BioRad XR + Imaging System, USA) was used to record the images. RNA from uninfected cell cultures and experimental control fish were utilized as negative controls.
Pathogenicity studies
In the present study, the pathogenicity of the rhabdovirus isolated from snakehead fish was assessed through experimental infection studies involving cultured carps, including rohu, common carp, and catfish. This investigation aimed to evaluate the virus’s ability to induce mortality in these fish species, building upon previous findings by John et al. (2021), which demonstrated the virus’s pathogenic potential using cell culture–grown virus.
Experimental fish
Experimental fish fingerlings of rohu (Labeo rohita), common carp (Cyprinus carpio), and catfish (Pangasius pangasius) were sourced from an adjacent national fish seed farm. The carps were housed in 100-l-capacity glass aquarium tanks, containing 40 l of fresh water, and were acclimatized for a period of 7 days until any non-specific mortalities subsided. Throughout the acclimatization period, the water temperature was maintained at 30 ± 1 °C, and the pH was kept at 8.2 ± 0.2. Commercial pelleted feed was given twice daily to the fish, and continuous aeration was provided in the tanks. Daily maintenance included siphoning out waste material, along with exchanging of 10–20% of the water volume.
Catfish fingerlings used in the pathogenicity study underwent similar maintenance protocols. They were housed in a closed recirculatory system with a water temperature of 32 ± 1 °C and a pH of 8.0 ± 0.2. The system included carbon filters for acclimatization and did not require additional aeration. The catfish were fed with commercial pelleted feed once daily, and daily maintenance involved siphoning out waste material and exchanging 10% of the water volume.
Experimental infection
In the infection study, 120 fish were allocated to each group: rohu, common carp, and catfish. At the initiation of the infection study, 30 fish were randomly sampled from each group, and their average sizes were recorded. The average lengths of rohu, common carp, and catfish were measured at 7.7 ± 0.2 cm, 7.37 ± 0.5 cm, and 5.97 ± 0.2 cm, respectively. Before the experiment, three fish each of rohu, common carp, and catfish were randomly selected, and the tissues such as the kidney and spleen were pooled separately for the three species of fish and tested for the presence of rhabdovirus (SHRV-In) by the RT PCR and cell culture isolation method. All the three species of fish were found to be negative for the viral infection.
For the infection protocol, two groups of 30 fish each were injected intraperitoneally in duplicate with 50 µl of the rhabdovirus-like agent (SNLKD1116) neat virus suspension per fish. Additionally, two control groups of 30 fish each were injected intraperitoneally in duplicate with 50 µl of SSN1 cell culture supernatant, following the same procedure as the virus isolates. Fish were then monitored daily for any behavioral changes or clinical signs. Sampling was conducted at intervals of 3, 7, 14, 21, and 28 days post-infection, with three fish sampled from each tank. These samples were assayed for the presence or absence of the rhabdovirus through tissue PCR using diagnostic primers and virus reisolation studies conducted in SSN1 cell lines. In the catfish experimental infection study, moribund or dead fish were collected for sampling. Cumulative survival rates were observed over a 10-day period. Survival analysis was performed using Kaplan-Meier survival curve analysis, as described by Goel et al. (2010).
Results
Biophysical and biochemical characteristics
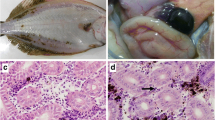
The optimal temperature range for viral replication was identified to be between 24 and 30 °C. Notably, the extent of cytopathic effect (CPE) in the culture was markedly higher at 27 °C (Fig. 1), which correlated with a higher viral titer observed in SSN1 cells (7.5 TCID50 ml−1), as illustrated in Fig. 1. Similar observations were made in snakehead kidney cells. At temperatures of 21 °C and 35 °C, viral replication was observed to be slower, and CPE was less extensive compared to 27 °C. The replication of the virus was found to be unaffected by treatment with 5-iodo-2-deoxyuridine (IUDR), as confirmed through the analysis of the known RNA virus, BSNV (blotched snakehead virus), which exhibited no loss of infectivity (Fig. 2). However, treatment with IUDR resulted in reduced infectivity of the known DNA virus SRDV. Acridine orange–stained SSN1 cells infected with the rhabdovirus-like agent displayed fluorescent red cytoplasmic inclusion bodies, indicating viral replication. Conversely, no such structures were observed in control uninfected SSN1 cells (Fig. 3). Treatment with organic solvents typically diminishes the infectivity of enveloped viruses by disrupting or fragmenting the viral membrane. Indeed, enveloped viruses tend to lose their infectivity upon exposure to organic solvents. Conspicuously, a complete loss of infectivity was observed when the viral suspension was treated with chloroform, suggesting the presence of essential lipids in the viral envelope (Fig. 2). Heat sensitivity assays conducted on SHRV-In titrated in SSN1 cells revealed that the 30-min exposure to 56 °C obliterated virus infectivity. Furthermore, stability testing at acidic (pH 3.0) and alkaline (pH 11.0) pH levels indicated that infectivity was lost under acidic conditions at pH 3.0. However, at pH 11.0, the titer remained at 5.5, indicating a twofold loss of infectivity. Notably, at pH 7, the infectivity remained stable at 7.3 TCID50/ml, indicating no loss of infectivity (Fig. 2). Transmission electron micrographs have shown that the virus particles were bullet-shaped with an average size of 164 nm length and 50 nm width (n = 4) (Fig. 4).
Analysis of structural proteins
The viral proteins from the purified virus preparation were analyzed using 12% SDS-PAGE. The analysis revealed five distinct bands, with their molecular masses ranging from 20 to 69 kDa (Fig. 5). The molecular masses of these five proteins were estimated to be 69, 57, 42, 28, and 20 kDa, respectively. A comparison with the structural proteins N, P, M, G, and L proteins of VHSV (viral hemorrhagic septicemia virus), IHNV (infectious hematopoietic necrosis virus), SHRV (snakehead rhabdovirus), and SVCV (spring viremia of carp virus), along with their corresponding deduced molecular weights from the nucleotide sequences, is given in Table 2.
SDS–PAGE analysis of structural proteins of rhabdovirus-like agent in 12% acrylamide gel stained with 0.1% Coomassie brilliant blue. Lane 1: Medium range molecular mass markers (GeNei™); Lane 2: Low-range molecular mass markers (GeNei™); Lane 3: Purified SNLKD1116. Five proteins of 68, 57, 42, 28, and 20 kDa are indicated
Pathogenicity studies
Experimental infection of catfish juveniles
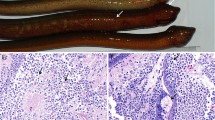
In the experiment involving catfish fingerlings, similar to the previous experiments, a cell culture supernatant containing the rhabdovirus-like agent was used for infection. Although no clinical signs were observed initially, mortality occurred at 48 and 72 h post-infection (p.i.), with some fish becoming moribund by day 7 (Fig. 6). Conversely, no mortality was observed in the control group injected with uninfected cell culture supernatant. Attempts were made to isolate the virus from pooled homogenates of the spleen and kidney collected from all catfish fingerlings at the third and seventh day post-infection. However, tissue extracts from virus-infected fingerlings failed to produce any cytopathic effect (CPE) in the inoculated cell cultures. Additionally, diagnostic PCR results for virus detection were inconclusive (Fig. 7). Interestingly, all moribund and dead fish samples exhibited a specific shorter band of around 360 bp, which was not detected in either the reaction control or samples from control fish. Although the specific expected band of 483 bp was not detected in the infected samples, the presence of these bands, along with the observed mortality, suggests the potential involvement of the virus in the mortality of the fishes.
PCR products obtained from rhabdovirus-like agent–infected catfish tissue samples analyzed by agarose gel electrophoresis. The target region of the primer is 483 bp (John et al. 2021). The amplified fragment is about 360 bp. Lane 1: Control fish sampled at 72 h p.i. Lane 2: Mouribund fish at 36 h p.i. Lane 3: Moribund fish at 60 h p.i. Lane 4: Fish sampled from infected viral tank 1 at 72 h p.i. Lane 5: Fish sampled from infected viral tank 2 at 72 h p.i. Lane 6: Negative control. Lane 7: Ladder 100 bp
Experimental infection of rohu
In the experimental setup, a clarified cell culture supernatant from virus-infected cells containing the rhabdovirus-like agent (SNLKD1116) was utilized for the infection of rohu and common carp fingerlings in two groups, each with duplicates. Intraperitoneal injection of the undiluted virus preparation could not induce any clinical signs or mortality in the rohu and common carp fingerlings throughout the 28-day duration of the experiment. Similarly, in the control group injected with uninfected cell culture supernatant, neither clinical signs nor mortality were noted in both species. Attempts were made to confirm the presence of the virus through virus reisolation from pooled homogenates of the spleen and kidney collected from all rohu and common carp fingerlings at sampling intervals of 3, 7, 14, 21, and 28 days. However, tissue extracts did not produce any cytopathic effect (CPE) in the inoculated cell cultures. Additionally, results from diagnostic PCR were consistently negative until the 28th day of both the experiments indicating the absence of infection in this species. These findings indicate the refractory nature of rohu and common carp fingerlings to the virus, as they did not support viral replication or induce clinical disease under the experimental conditions.
PCR amplification and sequence analysis of structural protein genes
The gene-specific primers for five genes of the virus isolate amplified expected amplicons for the five genes (Fig. 8). The consensus sequence of the N gene encoding the nucleocapsid protein of 564 bp fragment showed 91% identity. The product of the 311 bp fragment obtained by PCR targeting P gene on analysis showed 85% identity to snakehead rhabdovirus (AF147498). Analysis of the trimmed product sequence targeting the M gene of 567 bp showed 90% identity to the snakehead rhabdovirus (AF147498). Similarly, the 949 bp fragment of the G gene showed 91% identity. These products were further analyzed for protein sequence homology with the CLC main work bench and found that there were many synonymous nucleotide substitutions without any change in the amino acid sequences in the respective gene fragments. The protein fragment analysis by pairwise comparison of N, P, M, and G sequences showed that the amino acid identities with the snakehead rhabdovirus (AF147498) were at higher levels with 95.19%, 90.29%, 92.59%, and 95.57%, respectively (Fig. 9). The analysis showed that the amino acid identity of SHRV-In with VHSV and IHNV structural proteins was very low. In addition, the partial genome of 3492 bp created by joining the consecutively overlapping sequences was found to have about 85.44% identity to SHRV (Accession No.: AF147498). Nonetheless, our repeated trials with two sets of primers targeting the NV gene fragment did not produce any amplicon.
A PCR-amplified gene products of the structural protein genes of the rhabdovirus isolate (SNLKD1116) analyzed by agarose gel electrophoresis. A Lane 1: MRGRH24 primer targeting 1226 bp (N). Lane 3: MRGRH26 primer targeting 1424 bp (M). Lane 5: Primers targeting L polymerase block III sequences 483 bp (John et al. 2021). Lane 7: MRGRH27 primer targeting 692 bp (P). Lanes 2, 4, 6, and 8 are respective negative controls. Lane 9: Molecular weight marker of 100-mb ladder. B Lane 1: Negative control. Lane 2: MRGRH29 targeting 1057 bp (G). Lane 3: Similar molecular makers as in A
Virus phylogeny
Phylogenetic analysis of the sequences of four gene fragments was done using MEGA11. The analysis showed the grouping of the current virus isolate SHRV-In with SHRV and other novirhabdoviruses in all four analyses of N, P, M, and G genes (Fig. 10). The G gene, while showing 92% identity at the nucleotide level, had 96% identity at the amino acid level due to synonymous substitution. Similarly, phosphoprotein (P), matrix protein (M), and nucleoprotein (N) genes had 85, 90, and 91% identity at the nucleotide level, while at the amino acid level, the identity has increased to 90, 93, and 95%, respectively (Supplementary Figures S1—G gene, S2—P gene, S3—M gene, S4—N gene). A joined sequence of four genes was compared with the corresponding sequences of 14 other viruses and generated a single phylogenetic tree (Supplementary Figure S5), which had a similar grouping of the different virus strains similar to the four protein genes.
The phylogenetic relationship was inferred using the Maximum Likelihood method and the JTT matrix–based model. The bootstrap test with 1000 replicates was employed to assess the robustness of the inferred tree, with the percentage of trees in which associated taxa clustered together shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model, followed by selecting the topology with the superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 15 nucleotide sequences for all the four proteins N, P, M, and G corresponding to the genomic regions of the viral agent in the present study (SN Rhabdo), snakehead rhabdovirus (SHRV, AF147498), viral hemorrhagic septicemia virus (VHSV, Y18263), infectious hematopoietic necrosis virus (IHNV, L40883), hirame rhabdovirus (HIRRV, AF104985), spring viremia of carp virus (SVCV, U18101), perch rhabdovirus (PRV, JX679246), Arboretum virus isolate Lo-121 (ABTV, KC994644), eel virus European X (EVEX, FN557213), rabies lyssavirus (RVPV, JX276550), Chandipura virus (CHPV, GU212856), vesicular stomatitis New Jersey virus (VSVNJ, AY74804), Sonchus yellow net virus (SYNV, M87829), apple rootstock virus A (ApRVA, MH778545), and potato yellow dwarf virus (PYDV, KY564176). The evolutionary analyses were conducted in MEGA11
The nucleotide sequences obtained in the present study have been deposited in GenBank, and the corresponding accession numbers are as follows: glycoprotein, MH742781.1; matrix protein, MH742782.1; nucleoprotein, MH742783.1; phosphoprotein, MH742784.1.
Discussion
Biophysical and biochemical characteristics
The virus grew well in the cell lines from snakehead, both SSN1 and SNKD2a at 27 ℃. The virus was also found to grow in sea bass caudal peduncle cells but not in EPC and clownfish spleen cells at 27 ℃ (John et al. 2021). The rhabdoviruses isolated from ulcerated fishes from Thailand and Burma (UDRV-BP and UDRV-19) similarly grew well in snakehead fin cell lines but not on EPC (Frerichs et al. 1989; Kasornchandra et al. 1992). However, the snakehead rhabdovirus (SHRV) isolated from ulcerated snakehead fish in Thailand, for which the complete genome sequence is available, grew in EPC. SHRV unlike other fish rhabdoviruses had bacilliform morphology similar to plant rhabdoviruses (Kasornchandra et al. 1992; Dietzgen et al. 2012). Morphology of the present isolate (SNLKD1116, SHRV-In) and the other two viruses isolated from ulcerated fishes (UDRV-BP and UDRV-19) differed from SHRV by their bullet-shaped morphology (Frerichs et al. 1986; John et al. 2021).
Retention of infectivity up on treatment with IUDR and the presence of red cytoplasmic inclusion bodies in the virus-infected cell line indicate the presence of a single-stranded RNA virus multiplying in the cytoplasm (Rovozzo and Burke 1973). Complete loss of infectivity of the virus when treated with chloroform indicated that the virus has an envelope, which was fragmented or removed by the treatment with an organic solvent. These biochemical characteristics are similar to that of the rhabdoviruses (Dietzgen et al. 2017).
SHRV-In lost the infectivity within 30 min if exposure to 56 ℃. The virus lost infectivity at pH 3.0, but at pH 11.0, the infectivity was reduced by 2 logs while no loss of infectivity was noticed at pH 7.0 in the cell culture medium without FBS. Similar observations have been reported in ulcerated snakehead virus (UDRV), where infectivity was found to be poorly retained at pH 9 and deteriorated rapidly at pH 3 and pH 11 (Frerichs 1989).
Analysis of structural proteins
SHRV had five virion proteins with estimated molecular weights of > 150, 68, 42, 26.5, and 20 kDa, respectively (Kasornchandra et al. 1992). Analysis of structural proteins of tropical and temperate rhabdoviruses showed two different patterns forming two groups, group I with SHRV, VHSV, IHNV, and HRV showing five proteins of > 150 (L), 60–72 (G), 40.5–42 (N), 26.5–30 (P), and 20–22.5 (M) kDa bands and group II with pike fry rhabdovirus (PFRV), ulcerative disease rhabdoviruses (UDRV-BP and UDRV-19), and spring viremia of carp virus (SVCV) having four protein bands of > 150, 71–85, 43–53, and 22–24 kDa molecular weight, respectively (Nishizawa et al. 1991; Kasornchandra et al. 1992). The separation of the structural proteins of SHRV-In though did not show > 150 kDa protein band, probably due to a smaller number of copies per virion (Thomas et al. 1985) and higher concentration of SDS PAGE, other protein bands 57, 42, 28, and 20 kDa compared favorably with M, P, N, and G proteins of the group I rhabdoviruses.
Experimental infection studies
The experimental infection in rohu and common carp was negative, and no clinical signs or mortality was observed. However, catfish injected with the virus suffered mortality indicating the possible role of the virus in causing infection and death. The virus SHRV-In was highly pathogenic to the fingerlings of its natural hosts, snakehead fish, causing mortality 5 days post-infection (John et al. 2021). But other tropical rhabdovirus isolates did not cause any infection or mortality in earlier studies (Frerichs et al. 1993; Lio-Po et al. 2000). Temperate Novirhabdovirus species IHNV and VHSV on the other hand are highly pathogenic to a number of fishes (Hoffmann et al. 2005). Although rhabdoviruses are highly diverse and have a wide-ranging host spectrum, tropical novirhabdoviruses have a limited host range as indicated in the present study and the earlier works. Whether temperature has any specific role in the infectivity potential of the fish rhabdoviruses of the Novirhabdovirus genus would require further investigation.
Sequence analysis of structural protein genes
The rhabdovirus genome comprises at least five ORFs in the order 3′-N-P-M-G-L-5′ (Kuzmin et al. 2009; Dietzgen et al. 2012). All the above proteins could be detected in the current virus isolate. The presence of the L gene with 91.36% homogeneity with snakehead rhabdovirus (SHRV) for a 428 bp fragment of block III of L polymerase was earlier detected in this isolate (John et al. 2021). All amplified sequences of the gene fragments except that of the phosphoprotein (P) were without any nucleotide gaps in comparison with the snakehead rhabdovirus genome, which belongs to the Novirhabdovirus genus, indicating the high genomic similarity of the isolate, SNLKD1116. However, the lack of amplification of the NV gene distinguishes this isolate from SHRV (AF147498). The NV gene is an independent transcribing ORF situated between the G and L genes. It encodes a small non-virion protein of approximately 12–14 kDa, which is expressed in infected cells but remains undetectable in purified virions (Kurath and Leong 1985). The NV gene has been reported to be highly preserved among the Novirhabdovirus genus although there is less conservation among the sequences of different viruses (Kurath et al. 1997). The NV protein plays a significant role in inhibiting apoptosis and suppressing the host immune response (Ammayappan and Vakharia 2011; Kim and Kim 2013; Biacchesi et al. 2017). Reverse genetics studies involving NV gene deletion mutants indicated that the NV gene is required only in efficient replication but not for its viability (Biacchesi et al. 2000; Johnson et al. 2000). According to Alonso et al. (2004), while the virus may tolerate a complete deletion of the NV gene, the deletions in the G/NV junction could affect the abundance of both upstream and downstream mRNAs, which might result in the production of defective viruses. The NV gene shares a high degree of similarity between IHNV and HIRRV but differs widely from VHSV, and it has not been detected in SVCV, which is a Vesiculovirus that primarily infects carps (Kurath et al. 1997). The inability to amplify the NV gene with two sets of primers designed based on the snakehead rhabdovirus genome AF147498 indicates the possible extensive nucleotide differences in the rhabdovirus isolate and would require further studies. It could also be possible that the G–L intergenic region that accommodates the NV ORF is susceptible to a high degree of variations due to its non-structural ORF characteristics, which have only a low level of evolutionary constraint (Johnson et al. 2000; Alonso et al. 2004), could make SHRV-In grossly different from SHRV at least in this region of the genome.
The molecular weights of the four proteins N, P, M, and G of SHRV-In compare favorably with the molecular weights of other fish novirhabdoviruses. Through sequence comparison with other rhabdoviruses, we were able to confirm the high degree of homology and resemblance of the protein sequences with members of the Novirhabdovirus genus. Notably, the nucleoprotein and glycoprotein genes showed the highest sequence homology, approximately 95%, while the matrix protein and phosphoprotein genes displayed homologies of 92.6% and 90.3%, respectively, with their counterparts in SHRV. In contrast, the identity with VHSV and IHNV was substantially lower, ranging from 24.8 to 54% for the above mentioned four proteins.
Phylogenetic analysis of SHRV-In
The polymerase (L) gene fragment of 428 base pairs of the block III region exhibited a nucleotide identity of 91.36% with SHRV. However, this resulted in only a single amino acid change within the 142 amino acid sequence, with a high identity of 99.3%. This change was attributed to synonymous substitution of nucleotides (John et al. 2021). Rhabdoviruses experience strong selective pressure to minimize changes in the amino acid sequences, particularly within the polymerase gene, which is crucial for maintaining the functionality of the polymerase, which plays a pivotal role in initiating virus replication (Holmes et al. 2002; Bourhy et al. 2005). However, large-scale variations and high plasticity in the other proteins of the rhabdoviruses are reported for different species, and this has formed the basis of the diversity and complexity of their genomes and consequent ecologically manifold host range from mammals to several vertebrate species, arthropods, and plants (Dietzgen et al. 2017). The present isolate from snakehead fish isolated in India had close phylogenetic homology with the snakehead rhabdovirus (SHRV; AF147498) isolated from Thailand (Kasornchandra et al. 1992). Despite its close similarity with the SHRV, SHRV-In failed to amplify the NV gene with the primer sequences developed from the SHRV genome. Being geographically very distant and the snakehead fish being a freshwater inhabitant and practically impossible transmission through marine waters, it has to be seen whether the NV gene is absent in this isolate or exists with wide variation from the SHRV counterpart. With the NV gene being proved to be not essential in virus replication (Alonso et al. 2004), there could be a possibility that negligible selective pressure would exist for the sequence fidelity of this region of the genome. SVCV-infecting carps on the other hand do not carry the NV gene though this virus does not fall under the Novirhabdovirus genus (Kurath et al. 1997). Future studies would however be required to delineate this point. Current evidence places this isolate as an unclassified member of the Novirhabdovirus genus. Distinct clustering could be identified in the phylogenetic tree of the four gene fragments analyzed for different rhabdoviruses that were included in the present analysis. In all the analyses, the present isolate SHRV-In was clustered strongly with other novirhabdoviruses such as SHRV, VHSV, and IHNV. These results suggest that SHRV-In could be placed in the Novirhabdovirus genus in the family Rhabdoviridae.
Data availability
Sequence data is deposited in the GenBank and the accession numbers are as follows: glycoprotein, MH742781.1; matrix protein, MH742782.1; nucleoprotein, MH742783.1; phosphoprotein, MH742784.1. Additional data is provided as supplementary files.
Change history
04 July 2024
In this article, the caption of Figure 7 mentioned a reference John et al. (2017). This was corrected to John et al. (2021).
References
Ahne W, Jorgensen PEV, Olesen NJ, Wattanavijarn W (1988) Serological examination of a rhabdovirus isolated from snakehead, Ophicephalus Striatus. In Thailand with ulcerative syndrome. J Appl Ichthyol 4:194–196
Alonso M, Kim CH, Johnson MC, Pressley M, Leong JA (2004) The NV gene of snakehead rhabdovirus (SHRV) is not required for pathogenesis, and a heterologous glycoprotein can be incorporated into the SHRV envelope. J Virol 78:5875–5882
Ammayappan A, Vakharia VN (2011) Nonvirion protein of novirhabdovirus suppresses apoptosis at the early stage of virus infection. J Virol 85(16):8393–8402. https://doi.org/10.1128/JVI.00597-11
Biacchesi S, Thoulouze MI, Béarzotti M, Yu YX, Brémont M (2000) Recovery of NV knockout infectious hematopoietic necrosis virus expressing foreign genes. J Virol 74(23):11247–11253. https://doi.org/10.1128/jvi.74.23.11247-11253
Biacchesi S, Mérour E, Chevret D, Lamoureux A, Bernard J, Brémont M, Mediated (2017) NV Proteins of Fish Novirhabdovirus Recruit Cellular PPM1Bb Protein Phosphatase and Antagonize RIG-I-Mediated IFN Induction. Sci Rep 7:44025. https://doi.org/10.1038/srep44025
Bourhy H, Cowley JA, Larrous F, Holmes EC, Walker PJ (2005) Phylogenetic relationships among rhabdoviruses inferred using the L polymerase gene. J Gen Virol 86:2849–2858
Dietzgen RG, Calisher CH, Kurath G, Kuzman IV, Rodriguez LL et al (2012). In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ et al (eds) Virus taxonomy, ninth report of the International Committee on Taxonomy of Viruses. Elsevier, San Diego, pp 654–681
Dietzgen RG, Kondo H, Goodin MM, Kurath G, Vasilakis N (2017) The family Rhabdoviridae: mono- and bipartite negative-sense RNA viruses with diverse genome organization and common evolutionary origins. Virus Res 227(1):158–170
Feldman HA, Wang SS (1961) Sensitivity of various viruses to chloroform. Proceedings of the Society for Experimental Biology and Medicine 106:736–738
Frerichs GN (1989) Stability of snakehead (Ophiocephalus striatus) rhabdovirus under different environmental conditions. J Appl Ichthyol 5:122–126. https://doi.org/10.1111/j.1439-0426.1989.tb00483.x
Frerichs GN, Millar SD, Roberts RJ (1986) Ulcerative rhabdoviruses in fish in South-East Asia. Nature 322:216
Frerichs GN, Hill BJ, Way K (1989) Ulcerative disease rhabdovirus: cell-line susceptibility and serological comparison with other fish rhabdoviruses. J Fish Dis 12:51–56. https://doi.org/10.1111/j.1365-2761.1989.tb01290.x
Frerichs GN, Morgan D, Hart D, Skerrow C, Roberts RJ, Onion DE (1991) Spontaneously productive C-type retrovirus infection of fish cell lines. J Gen Virol 72:2537–2539
Frerichs GN, Millar SD, Chinabut S (1993) Clinical response of snakeheads, Ophicephalus striatus. To experimental infection with snakehead fish rhabdovirus and snakehead cell line retrovirus. Aquaculture 116:297–301
George MR, John KR, Mansoor M, Saravanakumar R, Sundar P, Pradeep V (2015) Isolation and characterization of a ranavirus from koi, Cyprinus carpio L., experiencing mass mortalities in India. J Fish Dis 38:389–403. https://doi.org/10.1111/jfd.12246
Goel MK, Khanna P, Kishore J (2010) Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res 1(4):274–278. https://doi.org/10.4103/0974-7788.76794
Hoffmann B, Beer M, Schütze H, Mettenleiter TC (2005) Fish rhabdoviruses: molecular epidemiology and evolution. Curr Top Microbiol Immunol 292:81–117
Holmes EC, Woelk CH, Kassis R, Bourhy H (2002) Genetic constraints and the adaptive evolution of rabies virus. Virology 292:247–257
John KR, George MR (2006) Development and characterisation of fish cell lines from warm water fishes. Completion report of the ICAR Project. Department of Aquaculture, Fisheries College and Research Institute, Tuticorin, India
John KR, George MR (2012) Viruses associated with epizootic ulcerative syndrome, an update. Ind Jou Virol 23:106–113
John KR, Richards RH (1999) Characteristics of a new birnavirus associated with a warm-water fish cell line. J Gen Virol 80:2061–2065
John KR, George MR, Mageshkumar P, Mansoor MM, Kaviarasu D, Sivasankar P, Selvamagheswaran M, Petchimuthu M (2021) Isolation and characterisation of a novel rhabdovirus from snakehead fish showing surface ulcerations and mortality from India: first report. Aquaculture 544:737120
Johnson MC, Simon BE, Kim CH, Leong JA (2000) Production of recombinant snakehead rhabdovirus: the NV protein is not required for viral replication. J Virol 74(5):2343–2350. https://doi.org/10.1128/jvi.74.5.2343-2350.2000
Kärber G (1931) Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche, Naunyn. Schmiedebergs Arch Exp Pathol Pharmakol 162:480–483. https://doi.org/10.1007/BF01863914
Kasornchandra J, Engelking HM, Lannan CN, Rohovec JS, Fryer JL (1992) Characterization of three rhabdoviruses from snakehead fish Ophicephalus Striatus. Dis Aquat Org 13:89–94
Kim MS, Kim KH (2013) The role of viral hemorrhagic septicemia virus (VHSV) NV gene in TNF-α- and VHSV infection-mediated NF-κB activation. Fish Shellfish Immunol 34(5):1315–1319. https://doi.org/10.1016/j.fsi.2013.02.026
Kurath G, Leong JC (1985) Characterization of infectious hematopoietic necrosis virus mRNA species reveals a nonvirion rhabdovirus protein. J Virol 53(2):462–468. https://doi.org/10.1128/JVI.53.2.462-468.1985
Kurath G, Higman KH, Björklund HV (1997) Distribution and variation of NV genes in fish rhabdoviruses. J Gen Virol 78(1):113–117. https://doi.org/10.1099/0022-1317-78-1-113
Kuzmin I, Novella I, Dietzgen R, Padhi A (2009) The rhabdoviruses: biodiversity, phylogenetics, and evolution. Infect Genet Evol 9:541–553. https://doi.org/10.1016/j.meegid.2009.02.005
Lio-Po GD, Traxler GS, Albright LJ, Leaño EM (2000) Characterization of a virus obtained from snakeheads Ophicephalus striatus with epizootic ulcerative syndrome, EUS in the Philippines. Dis Aquat Org 43:191–198
Lovy J, Lewis NL, Hershberger PK, Bennett W, Meyers TR, Garver KA (2012) Viral tropism and pathology associated with viral haemorrhagic septicaemia in larval and juvenile Pacific herring. Vet Microbiol 161:66–76
Nishizawa T, Yoshimizu M, Winton J, Ahne W, Kimura T (1991) Characterization of structural proteins of hirame rhabdovirus, HRV. Dis Aquat Org 10:167–172
Rovozzo G, Burke C (1973) A manual of basic virological techniques. Englewood Cliffs, N.J, Prentice-Hall
Schutze H, Enzmann PJ, Mundt E, Mettenleiter TC (1996) Identification of the non-virion (NV) protein of fish rhabdoviruses viral haemorrhagic septicaemia virus and infectious haematopoietic necrosis virus. J Gen Virol 77(6):1259–1263
Sivasankar P, John KR, George MR, Magesh Kumar P, Manzoor MM, Prince Jeyaseelan MJ (2017) Characterization of a virulent ranavirus isolated from marine ornamental fish in India. Virus Disease 28(4):373–382
Thomas D, Newcomb WW, Brown JC, Wall JS, Hainfeld JF, Trus BL, Steven AC (1985) Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol 54:598–607. https://doi.org/10.1128/JVI.54.2.598-607.1985
Walker PJ, Freitas-Astúa J, Bejerman N, Blasdell KR, Breyta R, Dietzgen RG et al (2022) ICTV Virus Taxonomy Profile: Rhabdoviridae 2022. J Gen Virol 103:001689
Wolf K (1988) Fish viruses and fish viral diseases. Cornell University Press, Ithaca, NY
Zeng WW, Wang Q, Wang YY, Liu C et al (2014) Genomic characterization and taxonomic position of a rhabdovirus from a hybrid snakehead. Arch Virol 159:2469–2473
Acknowledgements
The research formed a part of the MFSc (Master of Fisheries Science) program of the first author. The authors express their gratitude to the Tamil Nadu Dr. Jayalalithaa Fisheries University for their support and collaboration in conducting this study.
Funding
The research was conducted as part of Sub Project 21 within the framework of the National Surveillance Programme for Aquatic Animal Diseases (NSPAAD), which was funded by the National Fisheries Development Board, India. The project was nationally coordinated by the ICAR-National Bureau of Fish Genetic Resources (ICAR-NBFGR) under the reference number G/National Surveillance/2013–21.
Author information
Authors and Affiliations
Contributions
AJM carried out the investigation, analysis, experimental infection, and writing the original draft; KRJ conceptualized the work and carried out the molecular analysis, review, and editing; MRG conceptualized research work, reviewed the manuscript, and edited and undertook the overall administration and funding; PM conducted the cell culture investigation and experimental infection; MMM was involved with molecular investigation and analysis.
Corresponding author
Ethics declarations
Ethics approval
Not required.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abisha Juliet Mary, S., John, K.R., George, M.R. et al. Genomic characterization of the rhabdovirus-like agent isolated from snakehead fish and pathogenicity studies in cultured carps and catfish. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01570-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01570-9