Abstract
Astaxanthin, a natural ketone carotenoid, is among the environmentally friendly antioxidants and immunopotentiators. A 60-day feeding experiment was proceeded to assess the feasibility of astaxanthin-rich Haematococcus pluvialis as a growth promoter, antioxidant, and immunostimulant in the hybrid red tilapia (Oreochromis niloticus x O. mossambicus). Experimental diets containing grade amounts of Haematococcus pluvialis powder (0, 0.5, 1.0, and 1.5 g kg−1 feed) were formulated to be fed to red tilapia (Initial weight 27 ± 0.5 g) and designated as control, HP0.5, HP1, and HP1.5, respectively. The results indicated that the HP1 and HP1.5 promoted growth performance by decreasing FCR and increasing FBW, WG, WGR, and SGR confirmed by better intestinal morphology. Moreover, the HP1 and HP1.5 diets improved non-specific immunity via enhancing phagocytic activity, IgG and IgM contents, and nitric oxide, while decreasing MPO values compared to the control. Additionally, the H. pluvialis diets boosted antioxidant ability through elevating serum SOD and GSH activities, unlike the control group. The HP0.5, HP1, and HP1.5 diets also exerted hepatoprotective effects via histological sections as well as, suppressing liver enzymes (ALT, AST, ALP, and GGT) and reducing serum TG and cholesterol contents confirmed our data. Besides, a notable decrease in the serum levels of IFN-γ and IL-4 along with hepatic mRNA levels of TNF-α, IL-1β, IL-8, and caspase-3 with the increasing doses of H. pluvialis. These results proposed that a diet supplemented with 1 and 1.5 g kg−1 H. pluvialis is exhorted to augment the growth performance, hepatoprotection, antioxidant capacity, immunity, and anti-inflammatory response of red tilapia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most global and valuable cultured fish species are tilapia species with a total yield of 6.3 million metric tons in 2021, estimated at over 12 billion US$ (FAO 2023). In general, the extensive expansion of tilapia farming is related to their swift growth and preferable resistance to environmental stressors and diseases (Eissa et al. 2023a, 2023b). Red hybrid tilapia is considered the most genetically enhanced tilapia (GIFT) by crossbreeding the Nile tilapia, Oreochromis niloticus with Oreochromis mossambicus. These species have gained rapid popularity among Egyptian consumers because of their morphological attractiveness, higher marketability, and their fast growth (Aboelward et al. 2020). The wide tolerance to salinity (1–25 ppt) and higher densities increase its value in the aquaculture (Rahmah et al. 2020). In addition, red tilapia provides a favorable taste with lower off-flavor along with an increase in the long polyunsaturated fatty acids contents in comparison with other tilapia species (Banuelos-Vargas et al. 2021). According to these previous characteristics, formulating red tilapia diets using immunostimulant and antioxidant supplements remains a major challenge for red tilapia production strategies and limitations of antibiotic resistance and other drugs in intensive culturing techniques and a variety of infections (Griesh et al. 2024).
The exploitation of appropriate algal feed additives facilitates the prospective expansion of the tilapia farming industry for use as an alternative to antibiotics. The unicellular green microalga, Haematococcus pluvialis, from the family Haematococcaceae, is a major exporter of natural astaxanthin (3,3′-dihydroxy-β-carotene-4,4′-dione), with a 1.5–6.0% content in dry powder (Yu et al. 2021). Therefore, H. pluvialis has commercial significance as it can accumulate remarkable astaxanthin content under stressful light, elevated temperature and salinity, and nitrogen scarcity (Ambati et al. 2014), as well as the constancy and bioavailability of natural astaxanthin are superior to synthetic astaxanthin (Ma et al. 2017). The in vitro studies have proved that the antioxidant feature of astaxanthin was higher by 100–500 fold than other natural antioxidants (Elbahnaswy and Elshopakey 2023). As well, astaxanthin as a feed additive gives noteworthy benefits to the physiological health and growth of aquatic animals (Cerezal-Mezquita et al. 2022; Gervasi et al. 2018). Besides, the naturalistic red pigmentation of salmon, trout, crabs, and shrimp species mainly originates from carotenoid astaxanthin produced from H. pluvialis (Cerezal-Mezquita et al. 2022; Lim et al. 2018). The valuable roles of H. pluvialis have been established in the fish culture industry, especially in improving the immunity and antioxidant capacity along with increasing the survival rate promoting against stressors and microbial diseases in different aquatic animals (Elbahnaswy and Elshopakey 2023; Long et al. 2023). The positive impact of H. pluvialis as a feed supplement was investigated on the growth, anti-inflammatory status, metabolic capacity, and intestinal histology of juvenile Litopenaeus vannamei shrimp (Fang et al. 2022). Dietary H. pluvialis could effectively enhance the antioxidant ability and innate immunity parameters of the Chinese mitten crab (Wu et al. 2017).
To date, data about the beneficial effects of H. pluvialis are little obtainable as a dietary supplement in the red tilapia reared in raceways. Therefore, in this work, 60 days feeding trial was carried out to explore the impact of H. pluvialis on the growth achievement, chemical carcass composition, antioxidant capability, physiological status, pro-inflammatory and anti-inflammatory gene expression, and hepatic and intestinal morphology of juvenile red tilapia.
Materials and methods
Fish management and experimental design
The experiment was carried out at a confidential fish farm in West El-kantra- Ismailia Governorate, where hybrid red tilapia (O. niloticus × O. mossambicus) fingerlings were cultured. Before the start of the feeding trial, six hundred red tilapia juveniles with estimated average size (initial body weight of 27 ± 0.5 g) were reared in 12 concrete tanks (3 × 8 × 1.2 m) with a water capacity of 24/m3. Fish were randomly allotted in triplicates with a density of 50 fish per tank and supplemented the control diet for 2 weeks. During the acclimation period, red tilapias were assessed its healthy status through visual observation of clinical signs and some reflexes (ocular, defensive, and escape), along with microbial culturing to confirm fish was pathogen-free.
Over the 60-day feeding trial, water quality parameters were monitored twice a week. The maintained values were 26.5 ± 0.41 °C for water temperature; 2.5 ± 0.3 ppt for salinity; 6.7 ± 0.31 mg L−1 for D.O; 7.6 ± 0. 37 for pH; 0.4 ± 0.02 mg L−1 for ammonia nitrogen; 0.042 ± 0.01 mg L−1. The tanks were provided with filters and continuous aeration via air blowers.
Experimental diets and feeding protocol
Four isonitrogenous dietary regimes containing almost 30% crude protein were prepared (Table 1) according to previous research (Bombardelli et al. 2017). The supplemental 2% red H. pluvialis powder (Bioalgo (WF), Co. Ltd., Astalgae ®, China, and 2% astaxanthin content) was used. The product was incorporated in the supplemented diets at four levels (0, 0.5, 1.0, and 1.5 g kg−1 feed), designated as control, HP0.5, HP1, and HP1.5, respectively. The prepared diets were exposed to air at room temperature, mashed, and screened through 2–3 mm sieves, and kept in sterile plastic bags at 20 °C until used. During the trial, fish were supplied with test diets at 3% of the body weight containing different doses of H. pluvialis algae in the supplemented groups, twice a day (9:00 and 16:00 h) for 60 days. Uneaten feed and fecal wastes were removed via siphon feces were siphoned every morning, and about 25% of tank water was changed.
Sample collection
After 60 days of feeding, red tilapias in each tank were starved for 24 h. Then, all collected fish were prepared for the estimation of growth indices. Then, five fish from each group were randomly picked to be anesthetized with commercial clove oil solution (60 mg L−1) to obtain the blood from the heart puncture using 3 mm sterile syringes. Blood samples were kept in un-heparinized Eppendorf tubes to coagulate at room temperature to collect serum by using a centrifuge at 4000 rpm for 15 min. Then, serum samples were reserved at – 20 °C for analysis of immunological and biochemical indices as well as antioxidant capacity. Liver samples were removed and put in RNA later® (Sigma, USA) for analysis of mRNA expression and then kept at – 20 °C. At the same time, liver and intestine sections were removed and fixed in 10% neutral formaldehyde for histological examination.
Growth indices
At the termination of the feeding trial, the growth indices and survival rates were recorded by counting and weighing the fish in every group. The calculations were proceeded as follows:
Chemical analysis of diets
The chemical compositions of each diet were investigated according to the standard protocols of AOAC (Horwitz 2010). The analysis of moisture was done through heat drying in the ventilated oven at 105 °C until steady weight; crude lipid evaluation was followed by the Soxhlet ether-extraction method; crude protein was measured following the Kjeldahl method; ash was tested using a muffle furnace at 550 °C until constant weight. The nitrogen-free extract (NFE) was valued through the corresponding equation: NFE (g/kg) = 100 – (crude protein + crude lipids + ash + crude fiber).
Measurement of serum immunity indices, biochemical, and antioxidant capacity
The phagocytic activity was determined following the previous protocol (Kawahara et al. 1991). Phagocytic activity (PA) was assessed by dividing the number of phagocytic cells containing yeast by the total number of phagocytes multiplied by 100; meanwhile, the phagocytic index; phagocytic index (PI) = number of cells phagocytized/number of phagocytic cells (Dawood et al. 2020). As well, myeloperoxidase (MPO) activity was assessed using EnzChek assay Kits (Invitrogen™, Thermo Fisher Scientific, USA).
Following the manufacturer’s procedures, the serum concentrations of immunoglobulin M (IgM) and immunoglobulin G (IgG) were estimated using Fish Immunoglobulins ELISA kits bought from MyBioSource Co. (San Diego, California, USA) (Eissa et al. 2023b; Elbahnaswy et al. 2023). Serum nitric oxide levels were measured depending on the stated approaches of commercial kits (Bio-diagnostics, Cairo, Egypt) (Bryan and Grisham 2007). Serum tumor necrosis factor-alpha (TNF-α), interleukin-4 (IL-4), and interferon-gamma (IFN-γ) values were measured by ELISA Kit supplied by Quantikine company corresponding to previous protocols (Juhász et al. 2013; Nicola 1994; Swanson et al. 2001).
The serum samples of red tilapia were used to estimate antioxidant enzymes, such as superoxide dismutase (SOD), reduced glutathione (GSH), and catalase (CAT), as well as, lipid peroxidation indicator malondialdehyde (MDA) following the previously published protocols (Benzie and Strain 1996; Ellman 1959; Nishikimi et al. 1972; Ohkawa et al. 1979). Serum lipids including total cholesterol, triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured spectrophotometrically (Photometer BM Co., Germany, 5010) using commercial kits (Spinreact, Spain) according to the manufacturer’s instructions (Bucolo and David 1973; Naito and Kaplan 1984).
MyBioSource Assay Kits (MyBioSource Co., California, USA) was used to examine serum creatinine, urea, total bilirubin, total protein, and albumin levels were investigated according to the standard methods using an automated spectrophotometer (Perkin Elmer Co., Waltham, USA) (Anavekar et al. 2004; Prætorius and Poulsen 1953; Wedemeyer and Yasutake 1977; Young 1997). The globulin level was also determined according to a previous method (Reitman and Frankel 1957). The efficacy of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) was analyzed using an automated clinical spectrophotometer (Abbott Alcyon 300, USA) following the Pars-Azmon kit’s instructions (Pars Azmon, Tehran, Iran).
RNA extraction, cDNA production, and quantitative PCR
Liver Sects. (100 mg) from red tilapia of each group (control, HP0.5, HP1, and HP1.5) stored in RNA later solution, were subjected to total RNA extraction using Total RNA Extraction Kits (iNtRON Biotechnology, Inc., Gyeonggi-do, Korea) which stated the manufacturer’s instructions. The purity of RNA samples was calculated by UV–Vis Nanodrop spectrophotometer (Quawell Technology, Inc., San Jose, CA, USA). The first strand of complementary DNA (cDNA) containing 1 μg of total RNA was formed using a cDNA synthesis kit (Enzynomics Co. Ltd., Daejeon, Korea). The primer sequences of proinflammatory (IL-8, TNF-α, and IL-1β), anti-inflammatory IL-10, and caspase-associated genes, were used (Table 2) (Elbahnaswy et al. 2021; Zahran et al. 2021). Housekeeping gene beta-actin (β-actin) equalized the mRNA expressions of these genes. qRT-PCR was done using SYBR Green PCR Master Mix (Enzynomics Co. Ltd., Daejeon, Korea) for quantifying red tilapia cDNAs (1 μl) according to the manufacturer’s procedures via Quant studio 1 Real-Time PCR System (Applied Biosystems™, Thermo Fisher Scientific, Oslo, Norway). The thermocycling conditions were 95 °C for 15 min, followed by 45 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 20 s, followed by melt curve generation. The relative mRNA expression values of each target gene were normalized to β-actin mRNA transcripts using the 2−ΔΔCT method (Schmittgen and Livak 2008).
Examination of liver and intestinal histology
The excised liver and intestine samples were dried in ethanol, cleared in xylene, immersed in paraplast wax, and dissolved in 10% neutral formaldehyde (Zhao et al. 2020). Briefly, sections of 5–7 μm tissue thickness were then prepared using a rotary microtome, stained with hematoxylin and eosin, and investigated under a light microscope (Olympus CKX41, Tokyo, Japan). Also, the length and width of intestinal sections were statistically estimated.
Statistical analyses
GraphPad Prism version 8.0 (GraphPad Software, Inc., San Diego, California, USA) was implemented to perform statistics. Firstly, the normality verification using Shapiro–Wilk and Levene’s tests was performed. The findings were indicated as mean ± standard deviation (SD). All data were statistically inspected by one-way analysis (ANOVA) with post-hoc Tukey’s multiple range tests to detect whether H. pluvialis levels significantly affected the observed response. The notable difference was valued at p < 0.05.
Results
Growth and body chemical composition indexes
The results of the growth performance of red tilapia at the end of the 60-day feeding trial were summarized in Table 3. The final body weight, weight gain, weight gain rate, and specific growth ratio of fish were markedly increased with increasing dietary H. pluvialis levels up to HP1.5 (p < 0.05) compared with the control group. In addition, a notable decrease was observed in FCR levels as dietary H. pluvialis levels increased from HP0.5-HP1.5 compared with the control (p > 0.05), and the lowest FCR was detected in HP1 and HP1.5 diets (p < 0.05). During the experiment, there was no marked variation in the survival rates among the four diet groups (Table 3).
The whole-body composition of red tilapia fed a variety of H. pluvialis diets is revealed in Table 4. A significant increase was noticed in the body protein and ash in the dietary H. pluvialis groups (HP0.5, HP1, and HP1.5) when compared with the control (p > 0.05). A worthy decline in the lipids content of red tilapia fed by HP1 and HP1.5 diets, concerning the values shown in the control group (p < 0.05).
Serum immunological and pro-inflammatory cytokines indices and antioxidant capacity
The phagocytic activity and phagocytic index, as well as IgM content in HP0.5, HP1, and HP1.5 groups notably increased when compared to the control group (p < 0.05) (Table 5). As well, IgG content significantly increased in HP1 group (p < 0.05). Meanwhile, MPO levels markedly decreased with the higher levels of H. pluvialis, and the lowest increment was detected in HP1.5, when compared with the control group (p < 0.05). Nitric oxide values markedly increased in the HP1 group (p < 0.05). At the same time, IFN-γ and IL-4 levels were notably reduced in the serum of red tilapia-fed supplemented groups in comparison with the control one (p < 0.05). However, there was no disparity in TNF-α levels among all the groups (Table 5).
In comparison with control diet, the serum SOD and GSH activities manifested a notable increasing tendency in HP1 and HP1.5 supplemented groups (p < 0.05). Conversely, the serum levels of CAT showed no alterations in supplemented groups (HP1 and HP1.5) except for HP0.5 group when compared with control (p < 0.05). MDA level revealed no change between the control and dietary groups (Fig. 1).
Effects of H. pluvialis supplement (control group, HP0.5, HP1, and HP1.5) on serum superoxide dismutase (SOD, A), catalase (B), reduced glutathione (GSH, C), and malondialdehyde (MDA, D) levels in red tilapia. Data were represented as Mean ± SD. Data in the same row assigned with the different superscripts are significantly different (p < 0.05) using ANOVA Post Hoc (Tukey test)
Serum biochemical parameters and hepatic function
Liver (ALT, AST, ALP, and GGT) biomarkers in the serum of hybrid red tilapia were greatly affected (p < 0.05) by supplementary H. pluvialis when compared to control (Table 6). These enzymes were notably diminished (p < 0.05) in all dietary H. pluvialis levels up to 1.5 g kg−1. A similar trend has also been investigated regarding blood creatinine levels (p < 0.05); however, urea, total bilirubin, total protein, and albumin were not significantly affected by H. pluvialis supplementation. The highest globulin levels (p < 0.05) were acquired at 1 g kg−1 and 1.5 g kg−1 diet, respectively (Table 6).
Red tilapia fed the H. pluvialis diet at HP1 and HP1.5 had minimal levels of total cholesterol as well as elevated levels of TG than those of the control (p < 0.05). Meanwhile, the levels of TG were significantly decreased in the HP0.5 dietary group (p < 0.05). However, no considerable amendment was distinguished in the levels of HDL and LDL among all dietary groups (Fig. 2).
Effects of H. pluvialis supplement (control group, HP0.5, HP1, and HP1.5) on serum cholesterol (A), triglyceride (B), high-density lipoprotein, HDL (C), and low-density lipoprotein, LDL (D) levels of red tilapia. Data were represented as Mean ± SD. Data in the same row assigned with the different superscripts are significantly different (p < 0.05) using ANOVA Post Hoc (Tukey test)
Gene expression
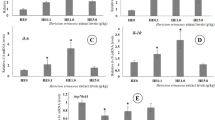
The hepatic expression of various genes (TNF-α, IL-1β, IL-8, IL-10, and caspase-3) in the red tilapia following dietary inoculation of the H. pluvialis was represented in Fig. 3. The significant downregulation of pro-inflammatory TNF-α, IL-1β, and IL-8 genes was displayed (p < 0.05) in fish-fed H. pluvialis doses at 1 and 1.5 g kg−1 diet (HP1 and HP1.5) when compared with the control diet (Fig. 3A–C). A similar trend was also observed in group HP0.5, except for IL-1β gene expression (Fig. 3B). On the other hand, the IL-10 mRNA expression was notably upregulated in all dietary groups containing H. pluvialis (p < 0.05), in comparison with the control group (Fig. 3D). Nevertheless, caspase-3 gene expression was markedly decreased in HP1 and HP1.5 supplemented diets (p < 0.05), with no change revealed in HP0.5 group (Fig. 3E).
Liver mRNA levels of proinflammatory (IL-8, TNF-α, and IL-1β), anti-inflammatory IL-10, and caspase genes of red tilapia-fed diet supplemented with H. pluvialis for 60 days. Four groups, the control group, HP0.5, HP1, and HP1.5 were tested. Data were expressed as Means ± SD (n = 3) and were normalized to the β-actin mRNA levels. Data in the same row assigned with the different superscripts are significantly different (p < 0.05) using ANOVA Post Hoc (Tukey test)
Histological examination
As for the liver, there was an improvement in the hepatic tissue structures, an improvement in the endothelium lining of the hepato-central veins, and a decrease in the inflammatory cells detected in supplemented groups. The best improvement was observed in groups HP1 (Fig. 4C), HP1.5 (Fig. 4D), and HP0.5 (Fig. 4B), in comparison with the control group (Fig. 4A). On the other hand, the third group (HP1), improving the normal sizes, numbers of Kupffer cells, appearance of hepatocytes, and pancreatic acinar cells were observed as shown in Fig. 4C, and in the fourth group (HP1.5), Kupffer cells were of medium size and number was shown in Fig. 4D, and in the second group (HP0.5), numerous and swelling of Kupffer cells and inflammatory cells was demonstrated was shown in Fig. 4B compared to the control group was shown in Fig. 1A.
As for the intestine, there was an improvement in the intestinal mucosal layers, as well as other intestinal tissues in the HP1 (Fig. 5C), and HP1.5 groups (Fig. 5D), and a notable increase in the measurable length and width of the intestinal villus (Fig. 6) which a noticed boost in the higher level of the substance in the HP1 group caused of the improving effect of astaxanthin.
Discussion
Haematococcus pluvialis is a significant microalgae species that produce astaxanthin, one of the most potent naturally occurring antioxidants (Shah et al. 2016). As far as the authors are aware, there is not enough data on the advantages of supplementing red tilapia raised in concrete tanks with H. pluvialis. A 60-day feeding trial was carried out in this study to check the effectiveness of H. pluvialis on the growth index, carcass chemical composition, antioxidant status, and anti-inflammatory potential of juvenile red tilapia.
In our research, red tilapia reared in raceways showed a significant increase in FBW, WG, WGR, and SGR of juvenile red tilapia as dietary H. pluvialis doses boosted from HP0.5–HP1.5 in comparison with the control group. These findings were similar to earlier research on tilapia (Oreochromis mossambicus) (Ju et al. 2017), large yellow croaker (Pseudosciaena crocea) (Li et al. 2014), and discus fish (Symphysodon haraldi) (Wang et al. 2016). The main explanation is due to the presence of a variety of important several nutrients in H. pluvialis, including minerals, vitamins, proteins (2–5%), fat (0.5%), carbohydrates (2–5%), and other bioactive substances (e.g., Carotenoids, Chlorophylls) that can promote the growth of fish (Yu et al. 2021). This might be also explained by the protein accumulation of hybrid tilapia that reflects the positive impact of the high protein content of H. pluvialis-supplemented diets; this could elucidate the improvement of fish growth. Similar effects were reported in hybrid red tilapia-fed H. pluvialis microalgae in the diet (Li et al. 2014), and in Nile tilapia-fed Nannochloropsis oculate at different levels (Zahran et al. 2023).
Additionally, this study exhibited a significant increase in the whole-body protein and ash along with a decrease in body lipids in the H. pluvialis groups (HP0.5, HP1, and HP1.5) unlike the control one. It has been reported that spotted sea bass (Lateolabrax maculatus) fed diets containing H. pluvialis (2, 4, 6, 8, and 10 g/kg) exhibited considerably lower total body lipid levels than fish fed the control diet (Yu et al. 2021). This outcome was comparable to those who discovered that juvenile golden pompano-fed diets containing astaxanthin had lower total body lipid contents (Xie et al. 2017). Similarly, astaxanthin has been shown to improve lipid utilization and lower lipid levels in red porgy Pagrus pagrus (Kalinowski et al. 2011).
In both vertebrates and invertebrates, phagocytosis is the first cellular defense mechanism by which cells adhere to the surface of recognized particles such as bacteria, and other microorganisms, and then engulf these recognized particles (Carbone and Faggio 2016). Immunoglobulin (Ig) is a glycoprotein produced by the proliferation and differentiation of B lymphocytes into plasma cells upon antigen stimulation (Burgos-Aceves et al. 2021). Myeloperoxidases (MPO) are peroxidase enzymes that oxidize a variety of halides and pseudohalides to produce numerous hypohalous acids by utilizing H2O2. In the current results, the phagocytic activity as well as IgM, IgG, and NO content were significantly elevated in the HP1 group (p < 0.05). Meanwhile, myeloperoxidase values markedly decreased with the gradual increase in H. pluvialis doses, and the lowest increment was revealed in HP1.5. In the same line, after treating common carp, Cyprinus carpio with astaxanthin supplemental diet at 50 and 100 mg kg−1 following Aeromonas hydrophila infection, a significant increase in serum phagocytic, respiratory burst, lysozyme, and bactericidal activities were noticed (Jagruthi et al. 2014). Moreover, rainbow trout that ingested astaxanthin had higher phagocytic indices and lysozyme activity (Amar et al. 2004). These results may help to explain why the fish fed on a diet high in astaxanthin may also enhance the bifacial microbiota in the digestive tract by enhancing the enzymes responsible for digesting fish, hence promoting the immune response (Elbahnaswy and Elshopakey 2023). As well, astaxanthin-treated human neutrophils elucidated a significant reduction in the generation of MPO and HClO in addition to a sharp decline in all reactive oxygen species (Guerra et al. 2012). Another result demonstrated the capacity of dietary astaxanthin from Haematococcus pluvialis (3 g/kg) to enhance the action of inducible nitric oxide synthase (i-NOS), a crucial indicator of oxidative stress which converts l-arginine to ultimately lead to increase production of NO in L. vannamei (Liu et al. 2022).
A previous study found that supplementing coral trout Plectropomus leopardus with 100–150 mg/kg of H. pluvialis astaxanthin could notably boost the IgM concentration in serum and liver, as well as the IgM mRNA expression in the liver both before and after challenge with Vibrio harveyi (Zhu et al. 2022). The inclusion of H. pluvialis astaxanthin in the meal considerably raised the total IgM content in the serum of Asian seabass, Lates calcarifer (Lim et al. 2019). The fact that supplemental astaxanthin was probably beneficial for fish Ig synthesis indicates that it may have an immunomodulatory effect on fish T lymphocyte activation, which is presumably the primary agent regulating the discrimination and proliferation of peripheral B cells that make Igs (Ashfaq et al. 2019).
The antioxidant enzymes demonstrated the functioning state of the body’s antioxidant system, indicating the body’s capacity to neutralize oxygen-free radicals and shield the fish’s tissues from oxidative damage (Shan et al. 2019). Nonetheless, a previous study found a connection between fish responsiveness in aquaculture and antioxidant defense (Guerriero et al. 2002). In this experiment, when compared to the control diet, the antioxidant enzyme activities of red tilapia, such as SOD, and GSH were significantly elevated by nutritional supplementation of H. pluvialis, which is the primary source of esterified astaxanthin. These were consistent with other findings that recorded significant decrease in the levels of CAT, SOD, and GPx in the liver of L. maculates with an increase in dietary H. pluvialis in Chinese mitten crab Eriocheir sinensis (Wu et al. 2017) and large yellow croaker Pseudosciaena crocea (Li et al. 2014). Earlier research has also demonstrated that dietary supplements containing astaxanthin from H. pluvialis (Sheikhzadeh et al. 2012) may enhance the activities of antioxidative enzymes (T-AOC and SOD) in O. mykiss. According to another research, feeding O. mykiss a meal containing 30 mg/kg H. pluvialis astaxanthin for 4 months significantly raised the activities of T-AOC, T-SOD, and GPx in the blood and liver with decreased MDA levels (Long et al. 2023). A previous study on Trachinotus ovatus showed the body’s antioxidant capacity may be enhanced by the addition of astaxanthin-rich Oedocladium carolinianum via the activation of the Nrf2-ARE signaling pathway (Zhao et al. 2022).
According to earlier research, serum AST and ALT activities are important indicators for evaluating liver function, and elevated levels of these enzymes in the serum may indicate hepatocyte dysfunction and liver injury (Ayyat et al. 2018). In this study, liver enzymes including ALT, AST, ALP, and GGT were notably decreased with increasing dietary H. pluvialis levels up to 1.0 g kg−1 diet and leveled off with a further increase to 1.5 g kg−1, compared to the control group. These decreases in liver enzyme activities are usually related to astaxanthin hepatoprotective actions as hepatic tissue was in good condition, as seen by intact cellular structures and the staining characteristics of the hepatocytes, and did not exhibit any congestion, inflammation, or vacuolization. Similarly, dietary H. pluvialis supplementation was observed to improve T. ovatus liver shape and decrease serum AST and ALT activity (Zhao et al. 2021). A minor elevation in blood creatinine level was also detected in this study; however, urea, total bilirubin, total protein, and albumin were not significantly affected by H. pluvialis supplementation. The primary cause of increased blood creatinine level is may be related to the negative effects of high astaxanthin or other elements in microalgae including heavy metals, toxins, and nucleic acids when high doses of algal were administrated (Spolaore et al. 2006).
Prior research has indicated that adding H. pluvialis or astaxanthin to the diet may improve the serum biochemical parameters of fish (Chimsung et al. 2013). The LDL-C/HDL-C ratio is assessed as an exponent of to transport capacity of cholesterol (Yun et al. 2011). In the current study, red tilapia fed the H. pluvialis diet at HP1 and HP1.5 had the lowest levels of cholesterol as well as the highest levels of TG than those of control. Meanwhile, the levels of TG were significantly decreased in the HP0.5 group with no considerable alterations observed in the levels of HDL and LDL among all dietary groups. Our results corroborate another study that showed a significant decrease of TG and cholesterol on days 1 and 4 in fish (Oncorhynchus mykiss) given 3 g kg−1 of H. pluvialis; however, these metabolites were significantly increased when fish were supplied with 10 g kg−1 of algae (Sheikhzadeh et al. 2012). Conversely, dietary H. pluvialis might lower triglyceride and cholesterol levels in large yellow croaker Pseudosciaena crocea (Li et al. 2014). Serum biochemical parameter measurements in the current investigation revealed that fish fed the astaxanthin-rich Oedocladium carolinianum diet (5%) had greater levels of HDL with lower levels of TG, cholesterol, and LDL than fish fed control diet (Zhao et al. 2022). It is unclear why the highest treatment groups have greater triglyceride levels. However, it may be attributed to the high fat content of the diet used to mix the algae was to be the cause of this rise.
The inflammatory cytokines present in fish can be categorized into two groups: pro-inflammatory cytokines (IL-8, TNF-α, and IL-1β) and anti-inflammatory cytokines (IL-10). By upregulating the genes concerning anti-inflammatory cytokines after downregulating pro-inflammatory cytokines-related genes, the inflammation of fish can be decreased (Ottinger et al. 2016). The treatment of oral astaxanthin in this study resulted in a decrease in serum IFN-γ and IL-4 levels along with a drop in hepatic TNF-α, IL-1β, and IL-8 mRNA levels with increasing H. pluvialis levels up to 1.5 g kg−1 diet. Additionally, the expression of hepatic anti-inflammatory IL-10 was statistically increased, suggesting that the inflammation in red tilapia can be lessened. A similar study also recorded that administration of 200, 400, and 800 mg/kg astaxanthin led to a decrease in TNF-α, IL-1β, and IL-8 serum levels with a rise in IL-10 levels in crucian carp (Wu and Xu 2021). Another finding clarified that astaxanthin could greatly suppress the expression of TNF-α, IL-1β, and IL-8, in the head kidney of snakeheads (Li et al. 2019). A recent report confirmed the anti-inflammatory effect of astaxanthin following exposure of Channa argus to LPS, demonstrated by a marked reduction in expression of NF-κB p65, IL-1, IL-8, and TNF-α in liver, kidney, spleen, and gut of fish (Zhu et al. 2022). The anti-inflammatory activity of astaxanthin is associated with its ability to block the NF-κB p65 pathway (Zhu et al. 2022).
The caspase family plays a crucial role in mediating apoptosis as well; caspase 3 serves as the essential executive molecule, commonly activated death protease, and is responsible for the precise cleavage of numerous important cellular proteins. Our findings showed that the caspase-3 gene was markedly decreased in HP1 and HP1.5-supplemented diets. Adding H. pluvialis (3.3, 6.7, and 13.3 g kg−1) to the diet also reduced the salinity stress response and apoptosis in post-larval white shrimp via controlling the caspase-3 mRNA levels, suggesting that the dietary H. pluvialis suppressed the death of post-larval white shrimp (Xie et al. 2018). Furthermore, the intake of 150 mg kg−1 astaxanthin reduced the high expression of caspase-3, caspase-9, and BAD, suggesting that astaxanthin mitigated HFD-induced apoptosis in largemouth bass (Xie et al. 2020).
Generally, the intestinal histomorphology notably contributed to the growth performance of fish species that are represented by higher intestinal villi height (Eissa et al. 2023b). In the current study, significantly higher intestinal villi height and intestinal mucosal layer thickness were shown in the dietary H. pluvialis groups, referring that H. pluvialis enhances the growth performance of red tilapia via improving the digestibility and absorption capacity of nutrients; subsequently, greater contact surface area between the intestine and nutrients was revealed in the supplemented groups. Consistent with our findings, a remarkable increase in the length of intestinal villi and thickness of the intestinal mucosal layer was observed following dietary inoculation of H. pluvialis in L. vannamei shrimp (Fang et al. 2022). Besides, our histological data exerted a beneficial effect of H. pluvialis on the liver health of red tilapia that was strengthened by transcriptional levels of anti-inflammation and apoptosis-related genes. Nevertheless, dietary astaxanthin-rich microalgal Oedocladium carolinianum was found to have a hepatoprotective impact by prohibiting the apoptosis and inflammatory response of trachinotus ovatus fish (Zhao et al. 2022).
Conclusion
Dietary supplementation of H. pluvialis astaxanthin could improve the growth efficacy, antioxidant ability, and innate immunity of hybrid red tilapia after two months of feeding, also the hepatoprotective effects were exerted. Considering these positive benefits of H. pluvialis, the optimal supplementation of dietary H. pluvialis levels should be between 1 and 1.5%.
Data availability
No datasets were generated or analysed during the current study.
References
Aboelward A, Eid A, KA M, Tonsy HD, Ayyat AN (2020) Effect of digestrom® on growth performance and feed utilization of red tilapia (O. niloticus× O. mossambicus). Egy J Aquac 10:65–83. https://doi.org/10.21608/eja.2020.23919.1016
Amar EC, Kiron V, Satoh S, Watanabe T (2004) Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunol 16:527–537. https://doi.org/10.1016/j.fsi.2003.09.004
Ambati RR, Phang S-M, Ravi S, Aswathanarayana RG (2014) Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs 12:128–152. https://doi.org/10.3390/md12010128
Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau J-L, White HD, Nordlander R, Maggioni A, Dickstein K (2004) Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351:1285–1295. https://doi.org/10.1056/NEJMoa041365
Ashfaq H, Soliman H, Saleh M, El-Matbouli M (2019) CD4: a vital player in the teleost fish immune system. Vet Res 50:1–11. https://doi.org/10.1186/s13567-018-0620-0
Ayyat MS, Ayyat AMN, Al-Sagheer AA, El-Hais AE-AM (2018) Effect of some safe feed additives on growth performance, blood biochemistry, and bioaccumulation of aflatoxin residues of Nile tilapia fed aflatoxin-B1 contaminated diet. Aquaculture 495:27–34. https://doi.org/10.1016/j.aquaculture.2018.05.030
Banuelos-Vargas I, de Oca GAR-M, Martinez-Montano E, Perez-Jimenez A, Mendoza-Gamboa OA, Estrada-Godínez JA, Hernandez C (2021) Antioxidant and immune response of juvenile red tilapia (Oreochromis sp) cultured at different densities in sea water with biofloc plus probiotics. Aquaculture 544:737112. https://doi.org/10.1016/j.aquaculture.2021.737112
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Bombardelli RA, dos Reis Goes ES, de Negreiros Sousa SM, Syperreck MA, Goes MD, de Oliveira Pedreira AC, Meurer F (2017) Growth and reproduction of female Nile tilapia fed diets containing different levels of protein and energy. Aquaculture 479:817–823. https://doi.org/10.1016/j.aquaculture.2017.07.031
Bryan NS, Grisham MB (2007) Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 43:645–657. https://doi.org/10.1016/j.freeradbiomed.2007.04.026
Bucolo G, David H (1973) Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19:476–482. https://doi.org/10.1093/clinchem/19.5.476
Burgos-Aceves MA, Abo-Al-Ela HG, Faggio C (2021) Physiological and metabolic approach of plastic additive effects: immune cells responses. J Hazard Mater 404:124114. https://doi.org/10.1016/j.jhazmat.2020.124114
Carbone D, Faggio C (2016) Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol 54:172–178. https://doi.org/10.1016/j.fsi.2016.04.011
Cerezal-Mezquita P, Espinosa-Álvarez C, Jáuregui-Tirado M, Jaime-Matus C, Palma-Ramírez J, Ruiz-Domínguez MC (2022) Physical-chemical characteristics of “Red Meal”, a novel non-defatted additive in the fish feed from cracked biomass of Haematococcus pluvialis. Anim Feed Sci Technol 285:115247. https://doi.org/10.1016/j.anifeedsci.2022.115247
Chimsung N, Lall S, Tantikitti C, Verlhac-Trichet V, Milley J (2013) Effects of dietary cholesterol on astaxanthin transport in plasma of Atlantic salmon (Salmo salar). Comp Biochem Physiol B Biochem Mol Biol 165:73–81. https://doi.org/10.1016/j.cbpb.2013.02.007
Dawood MA, Zommara M, Eweedah NM, Helal AI (2020) Synergistic effects of selenium nanoparticles and vitamin E on growth, immune-related gene expression, and regulation of antioxidant status of Nile tilapia (Oreochromis niloticus). Biol Trace Elem Res 195:624–635. https://doi.org/10.1007/s12011-019-01857-6
Eissa ME, Alaryani FS, Elbahnaswy S, Khattab MS, Elfeky A, AbouelFadl KY, Eissa E-SH, Ahmed RA, Van Doan H, El-Haroun E (2023b) Dietary inclusion of Pediococcus acidilactici probiotic promoted the growth indices, hemato-biochemical indices, enzymatic profile, intestinal and liver histomorphology, and resistance of Nile Tilapia against Aspergillus flavus. Anim Feed Sci Technol 306:115814. https://doi.org/10.1016/j.anifeedsci.2023.115814
Eissa E-SH, El-Sayed A-FM, Ghanem SF, Dighiesh HS, Abd Elnabi HE, Hendam BM, Elleithy AA, Eissa ME, Abd El-Aziz YM (2023a) Dietary mannan-oligosaccharides enhance hematological and biochemical parameters, reproductive physiology, and gene expression of hybrid red tilapia (Oreochromis niloticus x O. mossambicus). Aquaculture 740453. https://doi.org/10.1016/j.aquaculture.2023.740453
Elbahnaswy S, Elshopakey GE, Ibrahim I, Habotta OA (2021) Potential role of dietary chitosan nanoparticles against immunosuppression, inflammation, oxidative stress, and histopathological alterations induced by pendimethalin toxicity in Nile tilapia. Fish Shellfish Immunol 118:270–282. https://doi.org/10.1016/j.fsi.2021.09.015
Elbahnaswy S, Elshopakey GE, Shakweer MS, Eldessouki EA, Abdelwarith AA, Younis EM, Davies SJ, El-Son MA (2023) Bacterial Co-Infection as a Potential Threat to Farmed Flathead Grey Mullet (Mugil cephalus): Phenotypic and molecular diagnosis, histopathology, immunity response, and in vitro antibacterial evaluation. Fishes 8:357. https://doi.org/10.3390/fishes8070357
Elbahnaswy S, Elshopakey GE (2023) Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: a review. Fish Physiol Biochem 1–30. https://doi.org/10.1007/s10695-022-01167-0
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Fang H, Zhuang Z, Huang L, Niu J, Zhao W (2022) A newly isolated strain of Haematococcus pluvialis GXU-A23 improves the growth performance, antioxidant and anti-inflammatory status, metabolic capacity and mid-intestine morphology of juvenile Litopenaeus vannamei. Front Physiol 13:882091. https://doi.org/10.3389/fphys.2022.882091
FAO (2023) (Food and Agriculture Organization) Global aquaculture production quantity (1950 - 2021). FAO Rome Italy
Gervasi T, Pellizzeri V, Benameur Q, Gervasi C, Santini A, Cicero N, Dugo G (2018) Valorization of raw materials from agricultural industry for astaxanthin and β-carotene production by Xanthophyllomyces dendrorhous. Nat Prod Res 32:1554–1561. https://doi.org/10.1080/14786419.2017.1385024
Griesh AS, El-Nahla AM, Aly SM, Badran MF (2024) Role of vitamin E supplementation on the reproductive and growth performance, hormonal profile and biochemical parameters of female hybrid red tilapia. Thalassas: Int J Mar Sci https://doi.org/10.1007/s41208-024-00683-5
Guerra B, Bolin A, Otton R (2012) Carbonyl stress and a combination of astaxanthin/vitamin C induce biochemical changes in human neutrophils. Toxicol in Vitro 26:1181–1190. https://doi.org/10.1016/j.tiv.2012.06.010
Guerriero G, Di Finizio A, Ciarcia G (2002) Stress-induced changes of plasma antioxidants in aquacultured sea bass, Dicentrarchus labrax. Comp Biochem Physiol A Mol Integr Physiol 132:205–211. https://doi.org/10.1016/S1095-6433(01)00549-9
Horwitz W (2010) Official methods of analysis of AOAC International. Volume I, agricultural chemicals, contaminants, drugs/edited by William Horwitz. Gaithersburg (Maryland): AOAC International, 1997
Jagruthi C, Yogeshwari G, Anbazahan SM, Mari LSS, Arockiaraj J, Mariappan P, Sudhakar GRL, Balasundaram C, Harikrishnan R (2014) Effect of dietary astaxanthin against Aeromonas hydrophila infection in common carp, Cyprinus carpio. Fish Shellfish Immunol 41:674–680. https://doi.org/10.1016/j.fsi.2014.10.010
Ju ZY, Davis S, Ramm K, Steck M, Soller F, Fox BK (2017) Effects of microalgae-added diets on growth performance and meat composition of tilapia (Oreochromis mossambicus). Aquac Res 48:5053–5061. https://doi.org/10.1111/are.13322
Juhász K, Buzás K, Duda E (2013) Importance of reverse signaling of the TNF superfamily in immune regulation. Expert Rev Clin Immunol 9:335–348. https://doi.org/10.1586/eci.13.14
Kalinowski C, Robaina L, Izquierdo M (2011) Effect of dietary astaxanthin on the growth performance, lipid composition and post-mortem skin colouration of red porgy Pagrus pagrus. Aquac Int 19:811–823. https://doi.org/10.1007/s10499-010-9401-0
Kawahara E, Ueda T, Nomura S (1991) In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol 26:213–214. https://doi.org/10.3147/jsfp.26.213
Li M, Wu W, Zhou P, Xie F, Zhou Q, Mai K (2014) Comparison effect of dietary astaxanthin and Haematococcus pluvialis on growth performance, antioxidant status and immune response of large yellow croaker Pseudosciaena crocea. Aquaculture 434:227–232. https://doi.org/10.1016/j.aquaculture.2014.08.022
Li M-Y, Liu X-Y, Xia C-G, Wang G-Q, Zhang D-M (2019) Astaxanthin enhances hematology, antioxidant and immunological parameters, immune-related gene expression, and disease resistance against in Channa argus. Aquac Int 27:735–746. https://doi.org/10.1007/s10499-019-00362-w
Lim KC, Yusoff FM, Shariff M, Kamarudin MS (2018) Astaxanthin as feed supplement in aquatic animals. Rev Aquac 10:738–773. https://doi.org/10.1111/raq.12200
Lim KC, Yusoff FM, Shariff M, Kamarudin MS, Nagao N (2019) Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch, 1790). Aquaculture 512:734339. https://doi.org/10.1016/j.aquaculture.2019.734339
Liu Y, Zheng L, Xu B, Sagada G, Zhang J, Shao Q (2022) Effects of diets with varying astaxanthin from Yarrowia lipolytica levels on the growth, feed utilization, metabolic enzymes activities, antioxidative status and serum biochemical parameters of Litopenaeus vannamei. Fishes 7:352. https://doi.org/10.3390/fishes7060352
Long X, Wang L, Li Y, Sun W, Wu X (2023) Effects of long-term Haematococcus pluvialis astaxanthin feeding on the growth, coloration, and antioxidant capacity of commercial-sized Oncorhynchus mykiss. Aquac Rep 30:101603. https://doi.org/10.1016/j.aqrep.2023.101603
Ma N, Long X-W, Zhao L, Chang G-L, Wu X-G, Cheng Y-X (2017) Effects of dietary supplementation of synthetic astaxanthin on ovarian development, coloration and antioxidant capacity of adult female Chinese mitten crab, Eriocheir sinensis. Acta Hydrobiol Sin 41:755–765
Naito H, Kaplan A (1984) High-density lipoprotein (HDL) cholesterol. Clin Chem Toronto Princeton 1207–1213
Nicola N (1994) Guidebook to cytokines and their receptors. (No Title)
Nishikimi M, Appaji Rao N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854. https://doi.org/10.1016/S0006-291X(72)80218-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ottinger CA, Densmore CL, Robertson LS, Iwanowicz DD, VanderKooi SP (2016) Transforming growth factor-β1 expression in endangered age-0 shortnose suckers (Chasmistes brevirostris) from Upper Klamath Lake, OR relative to histopathology, meristic, spatial, and temporal data. Fish Shellfish Immunol 49:1–6. https://doi.org/10.1016/j.fsi.2015.12.019
Prætorius E, Poulsen H (1953) Enzymatic determination of uric acid with detailed directions. Scand J Clin Lab Invest 5:273–280. https://doi.org/10.3109/00365515309094197
Rahmah S, Liew HJ, Napi N, Rahmat SA (2020) Metabolic cost of acute and chronic salinity response of hybrid red tilapia Oreochromis sp. larvae. Aquac Rep 16:100233. https://doi.org/10.1016/j.aqrep.2019.100233
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Shah MMR, Liang Y, Cheng JJ, Daroch M (2016) Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci 7:531. https://doi.org/10.3389/fpls.2016.00531
Shan H, Wang T, Dong Y, Ma S (2019) Effects of dietary Ampithoe sp. supplementation on the growth, energy status, antioxidant capacity, and ammonia-N tolerance of the shrimp Litopenaeus vannamei: Continuous versus interval feeding. Aquaculture 509:32–39. https://doi.org/10.1016/j.aquaculture.2019.05.021
Sheikhzadeh N, Tayefi-Nasrabadi H, Khani Oushani A, Najafi Enferadi MH (2012) Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 38:413–419. https://doi.org/10.1007/s10695-011-9519-7
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96. https://doi.org/10.1263/jbb.101.87
Swanson MA, Lee WT, Sanders VM (2001) IFN-γ production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol 166:232–240. https://doi.org/10.4049/jimmunol.166.1.232
Wang L, Chen Z, Leng X, Gao J, Liu Y, Liu H, Song X (2016) Effect of Haematococcus pluvialis on growth, body color and antioxidation capacity of discus fish Symphysodon haraldi. Freshw Fish 46:92–97
Wedemeyer GA, Yasutake WT (1977) Clinical methods for the assessment of the effects of environmental stress on fish health. Department of the Interior, Fish and Wildlife Service
Wu S, Xu B (2021) Effect of dietary astaxanthin administration on the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). 3 Biotech 11:151. https://doi.org/10.1007/s13205-021-02700-3
Wu X, Zhao L, Long X, Liu J, Su F, Cheng Y (2017) Effects of dietary supplementation of Haematococcus pluvialis powder on gonadal development, coloration and antioxidant capacity of adult male Chinese mitten crab (Eriocheir sinensis). Aquac Res 48:5214–5223. https://doi.org/10.1111/are.13333
Xie S, Fang W, Wei D, Liu Y, Yin P, Niu J, Tian L (2018) Dietary supplementation of Haematococcus pluvialis improved the immune capacity and low salinity tolerance ability of post-larval white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 80:452–457. https://doi.org/10.1016/j.fsi.2018.06.039
Xie S, Yin P, Tian L, Yu Y, Liu Y, Niu J (2020) Dietary supplementation of astaxanthin improved the growth performance, antioxidant ability and immune response of juvenile largemouth bass (Micropterus salmoides) fed high-fat diet. Mar Drugs 18:642. https://doi.org/10.3390/md18120642
Xie J-J, Chen X, Liu Y-J, Tian L-X, Xie S-W, Niu J (2017) Effects of dietary astaxanthin on growth performance, hepatic antioxidative activity, hsp70, and hif-1α gene expression of juvenile golden pompano (Trachinotus ovatus). IJA_69–2017
Young DS (1997) Effects of drugs on clinical laboratory tests. Ann Clin Biochem 34:579–581. https://doi.org/10.1177/000456329703400601
Yu W, Lin H, Yang Y, Zhou Q, Chen H, Huang X, Zhou C, Huang Z, Li T (2021) Effects of supplemental dietary Haematococcus pluvialis on growth performance, antioxidant capacity, immune responses and resistance to Vibrio harveyi challenge of spotted sea bass Lateolabrax maculatus. Aquac Nutr 27:355–365. https://doi.org/10.1111/anu.13189
Yun B, Mai K, Zhang W, Xu W (2011) Effects of dietary cholesterol on growth performance, feed intake and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed high plant protein diets. Aquaculture 319:105–110. https://doi.org/10.1016/j.aquaculture.2011.06.028
Zahran E, Elbahnaswy S, Ibrahim I, Khaled AA (2021) Nannochloropsis oculata enhances immune response, transcription of stress, and cytokine genes in Nile tilapia subjected to air exposure stress. Aquac Rep 21:100911. https://doi.org/10.1016/j.aqrep.2021.100911
Zahran E, Elbahnaswy S, Ahmed F, Ibrahim I, Khaled AA, Eldessouki EA (2023) Nutritional and immunological evaluation of Nannochloropsis oculata as a potential Nile tilapia-aquafeed supplement. BMC Vet Res 19:65. https://doi.org/10.1186/s12917-023-03618-z
Zhao W, Fang H-H, Gao B-Y, Dai C-M, Liu Z-Z, Zhang C-W, Niu J (2020) Dietary Tribonema sp. supplementation increased growth performance, antioxidant capacity, immunity and improved hepatic health in golden pompano (Trachinotus ovatus). Aquaculture 529:735667. https://doi.org/10.1016/j.aquaculture.2020.735667
Zhao W, Fang HH, Liu ZZ, Huang MQ, Su M, Zhang CW, Gao BY, Niu J (2021) A newly isolated strain of Haematococcus pluvialis JNU35 improves the growth, antioxidation, immunity and liver function of golden pompano (Trachinotus ovatus). Aquac Nutr 27:342–354. https://doi.org/10.1111/anu.13188
Zhao W, Cui X, Wang Z-Q, Yao R, Xie S-H, Gao B-Y, Zhang C-W, Niu J (2022) Beneficial changes in growth performance, antioxidant capacity, immune response, hepatic health, and flesh quality of Trachinotus ovatus fed with Oedocladium carolinianum. Front Immunol 13:940929. https://doi.org/10.3389/fimmu.2022.940929
Zhu X, Hao R, Zhang J, Tian C, Hong Y, Zhu C, Li G (2022) Dietary astaxanthin improves the antioxidant capacity, immunity and disease resistance of coral trout (Plectropomus leopardus). Fish Shellfish Immunol 122:38–47. https://doi.org/10.1016/j.fsi.2022.01.037
Acknowledgements
This research was acknowledged by the owner of the special farm for help and support.
Author information
Authors and Affiliations
Contributions
Elsayed A. A. Eldessouki and Sayed Hemdan Eissa: Methodology, Investigation, Validation, Formal analysis. Gehad E. Elshopakey: Methodology, Investigation, Formal analysis, Validation, Writing-original draft, Writing-review & editing. Samia Elbahnaswy: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing-original draft, Writing-review & editing and Follow-up publication. Medhat S. Shakweer, Abdelwahab A. Abdelwarith, Elsayed M. Younis, Simon J. Davies, Amira Mili, Sameh A. Abdelnour, Yasmin M. Abd El-Aziz: Methodology, Formal analysis, and Writing-original draft.
Corresponding author
Ethics declarations
Ethics approval
Our trial has been accomplished with the approval of the Institutional Ethics Committee of the Faculty of Veterinary Medicine, Mansoura University, Egypt. It follows the general guidelines of the Canadian Council on Animal Care approved our experimental protocol (MU-ACUC (VM.R.24.04.162)).
Consent to participate
All authors have participated in this work.
Consent for publication
All authors have reviewed and approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Dietary H. pluvialis significantly improved red tilapia's growth performance and feed utilization.
• Dietary H. pluvialis elevated the antioxidant properties and immune parameters.
• Dietary H. pluvialis suppressed the expression of pro-inflammatory cytokines and apoptotic genes.
• Dietary H. pluvialis increased intestinal villous width and thickness of absorptive epithelium.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eldessouki, E.A.A., Elshopakey, G.E., Elbahnaswy, S. et al. Influence of astaxanthin-enriched Haematococcus pluvialis microalgae on the growth efficacy, immune response, antioxidant capacity, proinflammatory cytokines, and tissue histomorphology of hybrid red tilapia. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01524-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01524-1