Abstract
A 12-week feeding trial with common carp (Cyprinus carpio) was conducted to test the hypothesis that compound diets formulated on the basis of different soybean cultivars will have effects on growth parameters, feed utilization, and digestive system histology. Soybean grains were from the following cultivars: Alisa (SB-A), Balkan (SB-B), and Galeb (SB-G). The fourth tested diet contained grains from a mixture of different SB cultivars (SB-M). The results confirmed the tested hypothesis, as the fish from the group SB-A showed higher average weight gain, specific growth rates, and feed efficiency, as well as lower feed intake and feed conversion ratio, compared to the groups SB-B and SB-M. On the other hand, diets were expected to cause inflammation in the distal intestine of the fish, but this did not occur. Histological examination of the intestine and liver, performed at the end of weeks 1, 3, 6 and 12, showed no pathological changes. Most of the differences between the groups were found at the end of the trial. The group SB-A had a higher surface area of different intestinal layers compared to the groups SB-B and SB-G. The surface area of the goblet cells was greatest at most of the time points in SB-M. In the liver, the evaluation of the surface area of hepatocytes and their nuclei showed no significant differences between the groups. The differences in final body mass, which showed a maximum value of 18% between the groups, could be of significant importance for culture of this freshwater species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shortage of fishmeal (FM) remains one of the biggest concerns for the sustainability of aquaculture and the aquafeed industry (Hua et al. 2019). Especially in the last 30 years, a large number of papers has been published to find alternative ingredients to replace FM in fish diets. Novel ingredients from microalgae (Carneiro et al. 2020; Milledge, 2011; Siddik et al. 2024; Sørensen et al. 2017) or insects (Barroso et al. 2014; Belghit et al. 2018; Hua 2021; Mohan et al. 2022) have come into focus in recent years, but the majority of published studies have investigated the use of plants as an alternative to replace FM in fish diets (Aragão et al. 2022). Due to low production costs and global distribution, plant seeds are the ideal choice to replace FM. Among commercially available crops, soybean seed (SB) is one of the preferred options due to its balanced amino acid composition and high protein content (Gatlin et al. 2007). SB is usually offered in the form of soybean meal (SBM), which shows promising growth parameters in several fish species if inclusion rates are low to moderate. The disadvantage of SB and SBM as ingredients for fish feeds is that they contain several classes of antinutritional factors (ANFs), namely, saponins, lectins, phytosterols, and protease inhibitors (Francis et al. 2001; Krogdahl et al. 2010), which can cause problems in the metabolism and lead to inflammation of fish distal intestine and, in the worst case, mortality.
Carnivorous species in general, including fish from the economically important family Salmonidae, show a high degree of distal intestine enteritis induced by diets containing SBM (Agboola et al. 2022; Baeverfjord and Krogdahl, 1996; van den Ingh et al. 1991). The extent of SBM-induced enteritis is highly dependent on the fish species, and even two species of the same genus (Chinook salmon (Oncorhynchus tshawytscha) and pink salmon (O. gorbuscha)) respond differently to feeding diets in which 20% of FM is replaced by SBM (Booman et al. 2018). The effects of high levels of SBM in the diet of carnivorous fish and the development of intestinal pathology result in reduced growth and are also associated with changes in intestinal microbiota (Kononova et al. 2019; Zhang et al. 2022). Intestinal inflammation can be alleviated in several ways: (a) by reducing the amount of SBM in the diet (Agboola et al. 2022); (b) by including bacterial meal or lactic acid bacteria in the fish diet (Nimalan et al. 2023; Romarheim et al. 2011); (c) by incorporating plant extracts in diets (Fehrmann-Cartes et al. 2019); (d) by adding glutamine to fish diets (Gu et al. 2021); (e) by thermal processing (Krogdahl et al. 2010); (f) by fermentation (Novriadi et al. 2018; Refstie et al. 2005) or (g) by adding enzymes (Novriadi et al. 2019) to SBM-based diets. In addition, certain technological processes can be used to produce refined products from SB, such as soy protein concentrates which have no restrictions for feeding fish, including carnivores, in which intestinal inflammation does not occur (Kaushik et al. 1995; van den Ingh et al. 1991). When SB or SBM is processed in any of the above ways, its antinutrients are altered, but this also increases the cost of producing feed. The cost of producing fish feed is even more important in developing countries, e.g., in Asia, where fish protein accounts for between 15 and 53% of the total animal protein source for human consumption (Mohan Dey et al. 2005). In these countries, fish of lower economic value, such as cichlid or cyprinid fish, are usually produced. Common carp is an omnivorous and agastric fish species, but it is also prone to develop enteritis in the distal intestine when 20% of FM in its diet is replaced by SBM (Urán et al. 2008a). The inflammation lasts for a shorter duration compared to carnivorous species due to fast adaptation of its digestive system to the SBM-based diet (Urán et al. 2008a).
Ingredients are of utmost importance in feed formulation and SB genotype must be considered before the decision which SB cultivar or breeding line will be used for feed formulation. It is well known in plant science that the different SB genotypes have a wide variation in protein, lipid, and carbohydrate content (Medic et al. 2014; Thakur and Hurburgh, 2007; Vollmann et al. 2000) and in ANFs (Gu et al. 2010; Mittal et al. 2021; Vlahakis and Hazebroek 2000), but these facts have not been sufficiently explored in the aqua feed industry. As far as the authors are aware, the effects of different origins of SBM in fish feeds have been assessed in a single study, which found a different intensity of enteritis when Atlantic salmon (Salmo salar) were fed diets containing SBM purchased on different continents (Urán et al. 2009).
Considering these facts, the main objective of the present study is to test the hypothesis that the growth performance and functional histology of the liver and distal intestine of common carp depend on the inclusion of different SB cultivars in the diet.
Materials and methods
Experimental diets
To achieve the objective of the present study, four extruded diets were formulated (Table 1). Diets were based on FM, SBM, ground whole SB, wheat, and corn grains using the same formulation except for the alternative origin of SB. Three different SB cultivars were selected for the experimental diets: Alisa (SB-A), Balkan (SB-B), and Galeb (SB-G). The first two cultivars were developed by the Institute of Field and Vegetable Crops (Novi Sad, Serbia), registered in 2005 and 1994, respectively, and are grown throughout Serbia and some European countries such as Hungary and Bulgaria (Miladinović et al. 2006). The third cultivar, SB-G, was developed by Delta Agrar Company (Belgrade, Serbia) and is registered for use in Serbia, Romania, and Italy. The SB-M diet used comes from a commercially available mixture of different SB cultivars grown and collected at a major SB producer (Sojaprotein, Bečej, Serbia).
Experimental design
For conducting the 12-week nutritional trial, 1+ year-old common carp juveniles from the Center for Fishery and Applied Hydrobiology “Mali Dunav” of the Faculty of Agriculture, University of Belgrade, were used. Twenty-three fish were randomly placed in each of the 12 indoor conical-bottomed tanks (4 experimental groups in triplicate, 69 fish per group, 276 fish in total) with a volume of 120 L filled with dechlorinated tap water (flow-through system; exchange rate of 0.34 L min−1) with natural photoperiod (March–June). The fish were allowed to acclimatize to the new tanks for 20 days before the experiment began. During the acclimation period, they were fed daily ad libitum with FM diet that did not contain SB and SBM. Water temperature, dissolved oxygen concentration (DO), and oxygen saturation of the water in the tanks were automatically recorded every 10 min with a Stationary system (OxyGuard, Farum, Denmark). The average values of environmental parameters recorded during the experimental period were as follows: temperature, 22.3 (0.3) °C; DO, 8.8 (0.6) mgL−1; oxygen saturation, 99.1 (4.2) %. After the start of the experiment, fish were fed each day (except 1 day prior to the sampling and on the sampling days) using AGK belt feeders (Kronawitter, Wallersdorf, Germany), at a quantity of 3% of the total fish body mass calculated for each tank. The fish were measured every fortnight to be able to adjust the amount of given diet.
Growth performance and feed utilization
Growth performance and feed utilization were evaluated through the set of standard formulas:
where Wf is the final and Wi is the initial body weight (g), d is the number of feeding days, FI is the feed intake (g), PI is the protein intake (g), W is the body weight (g), and L is the fork length (cm).
Fish sampling
For histological examination of the distal intestine, fish were sampled at the end of weeks 1 (W01), 3 (W03), 6 (W06), and 12 (W12) after the start of the study. The rationale for this decision was based on the study by Urán et al. (2008a), which showed that the effects of enteritis in common carp last for about 40 days and peak after 7 days. The fish were not fed for 24 h before sampling. On the day of sampling, fish were taken randomly from each tank, anesthetized with clove oil, and measured to determine total length (cm) and weight (g). One fish per tank was taken for histological analysis at W01, W03, and W06 (three per group), while two fish per tank were taken at the end of the experiment (six per group). The fish were then sacrificed by a sharp blow to the head and dissected through an incision on the ventral side of the body. The distal part of the intestine, which was about 2 cm long, was cut off and immediately placed in 4% formaldehyde (Lach-Ner, Neratovice, Czech Republic). A liver sample was taken from the same fish sacrificed at the end of the experiment (at W12).
Histological processing
After 48 h of fixation in formaldehyde, the distal intestine and liver samples were transferred to 70% ethanol and later dehydrated in a graded ethanol series followed by xylene clearing, oriented, and embedded in paraffin in an automated tissue processor (Leica TP 1020, Nussloch, Germany). Samples were later sectioned to a nominal thickness of 5 μm (Leica SM2000R, Nussloch, Germany) and mounted on glass slides. The intestine was stained with a combination of Alcian blue 8G at pH 2.5 (AB) and periodic acid-Schiff (PAS), while the liver was stained using PAS only. All slides were later counterstained with hematoxylin, and stains were purchased from Merck (Darmstadt, Germany). The paraffin-embedded tissue was sectioned extensively and two complete cross-sections of the distal intestine and the liver were obtained from each sample. The sections were photographed using an optical microscope DM LB (Leica, Mannheim, Germany) equipped with the DFC 295 camera (Leica, Mannheim, Germany).
Quantification of intestinal tissue and structures
The cross-sections were blinded, photographed in a series of smaller photographs with a ×10 objective, and later assembled into one photograph using the MosaicJ plug-in (Thévenaz and Unser, 2007) installed in ImageJ v1.44p software (Schneider et al. 2012). To achieve an unbiased approach, a set of test points is superimposed on a whole image and the point-count intercept method (Gundersen et al. 1988) is used to quantify the surface profile area of the intestinal layers: (a) lamina epithelialis; (b) lamina propria; (c) tunica muscularis. The points were equally spaced 172 μm apart in the x and y axes (Fig. 1) and on average 720 points per section hit the tissue.
To estimate the surface areas (ā) on the cross-section of the slide, a surface area of 0.0296 mm2 (172 × 172 μm) was added to each test point (Gundersen et al. 1988). A simple approach was used to determine the surface area of the tunica mucosa: \({\stackrel{-}{a}}_{t.mucosa}={\stackrel{-}{a}}_{l.epithelialis}+{\stackrel{-}{a}}_{l.propria}\). Quantification of goblet cells and intraepithelial macrophages was performed by systematically, uniformly, and randomly taking photomicrographs of the intestinal folds with a ×40 objective (15 photomicrographs on average). Using the methodology described earlier, a test grid with a density of 13 × 17 points was placed over each photomicrograph (an average of 2555 points encountered on the tissue of a single fish), and the points intercepting goblet cells (Pgc) and epithelial cells (Pec) were counted. The surface area of the goblet cells was calculated as follows: \({\stackrel{-}{a}}_{goblet cells}={\stackrel{-}{a}}_{l.epithelialis}\times {P}_{gc}\times {\left({P}_{ec+gc}\right)}^{-1}\). In addition, two derived volume density estimates (\({\widehat{V}}_{V}(l. propria, t.mucosa)\) and \({\widehat{V}}_{V}\left(goblet cells, l.epithelialis\right)\)) were calculated as the number of points falling on the structure (Pa) divided by the total number of test points hitting the tissue layer (Pt):\({\widehat{V}}_{V}={P}_{a}\times {{P}_{t}}^{-1}\).
Quantification of hepatocytes
Liver samples from six animals per group were analyzed at W12. A series of ten microscopic images were taken per fish with a ×100 objective using the principle of systematic, uniform random sampling. To quantify the surface profile area of the cells and their nuclei, only hepatocytes with visible nucleoli were analyzed, as previously recommended (Rašković et al. 2019). As in the intestine, the method of point counting was used with a test grid superimposed on the microphotographs. The density of the test grid was 10 × 10 μm (100 µm2) for estimating the surface area of the hepatocytes and 3 × 3 μm (9 µm2) for the nuclei. On average, 102 hepatocytes were quantified per fish.
Quantification of PAS positivity in liver sections
To quantify the differences in glycogen content in the hepatopancreas, five microscopic images of PAS/H stained tissue sections per animal were analyzed at ×40 objective magnification using Image J software. The Colour Deconvolution plugin was used to convert RGB 256-bit images of PAS/H stained samples into two channels, one of them representing the PAS-staining. The mean gray level was measured for each PAS-channel image, and arbitrary values were calculated as 1000/grayscale level to obtain a direct proportionality between the PAS intensity and the values obtained. Six animals per group were studied.
Statistical analysis
All experimental datasets were first subjected to the Shapiro-Wilk test for the normality assumption and the Levene test for the homoscedasticity assumption. As all datasets met both assumptions, differences between experimental groups were tested using a one-way test ANOVA, followed by Duncan’s multiple range test (DMRT) as a post hoc test. Data in the manuscript are presented as means (standard deviation (SD)), while the significance level (α) was set at 5%.
Results
There were no statistical differences in body mass and Fulton’s condition factor between the experimental groups at the beginning of the trial (Table 2). Fish fed SB-A diet showed superior growth compared to fish fed SB-B and SB-M diets, as evidenced by significantly higher values for body mass, weight gain, and specific growth rate (p < 0.05) at the end of nutritional assays. The final Fulton’s condition factor of the fish fed with SB-A showed significantly lower values compared to the fish fed with SB-G (p < 0.05). The results of the feed utilization analyses were similar, as FCR, FER, PER, and feed intake were highest in the SB-A group compared to in the SB-B and SB-M groups (p < 0.05). No mortality was observed in either group during the course of the experiment.
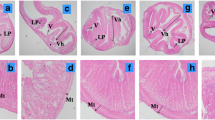
Histological assessment revealed no signs of inflammation of the distal intestine (Fig. 2). The intestinal tissue appeared normal and no differences were noted on the histological sections between groups. Supranuclear vacuoles and intraepithelial macrophages were present in all sampled fish. On the other hand, the estimation of the surface areas of the histological layers showed significant differences between the groups at the end of the experiment (W12; Fig. 3).
Histological sections of the distal intestine of common carp fed four experimental diets based on soybean cultivars (SB): (A) SB-A; (B) SB-B; (C) SB-G; (D) SB-M; The intestinal folds have a similar appearance in all groups: the lamina propria is located in the center of the intestinal fold, parallel to the long axis of the fold, while the lamina epithelialis is composed of enterocytes (columnar epithelial cells) and intraepithelial macrophages (double arrowheads). The enterocytes show a typical organization with abundant supranuclear vacuoles (asterisks) and dark blue–stained goblet cells. Note the presence of eosinophilic granulocytes in the l. propria (arrowheads), excessive mucus (arrows), and enlarged l. propria in the SB-M group (×400, AB/PAS, bar = 50 μm)
Quantitative assessment of the surface areas (mm2) of the histological layers in the cross-section of the distal intestine using the point-count intercept method. The graphs in the same row show the results of the fish sampled at the end of the following weeks: week 1 (A–E); week 3 (F–J); week 6 (K–O); week 12 (P–T). Different letters in a graph represent statistical differences between fish fed experimental diets based on soybean cultivars (SB) within the same intestinal layer (ANOVA, followed by DMRT, p < 0.05)
The average surface area of the l. propria was higher in the group SB-M than in the groups SB-B and SB-G at the W06 sampling (Fig. 3; p < 0.05). However, at the end of the experiment (W12), the SB-A group had higher average surface area compared to the groups SB-B and SB-G (Fig. 3; p < 0.05). The surface area of the l. epithelialis was smaller in the SB-G group than in the SB-A at W12 (Fig. 3; p < 0.05), while T. mucosa was largest in the SB-A group compared to the SB-B and SB-G groups at W12 (Fig. 3; p < 0.05). The same trend was observed for the t. muscularis at W12. Goblet cells surface area analysis showed the highest values in the SB-M group during W03 and W06, while at W12 no statistical differences between experimental groups were obtained (Fig. 3; p > 0.05). When comparing the mean volume density of the l. propria in relation to the t. mucosa, which generally decreases over time in all groups, no statistical difference was found between the test groups (p > 0.05; Fig. 4). Regarding the mean volume density of the goblet cells in relation to the l. epithelialis, the highest value was noticeable in the SB-M at the end of W01 and W12, compared to the SB-A and SB-B groups (p < 0.05; Fig. 4).
Mean volume density: (A) of l. propria in relation to t. mucosa and (B) of goblet cells in relation to l. epithelialis in the intestine of common carp fed five experimental diets based on soybean cultivars (SB) and sampled at different time points (weeks 1, 3, 6, and 12). Different letters represent statistical differences between fish fed experimental diets within the sampling time point (ANOVA, followed by DMRT, p < 0.05)
Histological analysis did not reveal significant histopathological alterations in the liver of the experimental groups at the end of W12 (Fig. 5). The hepatocytes of all experimental groups were mostly pale due to the large vacuolization and small amount of cytoplasm. Large nuclei with prominent nucleoli were present in the center of the cell or slightly eccentric. Histological and morphometric analysis of the hepatocytes showed no statistical difference between the experimental groups at the end of the study (Fig. 6). The size of the hepatocytes, expressed as the surface area, was similar in the groups. In like manner, no significant alterations in nuclear surface area were observed between the SB groups. However, the intensity of PAS staining was significantly higher in SB-A and SB-B groups compared to the other two groups (p < 0.05) (Fig. 6).
Histological appearance of the hepatocytes of the sampled fish at the end of the study (W12). The hepatocytes had a similar appearance in the majority of the sampled fish, with the exception of one individual in group SB-G and one individual in group SB-M, which are marked with arrows. Some sections contained red granules in the cytoplasm (arrowhead), which we believe to be a staining artifact (PAS; bar = 20 μm)
Discussion
Significant differences in growth performance were found between experimental diets, which depended solely on the source of SB. The differences in weight gain in the present study did not reflect levels of proteins and lipids in the experimental diets, implying that the probable reason for these differences was the difference among the SB cultivars. Namely, it was shown that different SB cultivars could vary in the concentrations of ANFs (Hoeck et al. 2000). Among them, lectins and trypsin inhibitors, known to impair growth and cause intestinal inflammation in fish, are normally reduced in the technological process of preparing extruded feed (Barrows et al. 2007), which was used in the present study, but this is not the case for saponins. Soy saponins are capable of reducing growth and inducing enteritis in fish (Gu et al. 2018; Krogdahl et al. 2015). They damage membranes and induce necrosis of enterocytes, which is associated with the release of pro-inflammatory cytokines, an increase in oxidative stress, and subsequent migration of lymphocytes into the intestinal mucosa (Gu et al. 2021; Zhou et al. 2023). In addition, saponins have a bitter taste (Wanka et al. 2019) and can cause palatability problems in fish, but feed intake was similar between groups, so the bitter taste was likely neutralized by other feed components.
Urán et al. (2009) found differences in the intensity of intestinal inflammation in Atlantic salmon when 20% of FM was replaced with six SBM diets derived from plants from four continents. Growth rate was not reported in this study, but inflammation was clearly dependent on the SBM selected. A similar study was conducted on Pacific white shrimp (Litopenaeus vannamei) fed diets containing different varieties of SBM from the state of Illinois, USA (Galkanda Arachchige et al. 2019). The results showed effects on body mass, weight gain, feed conversion ratio, total growth coefficients, and survival rate of shrimp after a 5-week feeding trial. These results support the differences in growth performance of common carp fed grains from different SB cultivars in the present study. Both studies confirm that the origin of SB and SBM can affect different aquaculture species and lead to their improved or deteriorated growth. The explanation for this phenomenon in Pacific white shrimp is either the different range of ANFs in SBs or the digestibility and absorption of nutrients (Galkanda Arachchige et al. 2019). Soybean varieties have also been tested as a factor in rat diets, and results showed an inverse correlation between soybean trypsin inhibitor concentration and lectin content and growth (Gu et al. 2010).
There are several possible explanations for why there were no signs of enteritis in the distal intestine of common carp in the first 21 days of the study, as expected in a previous study by Urán et al. (2008a): (1) In studies where FM was completely replaced by SBM in the diet of common carp, a smaller distal intestinal absorption area was observed after 90 days compared to FM (Marković et al. 2012). In the present study, the percentage of FM replaced by SB and SBM was probably insufficient to cause intestinal inflammation. Intestinal inflammation is a dose-dependent process, which is confirmed in studies where FM is gradually replaced by SBM in the diets of Atlantic salmon and turbot (Scophthalmus maximus) (Gu et al. 2016; Krogdahl et al. 2003). However, replacing 20% FM with SBM in common carp diets resulted in distal intestine inflammation (Urán et al. 2008a) which is a contradictory result compared to that in the present study. (2) Another reason could be the high content of brewer’s yeast (Saccharomyces cerevisiae) in all tested diets (160 g kg−1). Enteritis in Atlantic salmon is prevented when another single-cell protein source, a bacterial meal, is included in the feed (Romarheim et al. 2011). The positive effect on the growth of common carp was confirmed when FM was replaced in their diet by yeast protein concentrate at a level of 7.5–50%, while at the same time there was no adverse effect on distal intestinal morphology, except for an increased number of goblet cells (Omar et al. 2012). Another study in common carp showed improved growth performance when SBM was replaced with 5% yeast in the diet (Mareš et al. 2023). Yeast cell walls also act as an immunostimulant in the diet of Japanese sea bass (Lateolabrax japonicus) and have no effect on the histology of its digestive system (Yu et al. 2014). (3) The extrusion process, in which feed ingredients are mechanically sheared at high temperature and pressure in the presence of moisture, alters the structure of ingredients and content of ANFs, primarily endogenous trypsin inhibitors (Barrows et al. 2007), which cause inflammation of distal intestine in fish (Santigosa et al. 2010). In other studies, the use of extruded diets also leads to improved growth in rainbow trout (Oncorhynchus mykiss) and common carp (Božić et al. 2021; Oliva-Teles et al. 1994; Rašković et al. 2016a, b).
Although intestinal inflammation was not detected in the present study, the profile area of enterocytes (represented as the area of l. epithelialis on the cross-section of the intestine) and t. mucosa showed differences between fish fed experimental diets. SBM in fish diets causes shorter intestinal folds and/or lower enterocyte height in several omnivore and carnivore species (Wang et al. 2016; Wu et al. 2020; Zhu et al. 2021). A larger surface area of the mucosal profile is considered a beneficial feature of the intestine because nutrients can be absorbed more efficiently. When Atlantic salmon are fed an SBM diet, the number of enterocytes undergoing apoptosis in the epithelium is increased, but cells in the l. propria show no differences when immunolabeled with caspase-3 antibodies (Bakke-McKellep et al. 2007; Hofossæter et al. 2023). Loss of enterocytes leads to reduced metabolism and disruption of normal physiological digestive processes. In turbot fed SBM diet, the expression of anti-apoptotic gene B-cell lymphoma 2 (bcl-2) and three pro-apoptotic genes bcl-2-like protein 4 (bax), BH3 interacting-domain death agonist (bid), and caspase-3 (casp3) is upregulated in the distal intestine compared to fish fed FM diet, while diversity and dysbiosis of the intestinal microbiota were also detected in the SBM group (Li et al. 2022). The increase in the surface area of the l. propria is another marker of deteriorated intestinal health and is usually accompanied by infiltration of eosinophils, monocytes/macrophages, and neutrophils (Urán et al. 2008a), which were not found in greater abundance in the present study. The surface area of the l. propria was greater in the SB-A group, but when the values were normalized to the surface area of the t. mucosa and the volume density is calculated, there were no statistical differences. The SB-M group had the highest values for goblet cell surface area and volume density when normalized to l. epithelialis. These changes were subtle, but shorter mucosal folds, a wider l. propria, and a higher number of goblet cells are suggested as markers of inflammation of distal intestine in the scoring system published by Urán et al. (2008b). The proliferation of goblet cells allows for increased mucus production, which serves as protection for the epithelial lining of the intestine (Willora et al. 2022). Alterations in the t. muscularis were not part of the aforementioned scoring system and this layer is not the first choice in evaluating gut histology, but in the present study it is thickest in the fish with the highest weight gain (SB-A group).
The liver is the major organ of carbohydrate and lipid metabolism in animals and as such is the primary storage organ for energy. Therefore, planar morphometry of hepatocytes is often used as an indirect marker of liver function in nutritional assays (Rašković et al. 2011). Replacement of FM with SBM in dietary experiments can alter the size of hepatocytes and their nuclei (Matulić et al. 2020), but is highly dependent on the species studied and diet composition, particularly the ratio of FM replaced. When common carp are fed diets based on SBM and without FM, there are no significant differences in the size of hepatocytes or their nuclei (Marković et al. 2012). The histological appearance of the hepatocytes was typical compared to other nutritional assays in common carp (Božić et al. 2021; Kesbiç et al. 2023; Xie et al. 2021) or another cyprinid species, crucian carp (Carassius carassius) (Kasprzak et al. 2019). Although this type of vacuolization is sometimes referred to as steatosis, transmission electron microscopy of common carp hepatocytes revealed that the vacuoles usually contain lipid droplets and glycogen in different ratios as we have previously demonstrated (Rašković et al. 2016b) and are therefore defined as “normal hepatocytes of well-fed fish” (Couch, 1993). When steatosis occurs in fish liver, the nuclei of hepatocytes are displaced to the periphery of the cell and are normally in contact with the cell membrane (Caballero et al. 1999). Since this was not the case in the liver of herein analyzed experimental groups, we can conclude that in addition to the absence of histopathological changes (i.e., cell death foci, fibrosis, lymphocyte infiltration, etc.), we were not able to detect alterations in hepatocyte appearance, regarding their size and size of their nuclei, as well as the lipid droplets accumulation (i.e., steatosis). The only difference noted was the lower PAS staining intensity in SB-G and SB-M groups. This indicates a lower glycogen reserve in the hepatocytes of these groups. Glycogen depletion in hepatocytes is known to be caused by the increased presence of isoflavones in fish feed (Gu et al. 2015), which are found in high concentrations in soybean. There is a high probability that the soybeans used for the production of fish feed in these two groups had a higher content of isoflavones. However, in a study in which isoflavones were added to the feed of rainbow trout, contradictory results were shown. No PAS staining was observed either in the control group or in fish fed isoflavone-rich diets (Pastore et al. 2018), which is pointing to species-specific effects.
Conclusions
This study has proven that the growth of juvenile common carp is dependent on the choice of soybean cultivars incorporated to the diet. The differences in final body mass of 18% may be of great importance for the rearing of this freshwater species, but further studies are needed before this result can be used further. The histology of the intestine and liver did not show any pathological changes during the experiment at the selected sampling times. Inflammation of the distal intestine did not occur, contrary to the hypothesis tested, but the histomorphometric parameters showed a deterioration of intestinal health in some groups, which corresponded with poorer results in the growth parameters of the common carp.
Data availability
No datasets were generated or analyzed during the current study.
References
Agboola JO, Chikwati EM, Hansen JØ, Kortner TM, Mydland LT, Krogdahl Å, Djordjevic B, Schrama JW, Øverland M (2022) A meta-analysis to determine factors associated with the severity of enteritis in Atlantic salmon (Salmo salar) fed soybean meal-based diets. Aquaculture 555:738214. https://doi.org/10.1016/j.aquaculture.2022.738214
Aragão C, Gonçalves AT, Costas B, Azeredo R, Xavier MJ, Engrola S (2022) Alternative proteins for fish diets: implications beyond growth. Animals 12:1211. https://doi.org/10.3390/ani12091211
Baeverfjord G, Krogdahl A (1996) Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: a comparison with the intestines of fasted fish. J Fish Dis 19:375–387. https://doi.org/10.1046/j.1365-2761.1996.d01-92.x
Bakke-McKellep AM, Penn MH, Salas PM, Refstie S, Sperstad S, Landsverk T, Ringø E, Krogdahl Å (2007) Effects of dietary soyabean meal, inulin and oxytetracycline on intestinal microbiota and epithelial cell stress, apoptosis and proliferation in the teleost Atlantic salmon (Salmo salar L). Brit J Nutr 97:699–713. https://doi.org/10.1017/S0007114507381397
Barroso FG, de Haro C, Sánchez-Muros M-J, Venegas E, Martínez-Sánchez A, Pérez-Bañón C (2014) The potential of various insect species for use as food for fish. Aquaculture 422–423:193–201. https://doi.org/10.1016/j.aquaculture.2013.12.024
Barrows FT, Stone DAJ, Hardy RW (2007) The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchus mykiss). Aquaculture 265:244–252. https://doi.org/10.1016/j.aquaculture.2007.01.017
Belghit I, Liland NS, Waagbø R, Biancarosa I, Pelusio N, Li Y, Krogdahl Å, Lock E-J (2018) Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 491:72–81. https://doi.org/10.1016/j.aquaculture.2018.03.016
Booman M, Forster I, Vederas JC, Groman DB, Jones SRM (2018) Soybean meal-induced enteritis in Atlantic salmon (Salmo salar) and Chinook salmon (Oncorhynchus tshawytscha) but not in pink salmon (O. gorbuscha). Aquaculture 483:238–243. https://doi.org/10.1016/j.aquaculture.2017.10.025
Božić G, Rašković B, Stanković M, Poleksić V, Marković Z (2021) Effects of different feeds on growth performance parameters, histology of liver, distal intestine, and erythrocytes morphology of common carp (Cyprinus carpio L). Biologia 76:3769–3779. https://doi.org/10.1007/s11756-021-00882-y
Caballero MJ, López-Calero G, Socorro J, Roo FJ, Izquierdo MS, Férnandez AJ (1999) Combined effect of lipid level and fish meal quality on liver histology of gilthead seabream (Sparus aurata). Aquaculture 179:277–290. https://doi.org/10.1016/s0044-8486(99)00165-9
Carneiro WF, Castro TFD, Orlando TM, Meurer F, Paula DAJ, Virote BCR, Vianna ARCB, Murgas LDS (2020) Replacing fish meal by Chlorella sp. meal: effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture 528:735612. https://doi.org/10.1016/j.aquaculture.2020.735612
Couch JA (1993) Light and electron microscopic comparisons of normal hepatocytes and neoplastic hepatocytes of well-differentiated hepatocellular carcinomas in a teleost fish. Dis Aquat Organ 16:1–14. https://doi.org/10.3354/dao016001
Fehrmann-Cartes K, Coronado M, Hernández AJ, Allende ML, Feijoo CG (2019) Anti-inflammatory effects of aloe vera on soy meal-induced intestinal inflammation in zebrafish. Fish Shellfish Immun 95:564–573. https://doi.org/10.1016/j.fsi.2019.10.075
Francis G, Makkar HPS, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227. https://doi.org/10.1016/S0044-8486(01)00526-9
Galkanda Arachchige HSC, Qiu X, Stein HH, Davis A (2019) Evaluation of soybean meal from different sources as an ingredient in practical diets for Pacific white shrimp Litopenaeus vannamei. Aquac Res 50:1230–1247. https://doi.org/10.1111/are.13998
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza J, Stone E, Wilson D, Wurtele R (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
Gu C, Pan H, Sun Z, Qin G (2010) Effect of soybean variety on anti-nutritional factors content, and growth performance and nutrients metabolism in rat. Int J Mol Sci 11:1048–1056. https://doi.org/10.3390/ijms11031048
Gu M, Gu JN, Penn M, Bakke AM, Lein I, Krogdahl Å (2015) Effects of diet supplementation of soya-saponins, isoflavones and phytosterols on Atlantic salmon (Salmo salar, L) fry fed from start-feeding. Aquac Nutr 21:604–613. https://doi.org/10.1111/anu.12187
Gu M, Bai N, Zhang Y, Krogdahl Å (2016) Soybean meal induces enteritis in turbot Scophthalmus maximus at high supplementation levels. Aquaculture 464:286–295. https://doi.org/10.1016/j.aquaculture.2016.06.035
Gu M, Jia Q, Zhang Z, Bai N, Xu X, Xu B (2018) Soya-saponins induce intestinal inflammation and barrier dysfunction in juvenile turbot (Scophthalmus maximus). Fish Shellfish Immun 77:264–272. https://doi.org/10.1016/j.fsi.2018.04.004
Gu M, Pan S, Li Q, Qi Z, Deng W, Bai N (2021) Protective effects of glutamine against soy saponins-induced enteritis, tight junction disruption, oxidative damage and autophagy in the intestine of Scophthalmus maximus L. Fish Shellfish Immun 114:49–57. https://doi.org/10.1016/j.fsi.2021.04.013
Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394. https://doi.org/10.1111/j.1699-0463.1988.tb05320.x
Hoeck JA, Fehr WR, Murphy PA, Welke GA (2000) Influence of genotype and environment on isoflavone contents of soybean. Crop Sci 40:48–51. https://doi.org/10.2135/cropsci2000.40148x
Hofossæter M, Sørby R, Göksu AB, Mydland LT, Øverland M, Press CM (2023) Cyberlindnera Jadinii yeast as a functional protein source for Atlantic salmon (Salmo salar L.): early response of intestinal mucosal compartments in the distal intestine. Fish Shellfish Immun 137:108758. https://doi.org/10.1016/j.fsi.2023.108758
Hua K (2021) A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture 530:735732. https://doi.org/10.1016/j.aquaculture.2020.735732
Hua K, Cobcroft JM, Cole A, Condon K, Jerry DR, Mangott A, Praeger C, Vucko MJ, Zeng C, Zenger K, Strugnell JM (2019) The future of aquatic protein: implications for protein sources in aquaculture diets. One Earth 1:316–329. https://doi.org/10.1016/j.oneear.2019.10.018
Kasprzak R, Ostaszewska T, Kamaszewski M (2019) Effects of feeding commercial diets on the development of juvenile crucian carp Carassius carassius: digestive tract abnormalities. Aquat Biol 28:159–173. https://doi.org/10.3354/ab00717
Kaushik SJ, Cravedi JP, Lalles JP, Sumpter J, Fauconneau B, Laroche M (1995) Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 133:257–274. https://doi.org/10.1016/0044-8486(94)00403-B
Kesbiç OS, Acar Ü, Demirci B, Terzi F, Tezel R, Türker A, Güllü K, Erol HS (2023) Effects of replacement cold press poppy seed (Papaver somniferum) oil to fish oil at different proportions on the growth performance, blood parameters, and digestive tracks histopathology in juvenile common carp (Cyprinus carpio). Aquac Res 2023:3674258. https://doi.org/10.1155/2023/3674258
Kononova SV, Zinchenko DV, Muranova TA, Belova NA, Miroshnikov AI (2019) Intestinal microbiota of salmonids and its changes upon introduction of soy proteins to fish feed. Aquac Int 27:475–496. https://doi.org/10.1007/s10499-019-00341-1
Krogdahl Å, Bakke-McKellep AM, Baeverfjord G (2003) Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L). Aquac Nutr 9:361–371. https://doi.org/10.1046/j.1365-2095.2003.00264.x
Krogdahl Å, Penn M, Thorsen J, Refstie S, Bakke AM (2010) Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquac Res 41:333–344. https://doi.org/10.1111/j.1365-2109.2009.02426.x
Krogdahl Å, Gajardo K, Kortner TM, Penn M, Gu M, Berge GM, Bakke AM (2015) Soya saponins induce enteritis in Atlantic salmon (Salmo salar L.). J Agric Food Chem 63:3887–3902. https://doi.org/10.1021/jf506242t
Li C, Tian Y, Ma Q, Zhang B (2022) Dietary gamma-aminobutyric acid ameliorates growth impairment and intestinal dysfunction in turbot (Scophthalmus maximus L.) fed a high soybean meal diet. Food Funct 13:290–303. https://doi.org/10.1039/D1FO03034E
Mareš J, Poštulková E, Malý O, Zezula F, Šorf M, Všetičková L (2023) Brewer’s yeast as a diet supplement in carp aquaculture: impact on production coefficients and haematological and biochemical plasma parameters. Ital J Anim Sci 22:560–567. https://doi.org/10.1080/1828051X.2023.2214168
Marković Z, Poleksić V, Lakić N, Živić I, Dulić Z, Stanković M, Spasić M, Rašković B, Sørensen M (2012) Evaluation of growth and histology of liver and intestine in juvenile carp (Cyprinus carpio, L.) fed extruded diets with or without fish meal. Turk J Fish Aquat Sci 12:301–308. https://doi.org/10.4194/1303-2712-v12_2_15
Matulić D, Barišić J, Aničić I, Tomljanović T, Safner R, Treer T, Gao J, Glojnarić I, Čož-Rakovac R (2020) Growth, health aspects and histopathology of brown bullhead (Ameiurus nebulosus L.): replacing fishmeal with soybean meal and brewer’s yeast. Sci Rep 10:1104. https://doi.org/10.1038/s41598-020-57722-3
Medic J, Atkinson C, Hurburgh CR Jr (2014) Current knowledge in soybean composition. J Am Oil Chem Soc 91:363–384. https://doi.org/10.1007/s11746-013-2407-9
Miladinović J, Hrustić M, Vidić M, Đorđević V (2006) Trideset Godina oplemenjivanja soje u Naučnom institutu za ratarstvo i povrtarstvo u Novom Sadu. Ratar Povrt 42:297–316
Milledge JJ (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Bio 10:31–41. https://doi.org/10.1007/s11157-010-9214-7
Mittal P, Kumar V, Rani A, Gokhale SM (2021) Bowman-Birk inhibitor in soybean: genetic variability in relation to total trypsin inhibitor activity and elimination of Kunitz trypsin inhibitor. Not Sci Biol 13:10836. https://doi.org/10.15835/nsb13110836
Mohan Dey M, Rab MA, Paraguas FJ, Piumsombun S, Bhatta R, Ferdous Alam M, Ahmed M (2005) Fish consumption and food security: a disaggregated analysis by types of fish and classes of consumers in selected Asian countries. Aquac Econ Manag 9:89–111. https://doi.org/10.1080/13657300590961537
Mohan K, Rajan DK, Muralisankar T, Ganesan AR, Sathishkumar P, Revathi N (2022) Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: a review of past and future needs. Aquaculture 553:738095. https://doi.org/10.1016/j.aquaculture.2022.738095
Nimalan N, Sørensen SL, Fečkaninová A, Koščová J, Mudroňová D, Gancarčíková S, Vatsos IN, Bisa S, Kiron V, Sørensen M (2023) Supplementation of lactic acid bacteria has positive effects on the mucosal health of Atlantic salmon (Salmo salar) fed soybean meal. Aquac Rep 28:101461. https://doi.org/10.1016/j.aqrep.2022.101461
Novriadi R, Rhodes M, Powell M, Hanson T, Davis DA (2018) Effects of soybean meal replacement with fermented soybean meal on growth, serum biochemistry and morphological condition of liver and distal intestine of Florida pompano Trachinotus carolinus. Aquac Nutr 24:1066–1075. https://doi.org/10.1111/anu.12645
Novriadi R, Spangler E, Allen Davis D (2019) Comparative effect of advanced soy products or corn protein concentrate with porcine meal on growth, body composition, and distal intestine histology of Florida pompano, Trachinotus carolinus. J World Aquac Soc 50:433–447. https://doi.org/10.1111/jwas.12547
Oliva-Teles A, Gouveia AJ, Gomes E, Rema P (1994) The effect of different processing treatments on soybean meal utilization by rainbow trout, Oncorhynchus mykiss. Aquaculture 124:343–349. https://doi.org/10.1016/0044-8486(94)90407-3
Omar SS, Merrifield DL, Kühlwein H, Williams PEV, Davies SJ (2012) Biofuel derived yeast protein concentrate (YPC) as a novel feed ingredient in carp diets. Aquaculture 330–333:54–62. https://doi.org/10.1016/j.aquaculture.2011.12.004
Pastore MR, Negrato E, Poltronieri C, Barion G, Messina M, Tulli F, Ballarin C, Maccatrozzo L, Radaelli G, Bertotto D (2018) Effects of dietary soy isoflavones on estrogenic activity, cortisol level, health and growth in rainbow trout, Oncorhynchus mykiss. Aquac Res 49:1469–1479. https://doi.org/10.1111/are.13602
Rašković BS, Stanković MB, Marković ZZ, Poleksić VD (2011) Histological methods in the assessment of different feed effects on liver and intestine of fish. J Agric Sci 56:87–100. https://doi.org/10.2298/JAS1101087R
Rašković B, Čičovački S, Ćirić M, Marković Z, Poleksić V (2016a) Integrative approach of histopathology and histomorphometry of common carp (Cyprinus carpio L.) organs as a marker of general fish health state in pond culture. Aquac Res 47:3455–3463. https://doi.org/10.1111/are.12795
Rašković B, Ćirić M, Koko V, Stanković M, Živić I, Marković Z, Poleksić V (2016b) Effect of supplemental feeds on liver and intestine of common carp (Cyprinus carpio) in semi-intensive rearing system: histological implications. Biologia 71:212–219. https://doi.org/10.1515/biolog-2016-0017
Rašković B, Cruzeiro C, Poleksić V, Rocha E (2019) Estimating volumes from common carp hepatocytes using design-based stereology and examining correlations with profile areas: revisiting a nutritional assay and unveiling guidelines to microscopists. Microsc Res Techniq 82:861–871. https://doi.org/10.1002/jemt.23228
Refstie S, Sahlström S, Bråthen E, Baeverfjord G, Krogedal P (2005) Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture 246:331–345. https://doi.org/10.1016/j.aquaculture.2005.01.001
Romarheim OH, øverland M, Mydland LT, Skrede A, Landsverk T (2011) Bacteria grown on natural gas prevent soybean meal-induced enteritis in Atlantic salmon. J Nutr 141:124–130. https://doi.org/10.3945/jn.110.128900
Santigosa E, Sáenz de Rodrigáñez MÁ, Rodiles A, Barroso FG, Alarcón FJ (2010) Effect of diets containing a purified soybean trypsin inhibitor on growth performance, digestive proteases and intestinal histology in juvenile sea bream (Sparus aurata L). Aquac Res 41:e187–e198. https://doi.org/10.1111/j.1365-2109.2010.02500.x
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Siddik MAB, Sørensen M, Islam SMM, Saha N, Rahman MA, Francis DS (2024) Expanded utilisation of microalgae in global aquafeeds. Rev Aquac 16:6–33. https://doi.org/10.1111/raq.12818
Sørensen M, Gong Y, Bjarnason F, Vasanth GK, Dahle D, Huntley M, Kiron V (2017) Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS ONE 12:e0179907. https://doi.org/10.1371/journal.pone.0179907
Thakur M, Hurburgh CR (2007) Quality of US soybean meal compared to the quality of soybean meal from other origins. J Am Oil Chem Soc 84:835–843. https://doi.org/10.1007/s11746-007-1107-8
Thévenaz P, Unser M (2007) User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microsc Res Techniq 70:135–146. https://doi.org/10.1002/jemt.20393
Urán PA, Gonçalves AA, Taverne-Thiele JJ, Schrama JW, Verreth JAJ, Rombout JHWM (2008a) Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L). Fish Shellfish Immun 25:751–760. https://doi.org/10.1016/j.fsi.2008.02.013
Urán PA, Schrama JW, Rombout JHWM, Obach A, Jensen L, Koppe W, Verreth JAJ (2008b) Soybean meal-induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquac Nutr 14:324–330. https://doi.org/10.1111/j.1365-2095.2007.00534.x
Urán PA, Schrama JW, Jaafari S, Baardsen G, Rombout JHWM, Koppe W, Verreth JAJ (2009) Variation in commercial sources of soybean meal influences the severity of enteritis in Atlantic salmon (Salmo salar L). Aquac Nutr 15:492–499. https://doi.org/10.1111/j.1365-2095.2008.00615.x
van den Ingh TSGAM, Krogdahl Å, Olli JJ, Hendriks HGCJM, Koninkx JGJF (1991) Effects of soybean-containing diets on the proximal and distal intestine in Atlantic salmon (Salmo salar): a morphological study. Aquaculture 94:297–305. https://doi.org/10.1016/0044-8486(91)90174-6
Vlahakis C, Hazebroek J (2000) Phytosterol accumulation in canola, sunflower, and soybean oils: effects of genetics, planting location, and temperature. J Am Oil Chem Soc 77:49–53. https://doi.org/10.1007/s11746-000-0008-6
Vollmann J, Fritz CN, Wagentristl H, Ruckenbauer P (2000) Environmental and genetic variation of soybean seed protein content under Central European growing conditions. J Sci Food Agric 80:1300–1306. https://doi.org/10.1002/1097-0010(200007)80:9<1300::AID-JSFA640>3.0.CO;2-I
Wang L, Zhou H, He R, Xu W, Mai K, He G (2016) Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L). Aquaculture 464:87–94. https://doi.org/10.1016/j.aquaculture.2016.06.026
Wanka KM, Schulz C, Kloas W, Wuertz S (2019) Administration of host-derived probiotics does not affect utilization of soybean meal enriched diets in juvenile turbot (Scophthalmus maximus). J Appl Ichthyol 35:1004–1015. https://doi.org/10.1111/jai.13929
Willora FP, Vatsos IN, Mallioris P, Bordignon F, Keizer S, Martınez-Llorens S, Sørensen M, Hagen Ø (2022) Replacement of fishmeal with plant protein in the diets of juvenile lumpfish (Cyclopterus lumpus, L. 1758): effects on digestive enzymes and microscopic structure of the digestive tract. Aquaculture 561:738601. https://doi.org/10.1016/j.aquaculture.2022.738601
Wu X, Wang L, Xie Q, Tan P (2020) Effects of dietary sodium butyrate on growth, diet conversion, body chemical compositions and distal intestinal health in yellow drum (Nibea albiflora, Richardson). Aquac Res 51:69–79. https://doi.org/10.1111/are.14348
Xie M, Zhou W, Xie Y, Li Y, Zhang Z, Yang Y, Olsen RE, Ran C, Zhou Z (2021) Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture 543:736943. https://doi.org/10.1016/j.aquaculture.2021.736943
Yu HH, Han F, Xue M, Wang J, Tacon P, Zheng YH, Wu XF, Zhang YJ (2014) Efficacy and tolerance of yeast cell wall as an immunostimulant in the diet of Japanese seabass (Lateolabrax japonicus). Aquaculture 432:217–224. https://doi.org/10.1016/j.aquaculture.2014.04.043
Zhang W, Pang A, Tan B, Xin Y, Liu Y, Xie R, Zhang H, Yang Q, Deng J, Chi S (2022) Tryptophan metabolism and gut flora profile in different soybean protein induced enteritis of pearl gentian groupers. Front Nutr 9:1014502. https://doi.org/10.3389/fnut.2022.1014502
Zhou W, Lie KK, Chikwati E, Kousoulaki K, Lein I, Sæle Ø, Krogdahl Å, Kortner TM (2023) Soya saponins and prebiotics alter intestinal functions in Ballan wrasse (Labrus bergylta). Brit J Nutr 130:765–782. https://doi.org/10.1017/S000711452200383X
Zhu R, Duan J, Li L, Li M, Yu Z, Wang H-H, Quan Y-N, Wu L-F (2021) Effects of substituting fish meal with two types of soybean meal on growth, enzyme activities, and intestinal morphology in juvenile Rhynchocypris Lagowskii. N Am J Aquac 83:267–282. https://doi.org/10.1002/naaq.10190
Acknowledgements
The authors wish to express their gratitude to Zorica Radović for preparing samples for histological analysis and to two anonymous reviewers for their valuable comments and suggestions, which have helped improve the quality of our manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, grant numbers 451-03-65/2024-03/200116 and 451-03-66/2024-03/200045.
Author information
Authors and Affiliations
Contributions
Božidar Rašković: methodology, formal analysis, investigation, writing—original draft, visualization. Marko Stanković: formal analysis, investigation, writing—review and editing. Milica Markelić: methodology, investigation, writing—original draft, visualization. Vesna Poleksić: methodology, writing—review and editing. Gavrilo Božić: investigation, writing—review and editing. Snežana Janković: resources, writing—review and editing. Zoran Marković: conceptualization, writing—review and editing, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
The experiments were performed in accordance with the ethical conditions approved by the Serbian Ministry of Agriculture, Forestry and Water Management, and in accordance with the Serbian Animal Welfare Law.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rašković, B., Stanković, M., Markelić, M. et al. Growth, feed utilization, and quantitative histological assessment of the distal intestine and liver of common carp (Cyprinus carpio L.) fed formulated diets containing grains of different soybean cultivars. Aquacult Int 32, 6903–6921 (2024). https://doi.org/10.1007/s10499-024-01494-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-024-01494-4