Abstract
This study aimed to identify differences in management and cost–benefit characteristics of striped catfish (Pangasianodon hypophthalmus) nursery and grow-out farms in freshwater and brackish water areas in the Mekong River Delta (MRD) of Vietnam. One hundred ten striped catfish farms in both areas (50 nursery and 60 grow-out farms) were stratified and sampled for an interview to determine their management and cost–benefit characteristics. The results indicated that the survival rate and yield of fish nursed in brackishwater farms were higher than those in freshwater farms. Fingerling yields and production costs are positively and negatively correlated (p < 0.05) to the period-of-salinity effect, respectively. The net incomes of freshwater and brackishwater nursery farms do not differ significantly (p > 0.05). The fish yield of brackishwater grow-out farms was significantly lower than that of freshwater farms (p < 0.05). Management and cost–benefit parameters of grow-out farms in freshwater and brackishwater areas, if stocking fingerlings reared in the same areas, were not significantly different (p > 0.05). Water salinity strongly affected disease occurrence in the ponds, especially in nursery farms. In the MRD, to improve the climate-resilient farming of striped catfish, a salinity-tolerant strain is essential for nursery and grow-out farming in areas affected by salinity intrusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Striped catfish (Pangasianodon hypophthalmus) culture of the Mekong River Delta (MRD) distributed along the Mekong River from upstream (freshwater areas) to downstream (brackishwater areas) (Son et al. 2021). In 2021, striped catfish export turnover was US$ 1.62 billion, accounting for more than 18% of Vietnam’s US$ 8.89 billion total seafood export value (Vietnam Association of Seafood Exporters and Producers (VASEP) (2022)). In the period from 2008 to 2021, four up- and middle-stream territories (An Giang, Dong Thap and Vinh Long provinces, as well as Can Tho City) accounted for 77.6% of farming area and production, whereas the downstream provinces (Tien Giang, Ben Tre, Tra Vinh and Soc Trang), which were frequently affected by saline water intrusion, accounted for the remaining 22.4% (Compiled from Annual Reports of the Department of Agriculture and Rural Development in the MRD, Vietnam in 2021). With this distribution, striped catfish culture is sensitive to changes in the Mekong River’s flow regime and salinity intrusion. In the years 2016 to 2020, the MRD experienced saline water intrusion around 45–105 km from the coastline, causing losses in agriculture production and other economic activities (Hai et al. 2020; SIWRR 2020). The MRD is lowland with a maximum elevation of 4.0 m above mean sea level (IPCC 2007; Trieu and Phong 2015). In the future, water salinity in the coastal areas of the MRD has been projected to increase by tides and sea level rise, which could increase 0.33 m and 1.00 m by 2050 and 2100, respectively (USEPA 2012; UNFCCC 2003). Saline water intrusion can result in the expansion of brackishwater aquaculture areas, but it harms freshwater aquaculture systems, especially the striped catfish farming. The increased salinity of the water supply for freshwater fish farming reduces the scale of the farm, increases production costs, requires a salinity-tolerant seed and prolongs the culture period (Anh et al. 2018), which also harms striped catfish farming (Sebesvari et al. 2011). The vulnerability of aquaculture between inland and coastal areas varies, depending on the susceptibility and adaptability of the cultural systems (Islam et al. 2019). In addition, the development of hydro-electric dam systems in upstream areas has also changed the water flow regime of the Mekong River according to the seasons. These upstream hydro-electric dam systems can also contribute to slightly increased water flow during the dry season when discharging water for electric production and emergency releases that could contribute toward reducing saline water intrusion in the MRD. When there are no emergency water discharges, the water flow is lower than it was before the dam’s construction. Meanwhile, in the rainy season, the need for water storage for electricity production causes less water flow downstream (MRC 2022).
Striped catfish farming, especially on farms located in brackishwater areas, is highly dependent on freshwater resources (Phuong and Oanh 2010). Under the impact of climate change and the construction of hydro-electric dam systems, the farming of this species has faced challenges. To accommodate the above-mentioned negative effects and to suggest releasing innovation and adaption activities for striped catfish culture in the near future, this study aimed to determine differences in management and economic characteristics of striped catfish nursery and grow-out in the freshwater and brackishwater areas of the MRD in Vietnam.
Methodology
Data collection
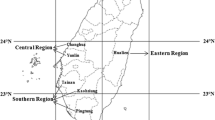
The study was carried out from January 2019 to December 2021 in seven territories of the MRD, Vietnam: An Giang, Dong Thap, Vinh Long, Tien Giang, Ben Tre and Soc Trang provinces, as well as Can Tho City. Sites and samples for interviews of farm operators were selected using a stratified sampling method based on two culture areas with different salinity conditions (Fig. 1). In areas without saline water intrusion (called ‘freshwater area’), the water supply was freshwater (or salinity of < 0.5 parts per thousand, or ppt) throughout the year. Meanwhile, the areas with saline water intrusion (called ‘brackishwater area’) experienced it yearly from August to October with low salinity. At each selected site, the farms were randomly selected; the sample consisted of 50 nursery farms (comprising 20 farms in brackishwater and 30 farms in freshwater) and 60 grow-out farms (comprising 30 in freshwater and 30 in brackishwater). Face-to-face interviews were conducted using a semi-structured questionnaire (Table 1). The questionnaire includes information such as the characteristics of the farm (e.g. farm age and size, settlement pond, nursery/grow-out pond, number of ponds, water depth); salinity condition (water salinity and number of months with salinity effect from water supply rivers/canals); disease syndromes; management parameters (larvae/fingerling sizes and sources, stocking density, culture period, feed and feeding, water exchange, economic feed conversion ratio (eFCR = total weight of feed fed per total weight of harvested fish), survival rate, harvested fish size, total yield and water use); and cost–benefit (key variables of total fixed cost, total variable cost, total cost, production cost, farm gate price, gross income, net income (NI) and net income per total cost (TC) ratio (NI/TC) of nursery/and grow-out farms).
Data analysis
Data collected were analysed using descriptive statistics (mean, standard deviation and frequency). Parametric correlation between parameters was analysed using bivariate and partial correlation (p < 0.05); meanwhile, a non-parametric correlation was determined using crosstab with chi-square (when ≥ 80% of cells (categories) had expected count ≥ 5) or likelihood ratio (when > 20% of cells (categories) had expected count < 5; p < 0.05). General description and discriminant analysis were also used to separate groups of farms with numbers of the month with salinity effects (p < 0.05). Differences in management and cost–benefit characteristics between freshwater and brackishwater groups of farms were analysed via independent t-test and covariance analysis (p < 0.05). Data were analysed using SPSS version 16.
Results
Salinity, water exchange, seed sources and diseases
Salinity and water exchange
In the brackishwater areas, farmers often measure salinity with a salinity metre before taking water into the pond, especially in dry-season months. If the salinity of the water supply (from rivers and canals) was greater than 5 ppt, pondwater exchange was not applied to control salinity as low as possible. If the salinity was greater than 5 ppt for longer a week, the amount of feed fed was reduced to maintain pondwater quality. Additionally, farmers evaluate poor water quality by simple observation such as bad smell, black colour or abnormal swimming behaviour of fish. In both nursery and grow-out farms, the amount of water exchange depends on the water quality of ponds, the availability of water in the rivers and canals and the tidal level. In months when the water source was saline, farms in brackishwater areas had exchanged water during low tide to obtain water with the lowest possible salinity instead of exchanging water during high tide.
Nursery farms
Farmers operated their farms year-round whenever fingerling demand for grow-out is needed. In brackishwater areas, the average duration of saline water intrusion was 7.7 months with an average salinity of 4.6 ± 1.7 ppt in the water supply (rivers and canals). Fish farmers followed one of three water-exchange schedules: once per day, once per week and as needed (i.e. when pondwater quality has deteriorated). These water-exchange schedules correlated in the nursery areas of freshwater and brackishwater (p < 0.05), which showed that with 12% water volume exchanged each time, the brackishwater nursery farms applied water exchange once per week and once per day rather than as needed (i.e. when pondwater quality had deteriorated). Farmers normally cannot predict the salinity of the water supply for the next water exchange. Meanwhile, nursery ponds in freshwater areas exchanged water once per day or whenever low water quality was observed or measured in the pond. As such, the freshwater nursery farms have high availability of suitable water sources when compared to that of brackishwater farms, and the freshwater nursery farm operators are more active in managing water exchange. The freshwater farms exchanged about 14% of the total pondwater each time, which was slightly higher than the rate for brackishwater farms.
Grow-out farms
The results showed that the farms in the brackishwater area were affected by saline water intrusion for 2–5 months with an average supply water salinity of 2.3 ± 2.7 ppt. The saline water occurred from December to April of the following year and tended to peak in March with an average of 2.5 ± 3.5 ppt. There are two nursing areas at sites Nos. 2 and 5 (Fig. 1). The nursing area at site No. 2 connects to the sea via the My Thanh River with high salinity, where limited freshwater flows from upstream, especially in the dry season. Site No. 5 has both nursing and grow-out areas; it is an islet that has low salinity because of freshwater flows from upstream (Fig. 2).
The once- or twice-daily frequency of water exchange for striped catfish ponds in freshwater and brackishwater areas did not show a significant correlation (p > 0.05). The percentages of farms with once-daily water exchange were 57% and 63% in freshwater and brackishwater areas, respectively. In the brackishwater area, farms with twice-daily water exchange accounted for 37% of total farms, whereas the percentage of farms with twice-daily water exchange was 43% in the freshwater area. Due to the limited volume of water for exchange in brackishwater areas, the volume of exchanged water averaged 29% of total pondwater volume each time, which was slightly lower than that of freshwater areas (33%) (Fig. 3).
Sources of larvae and fingerlings
Nursery farms used 2–3 days’ post-hatching larvae, whereas the grow-out used 20–30 g fingerlings. Larval sources were hatcheries in Dong Thap Province, which is a freshwater area. Dong Thap Province is also the main striped catfish larval production site in the MRD. Fingerlings for the grow-out farms were purchased from two sources: one nursed fingerlings in brackishwater (i.e. brackishwater fingerlings) and the other in freshwater (i.e. freshwater fingerlings). In brackishwater areas, grow-out farms used 66.7% brackishwater fingerlings and 33.3% freshwater fingerlings. By contrast, grow-out farms in the freshwater areas used only freshwater fingerlings (100%). It was found that grow-out farms in brackishwater used more fingerlings nursed in brackishwater conditions.
Diseases
Nursery farms
Bacillary necrosis of Pangasius (BNP, caused by Edwardsiella ictaluri) was found in both freshwater and brackishwater nursery farms. However, other diseases such as white liver and white gill (WLG, caused by ectoparasites and endoparasites) and red spot disease (RSD, a haemorrhagic disease caused by Aeromonas hydrophila, A. sobria and A. caviae) were reported in freshwater farms only. There is a relationship between the frequency of these disease occurrences and different areas of nursery farms (freshwater and brackishwater) (p < 0.05; Table 2).
Grow-out farms
BNP was the most common disease in all freshwater and brackishwater grow-out farms. RSD and WLG accounted for 97% and 60% of grow-out farms, respectively. The three above-mentioned diseases occurred in ponds with no significant correlation (p > 0.05) to the difference between freshwater and brackishwater farms. The incidence of swim bladder disease (SBD, a fungal disease caused by Fusarium sp.) was only 17% in freshwater areas, and this occurrence was significantly related to freshwater and brackishwater farms (p < 0.05; Table 2).
Diseases often break out in striped catfish farming when weather changes, especially during season changes (which tend to bring fluctuations in rainfall and temperature). With the exception of SBD (which has no specific treatment), all other mentioned diseases are treated with antibiotics (BNP, RSD) and antiparasitic drugs (SBD).
Management and cost–benefit analysis
Nursery farm
Discriminant analysis
The multivariate differences of nursery farm groups, based on the number of months with salinity effect, were extracted into two discriminant functions (namely functions 1 and 2). In discriminant analysis, two linear regression functions (1 and 2) are calculated based on management and cost–benefit parameters. Each farm (case) had score values of functions 1 and 2 (as coordinates in functions 1 and 2), which were estimated using coefficient values that participated in the function as well as standardised values of management and cost–benefit parameters. Parameters with large coefficient values that participated in functions 1 and 2 had a strong impact on the score values of farms. Next, the different functional score values of each farm were used to determine the corresponding coordinate axes of the farm’s distribution map (functions 1 and 2). In addition, any management and cost–benefit parameters have great absolute values of correlation coefficients with point values of functions that create discriminant between farm groups. Function 1 shared 86.4% of the variance explained and can significantly discriminate groups of nursery farms (p < 0.05). In this function, the management parameters have high loading of coefficients such as fingerling yield (− 1.25), farm gate price (− 1.45), total nursery area (− 0.67), eFCR (− 0.53), salinity (− 0.52), production cost (1.44), net income (1.22), larvae cost (0.77), age of pond (0.75), nursing period (0.70) and survival rate (0.53). Of these parameters, salinity and nursing period had the greatest coefficient correlation with function 1 (0.41 and 0.24, respectively); therefore, salinity and nursing period are important parameters in distinguishing among nursery farm groups in function 1 (Table 3).
Figure 4 indicates that salinity and nursing period are the main contributors to the variation of multivariates of nursery farms from different nursery areas and also shows that the nursery farm groups affected by salinity area tend to have higher values of variables with negative coefficients (and total values of function 1 below − 2 for each farm), whereas freshwater farms have total values for function 1 above 2. In the brackishwater area, the farm groups with different numbers of months with salinity effect did not differ significantly based on the value of function 1. Meanwhile, salinity and nursing period have a high correlation with function 1, indicating that the brackishwater groups have high salinity and shorter nursing periods compared to farms in freshwater areas. Function 2 explained 7.6% of the variance, but this function does not significantly discriminate the groups of nursing farms (p > 0.05; Table 3; Fig. 4); therefore, this function cannot be used to analyse multivariable differences among farm groups.
Farm construction characteristics
Because nursery farms nurse fish only from larvae to fingerling sizes, no ponds are used for grow-out. Farm size is the land area including nursery ponds, houses and other auxiliary items such as warehouses, internal canals and pond banks (used as vegetable gardens for family consumption). The ratio of the nursery and total farm is less than 50% in freshwater, whereas it is more than 70% in brackishwater (Table 4). The smaller ratio in the freshwater nursery farms is attributable to the larger space being used for vegetation. Nursery farms operated 2–4 crops per year; the number of crops depended on the fingerling demand of grow-out farms.
The average age of ponds in brackishwater areas (5.8 years) was significantly lower than in freshwater areas (9.4 years, p < 0.05). Nursery farms for striped catfish were first started in upstream provinces in the MRD (such as An Giang and Dong Thap) that are near to grow-out areas. There, farms in brackishwater areas are significantly larger, and with more ponds, than those in freshwater areas (p < 0.05).
Characteristics of techniques, cost and profit
In the nursery, larvae were fed twice daily with commercial pellets containing 28–42% crude protein in brackishwater farms and 30–41% in freshwater farms. The protein content of feeds used in the nursery farms depended on the availability of feed at the distribution agencies and farmers’ behaviour, rather than on the fingerlings’ protein requirements. The fingerlings nursed in brackishwater farms were fed at a rate of 5.5% body mass, which was significantly lower than that of freshwater farms (6.6%, p < 0.05). Although feeding rates were low, the nursing period in brackishwater was 2.3 months per crop, which was significantly shorter than that of freshwater areas (3.2 months per crop, p < 0.05). Management efficiency of nursery farms in brackishwater, as measured by indicators such as survival rate (%), fingerling yield (ton ha−1 crop−1) and fingerling sizes (ind. −1 kg−1), was significantly (p < 0.05) greater than that of freshwater nursery farms — especially fingerling yield, which averaged 30.1 ton ha−1 crop−1 for brackishwater farms, compared to 16.8 ton ha−1 crop−1 for freshwater farms (Table 4).
The feeding rate was adjusted based on health status and pondwater quality. However, the main purpose of a fish nursery is to achieve the highest survival rate at an acceptable growth rate, high fingerling quality and the highest production. Results showed that feed quality, feeding rate and nursing time were lower and shorter at brackishwater farms than at freshwater farms. However, key management efficiency (survival rate and fingerling yield) in brackishwater nursery farms was better than that of freshwater nursery farms. These findings indicate that management practices at brackishwater nursery farms are superior, leading to higher fingerling productivity.
Total variable cost (TVC), total cost (TC) and gross income of nursery farms in brackishwater were significantly greater than those of freshwater farms (p < 0.05). TVC, feed and interest costs of nursery farms in brackishwater were significantly higher than those of freshwater farms (p < 0.05). By contrast, lime and hired labour costs were significantly higher for freshwater farms than for brackishwater farms (p < 0.05). Farm gate price of fingerlings depended on many criteria, including fingerling quality, size and survival rate post-stocking in grow-out farms. The farm gate price of fingerlings nursed in brackishwater farms was lower than that of freshwater farms. The fingerling production cost and farm gate price of brackishwater farms were significantly lower (p < 0.05) than those of freshwater farms. No significant difference in net income was found between the two nursery farm groups (p > 0.05; Table 5). The insignificant difference in net income between freshwater and brackishwater nursery farms, despite differences in production costs, underscores the adaptability of nursery farmers in adjusting their techniques based on salinity conditions.
Salinity associated with fingerling yield and production cost
The results of bivariate and partial correlation analysis show the significant correlations (p < 0.05) between management parameters (input/cause variables) such as fingerling yield, months with salinity effect (‘period-of-salinity effect’), age of pond, nursing period, eFCR, fingerling size, survival rate, nursing area, nursing density and production cost, which are mapped in Fig. 5. The correlations are illustrated by connecting lines with coefficient value of bivariate and partial correlations in the first and second lines, respectively. Bivariate correlation provides a correlation between these variables, whereas partial correlation allows determining the salinity effect on the correlation among remaining variables, especially production cost and fingerling yield. This means that, if the coefficient values of two correlation types are not much different, the salinity effect has a negligible impact on the correlation between those variables (and vice versa).
Figure 5 indicates that the period-of-salinity effect variable is negatively correlated with production cost, but positively correlated with fingerling yield, meaning that with increased period-of-salinity effect, fingerling yield was increased whereas production cost was reduced. The results indicate that fingerling yield is negatively correlated with production cost (− 0.60); the correlation coefficient was slightly decreased (− 0.59) in the case of the salinity effect. This shows that under the conditions of salinity effect, production costs will decrease if fingerling yield increases.
Bivariate correlation versus partial correlation indicates that only three variables (eFCR, fingerling size and survival rate) are significantly correlated with fingerling yield and production cost, if the salinity effect is rejected. Results showed that fingerling yield is positively correlated with fingerling size and survival rate, but negatively correlated with the nursing period. Meanwhile, the increase in fingerling size (number of fingerlings per kg) and survival rate resulted in the reduction of production cost; and the increase of eFCR led to the increase of production cost. Thus, the salinity effect did not change the strength of correlation between these variables.
Conversely, when excluding salinity effect, nursery area, stocking density, nursing period and age of ponds, there was no significant correlation between fingerling yield and production cost (p > 0.05). This shows that the strength of the correlation between these variables and fingerling yield and production cost is greatly influenced by the salinity effect. Under the salinity effect, the increases in nursery area and stocking density contribute to the increase in fingerling yield (p < 0.05). The increase in the nursing period leads to decreased fingerling yield and increased production cost (p < 0.05; Fig. 5). The positive correlation between the period-of-salinity effect and fingerling yield suggests that understanding the timing of saline water intrusion is key to optimising nursery practices.
Grow-out farm
Discriminant of farm groups based on months of salinity effect
The discriminant procedure extracts two functions. Function 1 explained 91.2% of variance and significantly (p < 0.05) discriminated groups of grow-out farms. Function 1 has a high positive coefficient of average salinity (3.52), culture period (1.02), harvested fish size (0.89) and number of grow-out ponds (0.66) and a negative coefficient of farm size (− 2.09), warehouse cost (− 1.81), water consumption (− 1.09), source of fingerling (− 0.80), feed cost (− 1.18), energy cost (− 0.72), yield (− 0.64) and interest cost (− 0.59). These are key variables for distinguishing farm groups with different periods (months) of saline water intrusion. Through these coefficients, it is shown that causal variables such as salinity and farm size participate in the large discriminant function, followed by operational or management efficiency parameters (culture period and water consumption) and expenses (warehouse cost). Based on the standardised value of function 1 for each variable of each farm, Fig. 6 indicates that function 1 can significantly (p < 0.05) discriminate in five groups of farms (0, 2, 3, 4 and 5 months with salinity effect). However, the correlation of each variable with the value of function 1 was low (0.00–0.23), which means that the value variation of each variable was not strongly correlated with the value of function 1, except the salinity variable (correlation coefficient of 0.23) and source of fingerling used; that is, farmers in brackishwater areas tend to use fingerlings nursed in brackishwater areas (0.14; Table 6).
As a result of a low correlation coefficient, the farming groups are distributed separately along the function 1 axes, the farms with 0 months of salinity effect are located below − 5, due to the low value of variables with positive loading coefficient mentioned as salinity and management variables. Meanwhile, the variables with high negative loading (such as the variable of farm size, water consumption and costs) dominated in function 1; the remaining variables of the grow-out farms have low loading to function 1, indicating that fish farmers have adjusted their farming methods to achieve the best production efficiency that harmonises technology and net income. Figure 6 also shows that the groups in the brackishwater area did not differ in terms of farm characteristics, farming techniques and variable costs (these farms were distributed from 10 to 15 along function 1). In particular, the farms with 2, 3 and 4 months of salinity effect were distributed closely together (horizontal axes); these three groups comprise the grow-out farms of Cu Lao Dung (Soc Trang province, site 5) and Tieu Can (Tra Vinh province, site 4; Fig. 1). Function 2 explains 4.4% of the variance, despite strong contributions of gross income (− 3.08), yield (2.69), consumed water (− 1.57), culture period (1.36) and production cost (1.24) into the value of canonical function 2. However, function 2 does not significantly discriminate the groups of grow-out farms (p > 0.05); consequently, function 2 cannot be used to analyse multivariable differences between farm groups divided by the number of months with salinity effect, because the centroid variation of each group fluctuates slightly around zero (0) value of vertical axes (function 2).
Grow-out pond characteristics
Average pond age was 12 ± 5 years in freshwater areas and 11 ± 5 years in brackishwater areas, which shows that freshwater farms began grow-out activities before brackishwater farms did. Average farm size is larger in brackishwater areas, but the size of grow-out ponds was smaller compared to those of freshwater farms. However, grow-out pond parameters did not differ significantly (p > 0.05) between freshwater and brackishwater areas (Table 7).
Management and cost–benefit parameters
In Tables 8 and 9, the greater difference in the value of means presented in column [2] from the value of estimated means presented in column [3] indicates the stronger effect of fingerling sources compared to management parameters. If there is significant covariance (presented in column [4]) of fingerling sources to a certain management or cost–benefit parameter (in bold text), its estimated mean will be considered.
Management parameters
Fingerling size, stocking density and productivity were significantly lower for farms in brackishwater areas than those in freshwater areas (p < 0.05). This shows that in brackishwater areas with limited water resources for exchange due to salinity, the grow-out farms had lower stocking density and smaller fingerling size compared to farms in the freshwater areas. Commercial pellets for grow-out ponds in brackishwater and freshwater farms ranged from 26 to 28% crude protein. In general, fish were fed one to two times per day. Fish farmed in freshwater had a feeding frequency of 1.1 times/day, which was significantly lower than that of brackishwater (1.7 times/day, p < 0.05). If the covariance of fingerling source is not considered, the lower stocking density of brackishwater farms resulted in a significantly higher survival rate than in freshwater farms (p < 0.05). Consequently, in productivity terms, fish farmed in brackishwater areas showed a lower yield than in freshwater areas. Feeding rate, culture period, harvest fish size, eFCR and water consumption were not significantly different between the two groups (p > 0.05).
Covariance-based of freshwater and brackishwater fingerlings
The fingerling sources covariates significantly (p < 0.05) with management parameters of the two grow-out groups (freshwater and brackishwater) such as stocking density, fingerling size, survival rate, yield and eFCR. Table 8 indicates that there is a significant covariance (p < 0.05) between fingerling sources and the stocking density of two grow-out groups. Estimated stocking density was increased in brackishwater and decreased in the freshwater farms based on covariance between fingerling source and stocking density. Therefore, the covariance of fingerling sources and stocking density was decreased in the freshwater and increased in the brackishwater farms. If both farm groups used the same sources of fingerling, the stocking density of freshwater and brackishwater farms was not significantly different (p > 0.05). It was also demonstrated that in the brackishwater areas, the stocking densities of fingerling sourced from the brackishwater areas were 30.4 ± 13.5 ind. m−2, whereas it was 66.9 ± 23.4 ind. m−2 for fingerlings from the freshwater areas. Meanwhile, the grow-out farms in freshwater areas did not use fingerlings nursed in the brackishwater area, likely due to the long transport distance from the brackishwater area to the freshwater areas, as well as the high availability of fingerlings nursed in the freshwater area.
The fingerling size, survival rate, yield and eFCR of fish cultured in the two grow-out groups were also significantly covaried by fingerling sources (p < 0.05). If the covariance of fingerling sources are taken into account, the estimated means of these parameters for the freshwater and brackishwater grow-out groups were not significantly different (p > 0.05). In other words, if the same fingerling source is used, these parameters did not show a significant difference (Table 8). Results indicate that the variation between freshwater and brackishwater grow-out farms’ management characteristics (e.g. stocking density, fingerling size, survival rate, yield) is an important consideration for farmers when selecting the appropriate conditions for their farm operations. The importance of matching fingerlings to the target environment is highlighted by the finding that management-parameter efficiency not significantly different when fingerlings are sourced from the same salinity conditions as the grow-out farm.
Cost–benefit parameters
Generally, the TC of farms in the brackishwater area was significantly lower than that of farms in the freshwater area (p < 0.05). In terms of expense, feed and fingerling costs are respectively ranked as the first and second most expensive in total costs for both farm groups. The remaining costs of brackishwater farms were significantly lower than those of freshwater farms (p < 0.05), except for equipment and warehouse costs; this may be attributable to lower stocking density in brackishwater farms. For farms in the brackishwater area, only the energy cost was higher than that of farms in the freshwater area (p < 0.05). However, the efficiency of expenses and return, production cost, farm gate price and net income of farms in brackishwater and freshwater areas were not significantly different (p > 0.05).
There were covariances of fingerling sources to gross and net income, as well as to expenses such as fingerling, feed, energy and warehouse costs (p < 0.05). However, this covariance did not lead to a significant difference (p > 0.05) in these variables between the groups of brackishwater and freshwater farms. This means that the averages of such parameters for brackishwater and freshwater grow-out groups are not significantly different, regardless of the covariance of fingerling sources (Table 9). The estimation of net income, considering fingerling source covariance, is a valuable approach for assessing the economic performance of grow-out farms in different salinity conditions.
Discussion
Effects of salinity on the growth of striped catfish
Nursery
Larvae nursed in brackishwater have a shorter duration and higher survival rate, resulting in higher fingerling productivity compared to that of fish nursed in freshwater; this may be due to the advantages of management practices. Brackishwater nursery farms have low production costs and farm gate prices compared to freshwater nursery farms, and no significant difference was found between these two nursery groups’ net incomes. The effect of the period-of-salinity effect was positively correlated with fingerling yield and negatively correlated with production costs. This result is due to the eFCR, stocking density, nursery site, pond age and the effect of time with saline water intrusion affecting the nursing period. There was a slight difference in the stocking density between freshwater and brackishwater nursery farms. Larvae survival rates were higher in the brackishwater farms than in the freshwater farms.
Salinity is one of the important factors affecting the growth rate, survival rate and metabolism of fish (Amornsakun et al. 2017). In freshwater fishes, growth rates are faster in slightly brackishwater conditions due to isostatic energy savings (Gilles and Patrick 2001). In striped catfish, ion exchange (Na + , K+ and Cl−) of cell membranes tends to decrease at salinities close to the iso-osmotic point (9 ppt; Phuong et al. 2023). Freshwater fish with iso-osmotic salinities typically range from 10 to 12 ppt (Varsamos et al. 2005), whereby the fish saves considerable energy for growth (Gilles and Patrick 2001). Hai et al. (2022) reported that the best growth performance for striped catfish, from larvae to fingerling stages, occurs at salinities of 5–10 ppt; performance began to diminish at higher salinities. In the 0–20-ppt salinity range, alterations in gut genes involved in ion exchange and stress response occur that help fish adapt to water salinity (Hieu et al. 2022). In the 0–9-ppt salinity range, striped catfish larvae had the highest survival rate; digestive enzyme activities and plasma osmolality were not affected at 6 ppt salinity (Ha et al. 2021).
Grow-out
Brackishwater farms have limited availability of water for daily exchange. With high stocking density applied in the grow-out stage, the volume of water exchange is immense, thus resulting in lower stocking density in brackishwater farms than in freshwater farms. The survival rate of fish in brackishwater farms is higher than in freshwater farms. At 6 ppt salinity and 35 °C conditions, striped catfish had fast growth; salinity and temperature did not affect growth hormone at 0–12 ppt salinity (Phuc et al. 2015). Striped catfish had a growth rate of 0.5 g day−1 at 9 ppt salinity, which was higher than at the other salinities, ranging from 0 to 15 ppt, and the lowest FCR was found (Lam et al. 2011). According to Thao et al. (2013), the survival rate of striped catfish was highest in conditions of 2–10 ppt salinity; in particular, fish reared at 6 ppt salinity had especially high rates of growth performance and survival and low FCR. If the water salinity in the MRD does not exceed 10 ppt, striped catfish farming is not affected (Phuc et al. 2014). Salinity in excess of 10 ppt will reduce suitability for striped catfish farming in the Soc Trang and Ben Tre provinces of these study areas, as well as in other coastal areas of the MRD. Therefore, one of the sustainable solutions is to develop salinity-tolerant striped catfish strains (Anh et al. 2014).
The survey results showed that farms with water supply had an average salinity of 4.5 ± 2.6 ppt (< 9 ppt) and a maximum salinity period of 5 months per year, which also showed that there is evidence-based scientific and practical farming for the development of striped catfish in the brackishwater areas. With the same fingerling source, it would be expected that there is no difference in the management and cost–benefit characteristics between brackishwater and freshwater farming. The advantages of fingerlings nursed in areas close to grow-out farms are higher than those of fingerlings nursed in areas with different ecological conditions, such as freshwater areas. This shows that striped catfish fingerlings nursed in brackishwater areas are better for stocking in brackishwater grow-out farms. Research on the salinity tolerance of striped catfish fingerlings, which has been initially successful, shows a strong potential for fingerling nurseries in low-salinity areas to meet the fingerling demand for grow-out in the MRD area under salinity intrusion caused by climate change (Hai et al. 2022).
Diseases
Only BNP was reported in the nursing period of brackishwater farms, whereas various diseases (including BNP, WLG and RSD) were reported in freshwater farms. For the grow-out stage, fish from brackishwater farms were not infected by SBD, whereas BNP, RSD, WLG and SBD diseases were all detected in fish from freshwater farms. As Edwardsiella ictaluri is a causative agent of BNP, after 14 days of salinity increase, fish nursed in 11–14 ppt salinity were more sensitive to E. ictalurid, with significantly higher cumulative mortality than those nursed at lower salinities; mortality was not significantly different among groups of fish reared in salinities of 0, 2, 5 and 8 ppt (Tu et al. 2020). Fish gut responds to changes in different osmotic pressure conditions, and it plays a role in ionic balance in saline water (Takei and Hwang 2016), which leads to a 10- to 50-fold increase in water intake to compensate for water loss (Grosell 2006, 2010). Among striped catfish cultured in freshwater, the intestinal tract was dominated by phyla Bacteroidetes, Firmicutes, Proteobacteria and Verrucomicrobia; meanwhile, among those cultured in brackishwater (0–20 ppt; Hieu et al. 2022), Sulfurospirillum and Vibrio were the most abundant. Hieu et al. (2022) also reported that salinity gradients up to 10 ppt did not negatively affect immune responses. In general, the diversity of disease pathogens in striped catfish reared in brackishwater is lower than in freshwater conditions; this is due to changes in the ecological environment at different salinity levels, especially changes in osmotic pressure, which have affected the occurrence of disease in striped catfish farming.
Management and cost–benefit issues
Nursery farm
Nursery farms in freshwater and brackishwater areas have different characteristics in terms of age, farm size and feeding rate, which result in higher survival rates and fingerling yields for brackishwater farms compared to freshwater farms. However, brackishwater farms have higher total costs, but lower farm gate prices, compared to freshwater farms. Therefore, the net incomes and NI/TC ratios of the two farm groups were not significantly different (p > 0.05). These findings show that the nursery farmers in both freshwater and brackishwater areas have adjusted their techniques based on natural conditions (especially salinity) to improve their economic efficiency.
Grow-out farm
The result indicated that the management characteristics of grow-out farms in freshwater and brackishwater areas are significantly different (p < 0.05) in terms of stocking density, fingerling size, survival rate and yield. The advantages of freshwater farms are higher stocking density, larger fingerling size and higher yield, whereas brackishwater farms have smaller fingerling size and higher survival rate.
However, calibrating these parameters with the fingerling sources (freshwater and brackishwater), the efficiency of the management parameters of the two farm groups is not significantly different (p > 0.05). In other words, if grow-out farms in brackishwater areas are stocked with brackishwater fingerling, management efficiency is higher than that of freshwater fingerling. Fingerlings nursed in brackishwater are better adapted to brackishwater conditions. Similar to the above-mentioned issues, the total costs of brackishwater and freshwater farms are significantly different (p < 0.05) without considering the covariance of fingerling sources. If the total cost is estimated using the covariance of fingerling sources, the total cost and net income of the grow-out farms in the two farming areas are not significantly different (p > 0.05).
Estimated yields (with fingerling source covariance) ranged from 280 to 314 ton ha−1 crop−1; thus, the estimated net incomes of brackishwater and freshwater farms are US$ 79,600 and US$ 69,200 ha−1 crop−1, respectively. According to Hien et al. (2020), striped catfish yields and net income were 406 to 517 ton ha−1 crop−1 and US$ 59,483 to US$ 82,328 ha−1 crop−1 for brackishwater and freshwater farms, respectively. There is no significant difference in economic parameters between the two farming areas. Fish yield varies between brackishwater and freshwater farms, but the net incomes of these farming areas are only slightly different in absolute values. The comparison of yields and net income with a previous study by Hien et al. (2020), as well as the observation that fish yields in Hien’s study are lower than those in An Giang and Dong Thap provinces as measured by this study, provide useful context for the economic performance of the farms in the study areas.
Conclusions
The survival rate and productivity of fingerlings (10.2%, 30.1 ton ha-1 crop-1) nursed in brackishwater areas are higher than those nursed in freshwater areas (8.7%, 16.8 ton ha-1 crop-1). The period-of-salinity effect has positive (0.56) and negative (-0.36) correlations with fingerling yield and production cost, respectively, leaving no significant difference in net income between brackishwater and freshwater nursery farms. During the grow-out stage, low stocking density leads to lower fish yields (42.5 ind. m-2, 229 ton ha-1 crop-1) in brackishwater farms compared to freshwater farms (67.5 ind. m-2, 366 ton ha-1 crop-1). However, with the same fingerling sources, these two farming areas show no significant difference in management and cost–benefit characteristics. Brackishwater grow-out farms stocked with fingerlings nursed in brackishwater areas have better management efficiency compared to brackishwater farms stocked with fingerlings nursed in freshwater areas. There is no significant difference in economic parameters between the two farming areas, which is important for understanding the overall economic viability of striped catfish farming in both freshwater and brackishwater regions. Only a clinical sign of BNP disease is reported in brackishwater farms; no clinical sign of SBD was reported in brackishwater ponds. To support the adaptability of nursery and grow-out practices in response to salinity conditions, and the importance of matching fingerling sources to the target environment, a programme of selective breeding for salinity-tolerant strains of striped catfish is necessary to improve climate change adaptivity of this farming system in the MRD.
References
Amornsakun T, Vo VH, Petchsupa N, Pau TM, Hassan Bin A (2017) Effects of water salinity on hatching of egg, growth, and survival of larvae and fingerlings of snakehead fish, Channa striatus. Songklanakarin J Sci Technol (SJST) 39(2):137–142. https://doi.org/10.14456/sjst-psu.2017.16
Anh NL, Vinh HD, Bosma RH, Johan AJV, Rik L, De Silva SS (2014) Simulated impacts of climate change on current farming locations of striped catfish (Pangasianodon hypophthalmus, Sauvage) in the Mekong Delta, Vietnam. AMBIO 43:1059–1068. https://doi.org/10.1007/s13280-014-0519-6
Anh NL, Tung BVP, Bosma RH, Verreth J, Leemans R, De Silva SS, Lansink AO (2018) Impact of climate change on the technical efficiency of striped catfish, Pangasianodon hypophthalmus, farming in the Mekong Delta, Vietnam. J World Aquacult Soc 49(3):570–581
Gilles B, Patrick P (2001) How should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130(4):411–423. https://doi.org/10.1016/S1532-0456(01)00268-X
Grosell M (2006) Intestinal anion exchange in marine fish osmoregulation. J Exp Biol 209(15):2813–2827. https://doi.org/10.1242/jeb.02345
Grosell M (2010) The role of the gastrointestinal tract in salt and water balance. In: Grosell M, Anthony PF, Colin JB (eds) Fish physiology, Elsevier Inc., pp 135–164. https://doi.org/10.1016/S1546-5098(10)03004-9
Ha NTK, Em NT, Ngoc NM, Yasuaki T, Phuong NT, Huong DTT (2021) Effects of salinity on growth performance, survival rate, digestive enzyme activities, and physiological parameters of striped catfish (Pangasianodon hypophthalmus) at the larval stage. Can Tho Univ J Sci 13:1–9. https://doi.org/10.22144/ctu.jen.2021.011
Hai TN, Phuong NT, Hoa NV, Viet LQ, Khanh LV, Tao CT, Anh NTN, Thao NTT, Sorgeloos P (2020) Promoting coastal aquaculture for adaptation to climate change and saltwater intrusion in the Mekong Delta, Vietnam. World Aquacult 51(2):19–26
Hai DM, Yen DT, Liem PT, Tam BM, Son VN, Huong DTT, Hang BTB, Tran NTN, Hieu DQ, Patrick K, Phuong NT, Frederic F (2022) Selective breeding of saline-tolerant striped catfish (Pangasianodon hypophthalmus) for sustainable catfish farming in climate vulnerable Mekong Delta, Vietnam. Aquacult Rep 25. https://doi.org/10.1016/j.aqrep.2022.101263
Hien HV, Sanh NV, Phuong NT (2020) Analysis of factors affecting the linkage models in striped catfish (Pangasianodon hypophthalmus) farming in the Mekong Delta, Vietnam. Can Tho Univ J Sci 56(3):204–212. https://doi.org/10.22144/ctu.jvn.2020.070. (in Vietnamese)
Hieu DQ, Hang BTB, Jep L, Mutien-Marie G, Huong DTT, Yen DT, Liem PT, Tam BM, Hai DM, Son VN, Phuong NT, Frederic F, Patrick K (2022) Salinity significantly affects intestinal microbiota and gene expression in striped catfish juveniles. Appl Microbiol Biotechnol 106:3245–3264. https://doi.org/10.1007/s00253-022-11895-1
Intergovernmental Panel on Climate Change (IPCC) (2007) Climate change 2007: impacts, adaptation, and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Islam MM, Barman A, Kundu GK, Kabir MA, Paul B (2019) Vulnerability of inland and coastal aquaculture to climate change: evidence from a developing country. Aquac Fish 4(5):183–189. https://doi.org/10.1016/j.aaf.2019.02.007
Lam NC, Huong DTT, Son VN, Phuong NT (2011) Physiological changes and growth of tra catfish (Pangasianodon hypophthalmus) exposed to different salinities. Can Tho Univ J Sci 17(a):60–69 (in Vietnamese)
Mekong River Commission (MRC) (2022) The effects of Chinese dams on water flows in the Lower Mekong Basin. https://www.mrcmekong.org/news-and-events/news/the-effects-of-chinese-dams-on-water-flows-in-the-lower-mekong-basin/. Assessed 15 Dec 2022
Phuc NTH, Huong DTT, Mather PB, Hurwood DA (2014) Experimental assessment of the effects of sublethal salinities on growth performance and stress in cultured tra catfish (Pangasianodon hypophthalmus). Fish Physiol Biochem 40(6):1839–1848. https://doi.org/10.1007/s10695-014-9972-1
Phuc NTH, Linh TTK, Tri NM, Phuoc TT, Phuong Trang TT., NT, (2015) Effects of temperature and salinity interaction on growth performance and growth hormone level of tra catfish (Pangasianodon hypophthalmus) juvenile. Can Tho Univ J Sci 36(b):88–97 (in Vietnamese)
Phuong NT, Oanh DTH (2010) Striped catfish aquaculture in Vietnam: a decade of unprecedented development. In: De Silva SS, Davy, FB (eds) Success Stories in Asian Aquaculture. Springer, Dordrecht, pp131–147. https://doi.org/10.1007/978-90-481-30
Phuong NT, Ha NTK, Huong DTT (2023) Growth and feed intake of striped catfish (Pangasianodon hypophthalmus) fingerlings reared in different salinities. AACL Bioflux 16(1):496–507
Sebesvari Z, Le TTH, Fabrice GR (2011) Climate change adaptation and agrichemicals in the Mekong Delta, Vietnam. In: Stewart MA, Coclanis PA (eds) Environmental change and agricultural sustainability in the Mekong Delta. Advances in Global Change Research, 45. © Springer Science+Business Media B.V. 2011, pp 219–239. https://doi.org/10.1007/978-94-007-0934-8_13
Son VN, Hai DM, Quynh ND, Nghia LV, Truc PTT, Anh NQ, Huong DTT, Lebel B, Lebel L, Phuong NT (2021) Climate change-related risk adaptation in striped catfish, tilapia and shrimp farming systems in the Mekong Delta, Vietnam. Can Tho Univ J Sci 13(1):24–38. https://doi.org/10.22144/ctu.jen.2021.004
Southern Institute of Water Resources Research (SIWRR) (2020) Newsletter on water intake in March and forecast of saltwater intrusion in April and May 2020. http://www.siwrr.org.vn/docs/2018/files/Bantin_VKHTLMN.pdf. Accessed 28 Sept 2022
Takei Y, Hwang PP (2016) Homeostatic responses to osmotic stress. In: Schreck CB, Tort L, Farrell AP, Brauner CJ (eds) Fish physiology. Elsevier, pp 207–249. https://doi.org/10.1016/B978-0-12-802728-8.00006-0
Thao NL, Khoe VM, Toa HV, Ngan NH, Ha NTK, Phuong NT, Phuc NTH (2013) Effect of salinity on growth performance and cortisol level of cultured tra striped catfish (Pangasianodon hypophthalmus). Can Tho Univ J Sci 25(b):1–10 (in Vietnamese)
The United States Environmental Protection Agency (USEPA) (2012) Climate change indicators in the United States, 2012. Technical documentation
Trieu TTN, Phong NT (2015) The impact of climate change on salinity intrusion and Pangasius (Pangasianodon hypophthalmus) farming. Aquacult Int 23:523–534. https://doi.org/10.1007/s10499-014-9833-z
Tu TC, Phu TQ, Oanh DTH (2020) Effect of salinities on the susceptibility of striped catfish (Pangasianodon hypophthalmus) to Edwardsiella ictalurid. Can Tho Univ J Sci 56(1):45–51. https://doi.org/10.22144/ctu.jsi.2020.006. (in Vietnamese)
United Nations Framework Convention on Climate Change (UNFCCC) (2003) Vietnam initial national communication. The Socialist Republic of Viet Nam, Ministry of Natural Resources and Environment, Ha Noi
Varsamos S, Nebel C, Charmantier G (2005) Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol 141(4):401–429. https://doi.org/10.1016/j.cbpb.2005.01.013
Vietnam Association of Seafood Exporters and Producers (VASEP) (2022) Overview of seafood export 2021. https://vasep.com.vn/san-pham-xuat-khau/tin-tong-hop/xuat-nhap-khau/toan-canh-xuat-khau-thuy-san-2021-23691.html. Accessed 27 Sept 2022
Acknowledgements
This paper was the activity of the PANGAGEN project, supported by ARES-CCD (Acad´emie de Recherche et d′ Enseignement Sup´erieur — Commission de la Cooperation au D´eveloppement) and funded by the Belgian Development Cooperation (DRP/TPS 2017).
Author information
Authors and Affiliations
Contributions
Son, Phuong, and Nicolas: study design, field survey, data analysis, and MS writing Hai, Quynh, Hieu, Hien, Ha: field survey, data clearance/checking, data computing Yen, Huong, Frederic, Patrick, Nicolas: study design, MS reading All authors reviewed the MS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vo, S.N., Dao, H.M., Nguyen, Q.D. et al. Striped catfish farming in different salinity conditions in the Mekong Delta, Vietnam: a comparison of management and economic efficiencies. Aquacult Int 32, 4321–4344 (2024). https://doi.org/10.1007/s10499-023-01376-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01376-1