Abstract
Paraprobiotics, non-viable versions of probiotic microorganisms, offer a promising prophylactic strategy in aquaculture, addressing concerns about the safety and functionality of probiotics while harnessing similar health benefits for fish and shellfish. This study determined the dietary effects of paraprobiotic preparation from Bacillus amyloliquefaciens COFCAU_P1 to support the immune system and control Aeromonas hydrophila infection in Labeo rohita fingerlings. Paraprobiotic was prepared by heat and formalin inactivation of the probiotic B. amyloliquefaciens and subsequently analyzed by scanning electron microscopy. The cellular immunological responses viz. superoxide anion, myeloperoxidase activity, nitric oxide production, and leucocyte proliferation of rohu head-kidney (HK) leucocytes increased significantly with different doses (106, 107, and 108 cells ml−1) of both heat and formalin-inactivated preparations in vitro. Both preparations significantly enhanced the in vitro immune gene (IL-1β and IFN-γ) expression, indicating their immunostimulatory response at the molecular level. As the formalin-inactivated preparation showed a better immune response, it was selected for the subsequent in vivo experiment. Dietary administration of formalin-inactivated B. amyloliquefaciens at different doses (106, 107, and 108 cells g−1 feed) showed significantly higher responses in innate immune (respiratory burst, myeloperoxidase, and anti-protease activity) and biochemical parameters (total protein, albumin, globulin alkaline phosphatase activity, and glucose content). Resistance against experimental A. hydrophila infection was increased significantly after 30 days of feeding of the formalin-inactivated B. amyloliquefaciens. At the paraprobiotic dose of 1 × 108 cells g−1 feed, the maximum immune response and survivability against A. hydrophila infection were observed. It can be inferred from the results that formalin-inactivated B. amyloliquefaciens paraprobiotic can be used as a promising immunostimulant in aquaculture. The potency of B. amyloliquefaciens paraprobiotic to enhance immunity and survivability of rohu against experimental A. hydrophila infection is worth mentioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rising demand for food has made a large portion of the world population rely on fish and aquatic food as low-priced sources of protein. Therefore, the relevance of aquaculture has been prominent from both present and future perspectives. To meet such huge demand, aquaculture has been diversified, expanded, and intensified over the past few decades. However, intensified aquaculture practices and species transportation across countries are now becoming the cause of increasing disease outbreaks from existing and newly emerging pathogens. The disease is a major hindrance to the growth of aquaculture and is responsible for severe economic loss in many developing countries worldwide (Subasinghe et al. 2009). Antibiotics and different chemotherapeutics are used traditionally to prevent and control infectious diseases in aquaculture (Baticados and Paclibare 1992). However, indiscriminate use of antibiotics and chemotherapeutics is not advisable because of their harmful effects that can potentially develop antibiotic and drug-resistant pathogens (Choudhury and Kamilya 2019). Consequently, the use of probiotics in aquaculture is on the rise as a potent alternative to chemotherapeutics and antibiotics (Magnadottir 2010).
Probiotics are live microorganisms that are used as a feed supplement to maintain the microbial equilibrium in the gut of the host (Fuller 1989). When applied in sufficient amounts, these live microorganisms contribute numerous health benefits to the host. Despite the beneficial effects of probiotics, the application of live probiotic organisms is associated with safety, functionality, and applicability issues. Some of these major concerns include stringent storage requirements of probiotics as they affect the viability of microbes (Nayak 2010), the acquisition of virulence genes by probiotics from pathogenic microbes via horizontal gene transfer in the aquatic environment (Newaj-Fyzul et al. 2014), and potential risk to wild aquatic organisms, when live bacteria are released into fish pens or cages (Diaz-Rosales et al. 2006). Recent studies indicate that paraprobiotics, which are non-viable forms of live probiotic organisms, can provide similar beneficial attributes and overcome the constraints of applying live probiotics (Choudhury and Kamilya 2019).
The term “paraprobiotic” was defined by Taverniti and Guglielmetti (2011) as “non-viable microbial cells (intact or broken) or crude cell extracts (i.e., with complex chemical composition), which, when administered (orally or topically) in adequate amounts, confer a benefit on the human or animal consumer.” The killed/attenuated bacteria have beneficial effects and can be used for immunostimulation (Singh et al. 2017). However, very few research works have been done regarding the development of paraprobiotics and their application in aquaculture.
Bacillus amyloliquefaciens COFCAU_P1 is a host gut (Labeo rohita) derived autochthonous probiotic strain. The probiotic and disease-resistance properties (against Aeromonas hydrophila infection) of the strain COFCAU_P1 have already been demonstrated both in vitro and in vivo by our laboratory (Khan et al. 2021, 2022). However, the paraprobiotic properties of this strain have not been explored. Thus, the present study envisaged preparing paraprobiotics from COFCAU_P1 and investigating the immunomodulatory properties under in vitro conditions. Further, the dietary effect of the selected paraprobiotic (based on the in vitro results) on immune-biochemical response and susceptibility of L. rohita against the A. hydrophila challenge was also studied.
Materials and methods
Experimental fish
Fingerlings (16.05 ± 3.95 g, 10 ± 1.03 cm) of L. rohita were collected from a nearby fish farm and stocked in well-aerated fiberglass reinforced plastics (FRP) tanks (500 L) for 15 days for acclimatization before the experiment. Feeding the fish with a pelleted diet (at a rate of 3% of body weight) was done twice a day. During the acclimatization, water temperature (26 ± 3 °C) and other water quality indicators were kept stable.
Preparation of paraprobiotic
The strain COFCAU_P1, previously isolated from the intestine of L. rohita (Khan et al. 2021), was available in our laboratory. The strain was grown in nutrient broth at 30 °C for 48 h. To obtain the bacterial pellet, the suspension was centrifuged for 10 min at 6000 g at 4 °C and washed twice with sterile phosphate-buffered saline (PBS; pH 7.2). Three bacterial concentrations (1 × 108, 1 × 107, and 1 × 106 cells ml−1) were prepared and used further to prepare paraprobiotics.
Two types of inactivation methods (heat and formalin) were used for preparing paraprobiotics. In the heat inactivation method, bacteria were heat-treated at 60, 70, 80, 90, 100, and 110 °C for different periods (5, 7, and 10 min) following previous studies (Diaz-Rosales et al. 2006; Kamilya et al. 2015; Yan et al. 2016; Singh et al. 2017). In the formalin inactivation method, different concentrations of formalin (1, 1.5, 1.7, 2, and 2.2%) were selected for inactivation. Formalin was mixed with bacterial suspension and kept at 4 °C for different periods (12, 24, and 48 h) (Taoka et al. 2006; Newaj-Fyzul et al. 2007; LaPatra et al. 2014). The non-viability of the treated bacteria was examined by culturing them on nutrient agar plates. Each concentration, i.e., 1 × 106, 1 × 107, and 1 × 108 cells ml−1 of heat-inactivated preparations were designated as HP6, HP7, and HP8, whereas the formalin-inactivated preparations were designated as FP6, FP7, and FP8.
Scanning electron microscopy
Scanning electron microscopy (SEM) was done to detect the structural alternation in the inactivated bacterial cells following the method of Barros et al. (2021) with slight modification. After fixing in glutaraldehyde solution (2.5% phosphate buffer, pH 7.2) for 24 h, the control and inactivated samples were dried in a freeze-dryer. After fixing the samples in SEM stubs and coating them with a 6 nm thick layer of gold spray, the observation and capturing of images were done at 5 kV voltage using a scanning electron microscope (Zeiss, Germany).
In vitro immunological responses
Isolation of head-kidney leucocytes from L. rohita fingerlings
Head-kidney (HK) leucocytes were collected aseptically from L. rohita fingerlings following a previously described method (Kamilya et al. 2006). The Leibovitz’s L-15 medium (HiMedia, India) supplemented with 10% fetal calf serum (HiMedia), penicillin (100 IU ml−1), and streptomycin (100 µg ml−1) was used to prepare the leucocyte cell suspension. After washing the cell suspension twice by centrifugation at 1500 g for 10 min at 4 °C, the cell pellet was suspended again in L-15 medium. The cell suspension was then carefully decanted on top of the histopaque@1077 (Sigma-Aldrich, USA). Following centrifugation at 1500 g for 20 min at 4 °C, the leucocytes were drawn carefully from the interphase layer, transferred into a sterile centrifuge tube, and washed twice using sterile PBS (pH 7.4) by centrifugation at 1500 g for 10 min at 4 °C. Counting of the purified leucocytes was done using a hemocytometer (Neubaeur improved; Marienfeld, Germany), and the viability of the cells was examined by trypan blue exclusion test.
Superoxide anion (O 2 −) production
In the superoxide anion production assay (Monsang et al. 2021), nitroblue tetrazolium (NBT; HiMedia) was first dissolved in an L-15 medium to obtain a final concentration of 2 mg ml−1, and the medium was then sterilized by filtration. A hundred microlitres of leucocyte suspension (1 × 106 cells ml−1) were dispensed into 96-well microtiter plate wells. Three concentrations of each paraprobiotic preparation (100 µl) were added into the leucocytes seeded wells, followed by 50 µl of NBT. To ensure the specificity of the reaction, 300 U ml−1 of superoxide dismutase (Sigma-Aldrich) was added to positive control wells. Only PBS was used in negative control wells. Triplicate wells were used for each of the treatments. After incubating the microtiter plate at room temperature for 25 min, the supernatant was withdrawn from each well. The cells were then treated with 200 µl of 70% methanol for 1 min for fixation. The unreduced NBT was removed by washing the wells multiple times with 70% methanol. One hundred twenty microlitres of 2 M KOH and 140 µl of DMSO were added to each well to dissolve the reduced NBT, and the OD595 was recorded by a spectrophotometer (Thermo Scientific, USA).
Myeloperoxidase activity
To quantitate the myeloperoxidase activity of HK leucocytes, the method described by Kamilya et al. (2015) was followed. Head-kidney leucocytes (100 µl; 1 × 106 cells ml−1) were added in the wells of a microtiter plate, followed by 100 µl of each paraprobiotic preparation. Triplicate wells were used for each of the treatments, including a PBS control. After incubating the plate for 30 min at room temperature, cetyl trimethyl ammonium bromide (75 µl; 0.02%; HiMedia) was added to each well to lyse the leucocytes. Following this, 3,3′,5,5′-tetramethyl benzidine hydrochloride (50 µl; 20 mM; HiMedia) and hydrogen peroxide (25 µl; 5 mM; HiMedia) were added to each well and incubated for 2 min. After incubation, sulfuric acid (50 µl; 2 M) was added to terminate the reaction, and the OD450 was recorded by a spectrophotometer.
Nitric oxide (NO) production
The production of NO was determined following the method of Monsang et al. (2021) with minor modifications. After distributing HK leucocytes (100 µl; 1 × 106 cells ml−1) to the individual well of a microtiter plate, different concentrations of each paraprobiotic preparation and PBS (control) were added to the wells in triplicate, and the plate was incubated in a humidified 5% carbon dioxide incubator for 24–72 h at 25 °C. After incubation, 100 µl of supernatant was removed from each well, and 100 µl of 1% sulphanilamide (HiMedia) in 2.5% phosphoric acid was added. Finally, 100 µl of 1% N-naphthyl-ethyl diamine (HiMedia) in 2.5% phosphoric acid was added to it, and the OD540 was recorded by a spectrophotometer.
Leucocytes proliferation
The proliferative response of HK leucocytes was determined by the MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay as described previously (Kamilya et al. 2006). A hundred microlitres of HK leucocytes were seeded to the wells of a microtiter plate, followed by 100 µl of different concentrations of each paraprobiotic preparation. The mitogen concanavalin A (Con A; 50 µg ml−1) was added to each well. Triplicate wells were used for each of the paraprobiotic concentrations, only PBS (negative control), and only ConA (positive control). After incubating the plate for 72 h at 25 °C, 20 µl of 5 mg ml−1 filter-sterilized MTT solution was added to all the wells. The plate was again incubated for 4 h at 25 °C. Following the incubation, the culture media were removed, 200 µl of DMSO was added to each well, and it was mixed for 2 min. The OD595 of the solution was recorded by a spectrophotometer. The mean optical density of stimulated cultures was divided by the mean optical density of the non-stimulated cultures to derive the proliferative response as stimulation index (SI).
Immune gene expression
Isolation of total RNA and cDNA synthesis
A quantitative real-time polymerase chain reaction (qPCR) was employed to examine the mRNA expression of two immune-relevant genes, IL-1β and IFN-γ (Monsang et al. 2021). The gene-specific primers were used to carry out qPCR (Table 1). The housekeeping β-actin gene was used as an internal control to normalize the expression of the target genes. The HK leucocytes were incubated with different concentrations of each paraprobiotic preparation for four durations, viz. 1, 12, 24, and 48 h. After incubation, the total RNA was extracted using TRIzol reagent (Invitrogen, USA), and the concentration and purity of the isolated RNA were checked using a bio-spectrophotometer (Eppendorf, Germany).
After DNase treatment (Thermo-Scientific, USA), cDNA was synthesized from RNA using the iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad, USA). Briefly, 11 µl of the extracted RNA (2 µg) was mixed with 4 µl of 5 X iScript RT Supermix, and 5 µl of nuclease-free water was added to make a 20 µl mixture volume. The mixture was continuously incubated at 25 °C for 5 min, 46 °C for 20 min, and 95 °C for 1 min. The synthesized cDNA was stored at − 20 °C.
Gene expression
The qPCR assay of the previously transcribed cDNA as a template was done using SYBR green (Bio-Rad, USA). The qPCR was run using QuantStudio5 Real-Time PCR system (Applied Biosystem, USA) in 10 µl of reaction mixture containing 5 µl SYBR green, 1 µl cDNA, 1 µl of forward primer, 1 µl of reverse primer, and 2 µl of nuclease-free water. All the samples were amplified in triplicate, and the PCR amplification comprised 40 cycles of 95 °C for 10 min, 95 °C for 15 s, and 60 °C for 1 min. The relative expression of the selected genes was calculated using the 2−ΔΔCT formula (Pfaffl 2001).
In vivo immunomodulatory effects of paraprobiotic-supplemented diet
Experimental diets
The formalin-inactivated preparation showed a better immune response than the heat-inactivated preparation, so it was selected for the subsequent in vivo experiment. Three treatments (with different paraprobiotic concentrations designated as T1, T2, and T3) and a control (without paraprobiotic; designated as C) diet were prepared for the in vivo experiment. All the ingredients (as listed in Table 2), except vitamins, minerals, and paraprobiotic samples, were mixed and autoclaved for 15 min at 121 °C. After cooling, vitamins, minerals, and paraprobiotic for each treatment were mixed with the feed ingredients, and the dough was prepared. The dough was passed through a hand pelletizer to obtain pellets of uniform size (1 mm). The pellets were dried at 38 °C in a drying cabinet and stored at room temperature.
Feeding schedule
Triplicate tanks were randomly assigned to the three treatments (T1, T2, and T3) and control (C) groups. Well-acclimatized L. rohita fingerlings (16.05 ± 3.95 g, 10 ± 1.03 cm) were randomly distributed in all the tanks (15 fish per tank) and fed with the experimental diets at the rate of 3% of body weight twice a day. Enough aeration, water exchange (up to 25% daily), and siphoning of waste materials were done to maintain the water quality parameters. The feeding was continued for 30 days.
Sample collection
Fish samples were collected on days 0, 15, and 30 days after the start of the feeding. Collection of blood, plasma, and serum was accomplished from randomly selected and anesthetized (50 µl−1 of clove oil) fish. Blood was collected from the caudal vein using a sterilized 1 ml hypodermal syringe and 24-gauge needle. Blood samples of three fish from each tank were pooled, generating one pooled sample per tank, and, overall, three replicates per treatment. An aliquot of blood was kept with an anticoagulant (EDTA) to obtain plasma. After keeping at room temperature for 2 h, the sampled blood was centrifuged at 1500 g for 10 min to get serum (Kaur et al. 2018). Both the serum and plasma were stored at − 20 °C until they were used for further analysis. All the immunological and biochemical parameters, as described in the subsequent sections, were measured from three pooled blood samples from each treatment.
Immunological parameters
Superoxide anion production by blood leucocytes was estimated using NBT assay (Anderson and Siwicki 1995). A sample of 100 µl of the pooled blood was distributed in the wells of a microtiter plate. Each well was added with 100 µl of NBT (0.2%), and the plate was incubated at room temperature for 30 min. After incubation, 50 µl of the NBT-blood cell suspension was removed and poured into a microcentrifuge tube containing 1.0 ml of N, N-dimethylformamide solution. After centrifuging the mixture at 3000 g for 5 min, the OD540 of the supernatant was measured using a spectrophotometer.
To estimate the total myeloperoxidase content (Kaur et al. 2018), 10 µl of serum and 90 µl of PBS (pH 7.4) were mixed in the wells of a microtitre plate, followed by the addition of 35 µl each of 20 mM 3,3,5,5-tetramethyl benzidine hydrochloride (HiMedia) and 5 mM H2O2. After keeping the mixture for 2 min, the reaction was stopped by adding 35 µl of 4 M sulfuric acid, and the OD450 of the solution was measured.
To measure the serum anti-protease activity (Zuo and Woo 1997), 10 µl of serum and 100 µl of trypsin (200 µg ml−1) were mixed and incubated for 30 min at 25 °C. After the incubation, 1 ml of casein (2.5 mg ml−1 of PBS) was added, and the mixture was incubated for 15 min at 25 °C. Five hundred microlitres of 10% trichloroacetic acid were added to terminate the reaction. After centrifuging the solution at 10,000 g for 5 min, the OD280 of the supernatant was measured. The anti-protease activity was expressed in terms of trypsin inhibitory capacity using the following formula:
where X1 is the control activity without the serum, and X2 is the activity remaining after incubation with the serum.
Biochemical parameters
Commercially available biochemical parameter estimation kits were used for the measurement of the glucose content of the serum (Accurex Biomedical Pvt. Ltd., India), total serum protein, albumin content, serum glutamate pyruvate transaminase (SGPT), and serum glutamate oxaloacetate transaminase (SGOT) activity (Diatek Healthcare Pvt. Ltd., India), and serum alkaline phosphatase activity (ALP) (Medsource Ozone Biomedicals Pvt. Ltd., India).
Challenge study
A separate feeding experiment was conducted for the challenge study, as mentioned above. After the completion of feeding with the paraprobiotic-supplemented and control diets, each fingerling was intraperitoneally injected with 100 µl of LD50 dose (1 × 104.5 CFU ml−1) (Khan et al. 2022) of A. hydrophila ATCC 7966 and kept under observation for 14 days. Fish were fed with the control diet during the experimental period. The cause of infection was validated by isolating the bacteria from the dead fish. The mortalities were recorded, and the percentage of survival was calculated.
Statistical analysis
The statistical data analysis was performed using SPSS-20.0 (SPSS Inc., Chicago, IL, USA) software. All the observations are presented as mean ± standard error (SE). One-way analysis of variance (ANOVA) and Duncan’s test were performed to compare the means. To determine the significance, a probability level of 0.05 was chosen.
Results
Paraprobiotic preparation
No bacterial colonies were observed on the agar plate for heat and formalin-inactivated preparations. The absence of bacterial colonies confirmed the complete inactivation of bacteria in both methods.
From the SEM analysis of the live (Fig. 1A), heat-inactivated (Fig. 1B), and formalin-inactivated (Fig. 1C) probiotic bacteria, the morphological changes in bacterial cells were visualized. Smooth and intact cell surfaces were observed in live bacteria. However, roughness and damage on the cell surface (marked by black arrows) were observed in both the heat and formalin-inactivated bacterial samples. Cell debris and lysed cells (marked by white arrows) can also be observed in Fig. 1B and C.
In vitro immunological responses
Superoxide anion production
The superoxide anion production was significantly enhanced (P < 0.05) in rohu HK leucocytes treated with different concentrations of heat-inactivated and formalin-inactivated paraprobiotic preparations except in HP6 (Fig. 2A). The radical production was maximum in the FP8 group.
A Superoxide anion production, B myeloperoxidase activity, C nitric oxide production, and D leucocyte proliferation in HK leucocytes of L. rohita fingerlings. PBS was used as a negative control. SOD and conA were positive control for superoxide anion production and leucocyte proliferation measurement, respectively. HP6, HP7, and HP8: Heat-inactivated paraprobiotic with a concentration of 1 × 106, 1 × 107, and 1 × 108 cells ml−1, respectively. FP6, FP7, and FP8: Formalin-inactivated paraprobiotic with a concentration of 1 × 106, 1 × 107, and 1 × 108 cells ml−1, respectively. All the data (n = 3) are presented as mean ± SE. Different alphabetic superscripts indicate significant differences (P < 0.05) compared to the control
Myeloperoxidase activity
There was significantly higher (P < 0.05) myeloperoxidase activity in all the treatments except in HP6 and FP6 (Fig. 2B). The myeloperoxidase activity was more prominent in the leucocytes treated with formalin-inactivated than heat-inactivated preparation. Among the different concentrations, FP8 showed maximum myeloperoxidase activity.
NO production
NO production was significantly higher (P < 0.05) in all treatments except HP6 and FP6. The treatment group FP8 showed the maximum NO production (Fig. 2C).
Leucocyte proliferation
Leucocyte proliferation was significantly higher (P < 0.05) in HP8, FP7, and FP8 than in Con A-induced HK leucocytes (positive control). The maximum leucocyte proliferation was observed in FP8 (Fig. 2D).
Immune gene expression
Both the paraprobiotic preparations showed significantly higher (P < 0.05) fold changes in the IL-1β and IFN-ɣ expression in rohu HK leucocytes (Fig. 3A, B). The highest cytokine expression was noticed in FP8 compared to the control. The maximum expression of both IL-1β and IFN-ɣ for all the different concentrations was observed at 12 h and then decreased gradually at 24 and 48 h.
A Expression of IL-1β and B expression of IFN-γ at different sampling hours in rohu HK leucocytes after incubating with different concentrations of paraprobiotic. HP6, HP7, and HP8: Heat-inactivated paraprobiotic with a concentration of 1 × 106, 1 × 107, and 1 × 108 cells ml−1, respectively. FP6, FP7, and FP8: Formalin-inactivated paraprobiotic with a concentration of 1 × 106, 1 × 107, and 1 × 108 cells ml−1, respectively. All the data (n = 3) are presented as mean ± SE. Different alphabetic superscripts indicate significant differences (P < 0.05) compared to the control
In vivo immunomodulatory effects of paraprobiotic-supplemented diet
Superoxide anion production
A significantly higher superoxide anion production (P < 0.05) was observed in fish fed with all the different concentrations of formalin-inactivated paraprobiotic-supplemented diet compared to the fish fed with basal (control) diet on the 15th and 30th day. The fish fed the T3 diet showed the maximum superoxide anion production (Fig. 4A).
A Superoxide anion production by phagocytes of L. rohita, B myeloperoxidase activity, and C ani-protease activity in serum. Control: Fish fed with basal diet. Diet T1, T2, and T3: fish fed with a basal diet supplemented with formalin-inactivated B. amyloliquefaciens at the rate of 1 × 106, 1 × 107, and 1 × 108 cells g.−1 diet, respectively. All the data (n = 3) are presented as mean ± SE. Different superscript letters indicate statistically significant differences (P < 0.05)
Myeloperoxidase activity
A significantly higher (P < 0.05) myeloperoxidase activity was observed in fish fed with all the different concentrations of formalin-inactivated paraprobiotic-supplemented diet compared to the control diet on both the 15th and 30th day. In contrast, the maximum activity was noticed in fish of the T3 group on the 30th day (Fig. 4B).
Anti-protease activity
On the 15th day, the fish fed with all the different concentrations of formalin-inactivated paraprobiotic-supplemented diet showed significantly higher (P < 0.05) anti-protease activity than those provided with the control diet. However, on day 30th, fish in the T2 and T3 groups showed significantly higher (P < 0.05) anti-protease activity compared to control diet-fed fish (Fig. 4C).
Biochemical parameters
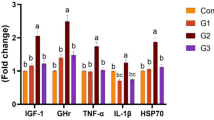
Except for 0 days, the serum glucose content was decreased significantly (P < 0.05) in fish fed with all the different concentrations of formalin-inactivated paraprobiotic-supplemented diet compared to the fish fed with the control diet (Table 3).
On the 15th day and 30th day, a significantly higher (P < 0.05) protein concentration was observed in fish fed with the T1, T2, and T3 diets compared to the control group fish (Table 4).
The serum albumin content of fish fed with the paraprobiotic-supplemented diets was significantly higher (P < 0.05) on the 15th day (Table 5). In contrast, the globulin content increased significantly on the 15th day and 30th day of feeding (Table 6).
On the 15th and 30th day, the alkaline phosphatase activity was significantly enhanced (P < 0.05) in fish fed with diets supplemented with formalin-inactivated (Table 7).
Challenge study
After injecting with A. hydrophila ATCC 7966, 53.33 ± 3.33%, 56.67 ± 3.33%, and 66.67 ± 3.33% survivability of L. rohita was noticed in fish fed with T1, T2, and T3 diets, respectively. The survivability of L. rohita in control was 46.67 ± 3.33%. Significantly enhanced (P < 0.05) survivability of challenged fish was observed only in the T2 and T3 groups (Fig. 5). The dead fish showed hemorrhages in the abdominal region and dropsy as prominent clinical signs.
Discussion
The published research findings suggest that non-viable microbial preparations can modify biological responses in fish and shellfish through immunological activation. Such reports are significant and promising, especially given the concerns about the possible safety issues related to releasing live probiotic microorganisms into the aquatic environment. Hence, the current study assessed the immune-biochemical response and disease-resistance ability of a paraprobiotic preparation from B. amyloliquefaciens COFCAU_P1.
Many researchers have applied various methods to inactivate probiotic bacteria and prepare paraprobiotics. The inactivation method, which exceeds minimum conditions, can disintegrate the physiology and morphology of bacterial cells, which is not desirable for preparing a paraprobiotic (Almada et al. 2021). Two inactivation methods (heat and formalin) were used to prepare paraprobiotic. According to Gould (1989), elevated temperature can influence several components of microorganism cell structure, including membrane integrity breakdown, nutrition and ion loss, ribosome aggregation, inactivation of key enzymes and protein coagulation, and DNA filament breakage, thus inactivating bacterial cells. In this study, live B. amyloliquefaciens COFCAU_P1 was inactivated after treatment at 110 °C for 10 min. According to Barros et al. (2021), the inactivation condition depends on probiotic strain, too. The duration and temperature needed for the complete inactivation of each probiotic strain may also vary. The inactivation of bacteria by formaldehyde occurs via the alkylation of the amino and sulfhydral groups of proteins and the ring nitrogen atoms of purine bases (Favero and Bond 1991). In this study, when live B. amyloliquefaciens COFCAU_P1 was treated with 2.2% (v/v) formalin for 48 h at 4 °C, no colony was found on nutrient agar, confirming the inactivation of the bacteria. A similar result was found when B. subtilis AB1 was treated with the same condition (2.2% formalin, 48 h) (Newaj-Fyzul et al. 2007).
The SEM analysis shows that the cell membrane of the live B. amyloliquefaciens COFCAU_P1 cell had a smooth surface and integrity before the treatments. However, following the treatments, they became rough and ruptured with the presence of cell debris. Similar morphological changes were observed in Lactobacillus acidophilus LA-5 after conventional and ohmic heating and in L. brevis after treatment with high-intensity pulsed electric field (Elez-Martinez et al. 2005; Barros et al. 2021).
Immune responses by HK leucocytes of L. rohita were measured in vitro to check the immunomodulatory potential of heat and formalin-inactivated paraprobiotic preparations and also to find out the better inactivation method which could be applied in feed as a supplement for the in vivo experiment. Macrophages are the primary phagocytic cells in fish, predominating in the head kidneys, and other organs. Macrophages are a crucial indicator of immunological function (Lunden et al. 2002). The respiratory burst is a phenomenon that occurs in both fish and mammals when the phagocyte cell membrane is stimulated. This stimulation activates the membrane-associated NADPH-oxidase, which increases oxygen consumption and triggers the release of reactive oxygen intermediates (Płytycz et al. 1989; Secombes 1996). Superoxide anion generation (a vital member of the ROI family) is regarded as one of the most critical microbicidal components of phagocyte activity (Secombes 1990). Macrophage activation factor (MAF) and lipopolysaccharide (LPS) also stimulate nitric oxide production, which is an important mechanism of the innate immune response (Neumann and Belosevic 1996; Secombes et al. 2001). When stimulated by various paraprobiotics, it had been observed that fish phagocytes produced superoxide anion and nitric oxide (NO) (Villamil et al. 2002; Salinas et al. 2006; Kamilya et al. 2015). In this study, significantly higher superoxide anion and nitric oxide production in rohu HK leucocytes suggested the activation of cellular immune response when formalin-inactivated paraprobiotic preparation was applied. Granules of neutrophil essentially produce the myeloperoxidase enzyme during the oxidative respiratory burst, which generates hypochlorous acid to counteract infections (Dalmo and Bøgwald 2008). A significantly higher myeloperoxidase activity was detected in rohu HK leucocytes when exposed to paraprobiotic preparations. The result of the myeloperoxidase activity was similar to previous research conducted by Kamilya et al. (2015) when they applied heat, formalin, and UV-inactivated B. amyloliquefaciens FPTB16 to catla HK leucocytes. Leucocyte proliferation confirmed the viability of the leucocytes incubated with paraprobiotic preparation and the ability of paraprobiotic preparation to promote the polyclonal activation of leucocytes. In addition, the leucocyte proliferation study indicates that the formalin-inactivated preparation was not harmful to fish because lymphocyte proliferation is by far the most often employed immunological response in immunotoxicology investigations (Desforges et al. 2016). Overall, it was observed that formalin-inactivated preparation increased the cellular immune response of rohu HK leucocytes.
The mRNA expression analysis of immune-relevant genes was performed to better understand leucocytes’ immunological responses after incubation with paraprobiotic preparations at the molecular level. After incubating rohu HK leucocytes with paraprobiotics for 48 h, a significant increase in two cytokine gene (IL-1β, IFN-ɣ) expressions was observed. Different leucocytes produce the pro-inflammatory cytokine IL-1β and participate in the immune response to tissue damage and microbial challenge by activating lymphocytes and promoting the production of other cytokines (Low et al. 2003). As a result, the IL-1β expression observed in this study would have a wide range of downstream consequences, including the release of additional cytokines (Biswas et al. 2012). The IFN-ɣ is a cytokine that plays an important role in mediating immunity against viral diseases in fish (Zou and Secombes 2011). Biswas et al. (2013) reported significant up-regulation in both IL-1β and IFN-ɣ in Japanese puffer fish (Takifugu rubripes) when HK cells were stimulated with paraprobiotic preparations of L. paracasei (strain 06TCa22) and L. plantarum (strain 06CC2) which supports the result of the present study where significant up-regulation of these cytokines was observed in rohu HK leucocytes when incubated with paraprobiotic preparations. Up-regulation of IFN-ɣ might be induced by the combined effect of other cytokines like IL-12 and IL-18 expression, which was reported by a previous study by Biswas et al. (2013). Cell wall components such as peptidoglycans, lipopolysaccharides, and β-glucans of bacteria and yeast are known to modulate the immune response. Regardless of past exposure to that organism, the innate immune system recognizes and binds these conserved pathogen-associated molecular patterns (PAMPs) shared by the major classes of pathogenic bacteria using the pathogen recognition receptors (PRRs) (Panigrahi et al. 2011). This could be the reason behind the immunostimulating effect of paraprobiotics.
The heat and formalin-inactivated paraprobiotics showed an enhanced immune response, but formalin-inactivated preparation showed a comparatively better immune response than heat-inactivated preparation. This was contrary to Kamilya et al. (2015), who reported that heat-inactivated B. amyloliquefaciens FPTB16 showed the best immunostimulatory effect on catla HK leucocyte. It has been observed that the method of inactivation employed to create paraprobiotic is critical to their future potency. This might be explained by the distinct mode of action of the various inactivation processes in creating non-viable cells, which could lead to additional health advantages. Furthermore, the inactivation circumstances (intensity) appear important for the effectiveness of paraprobiotics. This is because the more drastic the morphological and biochemical alterations in probiotic cells generated by the inactivation process, the less likely paraprobiotics are to provide health advantages (Almada et al. 2016; 2021; Deshpande et al. 2018; Ramkrishna et al. 2019). So, formalin-inactivated paraprobiotic showed a better immunostimulatory effect than heat-inactivated paraprobiotic because high temperatures may denature some proteins and molecules with immunostimulatory properties.
Based on the result of the in vitro experiment, formalin-inactivated preparation was selected to evaluate the immunomodulatory effect in L. rohita fingerlings after dietary administration. Different immunological and biochemical parameters were assessed to check the immunomodulatory effect and overall well-being of fish. Regarding immunological parameters, the study found that dietary supplementation with formalin-inactivated paraprobiotic preparation at various dosages significantly improved the immune response in L. rohita. The respiratory burst response of L. rohita increased significantly in all the treatments after dietary administration of paraprobiotic. The diet containing the paraprobiotic dose of 1 × 108 cells g−1 of feed showed the maximum response on the 15th and 30th days of feeding. Higher respiratory burst activity can be correlated with the enhanced ability of phagocytes to kill microbial pathogens (Sharp and Secombes 1993; Weir and Stewart 1993; Sahu et al. 2007). Respiratory burst activity was increased when formalin-inactivated and sonication-inactivated B. subtilis AB1 supplemented diet was fed to Oncorhynchus mykiss (Newaj-Fyzul et al. 2007). Similar results were also reported after dietary administration of heat-inactivated L. delbrueckii and B. subtilis, heat-inactivated L. plantarum, and heat-inactivated B. amyloliquefaciens FPTB16 to S. aurata, M. rosenbergii, and C. catla, respectively (Salinas et al. 2008; Dash et al. 2015; Singh et al. 2017).
Besides respiratory burst activity, the capacity of the enzyme secreted by phagocytic cells to destroy pathogens is determined by myeloperoxidase activity. Myeloperoxidase uses oxidative radicals to produce hypochlorous acid to kill pathogens (Klebanoff et al. 2013). They significantly increased myeloperoxidase activity on the 15th day of dietary administration of formalin-inactivated preparation in all the treatment groups, indicating immunocompetency of the paraprobiotic preparation. The result of the myeloperoxidase activity of the current study also supports the result of the previous study of Singh et al. (2017) where they reported increased myeloperoxidase activity after dietary administration of paraprobiotic B. amyloliquefaciens FPTB16.
Bacteria must degrade host proteins to enter, proliferate, and damage the host. Fish blood includes a variety of protease inhibitors such as α1-antiprotease, α2-antiplasmin, and α2-macroglobulin which help to limit bacteria’s ability to penetrate and proliferate (Ellis 2001; Mukherjee et al. 2019). So, anti-protease is an important tool in the humoral defense system in fish (Lange et al. 2001). In the current study, the anti-protease level increased significantly compared to the control when fed with paraprobiotic preparation. Increased α1-anti-protease level was also reported in O. mykiss after dietary administration of formalin-inactivated and sonication-inactivated B. subtilis AB1 (Newaj-Fyzul et al. 2007).
Therefore, after analyzing both cellular and humoral innate immunological parameters, the study suggests that formalin-inactivated paraprobiotic preparation also has potential immunostimulatory activity under in vivo conditions.
Glucose plays a key role in the bioenergetics of animals, and it is also an indicator of stress (Lucas 1996; Martínez-Porchas et al. 2009). In this study, the blood glucose levels dropped significantly in fish fed with paraprobiotic-supplemented diets. It may indicate that paraprobiotic preparation might have some role in managing stress in fish. Significantly reduced blood glucose levels were also observed in red sea bream when fed with heat-inactivated L. plantarum (Dawood et al. 2015). Contrarily, a significant enhancement in the glucose level was observed in O. mykiss after dietary administration of heat-inactivated Tsukamurella inchonensis (Nofouzi et al. 2018).
Protein is an essential component for the maintenance of the defense system (Anderson and Anderson 2002). A high level of innate immunity is associated with an enhanced level of total serum proteins (Wiegertjes et al. 1996). In this study, a significantly higher total protein level in all the treatment groups suggests that B. amyloliquefaciens COFCAU_P1 can activate the immune system. Increased serum protein was also observed in Nile tilapia and red sea bream fed with paraprobiotic Bacillus sp. NP5 and P. pentosaceus, respectively (Dawood et al. 2016; Mulyadin et al. 2021). However, no significant change in the total serum protein level was observed in O. mykiss after dietary administration of heat-inactivated T. inchonensis (Nofouzi et al. 2018).
Alkaline phosphatase is a metalloenzyme involved in immune system activity (Dong et al. 2015). In this study, formalin-inactivated paraprobiotic significantly increased the alkaline phosphatase activity compared to the control. Catla, fed with the paraprobiotic B. amyloliquefaciens FPTB16, also showed increased alkaline phosphatase activity ((Singh et al. 2017). On the other hand, no significant change in activity was reported in O. mykiss after dietary administration of heat-inactivated T. inchonensis (Nofouzi et al. 2018).
Fish survivability after experimental infection may be a vital gauge for measuring the host’s health while determining the efficiency of immune boosters like paraprobiotics (Cerezuela et al. 2012). In this work, dietary administration of formalin-inactivated paraprobiotic preparation enhanced the survivability of L. rohita, challenged with A. hydrophila ATCC 7966. A significant increase in survivability was observed in the T3 group after 30 days of paraprobiotic feeding. This result can be correlated with the in vivo immune response results in fish, where diet T3 showed the overall maximum immune response after 30 days of feeding. The increased immune response may have resulted in increased resistance of rohu to A. hydrophila infection. It may also be deduced that many immunomodulatory components present in the paraprobiotic improved the innate immunity of fish, resulting in greater resistance to the bacteria. Several studies also reported increased disease-resistance ability of different paraprobiotic against different bacterial infections (Irianto and Austin 2002; Newaj-Fyzul et al. 2007; Pan et al. 2008; Rodriguez-Estrada et al. 2013; Dash et al. 2015).
Conclusion
The present study indicates that both the heat and formalin-inactivated B. amyloliquefaciens COFCAU_P1 were able to elicit a cellular immune response. However, formalin-inactivated B. amyloliquefaciens COFCAU_P1 showed better immunostimulatory properties than the heat-inactivated preparation. The immune-biochemical responses were up-regulated throughout the feeding trial for 30 days. Resistance against A. hydrophila was also increased after feeding with the formalin-inactivated paraprobiotic-supplemented diet. We suggest that formalin-inactivated B. amyloliquefaciens COFCAU_P1 can be supplemented with fish feed at 1 × 108 cells g−1 to enhance the immune response in L. rohita.
Data Availability
All data supporting the findings of this study are available within the paper.
References
Almada CN, Almada CN, Martinez RC, Sant’Ana AS (2016) Paraprobiotics: evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci Technol 58:96–114. https://doi.org/10.1016/j.tifs.2016.09.011
Almada CN, Almada-Erix CN, Roquetto AR, Santos-Junior VA, Cabral L, Noronha MF, Gonçalves AESS, Santos P, Santo A, Martinez J, Lollo PC, Costa WKA, Magnani M, Sant’Ana AS (2021) Paraprobiotics obtained by six different inactivation processes: impacts on the biochemical parameters and intestinal microbiota of Wistar male rats. Int J Food Sci Nutr 72(8):1057–1070. https://doi.org/10.1080/09637486.2021.1906211
Anderson NL, Anderson NG (2002) The human plasma proteome. Mol Cell Proteomics 1:845–867. https://doi.org/10.1074/mcp.R200007-MCP200
Anderson DP, Siwicki AK (1995) Basic haematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian Aquaculture II. Asian Fisheries Society, Manila, Philippines, Fish Health Section, pp 185–202
Barros CP, Pires RP, Guimarães JT, Abud YK, Almada CN, Pimentel TC, Sant’Anna C, De-melo LDB, Duarte MCKH, Silva MC, Sant’ana MC, Freitas MQ, Cruz AG (2021) Ohmic heating as a method of obtaining paraprobiotics: impacts on cell structure and viability by flow cytometry. Int Food Res J 140:110061. https://doi.org/10.1016/j.foodres.2020.110061
Baticados MCL, Paclibare JO (1992) The use of chemotherapeutic agents in aquaculture in the Philippines. In Diseases in Asian Aquaculture I. Proceedings of the First Symposium on Diseases in Asian Aquaculture. p 531–546, November, 26–29, 1990, Bali, Indonesia. Asian Fisheries Society, Fish Health Section.
Biswas G, Korenaga H, Takayama H, Kono T, Shimokawa H, Sakai M (2012) Cytokine responses in the common carp, Cyprinus carpio L. treated with baker’s yeast extract. Aquaculture 356:169–175. https://doi.org/10.1016/j.aquaculture.2012.05.019
Biswas G, Korenaga H, Nagamine R, Takayama H, Kawahara S, Takeda KY, Dashnyam B, Kono T, Sakai M (2013) Cytokine responses in the Japanese pufferfish (Takifugu rubripes) head kidney cells induced with heat-killed probiotics isolated from the Mongolian dairy products. Fish Shellfish Immunol 34(5):1170–1177. https://doi.org/10.1016/j.fsi.2013.01.024
Cerezuela R, Guardiola FA, González P, Meseguer J, Esteban MÁ (2012) Effects of dietary Bacillus subtilis, Tetraselmis chuii, and Phaeodactylum tricornutum, singularly or in combination, on the immune response and disease resistance of sea bream (Sparus aurata L.). Fish Shellfish Immunol 33(2):342–349. https://doi.org/10.1016/j.fsi.2012.05.004
Choudhury TG, Kamilya D (2019) Paraprobiotics: an aquaculture perspective. Rev Aqua 11(4):1258–1270. https://doi.org/10.1111/raq.12290
Dalmo RA, Bøgwald J (2008) ß-glucans as conductors of immune symphonies. Fish Shellfish Immunol 25(4):384–396. https://doi.org/10.1016/j.fsi.2008.04.008
Dash G, Raman RP, Prasad KP, Makesh M, Pradeep MA, Sen S (2015) Evaluation of paraprobiotic applicability of Lactobacillus plantarum in improving the immune response and disease protection in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Fish Shellfish Immunol 43(1):167–174. https://doi.org/10.1016/j.fsi.2014.12.007
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2015) Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 442:29–36. https://doi.org/10.1016/j.aquaculture.2015.02.005
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2016) Effects of dietary killed Pediococcus pentosaceus on growth performance, feed utilization and blood characteristics of red sea bream, Pagrus major juvenile. Aquac Nutr 22:923–932. https://doi.org/10.1111/anu.12314
Desforges JPW, Sonne C, Levin M, Siebert U, De Guise S, Dietz R (2016) Immunotoxic effects of environmental pollutants in marine mammals. Environ Int 86:126–139. https://doi.org/10.1016/j.envint.2015.10.007
Deshpande G, Athalye-Jape G, Patole S (2018) Para-probiotics for preterm neonates-the next frontier. Nutrients 10(7):871. https://doi.org/10.3390/nu10070871
Diaz-Rosales P, Salinas I, Rodriguez A, Cuesta A, Chabrillon M, Balebona MC (2006) Gilthead seabream (Sparus aurata L.) innate immune response after dietary administration of heat-killed potential probiotics. Fish Shellfish Immunol 20(4):482–492. https://doi.org/10.1016/j.fsi.2005.06.007
Dong XQ, Zhang DM, Chen YK, Wang QJ, Yang YY (2015) Effects of antimicrobial peptides (AMPs) on blood biochemical parameters, antioxidase activity, and immune function in the common carp (Cyprinus carpio). Fish Shellfish Immunol 47(1):429–434. https://doi.org/10.1016/j.fsi.2015.09.030
Elez-Martinez P, Escola-Hernandez J, Soliva-Fortuny RC, Martín-Belloso O (2005) Inactivation of Lactobacillus brevis in orange juice by high-intensity pulsed electric fields. Food Microbiol 22(4):311–319. https://doi.org/10.1016/j.fm.2004.09.005
Ellis AE (2001) Innate host defence mechanisms of fish against viruses and bacteria. Dev Comp Immunol 25(8–9):827–839. https://doi.org/10.1016/S0145-305X(01)00038-6
Favero MS, Bond WW (1991) Chemical disinfection of medical and surgical materials. In: Block SS (ed) Disinfection, Sterilization, and Preservation. Lea and Febiger, Philadelphia, pp 617–641
Fuller R (1989) A review: probiotics in man and animals. J Appl Microbiol 66(5):365–378. https://doi.org/10.1111/j.1365-2672.1989.tb05105.x
Gould GW (1989) Heat induced injury and inactivation. In: Gould GW (ed) Mechanisms of Action of Food Preservation Procedures. Elsevier Applied Science, London, pp 11–42
Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25:633–642. https://doi.org/10.1046/j.1365-2761.2002.00422.x
Kamilya D, Ghosh D, Bandyopadhyay S, Mal BC, Maiti TK (2006) In vitro effects of bovine lactoferrin, mushroom glucan and Abrus agglutinin on Indian major carp, catla (Catla catla) head kidney leukocytes. Aquaculture 253(1–4):130–139. https://doi.org/10.1016/j.aquaculture.2005.07.038
Kamilya D, Baruah A, Sangma T, Chowdhury S, Pal P (2015) Inactivated probiotic bacteria stimulate cellular immune responses of catla, Catla catla (Hamilton) in vitro. Probiotics Antimicrob Proteins 7(2):101–106. https://doi.org/10.1007/s12602-015-9191-9
Kaur N, Kumar R, Kamilya D (2018) Modulation of systemic and mucosal immune responses of Catla catla (Hamilton, 1822) experimentally challenged with gill monogeneans. Fish Shellfish Immunol 74:567–572. https://doi.org/10.1016/j.fsi.2018.01.026
Khan MIR, Kamilya D, Choudhury TG, Tripathy PS, Rathore G (2021) Deciphering the probiotic potential of Bacillus amyloliquefaciens COFCAU_P1 isolated from the intestine of Labeo rohita through in vitro and genetic assessment. Probiotics Antimicrob Proteins 13(6):1572–1584. https://doi.org/10.1007/s12602-021-09788-2
Khan MIR, Kamilya D, Choudhury TG, Tripathy PS, Rathore G (2022) Dietary administration of a host-gut derived probiotic Bacillus amyloliquefaciens COFCAU_P1 modulates immune-biochemical response, immune-related gene expression, and resistance of Labeo rohita to Aeromonas hydrophila infection. Aquaculture 546:737390. https://doi.org/10.1016/j.aquaculture.2021.737390
Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM (2013) Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol 93(2):185–198. https://doi.org/10.1189/jlb.0712349
Lange S, Guđmundsdottir BK, Magnadottir B (2001) Humoral immune parameters of cultured Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol 11(6):523–535. https://doi.org/10.1006/fsim.2000.0333
LaPatra SE, Fehringer TR, Cain KD (2014) A probiotic Enterobacter sp. provides significant protection against Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) after injection by two different routes. Aquaculture 433:361–366. https://doi.org/10.1016/j.aquaculture.2014.06.022
Low C, Wadsworth S, Burrells C, Secombes CJ (2003) Expression of immune genes in turbot (Scophthalmus maximus) fed a nucleotide-supplemented diet. Aquaculture 221(1–4):23–40. https://doi.org/10.1016/S0044-8486(03)00022-X
Lucas A (1996) Physical concepts of bioenergetics. In: Lucas A (ed). Bioenergetics of aquatic animals. English edition, Taylor and Francis, France.
Lunden T, Lilius EM, Bylund G (2002) Respiratory burst activity of rainbow trout (Oncorhynchus mykiss) phagocytes is modulated by antimicrobial drugs. Aquaculture 207(3–4):203–212. https://doi.org/10.1016/S0044-8486(01)00760-8
Magnadottir B (2010) Immunological control of fish diseases. Mar Biotechnol 12(4):361–379. https://doi.org/10.1007/s10126-010-9279-x
Martínez-Porchas M, Martinez-Cordova LR, Ramos-Enriquez R (2009) Cortisol and glucose: reliable indicators of fish stress. Panam J Aquat Sci 4(2):158–178
Monsang SJ, Acharya A, Khan MIR, Kamilya D (2021) In vitro effects of Asparagus racemosus ethanolic root extract on cellular immune response and immune-related gene expression of Labeo rohita (Hamilton, 1822) leucocytes and anti-Aeromonas hydrophila activity. Aquac Res 52(10):4724–4734. https://doi.org/10.1111/are.15306
Mukherjee A, Chandra G, Ghosh K (2019) Single or conjoint application of autochthonous Bacillus strains as potential probiotics: effects on growth, feed utilization, immunity and disease resistance in Rohu, Labeo rohita (Hamilton). Aquaculture 512:734302. https://doi.org/10.1016/j.aquaculture.2019.734302
Mulyadin A, Widanarni W, Yuhana M, Wahjuningrum D (2021) Growth performance, immune response, and resistance of Nile tilapia fed paraprobiotic Bacillus sp. NP5 against Streptococcus agalactiae infection. Jurnal Akuakultur Indonesia 20(1):34–46. https://doi.org/10.19027/jai.20.1.34-46
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–4. https://doi.org/10.1016/j.fsi.2010.02.017
Neumann NF, Belosevic M (1996) Deactivation of primed respiratory burst response of goldfish macrophages by leukocyte-derived macrophage activating factor (s). Dev Comp Immunol 20(6):427–439. https://doi.org/10.1016/s0145-305x(96)00029-8
Newaj-Fyzul A, Adesiyun AA, Mutani A, Ramsubhag A, Brunt AB (2007) Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 103(5):1699–1706. https://doi.org/10.1111/j.1365-2672.2007.03402.x
Newaj-Fyzul A, Al-Harbi AH, Austin B (2014) Developments in the use of probiotics for disease control in aquaculture. Aquaculture 431:1–11. https://doi.org/10.1016/j.aquaculture.2013.08.026
Nofouzi K, Sheikhzadeh N, Varshoie H, Sharabyani SK, Jafarnezhad M, Shabanzadeh S, Ahmadifar E, Stanford J, Shahbazfar AA (2018) Beneficial effects of killed Tsukamurella inchonensis on rainbow trout (Oncorhynchus mykiss) growth, intestinal histology, immunological, and biochemical parameters. Fish Physiol Biochem 45(1):209–217. https://doi.org/10.1007/s10695-018-0555-4
Pan X, Wu T, Song Z, Tang H, Zhao Z (2008) Immune responses and enhanced disease resistance in Chinese drum, Miichthys miiuy (Basilewsky), after oral administration of live or dead cells of Clostridium butyrium CB2. J Fish Dis 31(9):679–686. https://doi.org/10.1111/j.1365-2761.2008.00955.x
Panigrahi A, Viswanath K, Satoh S (2011) Real-time quantification of the immune gene expression in rainbow trout fed different forms of probiotic bacteria Lactobacillus rhamnosus. Aquac Res 42(7):906–917. https://doi.org/10.1111/j.1365-2109.2010.02633.x
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45. https://doi.org/10.1093/nar/29.9.e45
Płytycz B, Flory CM, Galvan I, Bayne C (1989) Leucocytes of rainbow trout (Oncorhynchuss mykiss) pronephros: cell types producing superoxide anion. Dev Comp Immunol 13:217–224. https://doi.org/10.1016/0145-305x(89)90002-5
Ramkrishna S, Chellamboli C, Anupama D (2019) A revolution by probiotic and paraprobiotic in food industry: review. Res Rev Int J Multidiscip 4(9):72–81
Rodriguez-Estrada U, Satoh S, Haga Y, Fushimi H, Sweetman J (2013) Effects of inactivated Enterococcus faecalis and mannan oligosaccharide and their combination on growth, immunity, and disease protection in rainbow trout. N Am J Aquac 75(3):416–428. https://doi.org/10.1080/15222055.2013.799620
Sahu S, Das BK, Pradhan J, Mohapatra BC, Mishra BK, Sarangi N (2007) Effect of Magnifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo rohita fingerlings. Fish Shellfish Immunol 23(1):109–118. https://doi.org/10.1016/j.fsi.2006.09.009
Salinas I, Diaz-Rosales P, Cuesta A (2006) Effect of heat-inactivated fish and nonfish derived probiotics on the innate immune parameters of a teleost fish (Sparus auratus L.). Vet Immunol Immunopathol 111(3–4):279–286. https://doi.org/10.1016/j.vetimm.2006.01.020
Salinas I, Myklebust R, Esteban MA, Olsen RE, Meseguer J, Ringo E (2008) In vitro studies of Lactobacillus delbrueckii subsp. lactis in Atlantic salmon (Salmo salar L.) foregut: tissue responses and evidence of protection against Aeromonas salmonicida subsp. salmonicida epithelial damage. Vet Microbiol 128:167–177. https://doi.org/10.1016/j.vetmic.2007.10.011
Secombes CJ (1990) Isolation of salmonid macrophages and analysis of their killing activity. In: Stolen JS, Fletcher TC, Anderson D, Robertson BS, Muiswinkel BNV (eds) Techniques in fish immunology. Vol. I. NJ: SOS Publications, Fair Haven, p 137–154.
Secombes CJ (1996) The nonspecific immune system: cellular defences. In: Iwama G, Nakanishi T (eds) The fish immune system: organism, pathogen, environment. Academic Press, Toronto, pp 63–103
Secombes CJ, Wang T, Hong S, Peddie S, Crampe M, Laing KJ, Cunningham C, Zou J (2001) Cytokines and innate immunity of fish. Dev Comp Immunol 25(8–9):713–723. https://doi.org/10.1016/s0145-305x(01)00032-5
Sharp GJE, Secombes CJ (1993) The role of reactive oxygen species in the killing of the bacterial fish pathogen Aeromonas salmonicida by rainbow trout macrophages. Fish Shellfish Immunol 3:119–129. https://doi.org/10.1006/fsim.1993.1013
Singh ST, Kamilya D, Kheti B, Bordoloi B, Parhi J (2017) Paraprobiotic preparation from Bacillus amyloliquefaciens FPTB16 modulates immune response and immune relevant gene expression in Catla catla (Hamilton, 1822). Fish Shellfish Immunol 66:35–42. https://doi.org/10.1016/j.fsi.2017.05.005
Subasinghe R, Soto D, Jia J (2009) Global aquaculture and its role in sustainable development. Rev Aquac 1(1):2–9. https://doi.org/10.1111/j.1753-5131.2008.01002.x
Taoka Y, Maeda H, Jo JY, Kim SM, Park SI, Yoshikawa T, Sakata T (2006) Use of live and dead probiotic cells in tilapia Oreochromis niloticus. Fish Sci 72(4):755–766. https://doi.org/10.1111/j.1444-2906.2006.01215.x
Taverniti V, Guglielmetti S (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6(3):261–274. https://doi.org/10.1007/s12263-011-0218-x
Villamil L, Tafalla C, Figueras A, Novoa B (2002) Evaluation of immunomodulatory effects of lactic acid bacteria in turbot (Scophthalmus maximus). Clin Diagn Lab Immunol 9(6):1318–1323. https://doi.org/10.1128/CDLI.9.6.1318-1323.2002
Weir DM, Stewart J (1993) Immunology, 2nd edn. Longman Group, UK Ltd, p 372
Wiegertjes GF, Stet RJM, Parmentier HK, Vas Muiswinkel WB (1996) Immunogenetics of disease resistance in fish: a Comparable approach. Dev Comp Immunol 20:365–381. https://doi.org/10.1016/s0145-305x(96)00032-8
Yan YY, Xia HQ, Yang HL, Hoseinifar SH, Sun YZ (2016) Effects of dietary live or heat-inactivated autochthonous Bacillus pumilus SE5 on growth performance, immune responses and immune gene expression in grouper Epinephelus coioides. Aquac Nutr 22:698–707. https://doi.org/10.1111/anu.12297
Zou J, Secombes CJ (2011) Teleost fish interferons and their role in immunity. Dev Comp Immunol 35(12):1376–1387. https://doi.org/10.1016/j.dci.2011.07.001
Zuo X, Woo PTK (1997) Natural antiprotease in rainbow trout, Oncorhynchus mykiss and brook char, Salvelinus fontinalis and the in vitro neutralization of fish α 2-macroglobulin by the metallo-protease from the pathogenic haemoflagellates, Cryptobia salmositica. Parasitology 114:375–382. https://doi.org/10.1017/s0031182096008578
Funding
This work was supported by a grant from the Department of Biotechnology, New Delhi, India (Grant numbers BT/PR25008/NER/95/953/2017).
Author information
Authors and Affiliations
Contributions
Kallol Barui: Investigation, Validation; Visualization; Writing original draft; Tanmoy Gon Choudhury: Designing the experiment, Funding acquisition, Supervision, Writing – review & editing; Dibyendu Kamilya: Designing the experiment, Writing – review & editing; Arambam Ashwini Devi: Investigation, Validation; Visualization; Shongsir Joy Monsang: Involved in the acquisition and analysis of data; Gaurav Rathore: Funding acquisition, Supervision, Writing – review & editing; W. Malemnganbi Devi: Investigation, Validation; Visualization; Monalisha Kumar: Investigation, Validation; Visualization.
Corresponding author
Ethics declarations
Ethical approval
All experiments involving fish were performed in accordance with the standard guidelines and policies suggested by the Institutional Animal Ethics Committee (IAEC), College of Fisheries, Central Agricultural University, Imphal, Tripura, India (CAU-CF/48/1AEC/2018/02 dated 02/02/2022).
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Daniel Merrifield
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barui, K., Choudhury, T.G., Kamilya, D. et al. Paraprobiotic supplementation to fish feed: effects on the immune support system and control of Aeromonas hydrophila infection in Labeo rohita. Aquacult Int 32, 4225–4248 (2024). https://doi.org/10.1007/s10499-023-01372-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01372-5