Abstract
Aquaculture is considered one of the fastest-growing food production sectors, which provides about 41% of the world’s fish food. Different types of pathogens, including bacteria, viruses, amoebas, oomycetes, fungi, and ectoparasites, have emerged to cause serious fish diseases. Medicines such as antibiotics and chemicals as well as vaccines have been widely used to combat fish diseases. However, their undesirable use to treat fish in aquaculture develops drug resistance in the surrounding microbes, especially antibiotic resistance in fish-associated bacterial strains in the column water and sediment, is mostly the consequence of applying antibiotics in aquaculture. The rise of antibiotic resistance in aquaculture and multidrug-resistant pathogens in the food chain threatens public health, hampering infection treatment and compromising food safety. Addressing this challenge is vital to protect both public well-being and the aquaculture industry’s future. Basic control measures for sustainable, disease-free aquaculture, including alteration of antibiotic resistance genes into other animal pathogens, have been driven by the potential development of antibiotics with respect to environmental impact and consumer safety. Anyhow, vaccination is an effective control measure that overcomes drug resistance conditions in aquaculture; even some therapeutic and prophylactic methods are being simultaneously applied for the treatment of fish diseases. Mostly, antibiotics and probiotics do not show strong effects after the development of new mutant strains and drug-resistant pathogens. Therefore, various types of vaccines have been developed to treat aquatic pathogens effectively by using advanced molecular techniques. Previous studies on fish vaccines have typically lacked comprehensive assessments of vaccine efficacy across varying environments and species, often focusing on single pathogens while neglecting the potential influence of pathogen interactions within complex aquatic ecosystems. This review focuses on advancements in technology and prospects of vaccines used in the aquaculture industries for reducing antibiotics usage. It also discussed the vaccination from traditional to modern new-generation ones including DNA, monovalent, and polyvalent vaccines. The objective is to provide an overview of aquaculture’s major bacterial pathogens and how vaccines can substitute antibiotics to protect aquatic animals from various infectious diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the world’s population is growing, the need for food has been enhanced while the major development of traditional land-food production systems became constrained by limited land availability that created food shortages for human beings. Therefore, seafood is viewed as a better source of food to participate in the feeding of the fast-rising human population (Khan et al. 2011). Aquaculture is one of the fastest-growing sectors in food-producing industries as its production has surpassed that of wild catch fisheries (FAO 2017). In the last two decades between 2002 and 2022, world fisheries and aquaculture production increased by 41%, generating 184.6 million metric tons in 2022, which was higher than the reported record of 178.1 million tons in 2021. This marks a 52 million tons increase comparatively with 2002. Finfish including freshwater, diadromous, and marine fish accounted for 76% of the total catch in 2022, however, which was slightly decreased as reported 79% in 2000. Recently in 2022, marine fish produced included 39% of all species followed by freshwater fish (33%), mollusks (13%), and crustaceans (9%) (World Food and Agriculture – Statistical Yearbook 2021).
In aquaculture, production losses are crucial due to disease outbreaks, consequently influencing food insecurity and farmers’ livelihoods harmfully. According to the report of FAO (2022), the consequences of diseases affect poor nations severely, causing about half of all productivity losses, which accounts for 90% of all types of aquaculture. Disease-related income losses might reach $6 billion each year. For instance, the cost of infectious salmon anemia alone in Chile is $2 billion, which resulted in the layoff of 20,000 people (Lozano et al. 2018). Diseases have cost China, one of the world’s largest aquaculture producers, 15% of total fish production loss (Leung and Bates 2012). Various bacteria, viruses, parasites, and fungi constitute the prevalent pathogen disease agents in aquaculture (Dhar et al. 2014). The lack of effective treatment modules for bacterial infections created an urgent need for discovering and implementing effective ways of disease prevention and control (Wilhelm et al. 2006).
Commercial variables influence aquaculture, and stocking densities and rearing conditions are changed to maximize returns within reasonable risk bounds. It is essential to eradicate highly specific pathogens from the stock and to minimize the bacterial burden in the event of opportunistic diseases such as vibrios or motile aeromonads. Antibiotic usage in aquaculture has been a concern for decades. The House of Lords in the UK published “Swann Report” in 1969, which described the unnecessary use of antibiotics in aquaculture and highlighted its possible risks to the health of both animals and consumers. In addition, the resulting data collected from research in Europe and North America recommends that the use of antibiotics should be limited and regulated to certain therapeutic conditions (Aminov 2010).
Antibiotic resistance is a global public health problem. Because of antibiotic resistance and residual difficulties, the use of antibiotics is strictly controlled and regulated. Whenever an antibiotic is administered, regardless of the situation, then the development of resistant bacterial strains may develop. Therefore, it is critical to use antibiotics with high-level caution (Hoelzer et al. 2017).
Generally, disease resistance can be strengthened by employing antibacterial drugs extensively to stimulate the innate immune defense, or using particular vaccinations when specific vaccines are available. Vaccines are employed prophylactically in a high-risk or diseased situation, while they are used therapeutically when there is a disease outbreak in the system (Reinertsen and Haaland 1995). It is recommended to use specific vaccines according to the “vaccination strategy” for particular diseases to vaccinate, as well as vaccination technique, vaccine type, vaccination schedule, and usage of revaccination. Vaccination is even a cost-effective technique; however, it is getting a higher significant aspect of the treatment management of several hazardous disease conditions in aquaculture productions. Vaccination is an advantageous procedure against various diseases, but still, it has many negative impacts. Anyhow, infectious diseases required the strategic development of vaccine design due to the uncontrolled use of antibiotics in aquaculture, which can lead to an increase in problems like bacterial resistance, environmental challenges, and food safety hazards (Cabello et al. 2016). As a result, fish vaccination has become one of the most significant, simple, and successful methods of preventing and controlling bacterial diseases in fish (Sudheesh and Cain 2017). Several notable advancements have been achieved in the development of viable fish vaccines. However, just a few vaccinations against infectious bacterial illnesses for fish are currently commercially available (Dadar et al. 2017).
For commercial development and use of vaccines, it must consider applications of technique and protocols carefully that can be incorporated into the regular species-specific productions, which are relevant to the ecology and epidemiology of the particular disease, i.e., the geographic range of the disease, seasonal occurrence, and host are an essential consideration for vaccine development and commercialization (Toranzo et al. 2009). The production and development of vaccines have played an essential role in the management of infectious diseases in aquaculture to decrease antibiotic usage in fish. Vaccines are usually more acceptable to treat animals than antibiotics because of their high anticipation against drug resistance, and the protective impact of herd immunity extends to some proportion of unvaccinated animals (Assefa and Abunna 2018). In addition to implementing various techniques in aquaculture, illness surveillance and having sensitive and precise diagnostic tests are essential for ensuring fish health. Even a single strategy for aquaculture health prevention and control is ineffective. How vaccination imparts long-lasting positive effects on fish as compared to antibiotics can be a revelation for understanding the use of bacterial vaccines in fisheries. Therefore, it is important to establish a platform for information exchange on a national or regional level between farmers and responsible parties. This review will go through the disadvantages of antibiotics and will address many forms and applications of bacterial vaccines, which are widely used as a treatment in fish farming. We will discuss the use of conventional aquaculture vaccinations and their applications and will overview the most active molecular methods of novel vaccine development for aquaculture productions.
Globally, there has been an increase in multidrug resistance, which is a hazard to public health. Recent studies have revealed the rise of bacterial infections that are multidrug-resistant and come from various sources, increasing the need for effective antibiotic usage. Due to rapid use of antibiotics, antimicrobial susceptibility testing identified and screened newly emerging MDR strains (Raharjo et al. 2023; Algammal et al. 2023; Algammal et al. (2022a, b). Crucial public health consequences result from the rise of antibiotic-resistant bacterial infections in fish. It has an impact on food safety, the spread of antibiotic resistance, aquaculture, the possibility for zoonotic disease, ecosystems, treatment options, and international trade, underscoring the need for a comprehensive One Health strategy to address this problem. To reduce the hazards associated with MDR bacterial infections in fish and protect public health, developing new, powerful, and secure antimicrobial drugs is essential (Irshath et al. 2023; Pepi and Focardi 2021; Heuer et al. 2009). Anyhow, vaccines prevent and control diseases, reduce symptoms and productivity losses from infections, and occasionally assist in disease control (Roeder 2011). Vaccines can potentially decrease antimicrobial resistance by preventing infections and reducing the need for antibiotics. They also enable the use of targeted antibiotics and reduce disease burdens in communities through herd immunity (Lipsitch and Siber 2016). The distinct spectrum of antibiotics versus vaccines complicates efforts to reduce antibiotic use. Antibiotics are often used broadly in animal production based on clinical signs, while vaccines target specific infections or pathogen strains with a limited spectrum (Hoelzer et al. 2018).
Antibiotic use and limitations
To combat disease outbreaks in aquaculture, the best course of action is prevention. It is common practice to offer antibiotic-medicated food to diseased fish to reduce bacterial infections (Cabello 2006). However, this is generally pricey and useless because unwell fish may continue to be off-feed. Furthermore, excessive chemical use against microbes has led to drug resistance, posing significant challenges to public health and national security. It has also been reported that antibiotic resistance enables bacteria to withstand high antibiotic concentrations, giving a selective advantage to resistant community members. Resistant strains outcompete susceptible ones. A concerning issue is that antibiotics in aquaculture overlap with those used in human medicine, promoting resistance to these drugs (Pepi and Focardi 2021). However, due to the various impacts of antibiotics including short-period protection, repeated treatments during outbreaks of diseases, challenges of resistant strains, and the rise of harmful residues in carcasses, it is recommended not to depend on antibiotics as preventative and treatment measures in fish (Miranda and Zemelman 2002). The introduction and extensive regular use of an anti-Aeromonas salmonicida vaccination resulted in a significant reduction in antibiotic use in the farmed salmon culture (Morrison and Saksida 2013). Therefore, several studies have reported and suggested that using different bacterial and viral vaccinations in animal populations could result in a significant reduction in antibiotic usage (Murphy et al. 2017). Anyhow, due to the lack of significant research work, clearly recommending the use of vaccines in place of antibiotics has not been properly reported.
Antibiotic resistance in aquaculture
Evaluating the impact of environmental changes on aquaculture sustainability is essential. The multi-antibiotic resistance index (MAR) metrics of aquaculture resemble human clinical bacteria MAR indices. As temperature rises in aquaculture, the prevalence of emerging infectious diseases (EIDs) is expected to rise (Reverter et al. 2020). Hence, antibiotics are often used in fish farming to prevent infectious diseases that threaten aquaculture outputs (Marcos-López et al. 2010). The current extensive use of medications against microorganism in aquaculture and terrestrial contamination of streams promotes the diversity, evolution, and propagation of antibiotic-resistant bacteria of public health significance (Schar et al. 2018).
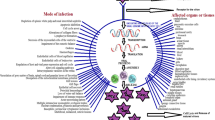
Anyhow, it is important to know that the calculated MAR indices derived from aquaculture may be limited due to inconsistent reporting of antimicrobial resistance across nations. However, the MAR index of aquaculture was calculated from the data of 40 different countries, which represented 93% of aquaculture production worldwide. Twenty-eight of the forty nations had investigated MAR indices greater than 0.2, which experts believed to have direct high-risk antibiotic contamination (Krumperman 1983). The worldwide MAR index of aquaculture-related bacteria was 0.25 (standard deviation = 0.01). Zambia (0.56), Mexico (0.55), and Tunisia (0.53) had the highest MAR values, while Canada (0.02), France (0.03), and the USA (0.08) had the least (Fig. 1) (Reverter et al. 2020).
The worldwide multi-antibiotic resistance (MAR) index is calculated using aquaculture microorganisms. Countries with insufficient data and no established MAR index are represented in white (Reverter et al. 2020)
The presence of antibiotic residues in aquatic habitats can promote the growth of resistant bacteria, promoting the spread of antibiotic resistance. This effect occurs even at lower concentrations than the MIC for routinely detected bacterial strains (Bengtsson-Palme and Larsson 2016). Antibiotic-resistant bacterial strains have been found in high concentrations around aquaculture sites where extensive antibiotics were used. This finding highlights the possibility of modified antibiotics exerting significant selective influence in aquaculture settings, enhancing antibiotic resistance among surrounding environmental bacteria (Zhu et al. 2017). Approximately 90% of aquatic bacteria found in natural environments are resistant to at least one antibiotic, with almost 20% resistant to multiple medicines. Multi-resistant bacteria can emerge when several antibiotics are used in aquaculture at the same time. Bacteria with genes encoding new antibiotic resistance pathways were also found (Lin et al. 2015). Resistance to antibiotics permits microorganisms to withstand high antibiotic concentrations, giving members of resistant colonies a selection advantage. Resistant bacterial strains outnumber those that are vulnerable. Some antibiotics used in aquaculture overlap with those used in human medical treatments, resulting in the development of antibiotic resistance (Romero et al. 2012). Overall, these data highlight the importance of immediate responsible measures at national and worldwide levels to reduce antibiotic usage and the global spread of MAR (Interagency Coordination Group on Antimicrobial Resistance (IACG) 2019).
Epigenetic modifications
The collection of molecular alterations known as the epigenome coordinates with the genome and controls how genes are expressed. Epigenetic alterations last longer and manifest symptoms earlier in contrast to DNA mutations. Aside from the programmed epigenetic regulation that supports cellular proliferation, epigenetic regulation may be altered by the environment, highlighting the need of studying epigenetic processes in detail (Sarropoulou et al. 2018). Epigenetics participates in the development of drug resistance in microorganisms especially influencing antibiotic resistance in bacteria (Ghosh et al. 2020). Antibiotic resistance driven by epigenetic inheritance in bacteria may be a process that drives evolution (Adam et al. 2008). In response to challenging environmental conditions, reversible epigenetic modifications can provide gene expression with a flexible and adaptable phenotype (Herrel et al. 2020), as well as boost the capacity for organisms to adapt to environmental change (O’Dea et al. 2016).
DNA methylation is an epigenetic remodeling method where methyl groups became detached from S-adenosyl methionine and makes a covalent bond to cytosine, and to guanosine, inside a dinucleotide CpG site (Moore et al. 2013). It has been demonstrated that epigenetic pathways are crucial for bacterial and archaeal resistance to heat stress (Blum and Payne 2019), having significant implications for microbial activity in the world dealing with climate change (McCaw et al. 2020). Recent research suggests that epigenetics play a crucial role in the development of drug resistance in bacteria. In the bacterial genome, the methylation of adenines and cytosines can alter mutation rates, regulating antibiotic sensitivity. Epigenetic processes reveal new roles in antibiotic resistance (Quandt et al. 2013). Some researchers are beginning to point to an epigenetic involvement with major implications for microbial activities in a warmer climate.
Epigenetic modifications in fish have the potential to impact antibiotic resistance by affecting different aspects of the fish’s microbiome and bacterial physiology. The relationship between epigenetic alterations in fish and antibiotic resistance constitutes an evolving area of investigation that provides insights into the intricate mechanisms responsible for the development of antibiotic resistance in fish populations. A comprehensive understanding of these mechanisms is vital for developing effective strategies to manage and mitigate antibiotic resistance in both aquaculture and wild fish populations (Consuegra et al. 2023; Pepi and Focardi 2021; Algammal et al. 2022a, b). Anyway, how epigenetic modifications can contribute to antibiotic resistance in fish is discussed below:
Genetic regulation
Epigenetic modifications possess the capacity to influence the expression of genes associated with antibiotic resistance. For instance, they can facilitate the overexpression of genes responsible for drug efflux pumps, which actively remove antibiotics from within the cells, thereby reducing the fish’s susceptibility to these drugs. Additionally, epigenetic changes can inhibit the expression of genes responsible for drug targets, ultimately diminishing the efficacy of antibiotics (Pepi and Focardi 2021; Wikumpriya et al. 2023).
Inheritance
Epigenetic modifications can be inherited from one generation to the next in fish. Consequently, if fish encounter antibiotics and undergo epigenetic changes that confer antibiotic resistance in their DNA, these changes can be passed down to their offspring. Over time, this can result in a fish population with a heightened prevalence of epigenetic modifications linked to antibiotic resistance (Liu et al. 2022).
Adaptive responses
Fish can exhibit epigenetic alterations as an adaptive response to antibiotic exposure. When exposed to antibiotics, fish with specific epigenetic changes that enhance their resistance to these drugs may gain a survival advantage. This can lead to the selection of fish carrying these advantageous epigenetic modifications within the population (Pepi and Focardi 2021).
Epigenetic flexibility
Fish possess the ability to display epigenetic plasticity, enabling them to adapt their epigenetic modifications in response to changing environmental conditions. If fish encounter antibiotics on a recurring basis, they may develop epigenetic modifications that confer antibiotic resistance as a protective mechanism (Moghadam et al. 2015).
Transgenerational impacts
Epigenetic modifications in fish can extend their effects across generations. Even if the initial exposure to antibiotics occurs in one generation, the resulting epigenetic changes can persist and affect subsequent generations. This cumulative effect can lead to an increasing prevalence of antibiotic resistance within fish populations over time (Li et al. 2022).
Biofilm formation
Epigenetic modifications may also influence the ability of bacteria to form biofilms. Biofilms are protective structures that bacteria can create on various surfaces, including fish tissues. These biofilms provide a physical barrier that shields bacteria from the impact of antibiotics. Epigenetic changes affecting bacterial genes involved in biofilm formation could promote antibiotic resistance by enhancing biofilm formation (Herrera et al. 2006; Shikongo-Nambabi et al. 2010).
Immune system function
Epigenetic changes in the fish’s immune system genes could impact the fish’s ability to mount an effective immune response against antibiotic-resistant bacteria. If epigenetic modifications reduce the fish’s immune defenses, it may become less effective at clearing bacterial infections, including those caused by antibiotic-resistant strains (Consuegra et al. 2023; Mukiibi et al. 2022; Bojarski et al. 2020).
Vaccination strategies
Bacterial infections are perhaps the major disease types in aquaculture that are the most concerning due to so many distinct primary and opportunistic pathogens implicated in disease outbreaks. Cultured fish are sensitive to a wide spectrum of bacterial infections at all stages of their development. Pathogens like Staphylococcus, Edwardsiella, Pseudomonas, Flavobacterium, Aeromonas, and other pathogens are mostly responsible for significant mortality and morbidity in several cultured freshwater fish species in various semi-intensive or intensive pond culture methods (Saikia et al. 2017). Bacterial infection continues to be a serious issue in rainbow trout, carp, tilapia, and catfish aquaculture (Rodger 2016). Infectious ailments continue to be the leading cause of death and morbidity in all cultured species, even though chemotherapeutic and preventive drugs have a negative impact like drug resistance and accumulation in the human body. To avoid substantial economic losses, illness outbreaks must be avoided, which necessitates the development of vaccines against key pathogens. With more strict antibiotic usage regulations and rising antibiotic resistance challenges (Mohamad et al. 2021), immunization is the most effective disease control technique. Since the first commercially approved fish vaccines were available (against enteric red mouth in 1976, followed by a vibriosis vaccine in 1977), much research has been conducted to develop vaccinations against many other major fish diseases. Recently, several potentially efficient vaccines that target various infectious agents have surfaced, providing hopeful immunization options for disease management in the aquaculture industry. Despite this, there are numerous essential infections for which no therapy or vaccination is present (Nayak 2020).
Vaccines for a wide variety of fish species are readily accessible, including Atlantic salmon, rainbow trout, sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata), tilapia (Oreochromis niloticus/mossambicus), amberjack (Seriola dumerili) and yellowtail (Seriola quinqueradiata) in Japan, catfish (Ictalurus punctatus), and Vietnamese (Pangasianodon hypophthalmus). Although live attenuated immunizations have been approved for use in catfish in the USA, most of these vaccines are based on whole-cell formulations that have been destroyed in formalin (Klesius and Pridgeon 2014). Canada has approved the use of a DNA vaccine to prevent infectious hematopoietic necrosis (IHN) in Atlantic salmon (Alonso and Leong 2013); similarly, a subunit vaccine (peptide; VP2) is used in Norway (against infectious pancreatic necrosis virus, IPNV); and a recombinant vaccine against infectious salmon anemia virus, ISAV, is used in Chile. Numerous multivalent vaccines designed specifically for Atlantic salmon are available, and micro-dose administration methods are being employed more frequently (i.e., 50 μl versus 100 μl). There are still few possibilities for vaccines that especially target carp and tilapia despite being well established as farmed species. The number of marketed vaccines for trout has also decreased after the monovalent furunculosis vaccine was taken off the market.
Fish species, immune system status, production cycle, life history, when disease occurs, farming technology (handling, mechanization, etc.), environment (e.g., temperature, salinity), stress factors, nutrition, and cost benefits are all significant considerations for the use of commercial vaccines in fish. The responsible use of medicines in agriculture alliance provides guidelines for the use of fish vaccinations. The bulk of commercial vaccinations contain adjuvants and are delivered intravenously (Adams and Subasinghe 2019).
Types of fish bacterial vaccines
There are many different kinds of vaccines available today, including subunit, DNA, synthetic peptide, recombinant vector, killed, attenuated, and DNA vaccines. Whole organism vaccines performed better than other forms of immunizations. However, the majority of immunizations do not entirely prevent infections (Dadar et al. 2016). Most traditional vaccinations include antigens that are so weak that they do not produce protection in the recipient. Furthermore, it may be difficult for developers to prevent emerging pathogens, the presence of antigenic shift and antigenic drift during host immune evasion by pathogenic organisms, besides microbes that cannot be grown in vitro, and the development of these vaccines is a slow and time-consuming process, which often poses difficulties in timely countering of starting to emerge and reemerging pathogens. As a result, enhanced vaccine design methodologies are being created to uncover additional forms of successful vaccinations (Gudding 2014).
Conventional fish vaccines
Traditionally, fish vaccines were made up of inactivated entire organisms (e.g., bacteria or viruses) with or without adjuvant (Tafalla et al. 2014), although there are now a variety of live attenuated or subunit protein vaccines available, frequently made with adjuvants. Recently, the majority of licensed vaccinations used in aquaculture were created using conventional methods and ideas that were comparable to those that Jenner and Pasteur first put out more than a century ago (Ulmer et al. 2012). In the 1990s, some modified live vaccinations were developed and commercialized for use in aquaculture. These vaccinations were effective, and their usage led to increasing commercial aquaculture output, while reducing the need for chemical therapies and feed-delivered antibiotics (Gudding and Muiswinkel 2013).
Components in preparation are shown on the left, production processes are shown in the middle, and administration channels are shown on the right. Injection-based immunization is shown by the fish receiving injections, while oral administration is represented by the fish-eating feed pellets. The arrangement of the fish in the aquarium serves as an example of immersion immunization (Fig. 2).
Various methods for developing fish vaccines (Ma et al. 2019a)
Inactivated/killed vaccine
The inactivated or killed vaccines are mostly made from virulent disease-causing bacterium by modifying to loss its characteristics of causing infection or reproduce. These modifications can be brought by using chemical, radiation, or physical methods in such a way that the microbial agents do not lose their antigenicity (Tlaxca et al. 2015). So far, the majority of bacterial vaccines used in aquaculture are inactivated vaccines generated from a broth culture of a particular strain(s) and then formalin-inactivated. Bacterins that contain both bacterial cells and extracellular products produce better outcomes. Some bacterial vaccines exhibit these characteristics as well, albeit not all immunizations reach appropriate protection levels when given as aqueous formulations by injection or immersion, such as those designed for salmonids against the Aeromonas salmonicida subspecies of salmonicida. Only an injectable vaccination with bacteria that has been given an oil adjuvant may achieve these levels (Toranzo et al. 2009).
Initially, killed vaccines were administered in vaccination of aquaculture productions by various experiments. It has been reported that the first commercially approved vaccine for fish was made by modifying Yersinia ruckeri, which were administered against enteric red mouth disease (Gudding and Muiswinkel 2013). Similarly, formalin-killed immersion vaccines were developed for the treatment of salmon and trout vibriosis (caused by Vibrio spp.) The first immersion-delivered salmonid vaccines were developed using a method that also created latent bacterial infections in Atlantic salmon (Salmo salar) (Shoemaker et al. 2009). These early immersion vaccines against A. salmonicida were ineffectual, as Bricknell et al. (1997) reported on the first injection-based bacterial immunization in Atlantic salmon. Currently, large-scale aquaculture vaccines, as Bricknell et al. (1997) reported the inactive form of immersion vaccines against A. salmonicids, which were administered for vaccination of Atlantic salmon.
Recently, enterprises of large-scale commercial aquaculture, who are experts in high-value species such as Atlantic salmon, are dominating the market, and depend mostly on lethal polyvalent injectable vaccinations including adjuvants and several antigens to protect aquaculture against many diseases (Evensen 2016). In Japan and Europe, they also use killed vaccinations in rainbow trout (Oncorhynchus mykiss), amberjack (Seriola dumerili), and yellowtail (Seriola quinqueradiata) against infections of Lactococcus spp. or/and Streptococcus spp. (Sommerset et al. 2005). A killed vibriosis vaccine, coupled with Photobacterium damselae (subsp. piscicida), is also accessible and utilized in the culture of European sea bream and sea bass. Furthermore, only a bacterin (ERM vaccine) as an oral vaccination is available commercially. A modified isolated pathogen of interest to autogenous vaccines has the characteristic of site-specific response, which can regulate aquaculture production for greater versatility. These vaccines are used in collective interaction with veterinary-client-patient (Yanong 2009). Besides, the frequently dead bacterial strains modified to inactivated autogenous homologous vaccines may afford manufacturers a profitable substitute for vaccinations as commercially. These vaccines may tailor against specific diseases as well as may provide a responsive treatment for emerging diseases when commercial vaccinations may ineffective and harmful to a given operation (Adams 2019).
The development of vaccines also has been problematic against some intracellular bacterium as for Piscirickettsia salmonis (salmonid rickettsial septicemia). This bacterium causes salmonid rickettsial septicemia (SRS, piscirickettsiosis) which has been reported as the most devastating aquaculture disease in Chile. However, the inactivated form P. salmonis bacterins are accessible due to their limited effectiveness, and research into novel vaccine development based on recombinant proteins is continuing (Kuzyk et al. 2001). While the long-term efficacy of the vaccines has yet to be established, these novel techniques may offer a remedy where even inactivated bacterins are ineffective (Table 1).
Live attenuated vaccines
Aquaculture might benefit greatly from live attenuated vaccinations (Table 2). However, live vaccine causes infection during vaccination. When vaccinated fish adopt resistant strain, the antigen might spread gradually and widely across the population over time (Desbois and Monaghan 2023). Live vaccinations also have the ability to boost the immune system of cellular components. The subspecies of Pfiesteria piscicida, including Aeromonas salmonicida (A. salmonicida), Edwardsiella ictaluri (E. ictaluri), Photobacterium damselae (P. damselae), and Edwardsiella tarda (E. tarda), have been studied for research purposes in laboratories. However, for practical applications, some issues like safety, longevity in fish and the environment, a potential return to virulence, and the potential for transmission to non-target species, such as wild fish, must be considered before deploying these live attenuated strains. The only vaccine currently authorized in the USA for the prevention of ESC (enteric septicemia of catfish) is specific to the bacterium E. ictaluri, which is recommended for 9-day-old catfish (Klesius and Shoemaker 1999). These are traditional vaccinations that are used to prevent illness in food-producing animals and humans (Shoemaker and Klesius 2014). They are prepared for virulence loss without killing the organisms by numerous laboratory passages and physical and chemical attenuation. Live vaccinations have been demonstrated in laboratory trials to be efficacious in fish. They stimulate mucosal, cellular, and humoral immune responses. The weakened pathogen multiplies in the intended host without causing any clinical symptoms (Shoemaker et al. 2009).
However, attenuated live vaccines are typically seen to be safe, and various issues need to be resolved to ensure that they do not revert to their original virulent state, maintain prolonged virulence, or become virulent in people who have already had vaccinations but have compromised immune systems. This situation could affect the efficacy of live vaccines as well as the regulatory process around them, along with the possibility of contamination with harmful bacteria. Currently, there are three live modified vaccines for aquaculture that are available in the US market including E. ictaluri vaccine against enteric septicemia of catfish (ESC), Flavobacterium columnare vaccine against columnaris also for catfish, and Arthrobacter vaccine against bacterial kidney disease (BKD) for salmonids (Klesius and Pridgeon 2014). A type of live Arthrobacter vaccine, known as Renogen, is used against BKD that contains non-pathogenic soil bacteria which can also give cross-protection from infectious Renibacterium salmoninarum (R. salmoninarum) (Shoemaker et al. 2009). The USA has approved two live bacterial vaccines that were developed using a sequential passage strategy and rifampicin at enhancing dose concentrations (Shoemaker et al. 2007). This technique was used to attenuate Vibrio anguillarum (V. anguillarum) for rainbow trout vaccination. This method has also been used to reduce the strength of Flavobacterium psychrophilum (F. psychrophilum) to attenuate, proving its security and effectiveness in salmonid species. The F. psychrophilum vaccine has been studied and improved under certain conditions after its formation. It has been demonstrated to provide substantial cross-protection against various strains of F. psychrophilum. This antibiotic mutagenesis approach was also practiced against other pathogens of fish including E. tarda in Japanese flounder and channel catfish, A. hydrophila and Flavobacterium spp. in Nile tilapia (Oreochromis niloticus) or carp species and channel catfish (Ictalurus punctatus), and V. anguillarum in Japanese flounder. Various compounds, including novobiocin and acriflavine dye, have been applied to attenuate E. ictaluri, Streptococcus iniae (S. iniae), Streptococcus agalactiae (S. agalactiae), and Aeromonas hydrophila (A. hydrophila); however, these vaccines have yet to be marketed (Laith et al. 2019). The Arthrobacter vaccination for BKD is considered more beneficial because of its individuality from gram-positive R. salmoninarum live strain; however, using rather a live A. davidanieli bacterium induces immunity to R. salmoninarum by cross-protection (Salonius et al. 2005). The application of this antigenic cross-reactivity microbe as an antigen can be successful, and additional ways for attenuating fish pathogens, such as antigenic cross-reactivity bacteria, the use of phylogenetic relations, and serial passages, have been investigated (Itano et al. 2006).
In the case of bacterial strains, molecular modification of pathogens has resulted in the generation of live vaccines against certain fish ailments. Some research on the effects of genetic recombination on the surface polysaccharides of Edwardsiella spp. has proved encouraging. The inhibition of polysaccharide production by inhibiting the O-polysaccharide (OPS) gene subsequently induces immunity after exposure to the virulent wild-type bacteria in catfish. Besides, other research has also generated attenuated Streptococcus spp. by removing virulence factors such as M-like proteins, phosphoglucomutase, and polysaccharides. Mutant strains of Vibrio mimicus (V. mimicus), Francisella asiatica (F. asiatica), and Vibrio alginolyticus (V. alginolyticus) are more examples of how genetic changes can result in attenuation (Ma et al. 2019a, b). These have protected fish and proved that live vaccination technology for aquaculture needs to be improved more.
DNA vaccines
A DNA vaccine is synthesized through modifications by identification and cloning a pathogen’s protective antigen. Some pathogenic fish viruses, such as VHSV and IHNV, have defensive antibodies that target their surface glycoprotein. In eukaryotic cells, the genes for glycoproteins and regulatory sequences make it possible to synthesize DNA vaccines, whereas in bacterial culture, plasmid is produced, purified, and administered through quality assurance testing before being utilized as a vaccine. After receiving the DNA vaccination, host cells absorb the genetic material to produce the glycoprotein. This circumstance causes the antigen to be detected by the fish’s immune system. DNA vaccines have the potential to substitute traditional vaccinations in aquaculture by inducing particular immune responses that includes antibodies, cytotoxic cells, and T-helper cells after DNA vaccination. However, only one microbial gene is encoded by a particular DNA sequence; hence, there is no reoccurrence to virulence, which is an important feature in aquaculture environmental safety. Therefore, the safety of host, surrounding environment, and consumer must be resolved before the use of DNA vaccines in aquaculture operations (Toranzo et al. 2009). The effect of DNA vaccines was also investigated against R. salmoninarum which causes bacterial kidney disease in salmon and stands out among the majority of DNA vaccines created for aquaculture that have mostly been administered for Bacterial diseases. However, this vaccination was revealed to be ineffective (Dhar et al. 2014). For the Piscirickettsia salmonis infection, which fish were immunized against, a more general strategy has been tried with a comprehensive plasmid DNA expression library. The level of protection was later discovered to be quite low, despite a pathogen-specific antibody response (Miquel et al. 2003). It has been reported that hybrid striped bass is shielded against Mycobacterium marinum by a DNA vaccine that encodes the naturally produced mycobacterial antigen Ag85A (Pasnik and Smith 2005).
A high vaccine dose may be instantly related to increased disease prevention. To make immunizations more effective, however, there are various techniques besides only increasing the amount of antigen/DNA that can be used. One of these strategies is the combination of a DNA vaccine with additional plasmids that encode regulatory proteins or the insertion of molecular adjuvant-coding genes into the DNA vaccine vector. This concept remains relatively unexplored in the context of fish, although there have been preliminary discoveries. According to a study, interferon regulatory factor-1 (IRF-1) may be employed as a vaccine adjuvant in Japanese flounder. IRF-1 is linked to cytokine signaling and host responses against pathogenic pathogens (Caipang et al. 2005). To demonstrate the complexity of developing effective vaccines, it may be necessary to implement a system of vaccinology approach in light of transcriptomics, epigenetics, proteomics, and metabolomics platforms along with bioinformatics (Hagan et al. 2015). Numerous institutions have the essential resources, knowledge, and expertise to facilitate such a strategy. After the completion of whole-genome sequencing programs, researchers now have improved insights into the transcriptome and proteome response brought on by vaccination for major aquaculture fish species. The new next-generation sequencing (NGS) technology could be useful in the study and development of fish vaccines. NGS is a recent technical advancement in nucleic acid sequencing that has changed the science of genomics. NGS, which is now accessible in nearly all molecular biology laboratories, gives a massive volume of sequence data at a reasonable cost. The research on fish disease has greatly benefited from this progress. NGS is increasingly being used to track aquatic viruses and bacteria, examine the virulence and development of illnesses, and identify as-yet-unidentified causative agents that result in mortality. In theory, the comprehensive information obtained from NGS could help to accelerate vaccine development and lead to highly effective vaccines. NGS provides the opportunity to evaluate epigenetic changes following vaccination. This can shed light on the different ways that different fish (such as non-responders) and lineages react to vaccines, as well as how vaccines might cause epigenetic changes that improve gene expression. Fish DNA vaccine research is currently in its early stages. However, studies using model antigens produce very encouraging outcomes, inducing both cellular and humoral immune reactions (Avarre 2020).
Monovalent and polyvalent vaccines
Polyvalent vaccines are considered the most effective vaccines due to its active characteristic in a particular condition especially when species-specific fish becomes susceptible simultaneously to many infectious diseases (Busch 1997; Ma et al. 2010). Furthermore, polyvalent vaccines need to cover all significant serotypes of every pathogen present within a specific geographic region. Illustrative instances of the effectiveness of polyvalent vaccines can be observed in the cases of salmonids and turbot. In these examples, polyvalent vaccines have demonstrated equivalent or enhanced protective capabilities to monovalent vaccines. Unfortunately, turbot fish could not be protected against infection by the utilization of a commercially available polyvalent vaccine designed for M. viscosa and salmon as one of its five antigens and a mineral oil adjuvant. It was reported that a particular anti-M. viscosa response was not affected significantly, but the resultant weight increase in vaccinated turbot was not lowered compared to controls 7 weeks after vaccination. Although both vaccinated and unvaccinated fish had high anti-M. viscosa antibody levels 5 weeks after vaccination. Another vaccine component brought a vaccination-induced antibody response against A. salmonicida. It is important to cautiously follow the procedure of polyvalent vaccines for vaccinations due to the possibility of antigen competition, particularly when providing these vaccines via injection. While monovalent vaccines stimulated effective protective immunity, polyvalent vaccines prompted stronger protection than monovalent vaccines, possibly due to more powerful and effective systemic immune responses elicited by multivalent vaccines (Björnsdóttir et al. 2004).
It is widely acknowledged that fish have a less broad and effective immune system than mammals (Klesius et al. 2004). On the other hand, monovalent vaccines generally have efficacy as polyvalent vaccines. However, as compared to monovalent furunculosis vaccines that only target furunculosis, vaccine formulations containing antigens from A. salmonicida, V. salmonicida, and V. anguillarum have high efficiency against furunculosis. It might be the result of immunological interactions between A. salmonicida and V. salmonicida (Hoel et al. 1997). Additionally, cross-protection between antigens of atypical furunculosis and furunculosis-causing bacteria has been reported. The vaccination against A. salmonicida subsp. salmonicida provided a cure for atypical furunculosis but did not treat classical furunculosis, indicating that the cross-protection appears to be unidirectional (Björnsdóttir et al. 2005).
Due to the random interactions between adjuvant and antigen, it is impossible to synthesize or develop a vaccine that gives protection against every infectious virus that a certain fish may come in contact with. Administrators of salmon farms must identify which infectious diseases are crucial and vaccinate their livestock accordingly (Table 3). Unfortunately, determining the immunological effect of synchronized vaccination is also difficult. It was proved that simultaneous vaccination of a polyvalent oil-AV and a DV enhances specific antibody formation; however, the particular anti-viral response may be disturbed (Skinner et al. 2010).
The majority of rainbow trout are protected against well-known bacterial infections including Aeromonas salmonicida subsp. salmonicida, Yersinia ruckeri, and Vibrio anguillarum by widely used vaccinations, but disease outbreaks still occur. Under experimental conditions, administration of the multicomponent vaccination resulted in protection against three specific bacterial diseases (yersiniosis, vibriosis, and furunculosis). It has been discovered that pentavalent vaccine IP infusion promotes particular antibody responses in trout against different bacterial antigens and controlled gene expression (Marana et al. 2019).
Nanovaccines
Nanovaccines for fish have emerged as a cutting-edge solution in aquaculture, revolutionizing disease prevention and health management strategies for aquatic species. These innovative vaccines utilize nanotechnology to optimize the delivery of antigens and adjuvants, enhancing the immune response in fish (Vinay et al. 2016). By employing nanoparticles like liposomes, polymeric nanoparticles, or virus-like particles (VLPs) as carriers, nanovaccines mimic pathogen structures, ensuring prolonged antigen exposure and promoting robust antibody production and cellular immunity (Celis-Giraldo et al. 2021; Jeong et al. 2020). Importantly, these vaccines reduce the stress associated with conventional vaccination methods, including handling and injection, thus improving the overall welfare of farmed fish. Additionally, nanovaccines can be tailored to target specific pathogens of concern in aquaculture, minimizing non-target effects. This precision is particularly significant in reducing antibiotic use, supporting environmentally sustainable practices, and mitigating antibiotic resistance challenges (Thompson et al. 2023; Mondal and Thomas 2022). Furthermore, as fish farmers increasingly recognize the economic benefits of disease prevention through nanovaccines, ongoing research and development efforts aim to refine formulations and address aquaculture-specific challenges (Du et al. 2022; Nasr-Eldahan et al. 2021; Fajardo et al. 2022). Nanovaccines for fish promise to revolutionize disease control in aquaculture and align with the growing emphasis on sustainable and responsible farming practices, promoting the health and productivity of aquatic populations.
Challenges of vaccinations in aquaculture
A vaccination strategy is an essential factor for overall fish health management. It is the most successful safety strategy for preventing the transmission of fish diseases by inducing defense mechanisms against bacterial disease outbreaks. As a result, a detailed understanding of diseases and pathogen profiles, as well as a basic economic background of operational costs, is required to design appropriate vaccination strategies (Mohd-Aris et al. 2019). Immunoprophylaxis is usually beneficial because it reduces disease-related economic losses. The same concept is true for vaccination usage in aquaculture, as it can also be utilized as a vaccine in the domain of aquaculture. However, with antibiotics and other chemical agents to treat disease outbreaks in fish farms, the development of the immune system along with other desirable advantages can also be achieved. Antibiotic use in aquaculture causes the development of antimicrobial resistance in both pathogenic and non-pathogenic microorganisms, potentially providing a threat to the environment, as well as the well-being of humans and animals (Mamun et al. 2019). As a result, long-term antibiotic use in commercial bioproduction, such as fish culture, is not appropriate.
Following the adoption of vaccination as a common technique for managing bacterial infections in farmed fish, antibiotic use has reduced dramatically, from 47 tons to around 1 ton of active substances in Norway (Gudding et al. 1999). During the rearing process, vaccinations are performed in many fish species against different diseases, as rainbow trout and salmon vaccinate against three to five diseases. As a result, production has increased dramatically over the last half-decade, an achievement that would have been impossible without the administration of such efficient vaccinations. Vaccines have proven to be a reliable and low-cost way to treat and prevent infectious fish diseases in aquaculture. Anyhow, vaccination has shown a significant reduction in losses associated with specific diseases, resulting in a decrease in antibiotic use. As demonstrated by the collaboration between immunologists and vaccinologists, it is critical to increase collaboration between basic and applied sciences to advance in the field of fish vaccinology. Understanding the immune systems of various fish species is crucial, with consideration of cellular and mucosal immunity.
Live vaccines have proven highly effective in defeating various diseases, including rainbow trout fry syndrome (RTFS), columnaris disease, Streptococcus infections, dropsy, bacterial kidney disease, fish septicemia, and ulcers. It is imperative to promote research aimed at developing secure live vaccines to enhance disease prevention. When it comes to DNA bacterial vaccines, they have yet to be studied further from the standpoint of bacterial disease protection. Compared to vaccines used in higher vertebrates, most fish vaccines require a higher antigen mass. However, the current availability of commercial vaccines remains limited due to the issues such as efficacy challenges and the complexities of producing substantial antigen quantities. These vaccines are essential for inducing a robust immune response, both at the local and systemic levels. Ensuring their protection from degradation until they reach the immune induction site is also crucial (Mutoloki et al. 2015).
Overall, bacterial vaccines designed for the aquaculture sector have exhibited superior accomplishments compared to their viral counterparts, a fact supported by the observable disparity in the quantity of commercially available salmonid vaccines within Norway (PHARMAQ AS. PHARMAQ – Products 2015). The aquaculture sector places considerable emphasis on extracellular bacteria due to the nature of viruses as viruses primarily infect host cells from the insides. However, there are a few exceptions to this such as Piscirickettsia salmonis, which is activated intracellularly and presents challenges in the development of vaccines (Sommerset et al. 2005). A powerful immune response to extracellular bacterial infections is mostly determined by humoral factors. Therefore, creating vaccines that elicit antibody responses to ensure the protection of aquatic creatures is challenging progress. Conversely, targeting intracellular pathogens needs a synergistic effort of diverse immune responses, which includes humoral, cytotoxic, and cell-mediated reactions. Designing a vaccine capable of eliciting such a comprehensive range of responses presents a challenging task. In contrast, bacterial vaccines possess certain advantages that contribute to their relatively simpler development compared to viral vaccines. Factors such as their larger size and the specific antigens/immunogens they comprise contribute to these differences (Villumsen et al. 2014). It is recommended to employ a strategy based on globally recognized and accepted principles for preventing, managing, and resolving issues in aquaculture while being tailored to local circumstances.
Conclusion
Vaccines have been routinely used as low-cost preventative strategies against contagious diseases in humans. For the production of human vaccines, numerous cutting-edge immunological methods, including genetic engineering and nanotechnology, have been used. However, due to several scientific, financial, and commercialization barriers, these advanced technologies could not be utilized in aquaculture. In aquaculture, commercial approval for vaccines remains limited, especially for precious species including salmonids, yellowtail, sea bream, sea bass, and catfish. Despite the challenges of commercialization, there has been a significant upswing in research dedicated to aquaculture vaccinology in recent years, resulting in the development of numerous effective immunization regimens. However, fish vaccinations may face limitations related to difficulty in administering vaccines to certain species, varying immune responses among different fish species, precise timing and environmental conditions, and achieving long-term protection against certain rapidly mutating pathogens. The innovative technique aided in the development of perfect vaccinations with precise biological and physical properties. The ideal fish vaccine ensures the safety of both the fish and the environment, proves economically viable for extensive production, offers straightforward administration, prompts robust immunity during periods of heightened vulnerability, and results in minimal adverse effects. Implementing vaccination strategies within aquaculture settings reduces the necessity for antibiotics and effectively shields fish populations against contagious illnesses, thereby diminishing the potential for the emergence of drug resistance. To prevent bacterial diseases in fish, various vaccines for freshwater and marine fish species have been formulated using advanced biotechnology techniques like next-generation sequencing, formulation of DNA vaccines, and live attenuated and killed vaccines, which are currently accessible in the commercial market. All of this progress in vaccine research is based on biotechnology-based techniques; however, it is always funding-dependent. Furthermore, government and stakeholder parties must need to resolve major aquaculture issues like infectious diseases by administration of the latest technologies. Therefore, there is a need for funding for sustainable aquaculture initiatives to identify the fundamental disease epidemiology and the immune systems across various farmed species, which can support and allow the development of relevant vaccines for practical applications. To deal with the rising number of infectious illnesses in aquaculture, vaccine research must expand. As a result, biotechnology offers a wide range of uses in aquaculture disease management. Novel views and methods for research could involve the exploration of bacterial vaccines, particularly those sourced from marine microorganisms, as potential alternatives within aquaculture, diverging from the conventional practice of employing the same antibiotics employed in human treatments. Regarding recommendations, it is crucial to decrease antibiotic utilization in aquaculture, elevate the standard of fish farm maintenance, and elevate facility hygiene standards. Furthermore, the effectiveness of fish vaccines in various settings and species should be evaluated in-depth in future studies, taking into account the intricate relationships between numerous infections. To further improve protection in aquaculture, research into novel vaccine formulations that take into account the multifactorial problems in aquatic habitats is also crucial.
References
Adam M, Murali B, Glenn NO, Potter SS (2008) Epigenetic inheritance-based evolution of antibiotic resistance in bacteria. BMC Evol Biol 8(52). https://doi.org/10.1186/1471-2148-8-52
Adams A (2019) Progress, challenges, and opportunities in fish vaccine development. Fish Shellfish Immunol 90:210–214. https://doi.org/10.1016/j.fsi.2019.04.066
Adams A, Subasinghe R (2019) Use of fish vaccines in aquaculture (including methods of administration), Veterinary Vaccines for Livestock, published by The Food and Agriculture Organization of the United Nations.
Algammal A, Hetta HF, Mabrok M, Behzadi P (2023) Editorial: Emerging multidrug-resistant bacterial pathogens “superbugs”: a rising public health threat. Front Microbiol 14. https://doi.org/10.3389/fmicb.2023.1135614
Algammal AM, Alfifi KJ, Mabrok M, Alatawy M, Abdel-Moneam DA, Alghamdi S, Azab MM, Ibrahim RA, Hetta HF, El-Tarabili RM (2022a) Newly emerging MDR B. cereus in Mugil seheli as the first report commonly harbor nhe, hbl, cytK, and pc-plc virulence genes and bla1, bla2, tetA, and ermA resistance genes. Infect Drug Resist 15:2167–2185. https://doi.org/10.2147/IDR.S365254
Algammal AM, Mabrok M, Ezzat M, Alfifi KJ, Esawy AM, Elmasry N, El-Tarabili RM (2022b) Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture 548:737643
Alonso M, Leong JAC (2013) Licensed DNA vaccines against infectious hematopoietic necrosis virus (IHNV). Recent Pat DNA Gene Seq 7(1):62–65. https://doi.org/10.2174/1872215611307010009
Aminov RI (2010) A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1. https://doi.org/10.3389/fmicb.2010.00134
Angelidis P, Karagiannis D, Crump EM (2006). Efficacy of a Listonella anguillarum (syn. Vibrio anguillarum) vaccine for juvenile sea bass Dicentrarchus labrax. Dis Aquat Org 11(7):19-24. https://doi.org/10.3354/dao071019
Assefa A, Abunna F (2018) Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet Med Int 2018:1–10. https://doi.org/10.1155/2018/5432497
Avarre J-C (2020) Next-generation sequencing: a revolution in the field of fish diseases. Bull Eur Assoc Fish Pathol 40(2):62–69
Bengtsson-Palme J, Larsson DJ (2016) Concentrations of antibiotics predicted to select for resistant bacteria: pro-posed limits for environmental regulation. Environ Int 86:140–149. https://doi.org/10.1016/j.envint.2015.10.015
Björnsdóttir B, Gudmundsdóttir S, Bambir SH, Gudmundsdóttir BK (2005) Experimental infection of turbot, Scophthalmus maximus (L.), by Aeromonas salmonicida subsp. achromogenes and evaluation of cross protection induced by a furunculosis vaccine. J Fish Dis 28(3):181–188. https://doi.org/10.1111/j.1365-2761.2005.00617.x
Björnsdóttir B, Gudmundsdóttir S, Bambir SH, Magnadóttir B, Gudmundsdóttir BK (2004) Experimental infection of turbot, Scophthalmus maximus (L.), by Moritella viscosa, vaccination effort and vaccine-induced side-effects. J Fish Dis 27:645–655. https://doi.org/10.1111/j.1365-2761.2004.00579.x
Blum P, Payne S (2019) Evidence of an epigenetics system in archaea. Epigenetics Insights 12. https://doi.org/10.1177/2516865719865280
Bøgwald J, Dalmo RA (2019) Review on immersion vaccines for fish: an update 2019. Microorganisms 7(12):627. https://doi.org/10.3390/microorganisms7120627
Bojarski B, Kot B, Witeska M (2020) Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 13(8):189
Bricknell IR, Bowden TJ, Lomax J, Ellis AE (1997) Antibody response and protection of Atlantic salmon (Salmo salar) immunised with an extracellular polysaccharide of Aeromonas salmonicida. Fish Shellfish Immunol 7:1–16
Busch RA (1997) Polyvalent vaccines in fish: the interactive effects of multiple antigens. Dev Biol Stand Aquat Animal Health Div 90:245–256
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8(7):1137–1144
Cabello FC, Godfrey HP, Buschmann AH, Dölz HJ (2016) Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect Dis 16(7):127–133. https://doi.org/10.1016/S1473-3099(16)00100-6
Caipang CMA, Hirono I, Aoki T (2005) Induction of antiviral state in fish cells by Japanese flounder, Paralichthys olivaceus, interferon regulatory factor-1. Fish Shellfish Immunol 19:79–91
Celis-Giraldo CT, López-Abán J, Muro A, Patarroyo MA, Manzano-Román R (2021) Nanovaccines against animal pathogens: the latest findings. Vaccines 9(9):988. https://doi.org/10.3390/vaccines9090988
Consuegra S, Webster TU, Anka I (2023) Microbiome, epigenetics and fish health interactions in aquaculture. Epigenetics in Aquaculture, pp 245–262
Dadar M, Dhama K, Vakharia VN, Hoseinifar SH, Karthik K, Tiwari R, Khandia R, Munjal A, Salgado-Miranda C, Joshi SK (2016) Advances in aquaculture vaccines against fish pathogens: global status and current trends. Reviews in Fisheries Science &Amp. Aquaculture 25(3):184–217. https://doi.org/10.1080/23308249.2016.1261277
Dadar M, Dhama K, Vakharia VN, Hoseinifar SH, Karthik K, Tiwari R, Khandia R, Munjal A, Salgado-Miranda C, Joshi SK (2017) Advances in aquaculture vaccines against fish pathogens: global status and current trends. Rev Fish Sci Aquac 25(3):184–217. https://doi.org/10.1080/23308249.2016.1261277
Delghandi MR, El-Matbouli M, Menanteau-Ledouble S (2020) Renibacterium salmoninarum—the causative agent of bacterial kidney disease in salmonid fish. Pathogens 9
Desbois AP, Monaghan SJ (2023) 13 Development of fish vaccines. Health Management for Sustainable Aquaculture, Fish Vaccines, p 209
Dhar AK, Manna SK, Allnutt FCT (2014) Viral vaccines for farmed finfish. Virusdisease 25(1):1–17. https://doi.org/10.1007/s13337-013-0186-4
Du Y, Hu X, Miao L, Chen J (2022) Current status and development prospects of aquatic vaccines. Front Immunol 13:1040336. https://doi.org/10.3389/fimmu.2022.1040336
Evensen Ø (2016) Development of fish vaccines: focusing on methods. In: Fish Vaccines, pp 53–74. https://doi.org/10.1007/978-3-0348-0980-1_3
Fajardo C, Martínez-Rodríguez G, Blasco J, Mancera JM, Thomas BN, De Donato M (2022) Nanotechnology in aquaculture: applications, perspectives and regulatory challenges. Aquac Fisheries 7(2):185–200. https://doi.org/10.1016/j.aaf.2021.12.006
FAO (2017) National aquaculture overview: India. Country profile fact sheets. FAO http://www.fao.org/fishery/factsheets/en
FAO (2022) The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome, FAO. https://doi.org/10.4060/cc0461en
Galindo-Villegas J, Mulero I, García-Alcazar A, Muñoz I, Peñalver-Mellado M, Streitenberger S (2013) Recombinant TNFα as oral vaccine adjuvant protects European sea bass against vibriosis: insights into the role of the CCL25/CCR9 axis. Fish Shellfish Immunol 35(4):1260–1271. https://doi.org/10.1016/j.fsi.2013.07.046
Ghosh B, Nguyen TD, Crosbie PBB, Nowak BF, Bridle AR (2016) Oral vaccination of first-feeding Atlantic salmon, Salmo salar L., confers greater protection against yersiniosis than immersion vaccination. Vaccine 34(5):599–608. https://doi.org/10.1016/j.vaccine.2015.12.044
Ghosh D, Veeraraghavan B, Elangovan R, Perumal V (2020) Antibiotic resistance and epigenetics: more to it than meets the eye. Antimicrob Agents Chemother 64(2). https://doi.org/10.1128/AAC.02225-19
Glenney GW, Petrie-Hanson L (2006) Fate of fuorescent microspheres in developing Ictalurus punctatus following prolonged immersion. Fish Shellfish Immunol 21(1):32–41. https://doi.org/10.1016/j.fsi.2005.10.003
Gudding R (2014) Vaccination as a preventive measure. In: Fish Vaccination, pp 12–21. https://doi.org/10.1002/9781118806913.ch2
Gudding R, Lillehaug A, Evensen Ø (1999) Recent developments in fish vaccinology. Vet Immunol Immunopathol 72(1–2):203–212. https://doi.org/10.1016/s0165-2427(99)00133-6
Gudding R, Muiswinkel WBV (2013) A history of fish vaccination: science-based disease prevention in aquaculture. Fish Shellfish Immunol 35(6):1683–1688. https://doi.org/10.1016/j.fsi.2013.09.031
Hagan T, Nakaya HI, Subramaniam S, Pulendran B (2015) Systems vaccinology: enabling rational vaccine design with systems biological approaches. Vaccine 33(40):5294–5301. https://doi.org/10.1016/j.vaccine.2015.03.072
Herrel A, Joly D, Danchin E (2020) Epigenetics in ecology and evolution. Funct Ecol 34:381–384. https://doi.org/10.1111/1365-2435.13494
Herrera FC, Santos JA, Otero A, García-López ML (2006) Occurrence of foodborne pathogenic bacteria in retail prepackaged portions of marine fish in Spain. J Appl Microbiol 100(3):527–536
Heuer OE, Kruse H, Grave K, Collignon P, Karunasagar I, Angulo FJ (2009) Human health consequences of use of antimicrobial agents in aquaculture. Clin Infect Di: an official publication of the Infectious Diseases Society of America 49(8):1248–1253. https://doi.org/10.1086/605667
Hoel K, Salonius K, Lillehaug A (1997) Vibrioantigens of polyvalent vaccines enhance the humoral immune response to Aeromonas salmonicida antigens in Atlantic salmon (Salmo salar). Fish Shellfish Immunol 7(2):71–80. https://doi.org/10.1006/FSIM.1996.0063
Hoelzer K, Bielke L, Blake DP, Cox E, Cutting SM, Devriendt B, Erlacher-Vindel E, Goossens E, Karaca K, Lemiere S, Metzner M, Raicek M, Collell Suriñach M, Wong NM, Gay C, Van Immerseel F (2018) Vaccines as alternatives to antibiotics for food producing animals. Part 1: challenges and needs. Vet Res 49(1). https://doi.org/10.1186/s13567-018-0560-8
Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A (2017) Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res 13:211. https://doi.org/10.1186/s12917-017-1131-3
Interagency Coordination Group on Antimicrobial Resistance (IACG) (2019) No time to wait: securing the future from drug-resistant infections. WHO
Irshath AA, Rajan AP, Vimal S, Prabhakaran VS, Ganesan R (2023) Bacterial pathogenesis in various fish diseases: recent advances and specific challenges in vaccine development. Vaccines 11(2):470
Itano T, Kawakami H, Kono T, Sakai M (2006) Live vaccine trials against nocardiosis in yellowtail Seriola quinqueradiata. Aquaculture 261:1175–1180. https://doi.org/10.1016/j.aquaculture.2006.09.006
Jaafar RM, Al-Jubury A, Chettri JK, Dalsgaard I, Kania PW, Buchmann K (2018) Secondary immune response of rainbow trout following repeated immersion vaccination. J Fish Dis 41(1):117–123. https://doi.org/10.1111/jfd.12682
Jeong KH, Kim HJ, Kim HJ (2020) Current status and future directions of fish vaccines employing virus-like particles. Fish Shellfish Immunol 100:49–57. https://doi.org/10.1016/j.fsi.2020.02.060
Jiang X, Zhang C, Zhao Y, Kong X, Pei C, Li L, Nie G, Li X (2016) Immune effects of the vaccine of live attenuated Aeromonas hydrophila screened by rifampicin on common carp (Cyprinus carpio L). Vaccine 8(34):3087–3092. https://doi.org/10.1016/j.vaccine.2016.04.075
Khan MA, Khan S, Miyan K (2011) Aquaculture as a food production system: a review. Biol Med 3:291–302
Klesius PH, Evans JM, Shoemaker CA (2004) Warmwater fish vaccinology in catfish production. Anim Health Res Rev 5(2):305–311. https://doi.org/10.1079/ahr200489
Klesius PH, Pridgeon JW (2014) Vaccination against enteric septicemia of catfish. In: Fish Vaccination, pp 211–225. https://doi.org/10.1002/9781118806913.ch18
Klesius PH, Shoemaker CA (1999) Development and use of modified live Edwardsiella ictaluri vaccine against enteric septicemia of catfish. Adv Vet Med:523–537. https://doi.org/10.1016/s0065-3519(99)80039-1
Krumperman PH (1983) Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol 46(1):165–170. https://doi.org/10.1128/aem.46.1.165-170.1983
Kuzyk MA, Burian J, Machander D, Dolhaine D, Cameron S, Thornton JC, Kay WW (2001) An efficacious recombinant subunit vaccine against the salmonid rickettsial pathogen Piscirickettsia salmonis. Vaccine 19(17–19):2337–2344. https://doi.org/10.1016/s0264-410x(00)00524-7
Laith A, Abdullah M, Nurhafizah W, Hussein H, Aya J, Effendy A, Najiah M (2019) Efficacy of live attenuated vaccine derived from the Streptococcus agalactiae on the immune responses of Oreochromis niloticus. Fish Shellfish Immunol 90:235–243. https://doi.org/10.1016/j.fsi.2019.04.052
Leung TLF, Bates AE (2012) More rapid and severe disease outbreaks for aquaculture at the tropics: implications for food security. J Appl Ecol 50(1):215–222. https://doi.org/10.1111/1365-2644.12017
Li X, Wang M, Liu S, Chen X, Qiao Y, Yang X et al (2022) Paternal transgenerational nutritional epigenetic effect: a new insight into nutritional manipulation to reduce the use of antibiotics in animal feeding. Animal Nutrition
Lin J, Nishino K, Roberts MC, Tolmasky M, Aminov RI, Zhang L (2015) Mechanisms of antibiotic resistance. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00034
Lipsitch M, Siber GR (2016) How can vaccines contribute to solving the antimicrobial resistance problem? mBio 7(3):428–416. https://doi.org/10.1128/mBio.00428-16
Liu C, Hu X, Cao Z, Sun Y, Chen X, Zhang Z (2019) Construction and characterization of a DNA vaccine encoding the SagH against Streptococcus iniae. Fish Shellfish Immunol 89:71–75. https://doi.org/10.1016/j.fsi.2019.03.045
Liu Z, Zhou T, Gao D (2022) Genetic and epigenetic regulation of growth, reproduction, disease resistance and stress responses in aquaculture. Front Genet 13:994471
Lozano I, Díaz NF, Muñoz S, Riquelme C (2018) Antibiotics in Chilean aquaculture: a review. InTech. https://doi.org/10.5772/intechopen.71780
Ma J, Bruce TJ, Jones EM, Cain KD (2019a) A review of fish vaccine development strategies: conventional methods and modern biotechnological approaches. Microorganisms 7(11):569. https://doi.org/10.3390/microorganisms7110569
Ma R, Yang G, Xu R, Liu X, Zhang Y, Ma Y, Wang Q (2019b) Pattern analysis of conditional essentiality (PACE)-based heuristic identification of an in vivo colonization determinant as a novel target for the construction of a live attenuated vaccine against Edwardsiella piscicida. Fish Shellfish Immunol 90:65–72
Ma Y, Zhang Y, Zhao D (2010) Polyvalent attenuated live vaccine for preventing and curing vibriosis of cultivated fish. Google Patents
Mamun MAA, Nasren S, Abhiman PB, Rathore SS, Sowndarya NS, Ramesh KS, Shankar KM (2019) Investigation of production, formation and characterization of biofilm cells of aeromonas hydrophila for oral vaccination of fish. Indian J Anim Res 54(5):563–569. https://doi.org/10.18805/ijar.B-3814
Marana MH, Sepúlveda D, Chen D, Al-Jubury A, Jaafar RM, Kania PW, Henriksen NH, Krossøy B, Dalsgaard I, Lorenzen N, Buchmann K (2019) A pentavalent vaccine for rainbow trout in Danish aquaculture. Fish Shellfish Immunol 88:344–351. https://doi.org/10.1016/j.fsi.2019.03.001
Marcos-López M, Gale P, Oidtmann BC, Peeler EJ (2010) Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom. Transbound Emerg Dis 57(5):293–304. https://doi.org/10.1111/j.1865-1682.2010.01150.x
McCaw BA, Stevenson TJ, Lancaster LT (2020) Epigenetic responses to temperature and climate. Integr Comp Biol 60(6):1469–1480. https://doi.org/10.1093/icb/icaa049
Mikkelsen H, Lund V, Larsen R, Seppola M (2011) Vibriosis vaccines based on various sero-subgroups of Vibrio anguillarum O2 induce specifc protection in Atlantic cod (Gadus morhua L.) juveniles. Fish Shellfish Immunol 30(1):330–339. https://doi.org/10.1016/j.fsi.2010.11.007
Miquel A, Muller I, Ferrer P, Valenzuela PD, Burzio LO (2003) Immunoresponse of Coho salmon immunized with a gene expression library from Piscirickettsia salmonis. Biol Res 36(3-4):313–323
Miranda CD, Zemelman R (2002) Bacterial resistance to oxytetracycline in Chilean salmon farming. Aquaculture 212(1–4):31–47. https://doi.org/10.1016/s0044-8486(02)00124-2
Moghadam H, Mørkøre T, Robinson N (2015) Epigenetics—potential for programming fish for aquaculture? J Mar Sci Eng 3(2):175–192
Mohamad A, Zamri-Saad M, Amal MNA, Al-saari N, Monir MS, Chin YK, Md Yasin IS (2021) Vaccine efficacy of a newly developed feed-based whole-cell polyvalent vaccine against vibriosis, streptococcosis and motile aeromonad septicemia in Asian seabass. Lates Calcarifer Vac 9(4):368. https://doi.org/10.3390/vaccines9040368
Mohamed LA, Soliman WS (2013) Development and efficacy of fish vaccine used against some bacterial diseases in farmed Tilapia. Nat Sci 11(6):120–128
Mohd-Aris A, Muhamad-Sofie MHN, Zamri-Saad M, Daud HM, Ina-Salwany MY (2019) Live vaccines against bacterial fish diseases: a review. 12(11):1806–1815. https://doi.org/10.14202/vetworld.2019.1806-1815
Mondal H, Thomas J (2022) A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac Int: journal of the European Aquaculture Society 30(4):1971–2000. https://doi.org/10.1007/s10499-022-00884-w
Moore LD, Le TD, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacology 38(1):23–38. https://doi.org/10.1038/npp.2012.112
Morrison D, Saksida SM (2013) Trends in antimicrobial use in Marine Harvest Canada farmed salmon production in British Columbia (2003-2011). Can Vet J-Revue Veterinaire Canadienne
Mukiibi R, Peñaloza C, Gutierrez A, Yáñez JM, Houston RD, Robledo D (2022) The impact of Piscirickettsia salmonis infection on genome-wide DNA methylation profile in Atlantic Salmon. Genomics 114(6):110503
Murphy D, Ricci A, Auce Z, Beechinor JG, Bergendahl H, Breathnach R, Bureš J, Duarte Da Silva JP, Hederová J, Hekman P, Ibrahim C, Kozhuharov E, Kulcsár G, Lander Persson E, Lenhardsson JM, Mačiulskis P, Malemis I, Markus-Cizelj L, Michaelidou-Patsia A, Jukes H (2017) EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J 15(1). https://doi.org/10.2903/j.efsa.2017.4666
Mutoloki S, Munang’andu HM, Evensen Y (2015) Oral vaccination of fish – antigen preparations, uptake, and immune induction. Front Immunol 6. https://doi.org/10.3389/fimmu.2015.00519
Nasr-Eldahan S, Nabil-Adam A, Shreadah MA, Maher AM, El-Sayed Ali T (2021) A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac Int: Journal of the European Aquaculture Society 29(4):1459–1480. https://doi.org/10.1007/s10499-021-00677-7
Nayak SK (2020) Current prospects and challenges in fish vaccine development in India with special reference to Aeromonas hydrophila vaccine. Fish Shellfish Immunol 100:283–299. https://doi.org/10.1016/j.fsi.2020.01.064
O’Dea RE, Noble DWA, Johnson SL, Hesselson D, Nakagawa S (2016) The role of non-genetic inheritance in evolutionary rescue: epigenetic buffering, heritable bet hedging and epigenetic traps. Current. Zoology 2(1):dvv014. https://doi.org/10.1093/eep
Pasnik DJ, Smith SA (2005) Immunogenic and protective effects of a DNA vaccine for Mycobacterium marinum in fish. Vet Immunol Immunopathol 103(3-4):195–206
Pepi M, Focardi S (2021) Antibiotic-resistant bacteria in aquaculture and climate change: a challenge for health in the Mediterranean area. Int J Environ Res Public Health 18(11):5723. https://doi.org/10.3390/ijerph18115723
PHARMAQ AS. PHARMAQ – Products. (2015). PHARMAQ.
Quandt EM, Deatherage DE, Ellington AD, Georgiou G, Barrick JE (2013) Recursive genomewide recombination and sequencing reveals a key refinement step in the evolution of a metabolic innovation in Escherichia coli. Proc Natl Acad Sci 111(6):2217–2222. https://doi.org/10.1073/pnas.1314561111
Raharjo HM, Budiyansah H, Mursalim MF, Chokmangmeepisarn P, Sakulworakan R, Debnath PP, Elayaraja S, Intan ST, Chuanchuen R, Dong HT, Mabrok M, Rodkhum C (2023) The first evidence of blaCTX-M-55, QnrVC5, and novel insight into the genome of MDR Vibrio vulnificus isolated from Asian sea bass (Lates calcarifer) identified by resistome analysis. Aquaculture 571:739500. https://doi.org/10.1016/j.aquaculture.2023.739500
Reinertsen H, Haaland H (1995) Sustainable fish farming: proceedings of the First International Symposium on Sustainable Fish Farming, Oslo, Norway, 28-31 August, 1994. Biology, Engineering
Reverter M, Sarter S, Caruso D, Avarre JC, Combe M, Pepey E, Pouyaud L, Vega-Heredía S, De Verdal H, Gozlan RE (2020) Aquaculture at the crossroads of global warming and antimicrobial resistance. Nature. Communications 11(1). https://doi.org/10.1038/s41467-020-15735-6
Rodger HD (2016) Fish disease causing economic impact in global aquaculture. In: BirkhäUser Advances in Infectious Diseases, pp 1–34. https://doi.org/10.1007/978-3-0348-0980-1_1
Roeder PL (2011) Rinderpest: the end of cattle plague. Prev Vet Med 102(2):98–106. https://doi.org/10.1016/j.prevetmed.2011.04.004
Romero J, Gloria C, Navarrete P (2012) Antibiotics in aquaculture – use, abuse and alternatives. Health Environ Aqua. https://doi.org/10.5772/28157
Saikia DJ, Chattopadhyay P, Banerjee G, Sarma D (2017) Time and dose dependent effect of Pseudomonas aeruginosa infection on the scales of Channa punctata (Bloch) through light and electron microscopy. Turkish J Fisher AquaSci 17:871–876
Salonius K, Siderakis C, MacKinnon AM, Griffiths S (2005) Use of Arthrobacter davidanieli as a live vaccine against Renibacterium salmoninarum and Piscirickettsia salmonis in salmonids. Dev Biol 121:189–197
Sarropoulou E, Kaitetzidou E, Papadaki M, Mylonas CC (2018). Role of epigenetics in fish, and the involvement of early rearing temperature. Published on MedAID H2020 project Blog: http://www.medaid-h2020.eu/index.php/2018/05/14/role-of-epigenetics-in-fish-and-the-involvementof-early-rearing-temperature/
Schar D, Sommanustweechai A, Laxminarayan R, Tangcharoensathien V (2018) Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: optimizing use and addressing antimicrobial resistance. PLoS Med 15(3):e1002521. https://doi.org/10.1371/journal.pmed.1002521
Shikongo-Nambabi MNNN, Kachigunda B, Venter SN (2010) Evaluation of oxidising disinfectants to control Vibrio biofilms in treated seawater used for fish processing. Water SA 36(3):215–220
Shoemaker CA, Klesius PH (2014) Replicating vaccines. In: Gudding R, Lillehaug A, Evensen O (eds) Fish Vaccination. John Wiley & Sons, Oxford, UK, pp 33–46
Shoemaker CA, Klesius PH, Drennan JD, Evans JJ (2011) Efficacy of a modified live Flavobacterium columnare vaccine in fish. Fish Shellfish Immunol 30(1):304–308. https://doi.org/10.1016/j.fsi.2010.11.001
Shoemaker CA, Klesius PH, Evans JJ (2007) Immunization of eyed channel catfish, Ictalurus punctatus, eggs with monovalent Flavobacterium columnare vaccine and bivalent F. columnare and Edwardsiella ictaluri vaccine. Vaccine 25(6):1126–1131. https://doi.org/10.1016/j.vaccine.2006.09.055
Shoemaker CA, Klesius PH, Evans JM, Arias CR (2009) Use of modified live vaccines in aquaculture. J World Aquacult Soc 40(5):573–585. https://doi.org/10.1111/j.1749-7345.2009.00279.x
Siwicki AK, Morand M, Terech-Majewska E, Niemczuk W, Kazun K, Glabski E (1998) Influence of immunostimulants on the effectiveness of vaccines in fish: in vitro and in vivo study. J Appl Ichthyol 14(5):225–227
Skinner LA, LaPatra SE, Adams A, Thompson K, Balfry SK, McKinley RS, Schulte PM (2010) Concurrent injection of a rhabdovirus-specific DNA vaccine with a polyvalent, oil-adjuvanted vaccine delays the specific anti-viral immune response in Atlantic salmon, Salmo salar L. Fish Shellfish Immunol 28(4):579–586. https://doi.org/10.1016/j.fsi.2009.12.017
Sommerset I, Krossøy B, Biering E, Frost P (2005) Vaccines for fish in aquaculture. Expert Rev Vaccines 4(1):89–101. https://doi.org/10.1586/14760584.4.1.89
Su FJ, Chen MM (2021) Protective efficacy of novel oral biofilm vaccines against Lactococcus garvieae infection in mullet, Mugil Cephalus. Vaccines (Basel) 9(8):844. https://doi.org/10.3390/vaccines9080844
Su FJ, Chen MM (2022) Protective efficacy of novel oral biofilm vaccines against Photobacterium damselae subsp. damselae infection in giant grouper, Epinephelus lanceolatus. Vaccines (Basel) 10(2):207. https://doi.org/10.3390/vaccines10020207
Sudheesh PS, Cain KD (2017) Prospects and challenges of developing and commercializing immersion vaccines for aquaculture. Int Biol Rev 1(1). https://doi.org/10.18103/ibr.v1i1.1313
Tafalla C, Bøgwald J, Dalmo RA, Munang’andu HM, Evensen Y (2014) Adjuvants in fish vaccines. In: Fish Vaccination, pp 68–84. https://doi.org/10.1002/9781118806913.ch7
Thinh NH, Kuo TY, Hung LT, Loc TH, Chen SC, Evensen O, Schuurman HJ (2009) Combined immersion and oral vaccination of Vietnamese catfish (Pangasianodon hypophthalmus) confers protection against mortality caused by Edwardsiella ictaluri. Fish Shellfish Immunol 27(6):773–776. https://doi.org/10.1016/j.fsi.2009.08.012
Thompson KD, Rodkhum C, Bunnoy A, Thangsunan P, Kitiyodom S, Sukkarun P, Yostawornkul J, Yata T, Pirarat N (2023) Addressing nanovaccine strategies for tilapia. Vaccines 11(8):1356. https://doi.org/10.3390/vaccines11081356
Tlaxca JL, Ellis S, Remmele RL (2015) Live attenuated and inactivated viral vaccine formulation and nasal delivery: potential and challenges. Adv Drug Deliv Rev 93:56–78. https://doi.org/10.1016/j.addr.2014.10.002
Toranzo AE, Romalde JL, Magariños B, Barja JL (2009) Present and future of aquaculture vaccines against fish bacterial diseases. In: Basurco B (ed) Rogers C. The use of veterinary drugs and vaccines in Mediterranean aquaculture. Zaragoza, CIHEAM, pp 55–176
Ulmer JB, Mason PW, Geall A, Mandl CW (2012) RNA-based vaccines. Vaccine 30(30):4414–4418. https://doi.org/10.1016/j.vaccine.2012.04.060
Vargas D, Vallejos-Vidal E, Reyes-Cerpa S, Oyarzún-Arrau A, Acuña-Castillo C, Imarai M, Reyes-López FE, Sandino AM (2021) The analysis of live-attenuated Piscirickettsia salmonis vaccine reveals the short-term upregulation of innate and adaptive immune genes in Atlantic salmon (Salmo salar): an in situ open-sea cages study. Microorganisms. 9(4):703. https://doi.org/10.3390/microorganisms9040703
Villumsen KR, Neumann L, Ohtani M, Strøm HK, Raida MK (2014) Oral and anal vaccination confers full protection against enteric redmouth disease (ERM) in rainbow trout. PLoS One 9(4):e93845. https://doi.org/10.1371/journal.pone.0093845
Vinay TN, Tanmoy GC, Anutosh P, Sanjay KG, Biplab S (2016) Nanovaccines: a possible solution for mass vaccination in aquaculture. World Aquacult 31
Wikumpriya GC, Prabhatha MWS, Lee J, Kim CH (2023) Epigenetic modulations for prevention of infectious diseases in shrimp aquaculture. Genes 14(9):1682
Wilhelm V, Miquel A, Burzio LO, Rosemblatt M, Engel E, Valenzuela S, Parada G, Valenzuela PD (2006) A vaccine against the salmonid pathogen Piscirickettsia salmonis based on recombinant proteins. Vaccine 24(23):5083–5091. https://doi.org/10.1016/j.vaccine.2006.03.027
World Food and Agriculture – Statistical Yearbook (2021) Policy Support and Governance. Food and Agriculture Organization of the United Nations https://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/1458575/
Yanong RP (2009) Use of vaccines in finfish aquaculture. EDIS 2009(1). https://doi.org/10.32473/edis-fa156-2008
Zhang D, Gao Y, Li Q, Ke X, Liu Z, Lu M, Shi C (2019) An effective live attenuated vaccine against Streptococcus agalactiae infection in farmed Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. https://doi.org/10.1016/j.fsi.2019.11.044
Zhang H, Chen M, Xu Y, Xu G, Chen J, Wang Y, Kang Y, Shan X, Kong L, Ma H (2020) An effective live attenuated vaccine against Aeromonas veronii infection in the loach (Misgurnus anguillicaudatus). Fish Shellfish Immunol. https://doi.org/10.1016/j.fsi.2020.05.027
Zhu Y-G, Zhao Y, Li B, Huang C-L, Zhang S-Y, Yu S, Chen Y-S, Zhang T, Gillings MR, Su J-Q (2017) Continental-scale pollution of estuaries with antibiotic resistance genes. Nat Microbiol 2:16270-1–16270-7. https://doi.org/10.1038/nmicrobiol.2016.270
Funding
This study was funded by the Technology Collaboration Program of “San Nong Jiu Fang” in Zhejiang Province (2022SNJF062), Ningbo Natural Science Foundation (2022J124), General Research Projects of Zhejiang Department of Education(Y202044159), Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02069-6).
Author information
Authors and Affiliations
Contributions
N.I. did the conceptualization, investigation, writing—original draft, and editing. Z.A. did the investigation and writing—original draft. K.W. did the writing—review and editing. C.S. did the writing—original draft preparation. C.M. did the project administration and resources. C.W. did the funding acquisition and resources. Q.W. did the supervision, investigation, writing—original drafts, editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Brian Austin
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Imtiaz, N., Anwar, Z., Waiho, K. et al. A review on aquaculture adaptation for fish treatment from antibiotic to vaccine prophylaxis. Aquacult Int 32, 2643–2668 (2024). https://doi.org/10.1007/s10499-023-01290-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01290-6