Abstract
The objective of this study was to evaluate the enzymatic protein hydrolysis of viscera Macrobrachium rosenbergii shrimp processing, as an alternative procedure for the hydrolysis of this residue. For this, shrimp residues were collected, the viscera were separated, and the samples were processed. The enzymes Alcalase® 2.4L and Flavourzyme® 1000L were added for the hydrolysis process, and 2 experimental designs were carried out. Subsequently, the design of the rotational central compound was carried out on the effect of three factors, each at five levels: temperature and concentration of enzymes. The temperature had a greater influence on the protein enzymatic hydrolysis of the shrimp viscera, obtaining values of the degree of hydrolysis (DH) of 2.11 ± 0.25% at the temperature of 67.8 °C, and concentration of the Alcalase® 2.4L and Flavourzyme® 1000L 0.50% enzymes. However, one of the control reactions had a DH of 2.06 ± 0.11% at a temperature of 61.0 °C, which was statistically equal to the value found for the enzymatic reaction. For the tests at 51.0 °C, an optimized reaction yield with DH of 1.88 ± 0.23% was obtained. Thus, it was possible to solubilize the protein present in shrimp viscera, making the hydrolyzate a source of protein for aquaculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, aquaculture production has grown significantly over the last 30 years. In 2018, the total production of aquatic animals was 82.1 million tons, yielding US$250.1 billion (FAO 2020), and providing more than 50% of fish for human consumption (FAO 2021). Shrimp farming is the aquaculture sector that shows the greatest growth in production, with emphasis on the marine species Litopenaeus vannamei, popularly known as Pacific white shrimp (FAO 2014). Freshwater shrimp farming also occupies a prominent place among aquaculture activities. The most recent data from the Food and Agriculture Organization of the United Nations (FAO 2018) showed a production of approximately 548,000 tons, with emphasis on the species Macrobrachium rosenbergii and Macrobrachium nipponense, with shrimp production contributing more than 60% (7.8 million tonnes) of world consumption (FAO-FIGIS 2017).
The industrial processing of shrimp produces numerous inedible parts. Approximately 50% of the animal is used for human consumption; the rest is discarded as inedible material, with cephalothorax being responsible for about 44% of this waste (Ferrer et al. 1996; Gernat 2001; Yadav et al. 2019; Santos et al. 2020). In addition to the cephalothorax and exoskeleton residues, there is also the disposal of shrimp viscera, where most of these processing residues overload sanitary landfills (Costa and Souza 2012). This reality implies a greater volume of waste, whose inadequate disposal can lead to environmental and human health problems (Heu et al. 2003; Zhang et al. 2018; Yadav et al. 2019). On the other hand, these by-products can also be configured as attractive proposals for the production of several value-added products, which can be explored in different biotechnological processes (Yadav et al. 2019; Santos et al. 2020).
Shrimp residues can be composed of parts of the cephalothorax, exoskeleton, and viscera, which can be an excellent protein source, presenting essential amino acids in their composition, providing excellent palatability and attractiveness to animal feed (Guimarães et al. 2008). These shrimp residues have been used in the production of chitosan, with applications in medicine and the food, pharmaceutical, and chemical industries. In addition to chitosan, carotenoids, protein hydrolysates, and glycosaminoglycans are also extracted from the cephalothorax and exoskeleton of shrimp (Cahú et al. 2012).
The chitin that makes up the shell of shrimp is used to extract chitosan (Alves et al. 2020) which is used in biomedicine, drug delivery, gene therapy, tissue engineering, and as a biomarker for bioimaging (Egladir et al. 2015; Muanprasat and Chatsudthipong 2017; Mitall et al. 2018), in food to reduce cholesterol (Panit et al. 2016; van der Gronde et al. 2016), as well as for environmental (Wang et al. 2009; Gupta et al. 2017) and agricultural (Wu and Liu 2008; Perez et al. 2016) applications, among others. On the other hand, chitin and pectin present in shrimp shells can decrease animal performance, depending on their concentrations, because they are not degraded by endogenous enzymes, affect nutrient digestibility, and modify the time the food remains in the digestive tract, as they are polymers of simple sugars, and resistant to hydrolysis in the gastrointestinal tract of non-ruminant animals (Brito et al. 2008). Therefore, it is important that these residues are reused, to avoid incorrect disposal in the environment and, also, as a way to use such residues as a source of substances of high economic and nutritional value (Kim and Venkatesan 2014).
One of the alternative processes for this use is the enzymatic hydrolysis of proteins found in fish by-products, which makes it possible to add significant nutritional value to them. The process involves the use of enzymes to cleave the peptide bonds of proteins, which can be used to change the chemical, functional, and sensory properties of these proteins, without harming their nutritional value (Pasupuleti and Braun 2010). Protein enzymatic hydrolysis has many advantages when compared to other methods, as it occurs under mild conditions of temperature, pH, and pressure; in addition, they produce more homogeneous hydrolyzates and the enzymes used in the process have substrate specificity, decreasing the probability of undesirable reactions that may result in the formation of toxic products (Duarte de Holanda and Netto 2006; Damodaran et al. 2010; He et al. 2013).
One of the criteria to quantify the proteolysis reaction is the degree of hydrolysis (DH) (Nielsen and Olsen 2002). This variable was defined as the number of cleaved peptide bonds or the number of free amino groups formed during the hydrolysis process, expressed in hydrolysis equivalence (Adler-Nissen 1986). The DH is also widely used as an indicator to compare different protein hydrolysates, being the main determinant of protein hydrolyzate properties (Mahmoud et al. 1992; Neves et al. 2004).
For aquaculture, protein represents the most expensive and important component in the production of feed (Savoie et al. 2011), as it is the nutrient that exerts the greatest influence on animal growth, as well as on weight gain, feed conversion, and carcass composition (Araripe et al. 2011). In a study of partial replacement of fishmeal by shrimp head protein hydrolysate for tilapia fingerlings, it was concluded that this ingredient is a promising protein source for the species at this stage, improving the growth rate with inclusion levels of up to 15% (Plascencia et al. 2002). Another study (Gisbert et al. 2018) indicated that the shrimp residual protein hydrolysate can be used as a supplement to increase the immune response of fish without affecting their zootechnical performance. Furthermore, protein hydrolysates in aquafeeds have been reported to increase intake, feed utilization, and somatic growth (Zheng et al. 2013; Khosravi et al. 2015) as well as improve skeletal development and digestive systems in fish larvae (Johannsdottir et al. 2014; Delcroix et al. 2015).
Given the above, the residues from the processing of shrimp can be an alternative source for the production of protein hydrolysates, allowing its valorization and still making use of its functional properties. Researches have already been carried out evaluating the effect of enzymatic protein hydrolysis of shrimp residues (Dey and Dora 2014; Gunasekaran et al. 2015; Zhang et al. 2016; Yen and May 2019); however, the effect of enzymatic hydrolysis in viscera only and using shrimp of the species M. rosenbergii has not been reported. Therefore, the objective of this work was to evaluate the enzymatic protein hydrolysis of the viscera resulting from the processing of shrimp M. rosenbergii as an alternative process to use this by-product.
Material and methods
Obtaining shrimp residue and proximate composition
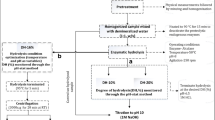
The residues of the shrimp M. rosenbergii (cephalothorax, carapaces, pereopods, and viscera) were obtained from a processing industry located in the west of the state of Paraná, Brazil. The samples were selected for the removal of carapaces, cephalothorax, and pereopods, leaving only the viscera. As the aim of the work was to produce a component that can be used as a supplement in animal feed, only the viscera were used for the enzymatic procedure, as the protein present in this material is digestible. The rest of the material (carapace, pereopods) is mainly composed of chitin, a material that can interfere with animal digestibility (Brito et al. 2008). Afterward, the samples were processed in a drying and sterilization oven at 55 °C for 72 h and stored in a freezer at − 3 °C until use for the production of hydrolysates. For enzymatic hydrolysis, commercial proteases Alcalase® 2.4L and Flavorzyme® 1000L (Novozymes, Bagsvaerd, Denmark) — supplied by Tovani Benzaquen Ingredientes (São Paulo, Brazil) — were used.
The proximate characterization of the residue in natura was carried out at the Federal University of Paraná (UFPR), Palotina Sector. For this, analyses were carried out to determine the dry matter (DM) contents submitted to drying at 105 °C in an oven, for 12 h; crude protein (CP), performed by the Kjeldahl method; ether extract (EE), in an extractor (ANKOM® XT10); and mineral matter (MM), obtained by burning the sample at 600 °C in a muffle furnace. The procedures were performed according to the Association of Official Analytical Chemists (AOAC 2010).
Enzymatic hydrolysis
The enzymatic hydrolysis tests were carried out using 100 mL of distilled water and 10 g of protein residues with the addition of enzymes and temperature adjusted according to the experimental design, for 2 h at 100 rpm in a shaker machine.
At the end of the process, an aliquot of the samples was sent for DH analysis, and the rest for enzymatic inactivation, which was placed in a water bath at 90 °C for 15 min, and, later, filtered in a polypropylene filter. Then, the total protein analysis was performed using the Kjedahl method (AOAC 2010).
The enzymatic hydrolysis tests were carried out according to experimental plans, simultaneously with control tests, carried out without the addition of enzymes.
Evaluation and optimization of enzymatic hydrolysis process conditions
Aiming to analyze the ranges of variables that influence the enzymatic hydrolysis of shrimp viscera, a 23 factorial design was initially created, in which the temperature variables (45, 55, and 65 °C) were evaluated according to the optimal conditions and to the concentration of the enzymes Alcalase® 2.4L and Flavorzyme® 1000L (0.75; 1.50 and 2.25% enzyme protein/substrate protein).
By determining the significant ranges of the variables on hydrolysis, a central composite rotational design (CCRD) was proposed, aiming at optimizing the process. The CCRD planning included 3 variables (temperature, Alcalase enzyme concentration® 2.4L and Flavorzyme enzyme concentration® 1000L), with levels (− 1 and + 1), axial points (− 1.68 and + 1.68), and four repetitions at the central point (0), resulting in 18 trials. The values of the variables were as follows: temperature (34.2, 41.0, 51.0, 61.0, and 67.8 °C) and concentration of the enzymes Alcalase® 2.4L and Flavorzyme® 1000L (0.08, 0.25, 0.50, 0.75, and 0.92% enzyme protein/substrate protein). The reference values of the studied variables were based on the data found in the literature [32, 33, 34, 35].
Determination of the degree of hydrolysis
The DH was estimated according to the method described by Hoyle and Merritt (1994) and Baek and Cadwallader (1995), expressed as the percentage of soluble proteins in trichloroacetic acid (TCA) concerning the amount of total initial protein; the protein soluble in TCA was obtained by the Lowry method (Lowry et al. 1951), in mg/mL, and the total protein by the Kjedahl method (AOAC 2010), in mg/mL, calculated using Eq. 1:
Psol = TCA-soluble protein (mg/ml).
Pt = total protein in the sample (mg/ml).
After the period of enzymatic hydrolysis, 1 mL aliquots of hydrolysate (soluble phase) were inactivated by adding 9 mL of 6.5% TCA solution and left to rest for 10 min. After filtration on quantitative paper (JP41 – Black Band), to remove the insoluble material precipitated by TCA, the content of soluble proteins in the filtrate was determined by the Lowry method (Lowry et al. 1951), expressed in milligrams of albumin. Sample absorbance readings were performed in a digital UV/Vis spectrophotometer at 625 nm.
Statistical analysis
All designs were performed in triplicate. A 23 factorial design with 12 trials was performed, 4 trials from the central point to investigate which variables were significant; for this, a Pareto chart and response surfaces were used. After verifying which variables were significant, a CCRD was carried out with 18 trials, 4 of which at the central point, to optimize the conditions for enzymatic hydrolysis; later, Pareto graph, response surfaces, and analysis of variance were performed to assess the significance of the results, using the F test.
Results
The results presented in Table 1 refer to the components that constitute the viscera from M. rosenbergii, through its chemical characterization. The crude protein content was 19.43%, ethereal extract 59.70%, and mineral matter 2.56%.
Table 2 shows the results of the 23 factorial design of soluble protein concentration and the corresponding soluble protein contents after enzymatic hydrolysis. It was possible to observe that the highest concentration of soluble protein was obtained in test 2 (1.20 ± 0.17 mg/mL), conducted at 65.0 °C and a concentration of 0.75% for both enzymes.
In Fig. 1, the Pareto chart is presented, where the values to the right of the red line were significant (p < 0.05). It is observed that temperature is the only significant variable that positively affects the response; that is, the higher the temperature values, the better the soluble protein concentration results. The concentration of the enzyme Flavorzyme® 1000L was negatively significant; that is, the enzyme did not increase in protein solubilization.
Figure 2a and b show the response surfaces that represent the behavior of the soluble protein concentration — through the interaction of the variables temperature and concentration of the enzymes Alcalase® 2.4L and Flavorzyme® 1000L, respectively.
Composite central rotational design
As observed in the 23 factorial design, the temperature was the only positively significant variable. Furthermore, it was verified on the response surfaces that lower enzyme concentrations were already favorable for good results of soluble protein concentration. Based on these data, the levels of the variables were modified in a new experimental design of the CCRD type, to optimize the experimental conditions for enzymatic hydrolysis of shrimp viscera.
Table 3 shows the matrix for CCRD with the variables used and the DH results after enzymatic hydrolysis. It was observed that in test 10, a higher result of DH 2.11 ± 0.25% was obtained, corresponding to a temperature of 67.8 °C and a concentration of 0.50% for both enzymes.
Figure 3 shows the Pareto graph for the model obtained in the CCRD planning of the enzymatic hydrolysis of shrimp viscera. The values that are to the right of the red line are significant (p < 0.05), and it is possible to observe that the quadratic temperature term was significant and had a positive effect. The interaction of the variable concentration of the enzyme Alcalase® 2.4L, in its quadratic form, and temperature in its linear form, as well as the concentration of the enzyme Flavorzyme® 1000L both in its linear form and in its quadratic form, was negatively significant.
By the analysis of variance, by the F test, comparing the calculated F value with the tabulated F, it is possible to affirm if the proposed model is valid and if the parameters of the equation fit the experimental data. Table 4 shows the analysis of variance of the enzymatic hydrolysis of shrimp viscera by the F test, where for the regression Fcalc > Ftab (4.13; 0.05) = 3.18, which denotes a significant result. For the lack of adjustment, Fcalc < Ftab (10.3; 0.05) = 8.79, since the lack of adjustment was not significant. Considering these results, the proposed model is valid, and it is possible to describe the empirical mathematical model of DH as a function of real variables and their interactions that were significant, presenting an adequate correlation coefficient (R2 = 0.831). The function that represents the process is presented in Eq. 2.
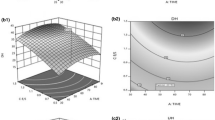
It was possible to observe that the highest DH results were found in the extreme temperatures evaluated and in the median concentrations of the enzyme Alcalase® 2.4L (Fig. 4).
Figure 5 shows the response surface for the DH values in relation to the variables Flavourzyme® 1000L concentration and reaction temperature. The highest DH results were found in two distinct regions: for higher concentrations of this enzyme and lower temperatures, and also for lower concentrations of this enzyme and higher temperatures.
Table 5 shows the DH values of the control reactions (without the addition of enzyme) and enzymatic reactions for comparison of results.
In test 2 of the control reaction, the DH obtained was 2.06 ± 0.11%, at a temperature of 61.0 °C. In test 10 of the enzymatic reaction, the DH value was 2.11 ± 0.25%, at a temperature of 67.8 °C and an enzymatic concentration of 0.50%. These results were statistically equal.
On the response surfaces shown in Fig. 6a and b, the highest and lowest temperatures used in the enzymatic reactions were fixed. In Fig. 6a, it can be seen that the highest DH values were obtained with the influence of Alcalase® 2.4L at the highest temperature; this may have occurred because the optimum conditions for the enzyme to act are in the temperature range between 50 and 70 °C, as mentioned above. On the response surface of Fig. 6b, the influence of Flavorzyme® 1000L was greater, as it acts at temperatures up to 50 °C. Through these surfaces, it is possible to verify how the temperature intervened in the enzymatic hydrolysis.
In the response surface shown in Fig. 7, at a temperature of 51.0 °C, for both enzymes, the concentrations were intermediate, where they reached the optimum point. For the temperature of 51 °C, the response surface was better adjusted so that the highest point of curvature was obtained at the median values of concentration and, as one moves away from this optimal point, the surface presents a smoother curve. When comparing this point of 1.88% of DH to the highest value obtained in the tests (2.11%) by the Tukey test, it was noted that they did not differ statistically (they did not present a significant mean difference). Therefore, the lowest temperature value is chosen. This result may have been achieved, as this temperature can be considered intermediate for both enzymes concerning the other temperatures tested — where for Flavorzyme® 1000L, the best temperature is up to 50 °C and for Alcalase® 2.4L between 50 and 70 °C, making joint action more favorable.
Discussion
According to Cahú et al. (2012) and Cheong et al. (2014), shrimp residues are rich in proteins and provide essential amino acids for animal feed supplements or human nutrition. The protein content in shrimp residues comprises of approximately 35% to 65% of its dry weight, depending on the types of processing and the species being processed (Mizani et al. 2005). From this protein content, essential and non-essential amino acids are 56.8% and 43.2%, respectively (Narayan et al. 2010). In the study of Synowiecki and Al-Khateeb (2000), using the shell residues from the processing of Crangon crangon shrimp, it was determined that the dry weight of the shrimp comprises 18% chitin, 43% protein, 29% ash, and 10% lipid. Guerard et al. (2007) used wild shrimp residues (a mixture of Penaeus brasiliensis and Penaeus subtilis) and the crude protein content in the residue was 51.9% (dry weight).
The differences observed between this work and studies in the literature are mainly due to the type of material used in the characterization. While in the present study only viscera were used, in the studies by Synowiecki and Al-Khateeb (2000) and Guerard et al. (2007), the authors used the carapace and the entire shrimp residue, respectively. Furthermore, factors such as species, age, the season of the year, and environmental conditions can also affect the composition of the crustacean and make comparisons between studies difficult (Guerard et al. 2007).
In the present work, the temperature had a positive influence on the hydrolysis process, as the concentration of soluble proteins from enzymatic reactions at higher temperatures showed superior results when compared to those from control reactions. However, at lower temperatures, the results of the control reactions were better than those of the enzymatic reactions; therefore, this may be due to commercial enzymes working best at temperatures between 50 and 70 °C (Jung et al. 2006; Novozymes 2001).
On the response surface shown, the temperature of 51 °C is more indicated, for both enzymes as were the nearby springs, where it will reach the high point of the planning. This result may have been more recent in the media, as this relationship is at other temperatures tested, where for our 50 °C and for Alcalase® 2.4L between 50 and 70 °C, making joint action more favorable.
Comparing the response surfaces that represent the behavior of the soluble protein concentration through the interaction of the temperature and concentration variables of the enzymes Alcalase® 2.4L and Flavorzyme® 1000L respectively, both surfaces show similar behavior, since at higher temperatures, the most favorable values of soluble protein concentration are reached, even using low concentrations of enzymes.
By increasing the temperature as well as the kinetic energy of the enzymes, the reaction rate becomes higher. The gradual increase in temperature favors the collisions between the active sites of the enzymes and the substrate, to increase the reaction rate (Fields 2001; Shuler and Kargi 2002). This fact was exemplified in the present work, in which higher temperatures benefited the reactions.
The shrimp protein hydrolysate produced in the present study was similar to that obtained from the head and husk of Penaeus monodon, using Alcalase with an enzyme:substrate ratio of 1.05% at 50 °C for 30 min, which resulted in a DH value of 2.3% (Dey and Dora 2014). Yen and May (2019) obtained a DH of 4.21% with the shrimp by-product hydrolysate from the shrimp processing industry, using Alcalase at a fixed concentration of 0.2% (4.8AU/kg protein) and 0.25% of Flavorzyme pH 6, at 55 °C for 5 h. Zhang et al. (2016) evaluated the optimization of the enzymatic hydrolysis process of northern pink shrimp residues — including heads, shells, and tails —where they observed that DH was maximized by 48.83 ± 0.30% using the enzyme:substrate ratio of 1.64%, hydrolysis time at 3.59 h, initial pH at 9, and temperature at 52.57 °C. Gunasekaran et al. (2015) obtained, after 3 h of head hydrolysis of Metapenaeus dobsoni at pH 8.2, the temperature of 45.4 °C, the enzyme:substrate ratio of 1.8%, and a DH of 42.44%. The differences observed between this study and those mentioned above can be explained by the longer time of the hydrolysis reaction by these authors, or also by the chemical and physical differences in the residues used.
For CCRD planning of enzymatic hydrolysis of shrimp viscera, the temperature was the only significant variable; however, according to Tavano et al. (2018), heat treatment can improve the proteolysis rate — although it can change the substrate accessibility for hydrolysis and the hydrolysate profile, as the enzymatic structure can be modified; that is, the denaturation of the enzyme occurs, which prevents its specific fitting with the substrate. Thus, there is an ideal temperature for each enzyme, at which the reaction rate is maximum (Fields 2001; Shuler and Kargi 2002). This may be one of the reasons why enzymes are not significant in the protein hydrolysis process.
However, in test 2 of the control reaction, the DH obtained was 2.06 ± 0.11%, at a temperature of 61.0 °C. In test 10 of the enzymatic reaction, the DH value was 2.11% ± 0.25%, at a temperature of 67.8 °C and an enzymatic concentration of 0.50%. Such results are statistically equal, which does not justify the use of enzymes, given that one of the limiting factors for the use of enzymes in industrial processes is the high cost of production and purification (Monteiro and Silva 2009; Nalinanon et al. 2009).
Although enzymatic processes are considered an advantageous alternative to accelerate the reactions, in this case, it was observed that the control reactions (without the addition of enzyme) achieved relevant DH results, being in some cases even greater than in the enzymatic reactions. This could be due to some endogenous hydrolytic enzymes that are found in biological material, such as proteases, lipases, and carbohydrases which, under certain conditions, can hydrolyze the residue (Senphan et al. 2018). Enzymes can be inhibited by substances that bind to the free enzyme or enzyme–substrate complex or compete for the enzyme’s catalytic site. The final result is a decrease or abrogation of enzyme activity (Monteiro and Silva 2009).
The shrimp hepatopancreas has vital functions as it is involved in several metabolic activities (Gibson and Barker 1979). This organ is involved in maintaining the balance of salts and ions; metabolism of proteins, lipids, and carbohydrates; removal of foreign bodies from the vascular system; and detoxification of metals and other organic substances; in addition, it secretes digestive enzymes that are released in the stomach, stores useful substances, and controls the body’s biochemical composition (Gibson and Barker 1979; Icely and Nott 1992). Furthermore, the hepatopancreas is a great source of proteases, mainly trypsin and chymotrypsin, which can hydrolyze several protein substrates (Sriket et al. 2012). These enzymes, already present in the substrate, may have competed with commercial enzymes for the active site, or some components may have inhibited their action, so the DH values of the enzymatic reactions and the control reactions may have remained close.
Numerous factors influence the properties and constituents of the final hydrolysate, such as the composition and variation of raw materials, enzyme specificity, reaction time, activity and concentration of endogenous enzymes, pH, and temperature (Opheim et al. 2015). According to Slizyte et al. (2005), the hydrolysis process can be negatively influenced by the lipid content, by the formation of protein/lipid complexes, more resistant to enzymatic disruption. These authors, when analyzing the composition of different fractions obtained after the hydrolysis of cod (Gadus morhua) with the enzymes Flavorzyme® and Neutrase®, observed that raw materials with a high lipid content resulted in a hydrolysate with a smaller amount of solubilized protein. Considering that in the present study the lipid content was 59.70%, this may have influenced the low DH values found.
High temperatures resulted in better DH; however, some precautions must be taken with high temperatures. Fluctuations in hydrolysis conditions, such as temperature, pressure, pH, salinity, or solvent concentration, can cause the denaturation of proteins, with the temperature being one of the main factors to denature protein molecules, which can lead to the loss of nutritional and functional value of the final hydrolysate (Franks et al. 1988; Tedford et al. 1998).
Through the DH results, it was verified that there was protein cleavage since DH is defined as the proportion of peptide bonds cleaved by the proteolytic enzyme. And, depending on the degree of hydrolysis, the molecular weight, and the primary structure of the peptides, the hydrolysate will have different applications (Liu et al. 2007; Balti et al. 2011; Amiza et al. 2012).
With the results obtained, it can be noticed that there was solubilization of the protein present in the shrimp viscera, both in the enzymatic reactions and in the control reactions (without the addition of enzymes). The in natura material presents 19.43% of DH; after hydrolysis, the results of soluble protein content were between 22 and 38%. This demonstrates the applicability of this protein in animal nutrition, avoiding the disposal of this residue, which could harm the environment.
Leduc et al. (2018) observed that shrimp hydrolysates can be used for animal feed and also pointed out that the shrimp hydrolysate Litopenaeus vannamei improved the organization of intestinal cells, increasing the height of the intestinal villi of fish Dicentrarchus labrax. This highlights the potential use of protein hydrolysate from the viscera of M. rosenbergii shrimp, as the material showed positive protein solubilization results.
Conclusion
In the process of enzymatic protein hydrolysis of shrimp viscera Macrobrachium rosenbergii, the temperature at 51.0 °C reached an optimized yield. The highest DH was 2.11 ± 0.25% in the enzymatic reaction at 67.8 °C and concentration 0.50% both for the enzyme Alcalase® 2.4L and for the enzyme Flavorzyme® 1000L. However, at 61.0 °C, the control reaction (without the addition of enzymes) presented a DH of 2.06 ± 0.11, being statistically equal to that obtained for the enzymatic reaction. This indicates that the utilized enzymes did not increase the percentage of cleaved peptide bonds for the shrimp viscera. However, solubilization of the protein present in this residue was observed, both in enzymatic reactions and in control reactions. In the tests carried out with the addition of enzymes, it was possible to obtain up to 61.66% of protein solubilization initially present in the material, while in the reactions without the addition of enzymes, this content did not exceed 58.38%.
Data availability
Not applicable.
Code availability
Not applicable.
References
Adler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier Applied Science, London, pp 365–404
Alves HJ, Gasparrini LJ, Silva FEB, Caciano L, de Muniz GIB, Ballester ELC, Cremonez PA, Arantes MK (2020) Alternative methods for the pilot-scale production and characterization of chitosan nanoparticles. Environ Sci Pollut Res 28:10977–10987. https://doi.org/10.1007/s11356-020-11343-5
Amiza MA, Kong YL, Faazaz AL (2012) Effects of degree of hydrolysis on physicochemical properties of Cobia (Rachycentron canadum) frame hydrolysate. Int Food Res J 19(1):199–206
Araripe MNBA, Lopes JB, Castro PL, Braga TEA, Ferreira AHC, Abreu MLT (2011) Redução da proteína bruta com suplementação de aminoácidos em rações para alevinos de tambatinga. Ver Bras De Zootec 40(9):1845–1850. https://doi.org/10.1590/S1516-35982011000900001
Association of Official Analytical Chemists (AOAC) (2010) Official methods of analysis of AOAC international, 18 th edn., Washington DC
Baek HH, Cadwallader KR (1995) Enzymatic hydrolysis of crayfish processing by-products. J Food Sci 60:929. https://doi.org/10.1111/j.1365-2621.1995.tb06264.x
Balti R, Jridi M, Sila A, Souissi N, Nedjar-Arroume N, Guillochon D, Nasri M (2011) Extraction and functional properties of gelatin from the skin of cuttlefish (Sepia officinalis) using smooth hound crude acid protease-aided process. Food Hydrocoll 25(5):943–950. https://doi.org/10.1016/j.foodhyd.2010.09.005
Brito MS, Oliveira CFS, Silva TRG, Lima RB, Morais SN, Silva JHV (2008) Polissacarídeos não amiláceos na nutrição de monogástricos – revisão. Acta Vet Bras 2 (4):111–117. https://doi.org/10.21708/avb.2008.2.4.917
Cahú TB, Santos SD, Mendes A, Cordula CR, Chavante SF, Carvalho LB Jr, Nader HB, Bezerra RS (2012) Recovery of protein, chitin, carotenoids processing waste. Process Biochem 47(4):570–577. https://doi.org/10.1016/j.procbio.2011.12.012
Cheong JY, Azwady AA, Nor Rusea G, Noormasshela UA, Shaziera AG, NurulAzleen AA, Muskhazli M (2014) The availability of astaxanthin from shrimp activity of carotenoprotein from shells of Pacific white shrimp extracted using hepatopancreas proteases. Food Biosci 5:54–63. https://doi.org/10.1016/j.fbio.2013.11.004
Costa SR, Souza PAR (2012) O impacto dos resíduos de pescado: o caso da “Feira do Bagaço no município de Parintins no Amazonas. DELOS: Desarrollo Local Sostenible 5(14):1–11.
Damodaran S, Parkin KL, Fennema OR (2010) Química de Alimentos de Fennema, 4ª. Artmed, São Paulo
Delcroix J, Gatesoupe FJ, Desbruyères E, Huelvan C, Le Delliou H, Le Gall MM, Zambonino-Infante JL (2015) The effects of dietary marine protein hydrolysates on the development of sea bass larvae, Dicentrarchus labrax, and associated microbiota. Aquac Nutr 21(1):98–104. https://doi.org/10.1111/anu.12139
Dey SS, Dora KC (2014) Optimization of the production of shrimp waste protein hydrolysate using microbial proteases adopting response surface methodology. J Food Sci Technol 51(1):16–24. https://doi.org/10.1007/s13197-011-0455-4
Duarte de Holanda H, Netto FM (2006) Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J Food Sci 71(5):298–303. https://doi.org/10.1111/j.1750-3841.2006.00040.x
Egladir MA, Uddin S, Ferdosh S, Adam A, Chowdhury AJK, Sarker Z (2015) Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal 23(4):619–629. https://doi.org/10.1016/j.jfda.2014.10.008
FAO - Food and Agricultural Organization of the United Nations (2014) The state of world fisheries and aquaculture - opportunities and challenges. Rome.
FAO - Food and Agricultural Organization of the United Nations (2018) The state of world fisheries and aquaculture - meeting the sustainable development goals. Rome. Licence: CC BY-NC-SA 3.0 IGO
FAO (2020) Food and Agricultural Organization of the United Nations.The state of world fisheries and aquaculture 2020. Sustainability in action. Romehttps://doi.org/10.4060/ca9229en
FAO (2021) Food and Agricultural Organization of the United Nations - FAO Aquaculture News, n° 23
FAO-FIGIS Food and Agriculture Organization of the United Nations (2017) Global Aquaculture Production 1950–2017
Ferrer J, Paez G, Marmol Z, Ramones E, Garcia H, Forster C (1996) Acid hydrolysis of shrimp-shell wates and the production of single cell protein from the hydrolysate. Bioresour Technol 57:55–60. https://doi.org/10.1016/0960-8524(96)00057-0
Fields PA (2001) Review: Protein function at thermal extremes: balancing stability and flexibility. Comp Biochem Physiol, Part A 129(2–3):417–431. https://doi.org/10.1016/s1095-6433(00)00359-7
Franks F, Hatley RHM, Friedman HL (1988) The thermodynamics of protein stability: cold destabilization as a general phenomenon. Biophys Chem 31(3):307–315. https://doi.org/10.1016/0301-4622(88)80037-1
Gernat AG (2001) The effect of using different levels of shrimp meal in laying hen diets. Poult Sci 80(5):633–636. https://doi.org/10.1093/ps/80.5.633
Gibson R, Barker PL (1979) The decapod hapatopancreas. Oceanogr Mar Biol: Ann Rev 17:285–346
Gisbert E, Fournier V, Solovyev M, Skalli A, Andree KB (2018) Diets containing shrimp protein hydrolysates provided protection to European sea bass (Dicentrarchus labrax) affected by a Vibrio pelagius natural infection outbreak. Aquac 495:136–143. https://doi.org/10.1016/j.aquaculture.2018.04.051
Guerard F, Sumaya-Martinez MT, Laroque D, Chabeaud A, Dufosse L (2007) Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem 42:1486–1491. https://doi.org/10.1016/j.procbio.2007.07.016
Guimarães IG, Miranda EC, Martins GP, Louro RV, Miranda CC (2008) Farinha de camarão em dietas para tilápia do Nilo (Oreochromis niloticus). Rev Bras de Saude e Prod Anim 9(1):140–149
Gunasekaran J, Kannuchamy N, Kannaiyan S, Chakraborti R, Gudipati V (2015) Protein hydrolysates from shrimp (Metapenaeus dobsoni) head waste: optimization of extraction conditions by response surface methodology. J Aquat Food Prod Technol 24(5):429–442. https://doi.org/10.1080/10498850.2013.787134
Gupta VK, Fakhri A, Agarwal S, Azad M (2017) Synthesis and characterization of Ag2S decorated chitosan nanocomposites and chitosan nanofibers for removal of lincosamides antibiotic. Int J Biol Macromol 103:1–7. https://doi.org/10.1016/j.ijbiomac.2017.05.018
He R, Girgih AT, Malomo SA, Ju X, Aluko RE (2013) Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J Funct Foods 5(1):219–227. https://doi.org/10.1016/j.jff.2012.10.008
Heu MS, Kim JS, Shahidi F (2003) Components and nutritional quality of shrimp processing by-products. Food Chem 82:235–242. https://doi.org/10.1016/S0308-8146(02)00519-8
Hoyle NT, Merritt JH (1994) Quality of fish protein hydrolysates from herring (Clupea harengus). J Food Sci 59:76–79. https://doi.org/10.1111/j.1365-2621.1994.tb06901.x
Icely JD, Nott JA (1992) Digestion and absorption: digestive system and associated organs. In: Harrison FW, Humes AG (eds) Microscopic anatomy of the invertebrates, vol 10. Decapod Crustacea. Wiley, New York, pp 147–201
Johannsdottir J, Heimisdottir HL, Hakonardottir K, Hrolfsdottir L, Steinarsson A, Imsland AK, Bjornsdottir R (2014) Improved performance of Atlantic cod (Gadus morhua L.) larvae following enhancement of live feed using a fish protein hydrolysate. Aquac Nutr 20(3):314–323. https://doi.org/10.1111/anu.12080
Jung W, Karawita R, Heo S, Lee B, Kima S, Jeon Y (2006) Recovery of a novel cabinding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process Biochem 41:2097–2100. https://doi.org/10.1016/j.procbio.2006.05.008
Khosravi S, Bui HTD, Rahimnejad S, Herault M, Fournier V, Jeong JB, Lee KJ (2015) Effect of dietary hydrolysate supplementation on growth performance, non-specific immune response and disease resistance of olive flounder (Paralichthys olivaceus) challenged with Edwardsiella tarda. Aquac Nutr 21(3):321–331. https://doi.org/10.1111/anu.12157
Kim S, Venkatesan J (2014) Seafood processing by-products: trends and applications (ed). Springer, New York
Leduc A, Zatylny-Gaudin C, Robert M, Corre E, Corguille GL, Castel H, Lefevre-scelles A, Fournier V, Gisbert E, Andree KB, Henry J (2018) Dietary aquaculture byproduct hydrolysates: impact on the transcriptomic response of the intestinal mucosa of European seabass (Dicentrarchus labrax) fed low fish meal diets. BMC Genomics 19(396):1–20. https://doi.org/10.1186/s12864-018-4780-0
Liu Z, Zeng M, Dong S, Xu J, Song H, Zhao Y (2007) Effect of an antifungal peptide from oyster enzymatic hydrolysates for control of gray mold (Botrytis cinerea) on harvested strawberries. Postharvest Biol Technol 46(1):95–98. https://doi.org/10.1016/j.postharvbio.2007.03.013
Lowry OH, Rosenbrough NJ, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Mahmoud MI, Malone WT, Cordle CT (1992) Enzymatic hydrolysis of casein: effect of degree of hydrolysis on antigenicity and physical properties. J of Food Sci 57(5):1223–1229. https://doi.org/10.1111/j.1365-2621.1992.tb11304.x
Mitall H, Ray SS, Kaith BS, Bhatia JK, Sharma J, Alhassan SM (2018) Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur Polym J 109:402–434. https://doi.org/10.1016/j.eurpolymj.2018.10.013
Mizani M, Aminlari M, Khodabandeh M (2005) An effective method for producing a nutritive protein extract powder from shrimp-head waste. Food Sci Technol Int 11(1):49–54. https://doi.org/10.1177/1082013205051271
Monteiro VN, Silva RN (2009) Aplicações industriais da biotecnologia enzimática. Revista Processos Químicos 3(5):9–23
Muanprasat C, Chatsudthipong V (2017) Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Therapeut 170:80–97. https://doi.org/10.1016/j.pharmthera.2016.10.013
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2009) Partitioning of protease from stomach of albacore tuna (Thunnus alalunga) by aqueous two-phase systems. Process Biochem 44:471–476. https://doi.org/10.1016/j.procbio.2008.12.018
Narayan B, Velappan SP, Zituji SP, Manjabhatta SN, Gowda LR (2010) Yield and chemical composition of fractions from fermented shrimp biowaste. Waste Manag Res 28(1):64–70. https://doi.org/10.1177/0734242X09337658
Neves RAM, Mira NVM, Marquez LUM (2004) Caracterização de hidrolisados enzimáticos de pescado. Ciênc Tecnol Aliment 24(1):101–108. https://doi.org/10.1590/S0101-20612004000100019
Nielsen PM, Olsen HS (2002) Enzymic modification of food protein. In: “Enzymes in food technology”. Robert J. Whitehurst and Barry A. Law (eds.) Sheffield Academic Press, Cap 6:109–143.
Novozymes (2001) Ficha Técnica da enzima Alcalase® 2.4L. Special Food/2001 0828103. pdf.http://www.ebiosis.co.kr/Novozymes%20Product%20Sheet/Alcalase%202.4L.pdf/
Opheim M, Šližytė R, Sterten H, Provan F, Larssen E, Kjos NP (2015) Hydrolysis of Atlantic salmon (Salmo salar) rest raw materials—effect of raw material and processing on composition, nutritional value, and potential bioactive peptides in the hydrolysates. Process Biochem 50(8):1247–1257. https://doi.org/10.1016/j.procbio.2015.04.017
Panit N, Wichaphon J, Lertisiri S, Niamsiri N (2016) Effect of physical and physicochemical characteristics of chitosan on fat-binding capacities under in vitro gastrointestinal conditions. LWT-Food Sci Technol 71:25–32. https://doi.org/10.1016/j.lwt.2016.03.013
Pasupuleti VK, Braun S (2010) State of the art manufacturing of protein hydrolysates. In: Pasupuleti VK, Demain AL. (Ed.). Protein hydrolysates in biotechnology. Dordrecht: Springer, 11–32. https://doi.org/10.1007/978-1-4020-6674-0_2
Perez JJ et al. (2016) Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr Polym 148:134–142. https://doi.org/10.1016/j.carbpol.2016.04.054
Plascencia JM, Olvera NMA, Arredondo FJL, Hall GM, Shirai K (2002) Feasibility of fishmeal replacement by shrimp head silage protein hydrolysate in Nile tilapia, (Oreochromis niloticus), diets. J Sci Food Agric 82(7):753–759. https://doi.org/10.1002/jsfa.1092
Santos VP, Marques NSS, Maia PC, Lima MABD, Franco LDO, Campos-Takaki GMD (2020) Seafood waste as an attractive source of chitin and chitosan production and its applications. Int J Mol Sci 21(12):4290. https://doi.org/10.3390/ijms21124290
Savoie ALE, François NR, Lamarre SG, Bilier PU, Beaulieu L, Cahu C (2011) Dietary protein hydrolysate and trypsin inhibitor effects on digestive capacities and performance during early-stages of spotted Wolf fish: suggested mechanisms. Comp Biochem Physiol 158(4):525–530. https://doi.org/10.1016/j.cbpa.2010.12.017
Senphan T, Benjakul S, Kishimura H (2018) Characteristics and antioxidative shell wastes through microbial fermentations, Aeromonas hydrophila and cell disruptions. Int J Agric Biol 16(2):277–284. 13–303/2014/16–2–277–284.
Shuler ML, Kargi F (2002) Bioprocess Engineering, 2ª. Prentice-Hall, New Jersey
Slizyte R, Dauksas E, Falch E, Il S, Rustad T (2005) Yield and composition of different fractions obtained after enzymatic hydrolysis of cod (Goadus morhua) by products. Process Biochem, London 40(3–4):1415–1424. https://doi.org/10.1016/j.procbio.2004.06.033
Sriket C, Benjakul S, Visessanguan W, Hara K, Yoshida A, Liang X (2012) Low molecular weight trypsin from hepatopancreas of fresh water prawn (Macrobrachium rosenbergii): characteristics and biochemical properties. Food Chem 68(2):147–152. https://doi.org/10.1016/S0308-8146(99)00165-X
Synowiecki J, Al-Khateeb NAAQ (2000) The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem 68(2):147–152. https://doi.org/10.1016/S0308-8146(99)00165-X
Tavano OL, Berenguer-Murcia A, Secundo F, Fernandez-Lafuente R (2018) Biotechnological applications of proteases in food technology. Compr Rev Food Sci Food Saf 17(2):412–436. https://doi.org/10.1111/1541-4337.12326
Tedford LA, Kelly SM, Price NC, Schaschke CJ (1998) Combined effects of thermal and pressure processing on food protein structure. Food Bioprod Process 76(2):80–86. https://doi.org/10.1205/096030898531837
Van Der Gronde T, Hartog A, Van Hess C, Pellikaan H, Pieters T (2016) Systematic review of the mechanisms and evidence behind the hypocholesterolaemic effects of HPMC, pectin and chitosan in animal trials. Food Chem 199:746–759. https://doi.org/10.1016/j.foodchem.2015.12.050
Wang J-P, Chen Y-Z, Yuan S-J, Sheng F-P, Yu H-Q (2009) Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Res 43(20):5267–5275. https://doi.org/10.1016/j.watres.2009.08.040
Wu L, Liu M (2008) Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr Polym 72(2):240–247. https://doi.org/10.1016/j.carbpol.2007.08.020
Yadav M, Goswami P, Paritosh K, Kumar M, Pareek N, Vivekanand V (2019) Seafood waste: a source for preparation of commercially employable chitin/chitosan materials. Bioresour Bioprocess 6(8):1–20. https://doi.org/10.1186/s40643-019-0243-y
Yen DT, May NT (2019) Optimization of enzymatic hydrolysis process from shrimp by-product for shrimp sauce production. Vietnam J Sci Technol 57(3B):97–104. https://doi.org/10.15625/2525-2518/57/3B/14426.
Zhang K, Zhang B, Chen B, Jing L, Zhu Z, Kazemi K (2016) Modeling and optimization of Newfoundland shrimp waste hydrolysis for microbial growth using response surface methodology and artificial neural networks. Mar Pollut Bull 109(1):1–8. https://doi.org/10.1016/j.marpolbul.2016.05.075
Zhang A, Wei G, Mo X, Zhou N, Chen K, Ouyang P (2018) Enzymatic hydrolysis of chitin pretreated by bacterial fermentation to obtain pure N-acetyl-d-glucosamine. Green Chem 20(10):2320–2327. https://doi.org/10.1039/C8GC00265G
Zheng K, Liang M, Yao H, Wang J, Chang Q (2013) Effect of size-fractionated fish protein hydrolysate on growth and feed utilization of turbot (Scophthalmus maximus L.). Aquac Res 44(6):895–902. https://doi.org/10.1111/j.1365-2109.2012.03094.x
Acknowledgements
Sabrina Aparecida Fabrini is thankful for the scholarship granted by the Coordination for the Improvement of Higher Education Personnel – CAPES; the work team thanks the company Tovani Benzaquen Ingredientes (São Paulo – BR), for supplying the enzymes. Eduardo Luis Cupertino Ballester thanks CNPQ for the research productivity grant – Level 1D (award number: 311456/2020-0).
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by Sabrina Aparecida Fabrini, Marlise Teresinha Mauerwerk, Eduardo Luis Cupertino Ballester, Raquel Stroher, and Fabiano Bisinella Scheufele. The first version of the manuscript was written by Sabrina Aparecida Fabrini and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study did not require any approval from the ethics committee for human or animal because this research involves only material from processing of shrimp.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fabrini, S.A., Stroher, R., Scheufele, F.B. et al. Optimization of the enzymatic hydrolysis process of shrimp viscera (Macrobrachium rosenbergii) with two commercial enzymes, aiming to produce an alternative protein source for aquaculture feed formulation. Aquacult Int 31, 807–825 (2023). https://doi.org/10.1007/s10499-022-01010-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-01010-6