Abstract
Brachyuran crustaceans are distributed worldwide and are highly valued in aquaculture. Viral infections are the most important limiting factor for the survival of these animals and, consequently, for the success of commercial farming. In this article, we provide a systematic review of the data related to viral pathogens, especially those that affect brachyuran crustacean aquaculture technically and economically, and the methods used to diagnose and control the diseases caused by these pathogens. The studies (n = 73), published between 1977 and June 2020, covered 32 virus species, most of which were from the Reoviridae (36%) and Nimaviridae (33%) families, with a focus on Callinectes sapidus reovirus 1 (CsRV1—20%) and White spot syndrome virus (WSSV—48%). The highest mortality rates due to acute events were reported in experimental trials with the blue crab Callinectes sapidus infected with CsRV1 or WSSV and with the mud crab Scylla spp. infected with Mud crab reovirus, Mud crab dicistrovirus-1, Mud crab tombus-like virus, or WSSV. The most commonly used techniques to diagnose the presence of viral pathogens are transmission electron microscopy (38.4%) and histopathology (34.2%). However, the use of molecular methods, such as polymerase chain reaction (PCR) and its variants, has increased in recent years to aid in the diagnosis of brachyuran crustaceans. Finally, improvements in cultivation management and the use of RNA interference therapies and herbal remedies have been investigated and provide valuable information for future strategies to contain and control viral infections in brachyuran crustacean aquaculture.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brachyuran crustaceans are distributed worldwide and are important fishery resources in many countries. Between 2010 and 2018, about 2 million tonnes were landed annually worldwide (FAO 2020). Several crab species are very valuable for aquaculture. Globally, about 1.2 million tonnes of these organisms were produced, with production increasing by about 34% in the last 10 years, reaching an estimated global production value of USD 12 million in 2019 (FAO 2020). In addition to the farming of hard-shelled brachyurans, there is a significant and growing industry for the production of soft-shelled crabs. These are animals that have just shed their shells and are still covered by a soft shell. Thanks to this characteristic, they can be eaten whole, making them a high-value gastronomic delicacy (Hungria et al. 2017; Tavares et al. 2018).
A major challenge to the sustainability of brachyuran crustaceans is sanitary, as the organisms can be infected and killed by a variety of pathogenic microorganisms, including protozoa (Messick and Shields 2000; Stentiford and Shields 2005), bacteria (Thibodeaux et al. 2009), and fungi (Shields and Overstreet 2003). However, viral pathogens are the most important group of infectious agents limiting the survival and farming success of crabs on a commercial scale (Shields and Overstreet 2003; Kennedy and Cronin 2007; Perazzolo et al. 2012). Data from several North American states indicate that crab mortality caused by a single viral pathogen can cause economic losses more than USD 2 million in soft-shell crab production facilities (Spitznagel 2019).
Detailed knowledge of the viral pathogens that infect brachyuran crustaceans is therefore essential for planning investments and developing strategies to mitigate the effects of viral infections in aquaculture (Shields 2003; Shields and Overstreet 2003). The aim of this review was to systematically compile data on viral pathogens that technically and economically affect brachyuran crustacean aquaculture and methods for diagnosing and controlling diseases caused by these pathogens. In addition, we have developed a flowchart to help diagnose farmed animals suspected of having a viral infection.
Materials and methods
The systematic literature review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta-analysis) guidelines (Moher et al. 2009), using Google Scholar as the search platform. The protocol for obtaining bibliographic references was applied in four phases: (1) search term selection, (2) pre-identification, (3) selection, and (4) eligibility (Fig. 1). The search focused on scientific articles, book chapters, technical reports, manuals, dissertations, and theses published until June 2020 that contained the following combination of terms in the title, abstract, or keywords: “callinectes OR scylla OR portunus OR carcinus OR eriocheir AND aquaculture AND virus.” The combination of search terms chosen for the search and selection of the bibliography related to viral pathogens was determined based on the most commonly cultivated genera of brachyuran crustacean in the world according to FAO Fisheries and Aquaculture Department database (FAO 2020).
Protocol flowchart for searching and obtaining the bibliographic references analyzed in the systematic review, based on PRISMA guidelines, applied to the compilation of data on viral pathogens that affect the cultivation of brachyuran crustaceans, as well as methods of diagnosis and disease control caused by these pathogens
The titles and abstracts of the retrieved documents were evaluated according to the following criteria: (1) the management to viral pathologies in cultured brachyuran crustaceans, (2) detection of viral pathogens in cultured brachyuran crustaceans, and (3) information on methods to control the effects of viral pathogens on brachyuran crustaceans. Exclusion criteria were (1) studies on other groups of crustaceans, such as shrimp and lobsters; (2) studies on brachyuran crustaceans that are not cultured in aquaculture; (3) studies that did not report viral infections in cultured brachyuran crustaceans; and (4) review articles and meta-analyses. There were no language restrictions in the selection of studies. All bibliographic references and PDF documents were imported using the automatic reference manager Mendeley 1.19.2 (Elsevier, The Netherlands). Duplicates were then removed, and the pre-selected texts read, resulting in the final selection of 73 documents of interest. They fully met the criteria set for this review.
The documents in PDF format selected at the end of the protocol were analyzed with the program R to observe the words most frequently used by the respective authors in the selected texts. A correlation analysis was carried out, taking into account all the main words used by the authors in each document, using special algorithms. For this, words with a frequency of ≥ 0.03% of the total number of words in each text were selected. Subsequently, each word was correlated with all other words by the Phi coefficient (ϕ), which corresponds to Pearson’s correlation coefficient for dichotomous variables.
Based on the publications selected during the systematic review, the flowchart with information on the clinical signs caused by the main viral agents in Callinectes sapidus was created using the LucidChart diagramming application (LucidChart 2020). The flowchart was created only for the diagnosis of the main viral pathogens in C. sapidus, as this species is geographically widespread and is the most important species explored in crabs farming in the Americas (Williams 1973; Melo 1996; Hungria et al. 2017; Tavares et al. 2018).
Results
The main pathogens of brachyuran crustaceans
Table 1 shows all virus species identified in brachyuran crustaceans of commercial interest in aquaculture between 1977 and 2020. A total of 32 virus species were recorded, of which nine were related to the Reoviridae; seven to the Baculoviridae family; five to the Rhabdoviridae; two to the Bunyaviridae, Dicistroviridae, and Parvoviridae; and one member each was related to the Herpesviridae, Nimaviridae, Roniviridae, Tombusviridae, and Picornaviridae families.
Among the 73 studies analyzed, the most studied virus families were Reoviridae (36%) and Nimaviridae (33%), particularly Callinectes sapidus reovirus 1 (CsRV1) and White spot syndrome virus (WSSV), which were represented in about 20 and 48% of the studies, respectively. Members of the family Baculoviridae, Bunyaviridae, Dicistroviridae, Parvoviridae, Roniviridae, and Tombusviridae were registered only once and were not studied further.
The most studied species of brachyuran crabs were the blue crab C. sapidus (59%) and the mud crab Scylla serrata (33%), both species of great importance for North American and Asian aquaculture. In addition, most studies of viral pathogens in brachyuran crustaceans have been conducted in the USA (30%), China (33%), India (10%), and Australia (5%). Although brachyuran crustaceans are considered an important fishery and aquaculture resource in South American countries, little is known about viral pathogens affecting these organisms on the continent.
RNA viruses
Viruses related to the Reoviridae
The Reoviridae is the largest and best-studied family among all double-stranded RNA (dsRNA) virus families (Mertens 2004). The viral particles of the reovirus have icosahedral symmetry, with diameters ranging from 60 to 85 nm and can be divided into two subfamilies, the “turreted” and the “non-turreted” viruses, depending on the presence of projections at the 12 vertices (Attoui et al. 2012). The dsRNA genome of reoviruses is segmented, and the number of segments varies between 9 and 12 (Attoui et al. 2012). In addition to structural classification, the degree of sequence divergence, especially for the most conserved genome segments and proteins, is also an important criterion for the classification of genera and species (Flowers et al. 2016; Zhao et al. 2021b).
Among the 15 genera of reoviruses, the only genus that infects crustaceans and is recognized by the International Committee on Taxonomy of Viruses (ICTV) is Cardoreovirus, which has 12 genome segments and infects the Chinese crab Eriocheir sinensis (Zhang et al. 2004; Attoui et al. 2012). However, since the first discovery of a reovirus in the marine crab Macropipus depurator in 1966, nine reoviruses have been reported in five crab species important for aquaculture, of which eight reoviruses have negative impacts on crops: W2, CsRV1, Eriocheir sinensis reovirus 905 (EsRV 905), E. sinensis reovirus 816 (EsRV 816), E. sinensis reovirus WX-2012 (EsRV WX-2012), Mud crab reovirus (MCRV) or Scylla serrata reovirus (SsRV), Swimming crab reovirus (SCRV), and Gill virus of Carcinus mediterraneus.
W2
About 20 years after the first discovery of a reovirus in a marine invertebrate, W2 reovirus was detected in C. mediterraneus from Prevost Lagoon and along the Mediterranean coast near Montpellier, France (Mari and Bonami 1987, 1988b). W2 virus has a paraspheric shape and no envelope, measures 65 to 70 nm in diameter, and forms unusual viral structures (rosettes) in the connective tissues of the hepatopancreas, digestive tract, gills, and hemocytes (Mari and Bonami 1988b). W2 is a 12-segment dsRNA virus with an electrophoretic pattern of 1/5/6 (Mari and Bonami 1988b). In experiments under controlled conditions (Mari and Bonami 1987, 1988b), crabs infected with W2 showed clinical signs of disease including lethargy, loss of appetite, weakness, and death after 20 days of rearing in a recirculating system. Although there are reports of deaths due to W2 infection, the discovery and study of this reovirus is still very recent, so there are no detailed reports in the literature on the prevalence, pathogenicity, and repeated detection of this reovirus in recent years.
CsRV1
At the same time as the first reoviruses were described in the Mediterranean, the pathogenic reovirus RLV (Reo-like virus) was identified in C. sapidus collected in the Atlantic Ocean, along the Chesapeake Bay in Maryland and Chincoteague Bay in Virginia, USA (Johnson 1977). Between 2005 and 2007, a reovirus was again detected in C. sapidus caught in the estuaries of the US Atlantic coast and named C. sapidus reovirus (CsRV) (Bowers et al. 2010; Tang et al. 2011). More recently, CsRV was renamed CsRV1 when a second reovirus was discovered in C. sapidus, collected in southern Brazil and tentatively named CsRV2 (Flowers et al. 2016; Zhao et al. 2021a).
CsRV1 is an unenveloped virus with an icosahedral capsid about 55 nm in diameter, and the virions are frequently associated with microtubules and microfilament structures and form a paracrystalline arrangement (Johnson 1983; Bowers et al. 2010; Zhao et al. 2021b). The CsRV1 genome consists of 12 dsRNA segments ranging in size from 4.3 to 1.1 kb, with an electrophoretic pattern of 1/5/6 (Bowers et al. 2010). Flowers et al. (2016) propose that CsRV1 belongs to a new genus of the Reoviridae family, called Crabreovirus, which has not yet been recognized as a genus by ICTV. CsRV1 infects tissues of mesodermal and ectodermal origin such as hemocytes, epidermis, nervous system, digestive tract, gills, muscles, and hepatopancreas and produces basophilic cytoplasmic inclusions that increase cell volume (Bowers et al. 2010; Chung et al. 2015). Infected hemocytes invade the glial cells of the cerebral and thoracic ganglia, which become necrotic (Johnson 1977, 1984). Due to neuronal necrosis, crabs become sluggish, show tremors, and eventually complete paralysis (Shields and Overstreet 2003).
In recent years, CsRV1 has become a major concern for crab aquaculture in the USA, as it is one of the species associated with episodes of high and rapid mortality of C. sapidus in soft-shell crab production systems (Spitznagel et al. 2019). Figure 2 shows the result of the correlation analysis performed based on the most frequently used terms by the authors and assessed in the selected documents using the PRISMA method. It was found that the selected articles were mainly concerned with on aspects related to the presence of CsRV1 reovirus in C. sapidus in soft crab production facilities, mainly in the states of Maryland, Virginia, and Florida. The reovirus was detected in more than 50% of dead or dying crabs in crab farms but was present in less than 5% of healthy crabs and 7% of crabs that successfully molted (Bowers et al. 2010; Spitznagel et al. 2019). Furthermore, experimental CsRV1 infections by intramuscular injection in C. sapidus caused 100% mortality after 10 to 13 days and resulted in the appearance of viral inclusions in hemocytes (Johnson 1977; Bowers et al. 2010).
Main terms used (≥ 0.05%) and Phi correlation (Φ ≥ 0.7) between them, in the 73 main scientific documents on viral pathogens in brachyuran crustaceans published between 1977 and June 2020. Note that the most cited viruses in the documents analyzed are the white spot virus “WSSV” and the Callinectes sapidus reovirus “CsRV.” The latter is correlated with terms that are related to the host, places where this virus was detected, and the main authors who published information about the reovirus
MCRV or SsRV
According to Zhao et al. (2021b), half of the reoviruses infecting brachyuran farmed crabs have been identified in China, including MCRV, also called SsRV, which infects the mud crab S. serrata (Weng et al. 2007; Huang et al. 2012; Deng et al. 2012). The MCRV genome consists of 12 dsRNA segments ranging in size from 4.6 to 1.1 kb, with an electrophoretic pattern of 1/5/6 (Weng et al. 2007; Chen et al. 2011b). This cytoplasmic virus infects connective tissue cells of the hepatopancreas, gills, and mud crab gut (Jithendran et al. 2009). Deng et al. (2012) propose that MCRV belongs to the genus Crabreovirus together with CsRV1.
According to Weng et al. (2007), MCRV virus causes high mortality and large economic losses in mud crab farming in Guangdong Province, China. The authors proved by histopathological analysis and tests with intramuscular injections, oral inoculation, immersion, and cohabitation that MCRV is the causative agent of sleeping sickness (SD) of S. serrata. The main clinical signs of the disease are sluggishness, loss of appetite, and a slight grayish coloration of the body (Weng et al. 2007). In addition, the authors observed that animals naturally infected with MCRV had atrophied hepatopancreas, empty gut, and yellowish gills. Reovirus is highly pathogenic to mud crabs, and transmission appears to be via the gut and body surface, as infection in experimentally infected S. serrata by intramuscular injection, immersion, or oral inoculation caused a mortality rate of 100% after 10 days and a mortality rate of 80% in cohabitation after 20 days (Weng et al. 2007). The characteristic signs of reovirus infection were observed from the fourth day after experimental infection (Weng et al. 2007).
EsRV
Since the first report, in 1994, in Jiangsu Province, China, tremor disease (TD) has been considered one of the most serious diseases of E. sinensis, causing great economic losses due to the high in crab farms (Zhang et al. 2002). Crabs with TD have leg tremors, sluggishness, and loss of appetite. The mortality rate can be as high as 70%, but to date, the causative agent of TD is unknown (Chen et al. 2011a, b). In studies to identify the causative agent of TD, Zhang et al. (2004) characterized the first E. sinensis reovirus, as EsRV905. EsRV905 develops in the cytoplasm of connective tissue cells of the hepatopancreas and gills and forms intracytoplasmic viral inclusions and necrotic lesions (Zhang et al. 2004). In experimental infections, purified EsRV905 caused about 30% mortality in crabs, but no typical signs of tremors were observed in E. sinensis, suggesting that EsRV905 can infect and replicate in E. sinensis but may not be the cause of TD in this crab (Zhang et al. 2004).
Later, Zhang and Bonami (2012) discovered a second type of reovirus isolated from E. sinensis in China, named EsRV816. Similar to EsRV905, EsRV816 develops in the cytoplasm of connective tissue cells of all organs, especially the hepatopancreas, gills, heart, and intestine, and forms eosinophilic inclusion bodies (Zhang and Bonami 2012). After experimental infection by injection of the purified virus, no signs of disease such as leg tremors were observed, but 28% of the crabs died 20 days after infection (Zhang and Bonami 2012).
A third virus, EsRVWX-2012, was also identified in E. sinensis crabs showing signs of TD, but no experimental infection was performed (Shen et al. 2015). EsRV905, EsRV816, and EsRVWX-2012 have non-enveloped icosahedral virus particles and infect the connective tissue of E. sinensis. However, EsRV905 and EsRV816 have particle sizes of 55 and 60 nm in diameter, respectively (Zhang et al. 2004; Zhang and Bonami 2012), while EsRVWX-2012 is larger at 60–70 nm in diameter (Shen et al. 2015). In addition to size differences, they also show differences in electrophoresis patterns. EsRV905 is 3/4/5, and EsRVWX-2012 is 1/5/6 with 12 segments, while EsRV816 has 10 segments with a 5/3/2 electrophoresis pattern (Zhang and Bonami 2012; Zhao et al. 2021b).
Based on its ultrastructure, physicochemical properties, and phylogenetic relationships, EsRV905 was placed in the new genus Cardoreovirus by ICTV (Attoui et al. 2012). Interestingly, EsRV816 showed very low homology to the putative RNA-dependent RNA polymerase (RdRp) sequences of EsRV905. Based on the phylogenetic relationships of viral polymerase, EsRV816 was classified into a new genus distinct from Cardoreovirus, Crustareovirus (Zhang et al. 2004; Zhang and Bonami 2012; Zhao et al. 2021b). EsRVWX-2012 was classified as a member of the genus Crabreovirus (Shen et al. 2015; Zhao et al. 2021b).
SCRV
Aquaculture of the swimming crab P. trituberculatus has suffered significant economic losses in China due to an epidemic that has caused high mortality in recent years (Zhang et al. 2015). Conventional molecular epidemiological studies and artificial infection tests have shown that the causative agent of the disease is SCRV (Li 2012; Li et al. 2012). The SCRV particle is icosahedral and non-enveloped and measures 30 ± 10 nm, which is considered smaller than most reoviruses (Zhang et al. 2015). Although the virus acronym can be confused with viruses infecting S. serrata, there is no evidence that SCRV can infect the mud crab. SCRV can cause severe hemorrhage in P. trituberculatus, resulting in mortality of up to 100% in as little as 10 days (Li 2012; Li et al. 2012; Fang et al. 2015). No experimental infection has been performed for SCRV, and further details on the structure of the genome are also not available in the literature.
Gill virus of C. mediterraneus
According to Johnson (1983), the gill virus of C. mediterraneus is paraspheric, non-enveloped, and 55 nm in diameter. The virus has been associated with the Reoviridae family. However, few details about the structure of the genome are found in the literature (Bonami et al. 1976; Johnson 1983). The virus infects and destroys epithelial cells, especially those of the gills. Inoculation of purified virus by intramuscular injection into healthy C. mediterraneus resulted in mortality in only 8 days (Johnson 1983). Crabs are also fatally infected with reovirus by the presence of tissues from infected diseased animals in culture ponds (Bonami et al. 1976). However, there are no detailed reports in the literature on the prevalence, pathogenicity, and repeated detection of this reovirus in recent years.
Viruses related to the Rhabdoviridae
The family Rhabdoviridae comprises 30 genera of enveloped, typically bacilliform viruses with single-stranded negative-sense RNA genomes (Walker et al. 2018). In addition to plants and vertebrates, members of the Rhabdoviridae family are also found in invertebrates, including crustaceans (Johnson 1984). Four viruses related to the Rhabdoviridae family have been detected in brachyuran crustaceans, Rhabdo-like virus A (RhVA), Rhabdo-like virus B (RhVB), Y-organ virus of C. mediterraneus, and Enveloped helical virus (EHV). Of the known rhabdoviruses, only RhVA has harmful effects on aquaculture.
RhVA
In his studies of the neuromuscular connections of the gastric muscles of the blue crab C. sapidus, Jahromi (1977) first identified a virus similar to the Rhabdoviridae found mainly in glial cells. Later, Yudin and Clark (1979) detected the same virus in the cells of the mandibular gland (ecdysial gland) of C. sapidus and initially named it EGV-2, due to its association with the ecdysial gland (Jahromi 1977; Yudin and Clark 1979). Years later, reovirus was renamed Rhabdo-like virus A (Johnson 1983; Shields and Overstreet 2003). RhVA is a bacilliform virus that is much smaller than other species in the family Rhabdoviridae. It has a diameter of 25 to 30 nm and a length of 100 to 150 nm and forms a paracrystalline arrangement in the cytoplasm of cells (Johnson 1983; Shields and Overstreet 2003). According to Johnson (1977), RhVA develops in the endoplasmic reticulum and infects hemopoietic tissue cells, hemocytes, connective tissue cells, reserve cells associated with connective tissue, epidermis, epicardial tissue, and glial cells of ganglia and nerves.
The manifestation of RhVA infection is associated with host stress, mainly due to transport and maintenance stress in cultivation systems (Jahromi 1977), physiological stress resulting from removal of the eyestalk (Yudin and Clark 1979), and by coinfection with other viruses, such as CsRV1, Baculo-B, CBV, and EHV (Johnson 1983, 1984). Experimental infection by injection of RhVA and CsRV1 resulted in mortality of C. sapidus after only 3 days under laboratory conditions (Johnson 1983; Shields and Overstreet 2003). In general, RhVA infection does not cause clinical signs in C. sapidus. However, Johnson (1983) observed that animals infected with RhVA and CsRV1 simultaneously showed paralysis.
Viruses related to the Bunyaviridae
The order Bunyavirales was recently introduced to group viruses with single-stranded negative sense RNA (-ssRNA), consisting of 2 to 4 segments (Maes et al. 2019). The order comprises 10 families. To date, however, there is only one viral family of bunyaviruses that infects crustaceans (Cruliviridae), and it contains a single member officially recognized by the ICTV, the Wenling lincruvirus (Maes et al. 2019). However, there are several viruses similar to bunyavirus that have been tentatively proposed to infect brachyuran crabs, such as Crab haemocytic virus (CHV) (Johnson 1983) and Carcinus maenas portunibunyavirus 1 (CmPBV1) (Bojko et al. 2019).
CHV
CHV is a spherical virus 55 to 80 nm in diameter found in the intracytoplasmic membrane of hemocytes and sometimes associated with the Golgi complex of the European crab C. maenas (Johnson 1983). CHV infection causes coagulation disorders, a reduction in the number of circulating hemocytes and can lead to death of C. maenas (Bang 1971; Hoover 1977). The main clinical sign of infection is seen when the hemolymph of the crab is removed and clots do not form (Bang 1971; Hoover 1977). Although it causes deleterious effects and even death of C. maenas, details of the CHV genome, prevalence, and effects on survival of the species are still unknown.
CmPBV1
CmPBV1 is the most recent virus detected in a brachyuran crustacean important for aquaculture, C. maenas. CmPBV1 was detected and sequenced by Bojko et al. (2019) in samples of C. maenas collected in the Faroe Islands, Denmark. The viral particles of CmPBV1 are spherical and 96.6 ± 12.2 nm in diameter, and their genome consists of three single-stranded RNA segments with negative-sense (Bojko et al. 2018, 2019). In previous studies, Bojko et al. (2018) observed that CmPBV1 was present at a prevalence of 1.1% in C. maenas collected off the coast of Nesvík, Faroe Islands. Infection resulted in eosinophilic inclusions in branchial epithelium, connective tissue, amebocytes, and hemocytes caused by the development of paracrystalline arrays. Although the effects of infection with CHV and the bunyavirus CmPBV1 on crab survival have not been described, Zhang et al. (2004) found, in a study of the reovirus EsRV905, that coinfection with EsRV905 and a bunyavirus-like virus increased the mortality rate of E. sinensis by up to 30%.
Viruses related to the Dicistroviridae
Mud crab dicistrovirus-1 (MCDV)
In studies to discover the causative agent of mortality in S. paramamosain mortality, Guo et al. (2013) discovered MCRV reovirus and found an additional virus, about 30 nm in diameter, that infects the mud crab simultaneously. The virus is icosahedral, non-enveloped, and 30 nm in diameter and has a genome of positive single-stranded RNA (ssRNA) (Zhang et al. 2011; Guo et al. 2013) and was named Mud crab dicistrovirus-1 (MCDV). The results of phylogenetic analysis by Guo et al. (2013) suggest that MCDV has a closer genetic relationship with Taura syndrome virus (TSV) than other dicistroviruses and that MCDV is a new member of the family Dicistroviridae, assigned to the genus Aparavirus (Guo et al. 2013).
In experimental infection of S. paramamosain, the intramuscular injection of MCDV by Guo et al. (2013) resulted in 100% mortality in only 7 days, while no crabs died in the control group. MCDV was detected in various organs such as gills, intestine, stomach, heart, hepatopancreas, gonads, muscles, hemolymph, and thoracic ganglia of S. paramamosain (Guo et al. 2013). In addition, the crabs showed clinical signs of infection, such as loss of appetite and behavioral changes, such as no responding to touch and staying on the sand surface during the day.
Viruses related to the Picornaviridae
Chesapeake bay virus (CBV)
Chesapeake bay virus (CBV) was first detected in juvenile C. sapidus collected from Tangier Sound, in the Chesapeake Bay during the summer (Johnson 1978, 1983). CBV is a single-stranded, icosahedral, and non-enveloped RNA virus about 30 nm in diameter that occurs in the cytoplasm of ectodermal cells, sometimes forming paracrystalline arrays (Johnson 1983). CBV infects neurosecretory cells, neurons, epidermis, gill epithelia, and foregut and hindgut epithelia (Shields 2003; Shields and Overstreet 2003). According to Johnson (1983), CBV can cause destruction of the central nervous system, branchial epithelium, and neurosecretory cells, leading to difficulties in gas exchange, osmotic control, and various vital processes in the blue crab, and can even lead to death.
In addition, omatids are often severely damaged, leading to blindness of the blue crab and behavioral changes in the host, such as swimming without orientation and remaining in a position with the head facing the abdomen. These behavioral changes allow infected crabs to be easily preyed upon, promoting cannibalism (Johnson 1983; Shields and Overstreet 2003). CBV is a virus that is pathogenic to juvenile and adult C. sapidus and can kill the host within 2 weeks to 2 months under laboratory conditions (Johnson 1983; Messick and Sindermann 1992a). There is also evidence that CBV may have a synergistic effect with other viruses such as CsRV1, RhVA, or EHV (Johnson 1983).
Viruses related to the Roniviridae
Sigh disease (SD) is widespread in E. sinensis farms in Chinese provinces of Jiangsu, Zhejiang, Anhui, and Shanghai (Zhang and Bonami 2007). The disease is characterized by sighing sounds that can be clearly heard in affected crabs due to slow blistering during breathing. In investigations into the causative agent of SD, Zhang and Bonami (2007) found a Roniviridae-like virus that grows in connective tissue cells and named it E. sinensis ronivirus (EsRNV).
EsRNV is an enveloped bacilliform virus with a diameter of 24 to 42 nm and a length of 60 to 170 nm, and the nucleocapsids are 16 to 18 nm in diameter and 150 to 250 nm long, forming parallel arrays (Zhang and Bonami 2007). The inclusion bodies in the cytoplasm observed with the light microscopy are 200 to 800 nm in size. In the study by Zhang and Bonami (2007), EsRNV was detected in lymphoid organs, gill connective tissue, hepatopancreas, heart, and intestine. Light microscopic analysis revealed basophilic inclusion bodies in the cytoplasm of lymphoid organs and intestinal cells, as well as apoptosis in these organs. Eosinophilic foci of necrotic connective tissue cells have also been described (Zhang and Bonami 2007). These features can be considered typical for members of the Roniviridae family.
Ronivirus was experimentally inoculated into E. sinensis by intramuscular injection and caused 100% mortality after 17 days, with deaths beginning on the thirteenth day (Zhang and Bonami 2007). Signs of SD were observed in about 30% of the animals infected with EsRNV, confirming that the ronivirus is the etiological agent of SD. In addition, the crabs showed sluggishness and anorexia.
Viruses related to the Tombusviridae
Members of the family Tombusviridae are envelope-less and have single-stranded RNA (ssRNA) genomes with positive sense (Hyodo and Kaido 2021). In addition to the detection of MCDV dicistrovirus, Gao et al. (2019) identified an icosahedral virus with a diameter of 72 nm not previously detected by molecular biology methods, the Mud crab tombus-like virus (MCTV), when studying individuals of S. paramamosain. In an experimental infection of S. paramamosain from a farm in Guangdong Province, China, Gao et al. (2019) observed that the viral load of MCTV in the hepatopancreas and hemocytes of the crabs increased significantly during the course of infection, but clinical signs of infection were not reported. Moreover, 60% of the crabs died after 96 h of MCTV infection, confirming the pathogenicity of this virus in S. paramamosain (Gao et al. 2019).
DNA viruses
Viruses related to the Baculoviridae
Members of the family Baculoviridae are double-stranded, enveloped, occluded, or non-occluded DNA viruses that infect the nucleus of the host cell (Anderson and Prior 1992). Baculoviruses are the largest group of viruses known in decapod crustaceans (Johnson 1988; Anderson and Prior 1992; Shields and Overstreet 2003). Eight baculoviruses have been detected in five crustacean species of aquaculture concern, including Baculovirus-B viruses (Baculo-B), C. mediterraneus hepatopancreas baculovirus, Penaeid rod-shaped DNA virus (PRDV), and Tau viruses (T virus), which have a negative impact on brachyurans farming.
Baculo-B
Baculo-B is a non-occluded, enveloped virus that shares many similarities with Baculovirus-A, except that it infects hemocytes and hematopoietic cells and often produces hyperchromatic areas in the center of the nucleus and hypertrophied homogeneous or contoured chromatin nuclei (Johnson 1983). Baculo-B viral particles have an ovoid shape, about 100 nm in diameter, and 335 nm in length, with conical and rounded ends (Shields and Overstreet 2003). The virions tend to form ordered matrices in the nucleoplasm, and their development is associated with intranuclear vesicles (Johnson 1983). Baculo-B has been detected in C. sapidus in Chesapeake and Chincoteague bays, Maryland and Virginia, respectively (Johnson 1983; Messick 1998). Experimentally infected crabs showed signs of disease, but only coinfection of Baculo-B with CsRV1 and RhVA viruses resulted in mortality (Johnson 1983; Messick 1998).
Baculovirus of the hepatopancreas of C. mediterraneus
A baculovirus was detected in the nucleus of cells of the hepatopancreas and midgut epithelium of C. mediterraneus collected off the coast of France (Pappalardo and Bonami 1979). The viral particles of baculovirus are bacilliform, enveloped, and measure 80 to 90 nm in diameter and 340 to 380 nm in length. Unlike other baculoviruses, the C. mediterraneus baculovirus is curved when in the envelope, but when the envelope is ruptured by osmotic shock, the virion remains straight. Infection with baculovirus in C. mediterraneus causes hypertrophy of the nucleus of epithelial cells and can be fatal to infected animals (Johnson 1983). Under laboratory conditions, this virus was transmitted to C. mediterraneus orally, by eating infected tissue and by intramuscular injection.
PRDV
The PRDV is an enveloped, non-occluded, ovoid virus with a diameter of about 84 nm and a length of 226 nm (Inouye et al. 1996). This virus is the causative agent of penaeid acute viraemia (PAV), a disease known to cause massive mortality in Penaeus japonicus shrimp farms. In the study by Momoyama et al. (1999), the pathogenicity of PRDV was investigated by infection testing using intramuscular and oral inoculation methods in juveniles of six crustacean species, including Penaeus spp. and the crab P. trituberculatus. All juvenile shrimp Penaeus spp. tested died 2 to 7 days after inoculation, resulting in up to 100% mortality. On the other hand, P. trituberculatus were significantly less sensitive to the virus compared to the other species tested. Two days after intramuscular injection, mortality was 10% and 30 days after oral inoculation, and mortality was only 4%. The crabs infected with PRDV showed hypertrophy of the nuclei of the epidermal cuticular cells of the stomach. However, no obvious clinical signs were observed in the animals.
T virus
The T virus, designated by the Greek letter tau, was first observed in samples of C. mediterraneus crabs in the lagoon Thau, France (Pappalardo et al. 1986). The T virus has the same general characteristics as the non-occluded baculoviruses, except for the arcuate rather than the rod-shaped particles typical of baculoviruses. The average particle size is 80 nm in diameter and 350 nm in length and forms parallel arrays in the nucleoplasm. Experimental transmission of the T virus caused lesions in cells (E, R, F, and B) of the hepatopancreas and midgut epithelium of C. mediterraneus. The first sign of infection was nuclear hypertrophy associated with disappearance of nucleoli and chromatin margination (Pappalardo et al. 1986). Infected crabs showed a decrease in aggressiveness, followed by an increase in lethargy and loss of appetite, leading to immobility and death a few days later. The mortality rate of infected animals was 100% after 25 days, compared to 20% in the control group (Pappalardo et al. 1986).
Viruses related to the Herpesviridae
Bi-facies virus (BFV)
The BFV, formerly known as herpes-like virus (HLV), is an icosahedral, enveloped virus consisting of a double-stranded DNA genome that infects the nucleus or cytoplasm of hemocytes and hemopoietic cells of C. sapidus (Johnson, 1977; Shields and Overstreet 2003). BFV virus can also infect connective tissue and gill epithelial cells, but has not been observed in muscle, heart, intestinal epithelial, gonadal, or nervous tissue (Johnson 1983). The full development of the bi-facies virus takes place in the nucleus, where this virus exhibits two types of development leading to two final forms. Type A enveloped particles with two envelopes 197 to 233 nm in diameter and type B particles with only one envelope 174 to 191 nm in diameter (Johnson 1988).
The experimental infection tests carried out by Johnson (1978) by intramuscular injection of hemolymph from a dying crab and oral ingestion of infected tissue as food resulted in death after 30 to 40 days. Infected crabs showed no clinical signs of infection until shortly before death, when they became inactive and stopped eating. Bi-facies virus has also been detected in wild populations of C. sapidus in Assawoman, Delaware, and Chincoteague Bays on the US East Coast with a prevalence of 13% (Johnson 1983). Naturally, infected crabs, however, can survive for at least 60 days (Johnson 1983).
Viruses related to the Parvoviridae
Parvo-like virus of C. mediterraneus
Mari and Bonami (1988a) have detected a small viral pathogen in the crab C. mediterraneus. The virus has some similarities to members of the family Parvoviridae due to its characteristics and physicochemical properties. The authors named it PC84, an icosahedral, non-enveloped virus with a diameter of about 23 to 27 nm (Mari and Bonami 1988a). This parvovirus infects the nucleus and cytoplasm of fibroblast and myoepithelial cells in the connective tissue of the hepatopancreas, midgut, and gills of C. mediterraneus, causing necrosis and defense reactions in some areas, characterized by the accumulation of degenerative cells such as nodules. Mari and Bonami (1988a) also visualized and found that the nuclei of infected cells did not have chromatin but an amorphous electron-dense material with virus particles in lighter areas. Experimental infection of C. mediterraneus by intramuscular injection of PC84 caused signs of weakness and loss of appetite and resulted in death of the animals 10 to 25 days after inoculation (Mari and Bonami 1988a).
Viruses related to the Nimaviridae
WSSV
There are many viruses that have a broad host range. One of these is WSSV, which was formerly known as baculovirus but was later classified by the ICVT as a species of the genus Whispovirus, which belongs to the family Nimaviridae (Vlak et al. 2005; Wang et al. 2019). WSSV has been known to penaeid shrimp researchers and farmers for more than three decades and is still considered the pathogen that most affects the sustainability and growth of shrimp farming worldwide. This virus is the causative agent of white spot disease (WSD), which causes high mortality in shrimp and damage to shrimp farming worldwide (Stentiford et al. 2009, 2010; Lightner 2011; Bateman et al. 2012).
WSSV is an enveloped, non-occluded, rod-shaped virus 70 to 150 nm in diameter and 275 to 380 nm in length with a tail-like appendage and a striated nucleocapsid (Durand et al. 1997; Vlak et al. 2005). WSSV infects all tissues of mesodermal and ectodermal origin, such as gills, lymphoid organs, cuticular epithelium, and subcutaneous connective tissue (Lightner 1996). The nuclei of infected cells become hypertrophic with marginalized chromatin and contain inclusion bodies that are highly eosinophilic in early stages of infection and basophilic in advanced stages of infection (Lightner 1996). Although the virus is most commonly associated with penaeid shrimp farmed in tropical regions, it is also capable of infecting, sickening, and killing a wide range of other decapod crustaceans, including crabs important to aquaculture such as C. maenas, E. sinensis, S. serrata, S. olivacea, S. paramamosain, S. tranquebarica, P. pelagicus, P. sanguinolentus, P. trituberculatus, C. sapidus, C. arcuatus, and C. danae.
Exposure tests based on natural (food) and artificial (intramuscular injection) routes of WSSV exposure in Cancer pagurus, C. maenas, and E. sinensis have shown that the species tested are considered susceptible to WSSV infection and that the virus is able to replicate and remain virulent, except in the case of C. maenas (Bateman et al. 2012) which did not suffer significant mortality when exposed to WSSV. In this case, the presence of the characteristic intranuclear inclusion bodies was observed only when injected intramuscularly, but with a much lower severity than in other species. In contrast, E. sinensis suffered from high mortality regardless of whether the animals were infected by feeding or by intramuscular injection, and showed the classic WSD pathology, except for white spots on the cuticle and a reddish coloration of the body. Later studies have also confirmed the pathogenicity and virulence of WSSV in E. sinensis (Ding et al. 2015, 2017a). According to Ding et al. (2015), injections of WSSV caused 100% mortality in only 10 days in tank-reared E. sinensis in eastern China.
Experimental WSSV infection studies have also confirmed pathogenicity and virulence in another important species in Asian aquaculture, the mud crab, S. serrata (Supamattaya et al. 1998; Ika Maharani 2005; Liu et al. 2011b; Raja et al. 2019). In the studies by Liu et al. (2011a, b), a tenfold serial dilution of a WSSV inoculum was injected intramuscularly into the mud crab. After 10 days, the mortality rate ranged from 38.9 to 100% for the most dilute or concentrated dose. The gill epithelial cells of the infected crabs showed hypertrophied nuclei with basophilic inclusions, and the infected animals showed signs of weakness, lethargy, reduced feed intake, loosening of the caecum, delayed hemolymph coagulation, and yellowish hemolymph. In the study by Raja et al. (2019), not only was there a high mortality rate in WSSV-infected mud crabs, but there was also a 60% reduction in total hemocyte count (THC) in infected crabs compared to healthy animals. Oral and water-based exposure tests conducted by Ika Maharani (2005) have also shown that WSSV not only causes mortality in S. serrata juveniles (72%), but can also affect crabs at zoea stage 1 (80%) and zoea stage 3 (40%), jeopardizing the success of larval rearing.
WSSV has also been detected in other species of the genus Scylla, such as S. olivacea (Norizan et al. 2019), S. paramamosain (Somboonna et al. 2010; Wang et al. 2017, 2018; Norizan et al. 2019; Kong et al. 2020; Lin et al. 2020; Ren et al. 2020), and S. tranquebarica (Rajendran et al. 1999; Jithendran et al. 2009; Norizan et al. 2019). Among these species, S. olivacea appears to be more susceptible to WSSV than S. paramamosain. According to Somboonna et al. (2010), WSSV injections (1 × 106 virus copies/g) caused 100% mortality in S. olivacea after 7 days. The S. paramamosain crabs survived 2 weeks after injection. For Somboonna et al. (2010), these results suggest that susceptibility to white spot disease in the genus Scylla is species and possibly dose-dependent. For mud crab farmers, the results suggest that to avoid losses during seasonal WSSV outbreaks, cultivation of S. paramamosain may be preferable to S. olivacea.
WSSV has also been detected in P. pelagicus (Lo et al. 1996; Kou et al. 1998a; Wang et al. 1998; Waikhom et al. 2006), P. sanguinolentus (Lo et al. 1996; Wang et al. 1998; Waikhom et al. 2006), and P. trituberculatus (Kou et al. 1998a; Meng et al. 2009). In experimental infection trials by intramuscular injection and ingestion of contaminated tissue, the virus caused 100% mortality in P. pelagicus in only 9 days, and the infected animals were apparently healthy and fed until the moment of death (Supamattaya et al. 1998). The crabs P. sanguinolentus and P. pelagicus also showed varying susceptibility to WSSV depending on the isolate. Mortality ranged from 50 to 100% after feeding on the tissues from shrimp infected with different South Indian isolates (G9 and A117), while shrimp fed isolate G27 showed no mortality (Waikhom et al. 2006).
Other studies have also highlighted the potential of WSSV to infect wild or cultivated populations of C. sapidus and cause adverse effects (Krol 2002; Shields and Overstreet 2003; Costa et al. 2012b; Powell et al. 2015), C. arcuatus (Galavíz-Silva et al. 2004), and C. danae (Costa et al. 2012b). Most studies confirmed that Callinectes spp. can serve as a biological reservoir for WSSV. On the other hand, WSSV exposure tests by natural means, ingestion of infected tissues, resulted in mortality rates of 66% in C. sapidus (Krol 2002), while intramuscular injection resulted in mortality rates of up to 100% (Blaylock et al. 2019). In addition, injected animals had a higher viral loads than animals fed infected tissue (Blaylock et al. 2019).
Techniques for detecting viral pathogens in brachyuran crustaceans
The most common clinical signs caused by viral infections in brachyuran crustaceans are weakness, lethargy, and decreased food intake (Bonami and Zhang 2011; Johnson 1977; Yago 1966). Some viruses can cause other specific clinical signs that may help in diagnosis, such as tremors and paralysis, as in the late stages of CsRV1 infection in C. sapidus (Shields and Overstreet 2003), yellowish hemolymph and looseness of appendages in WSSV infection (Liu et al. 2011b), and changes in swimming and posture in CBV infection (Johnson 1983; Shields and Overstreet 2003).
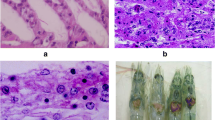
However, clinical signs alone are not decisive for the diagnosis of infections by viral pathogens in crabs. Some viruses may not cause obvious clinical signs, or these may only occur in more severe infections. Another problem is that the signs are not specific and may also be caused by other non-viral infectious agents, so it is necessary to use reliable and validated techniques for diagnosis. Figure 3 shows in the form of a flowchart how the combination of clinical signs, histopathological analysis, and electron microscopy analysis can contribute to the detection of specific viral pathogens that affect the cultivation of C. sapidus technically and economically. Transmission electron microscopy (TEM) and histopathology have been among the most commonly used techniques in the investigation of viral pathogens in brachyuran crustaceans from the earliest studies to the present day. Of the documents analyzed in this review, 38.4% used TEM to detect viruses and 34.2% used histopathology. Both techniques are reliable for the detection of viruses in crustaceans and essential for discovery and characterization of the biology of new viruses, making them standard tools for the diagnosis of WSSV infection according to the World Organization for Animal Health (OIE 2018). However, according to Pappalardo et al. (1986), TEM showed limitations in detecting Tau virus particles in C. mediterraneus. In the study, the virus was detected in only 55% of the experimentally infected animals, although all crabs died with typical signs of the disease. Figure 4 shows transmission electron micrographs of viral morphology representative of the major viral pathogens of C. sapidus discussed in the previous flowchart.
Transmission electron micrographs of the main viral agents that affect Callinectes sapidus. A Callinectes sapidus reovirus 1 (CsRV1) virus particles (Zhao et al. 2021b); B White spot syndrome virus (WSSV) enveloped virions (black arrow with white borders) and the nucleocapsid (white arrow), scale bar = 10 μm (Ding et al. 2015); C bi-facies virus (BFV) viral particles (scale bar not disclosed by the authors) (Shields and Overstreet 2003); D paracrystalline arrays of Chesapeake Bay virus (CBV) in the cell cytoplasm (scale bar not disclosed by the authors) (Shields and Overstreet 2003); E Rhabdo-like virus A (RhVA—black arrow with white borders) in the endoplasmic reticulum of the cell additionally infected with reo-like virus (RLV—white arrow) (Shields and Overstreet 2003); F Baculovirus B present in the cell nucleus (black arrow with white borders) (Shields and Overstreet 2003)
After the development of the molecular technique based on the polymerase chain reaction (PCR) as late as the 1980s (Mullis et al. 1986), it was used in studies on viral pathogens (26%) (Fig. 5). In addition to molecular PCR, a wide range of techniques were used to diagnose the presence of pathogenic viruses in brachyuran crustaceans in studies published between 1977 and 2020, including in situ hybridization (6.8%), genomic sequencing (11%), RT-qPCR or qPCR (32.9%), RT-LAMP (1.4%), and immunoassay tests (5.5%).
In situ hybridization is a cytogenetic technique based on the detection of small DNA or RNA fragments in tissue samples using specific probes. Complementary nucleotide sequences are labeled with fluorochromes and visualized by light microscopic fluorescence or epifluorescence (Chang et al. 1998). According to Chang et al. (1998), this technique has already proven successful for the detection of WSSV in shrimps, crabs, and lobsters, including P. sanguinolentus, and allows the identification of the virus in the target organs and tissues of the hosts. Liessmann et al. (2005), Momoyama et al. (1999), and Tang et al. (2011) also used in situ hybridization to determine the presence of the baculoviruses SBV and PRDV and the reovirus CsRV1 in S. serrata, P. trituberculatus, and C. sapidus, respectively. In situ hybridization offers high specificity, where the positive reaction occurs only with the target virus, and can therefore distinguish between viruses that are morphologically similar (Tang et al. 2011). However, this technique requires that the region infected by the virus be identified by visualizing the stained areas, which is less sensitive than PCR-based tests.
With the development of high-throughput PCR and next-generation sequencing (NGS), several viral genomes have been detected in brachyuran crustaceans, including WSSV (Galavíz-Silva et al. 2004; Bateman et al. 2012; Ding et al. 2015; Gunasekaran et al. 2018), CsRV1 (Bowers et al. 2010; Rogers et al. 2015a), PRDV (Momoyama et al. 1999), and SBV (Liessmann et al. 2005). Although PCR is widely used in studies, this technique alone is not suitable for quantifying the viral load present in the host, as it only detects the presence or absence of the viral genome by amplification of specific DNA fragments and visualization by electrophoresis (Bateman et al. 2012). In reoviruses, the pattern (number and size) of genome segments is also a criterion for identifying different species in a sample. It can be analyzed by agarose gel electrophoresis after RNA extraction without the need for amplification of the genome fragments (Bowers et al. 2010).
In contrast, real-time PCR (qPCR) is a variant of the PCR technique in which amplification and detection occur simultaneously and quantitative results can be obtained with higher precision, including determination of WSSV viral load (Meng et al. 2009; Liu et al. 2011a; Powell et al. 2015; Ding et al. 2017a; Huang et al. 2018; Ma et al. 2019; Kong et al. 2020), CsRV1 (Chung et al. 2015; Rogers et al. 2015a; Flowers et al. 2016, 2018; Spitznagel et al. 2019), MCRV (Jithendran et al. 2009; Deng et al. 2019b), MCTV (Gao et al. 2019), EsRV WX-2012 (Ma et al. 2016), SBV (Owens et al. 2010), and SCRV (Zhang et al. 2015). The qPCR may also include a reverse transcription step (RT-qPCR), which is used when the virus is RNA, as in reoviruses (Bowers et al. 2010; Flowers et al. 2016).
Although widely used, PCR, qPCR, and RT-qPCR techniques require the use of expensive and sensitive equipment, and sample preparation is tedious and time-consuming. However, these techniques have been modernized, and Ma et al. (2016) developed a reverse-transcription-loop-mediated (RT-LAMP) isothermal amplification assay with high specificity, sensitivity, and rapidity for the detection of EsRV WX-2012 in E. sinensis, which can be used to diagnose the virus in crabs from aquaculture farms where diagnostic equipment is usually not available. RT-LAMP eliminates the DNA extraction step and the use of a thermal cycler. In addition, the method had a detection limit of 15 pg and 100 times higher sensitivity than the conventional RT-PCR (Ma et al. 2016). Another advantage is that the result can be visualized with the naked eye without the need for additional reagents or electrophoresis steps.
To further facilitate the diagnosis of viral pathogens in crab farms, Zhang et al. (2015) and Fan et al. (2017) have developed a rapid immunochromatographic strip test for the differential and qualitative detection of SCRV virus in P. trituberculatus and SsRV in S. serrata, respectively. Both tests use a nitrocellulose membrane impregnated with specific antibodies that react with the viral antigen in the hemolymph sample of an infected animal. The antigen–antibody interaction of the virus-positive sample is visible through the red dye (colloidal gold) on the test strip. The test developed by Zhang et al. (2015) uses specific immunoglobulin Y (IgY) antibodies against SCRV proteins isolated in chicken eggs after virus inoculation. The test by Fan et al. (2017) uses specific polyclonal (pAb) and monoclonal (mAb) antibodies against SsRV capsid protein (p29), isolated from virus-infected rabbits. The strip test for SsRV detection had an overall sensitivity of 71% compared to RT-PCR for detection in mud crabs, while the SCRV test successfully detected the reovirus in all artificially infected P. trituberculatus samples.
Control of viral pathogens in cultured brachyuran crustaceans
Although viral infections currently pose the greatest threat to the success and sustainability of crustacean aquaculture worldwide (Perazzolo et al. 2012), the prophylaxis and control of viral infections in brachyurans is still essentially limited to the application of appropriate management practices and the reduction of stress conditions. In practice, the factors that determine the health status of brachyuran crustaceans are still poorly understood (Shields and Overstreet 2003; Perazzolo et al. 2012), and science is only taking the first effective steps to understand and control this problem.
Among the management methods used, controlling the temperature of the growing water is the most effective, as temperature has a major impact on both the host and the pathogen, and its variation usually creates favorable conditions for viral replication (Lavilla-Pitogo et al. 2007; Chung et al. 2015) and increases the infectivity and pathogenicity of the virus. The studies conducted by Chung et al. (2015b) showed that viral replication significantly increased in hemocytes and in various tissues when CsRV1-infected C. sapidus were exposed to an increase in temperature from 10 to 23 °C. Similar results were also observed by Zhang and Bonami (2012) in E. sinensis infected with EsRV816. In that study, 28.5% of the crabs died after 20 days at 28 °C. In the group kept at 20 °C, no crab died, indicating an influence of temperature on the infectivity and pathogenicity of the virus. In the study conducted by Lavilla-Pitogo et al. (2007), S. serrata exposed to a temperature of 19 to 21 °C tested positive for WWS by PCR after 15 days of culture. At temperatures of 27 to 29 °C, WSSV was only detected after 25 days, suggesting that in this case, exposure to lower temperatures may increase infection.
Because crustaceans lack efficient and specific immunological memory, as they lack lymphocytic cells, antibodies, and memory cells as in vertebrates, vaccination is impractical or even ineffective in brachyuran crustaceans (Rowley and Pope 2012). Therefore, it is crucial to find strategies to control viral infections and minimize the large losses suffered by the industry (Shields and Overstreet 2003). In this context, there have been important advances in understanding a number of antiviral mechanisms, such as the suppression of viral infections by the RNA interference (RNAi) system and the use of therapeutic or preventive agents, in the last decade (Fang et al. 2015; Wang et al. 2017, 2020; Huang et al. 2017; Deng et al. 2019b, a; Ma et al. 2019; Kong et al. 2020; Ren et al. 2020).
The RNAi system for suppression of viral infections is a promising approach to combat viral diseases in shellfish. RNAi is a cellular mechanism responsible for post-transcriptional gene silencing that acts on messenger RNA (mRNA). In this mechanism, a double-stranded RNA (dsRNA) of 21–25 nucleotides incorporated into an intracytoplasmic complex binds to a complementary nucleotide sequence located in the target mRNA and causes viral silencing by inhibiting translation and/or degradation of the mRNA (Perazzolo et al. 2012). Laboratory experiments have shown that crabs injected with specific anti-miRNA oligonucleotides reduced viral replication following a WSSV challenge (Huang et al. 2017, 2018). In the study by Huang et al. (2018), results showed that WSSV utilized microRNA-7 (miR-7) to increase viral replication during E. sinensis infection. However, replication was inhibited by down-regulation of miR-7 after injection of an anti-miRNA oligonucleotide (AMO-miR-7: 5′-TAGGCAGATTAGTGATTTCTT-3′) 24 to 48 h after infection. Although RNAi technology continues to be explored, there is no field trial data yet for the RNAi approach (OIE 2018).
Epigallocatechin-3-gallate (EGCG), the most abundant polyphenol extracted from green tea, a popular beverage worldwide, has potential antibacterial and anticancer properties (Jankun et al. 1997). According to Wang et al. (2017), EGCG can not only inhibit the replication of WSSV, but also increase the survival of innate immunity of S. paramamosain, which is a potential new therapeutic or preventive approach to combat WSSV. In the study by Wang et al. (2017), treatment with EGCG injections (1 mg/kg) significantly increased crab survival and decreased viral copy number of WSSV at 24, 48, and 72 h post-challenge, compared to animals in the control group. According to the authors, EGCG treatment can induce certain immunological signalling pathways, such as those of phenoloxidase and JAK-STAT, and increase the activity of profenoloxidase. The results of this study can be used for future antiviral research aimed at protecting the cultured brachyuran crustaceans from infection with WSSV and other viruses.
Conclusions
Numerous viral pathogens are closely associated with high mortality rates in brachyuran crustaceans of interest to aquaculture. These include viruses such as WSSV, which have had a negative impact on the penaeid shrimp aquaculture industry for decades. Of the virus species described in this review that have been detected in various hosts of brachyuran, most affect the animals in a systemic manner and infect tissues of mesodermal and ectodermal origin, likely contributing to the pathogenesis of these infections. In addition, experimental tests of animal exposure to various viral pathogens have shown that mortality rates were high following viral inoculation by intramuscular injection, but also by simple transmission, e.g., orally through consumption of infected tissues or via water—conditions that are common in an agricultural environment.
Since the description of the first brachyuran crustacean viruses, in the late 1970s, the discovery of new viruses has accelerated with the development of new molecular detection techniques, but the traditional techniques have not been replaced and are still considered useful tools for identifying viral diseases. The flowchart presented in this study, based on traditional diagnostic techniques, can be used as a guide for diagnosing viral diseases in farmed crabs. In addition, it is likely that the increase in studies using traditional detection methods in combination with molecular techniques, especially those using metagenomic approaches, will have an increasing impact on the identification and characterization of new viruses, which will certainly expand the diversity of viruses presented here to a wider range of crustaceans. This scenario may be daunting for the brachyuran crustacean aquaculture industry, as the usual preventive measures, such as vaccination, are not feasible or effective. However, in the search for a solution to this problem, new approaches have been explored to increase the survival rate of farmed animals. These include improving on-farm management practices and exploring the use of RNAi therapies and botanicals, which should be a topic for future research.
In this context, the issues addressed in this review clearly show that, despite the recent expansion of global production of brachyurans in aquaculture, scientific knowledge of viral pathogens, advances in diagnostic techniques, and the urgent search for disease control strategies are essential for this production sector to reach sustainable levels in the long term. Finally, the bottlenecks of global brachyuran aquaculture can only be addressed in practice if synergies are created between brachyuran aquaculture professionals and experts in pathology, epidemiology, therapy, and public policy makers in the field of food safety.
Data availability
The datasets generated during and/or analyzed during the current study are available on the request from the corresponding author.
References
Anderson IG, Prior HC (1992) Baculovirus infections in the mud crab, Scylla serrata, and a freshwater crayfish, Cherax quadricarinatus, from Australia. J Invertebr Pathol 60:265–273. https://doi.org/10.1016/0022-2011(92)90008-R
Attoui H, Mertens PPC, Becnel J, et al (2012) Family Reoviridae. In: LEFKOWITZ EJ, Dempsey DM, Hendrickson RC, et al. (eds) Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. New York: Elsevier Academic press, pp 541–637
Bang FB (1971) Transmissible disease, probably viral in origin, affecting the amebocytes of the European shore crab, Carcinus maenas. Infect Immun 3:617–623. https://doi.org/10.1128/iai.3.4.617-623.1971
Bateman KS, Tew I, French C et al (2012) Susceptibility to infection and pathogenicity of White Spot Disease (WSD) in non-model crustacean host taxa from temperate regions. J Invertebr Pathol 110:340–351. https://doi.org/10.1016/j.jip.2012.03.022
Blaylock RBR, Curran SSS, Organisms JL, Lotz JM (2019) White spot syndrome virus (WSSV) in cultured juvenile blue crabs Callinectes sapidus: oral versus injection exposure, and feeding frequency effects. Dis Aquat 133:147–156. https://doi.org/10.3354/dao03334
Bojko J, Stebbing PD, Dunn AM et al (2018) Green crab Carcinus maenas symbiont profiles along a North Atlantic invasion route. Dis Aquat Organ 128:147–168. https://doi.org/10.3354/dao03216
Bojko J, Subramaniam K, Waltzek TB et al (2019) Genomic and developmental characterisation of a novel bunyavirus infecting the crustacean Carcinus maenas. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-49260-4
Bonami JR, Zhang S (2011) Viral diseases in commercially exploited crabs: a review. J Invertebr Pathol 106:6–17. https://doi.org/10.1016/j.jip.2010.09.009
Bonami J-R, Comps M, Veyrunes JC (1976) Etude histopathologique et ultrastructurale de la paralysie virale du crabe Macropipus depurator. L Rev Trav Inst Pêches Marit 40:139–146
Bowers HA, Messick GA, Hanif A et al (2010) Physicochemical properties of double-stranded RNA used to discover a reo-like virus from blue crab Callinectes sapidus. Dis Aquat Organ 93:17–29. https://doi.org/10.3354/dao02280
Chang PS, Chen HC, Wang YC (1998) Detection of white spot syndrome associated baculovirus in experimentally infected wild shrimp, crab and lobsters by in situ hybridization. In: Aquaculture. pp 233–242
Chen A, Jiang Y, Qian D et al (2011a) Trembling disease of Chinese mitten crab. China Fish 11:57
Chen JG, Yang JF, Lou D, et al (2011b) A Reo-like virus associated with high mortality rates in cultured mud crab, Scylla serrata, in East Chinao,
Chung JS, Pitula JS, Schott E et al (2015) Elevated water temperature increases the levels of reo-like virus and selected innate immunity genes in hemocytes and hepatopancreas of adult female blue crab, Callinectes sapidus. Fish Shellfish Immunol 47:511–520. https://doi.org/10.1016/j.fsi.2015.09.027
Costa SW, Fraga APM, Zamparetti AS et al (2012a) Presence of the white spot syndrome virus (WSSV) in wild decapods crustaceans in coastal lagoons in southern Brazil. Arq Bras Med Vet e Zootec 64:209–216. https://doi.org/10.1590/S0102-09352012000100030
Costa SW, Fraga APMM, Zamparetti AS et al (2012b) Presença do vírus da síndrome da mancha branca em crustáceos decápodes silvestres em lagoas costeiras no Sul do Brasil. Arq Bras Med Vet e Zootec 64:209–216. https://doi.org/10.1590/S0102-09352012000100030
Deng XX, Lü L, Ou YJ et al (2012) Sequence analysis of 12 genome segments of Mud crab reovirus (MCRV). Virology 422:185–194. https://doi.org/10.1016/j.virol.2011.09.029
Deng H, Xu X, Hu L et al (2019a) A janus kinase from Scylla paramamosain activates JAK/STAT signaling pathway to restrain Mud crab reovirus. Fish Shellfish Immunol 90:275–287. https://doi.org/10.1016/j.fsi.2019.03.056
Deng H, Zhang W, Li JJ et al (2019b) A signal transducers and activators of transcription (STAT) gene from Scylla paramamosain is involved in resistance against Mud crab reovirus. Fish Shellfish Immunol 94:580–591. https://doi.org/10.1016/j.fsi.2019.09.045
Ding Z, Yao Y, Zhang F et al (2015) The first detection of white spot syndrome virus in naturally infected cultured Chinese mitten crabs, Eriocheir sinensis in China. J Virol Methods 220:49–54. https://doi.org/10.1016/j.jviromet.2015.04.011
Ding Z, Wang S, Zhu X et al (2017a) Temporal and spatial dynamics of white spot syndrome virus in the Chinese mitten crab, Eriocheir sinensis. Aquac Res 48:2528–2537. https://doi.org/10.1111/are.13089
Ding ZF, Cao MJ, Zhu XS et al (2017b) Changes in the gut microbiome of the Chinese mitten crab (Eriocheir sinensis) in response to White spot syndrome virus (WSSV) infection. J Fish Dis 40:1561–1571. https://doi.org/10.1111/jfd.12624
dos Tavares CPS, Silva UAT, Pereira LA, Ostrensky A (2018) Systems and techniques used in the culture of soft-shell swimming crabs. Rev Aquac 10:913–923. https://doi.org/10.1111/raq.12207
Durand S, Lightner DV, Redman RM, Bonami JR (1997) Ultrastructure and morphogenesis of White Spot Syndrome Baculovirus (WSSV). Dis Aquat Org 29:205–211. https://doi.org/10.3354/dao029205
Fan D, Liu J, Xu M et al (2017) A convenient immunochromatographic test strip for rapid detection of Scylla serrata reovirus. Virol Sin 32:335–337
Fang J, Li D, Xu R et al (2015) Tubulin mediates Portunus trituberculatus reovirus infection. Aquaculture 448:196–202. https://doi.org/10.1016/j.aquaculture.2015.06.001
FAO (2020) Global Capture and Aquaculture Production (FishStat). In: Rome, Italy. http://www.fao.org/figis/servlet/SQServlet?file=/work/FIGIS/prod/webapps/figis/temp/hqp_5215617422254205836.xml&outtype=html. Accessed 27 Jun 2020
Flowers EM, Johnson AF, Aguilar R, Schott EJ (2018) Prevalence of the pathogenic crustacean virus Callinectes sapidus reovirus 1 near flow-through blue crab aquaculture in Chesapeake Bay, USA. Dis Aquat Organ 129:135–144. https://doi.org/10.3354/dao03232
Flowers EM, Bachvaroff TR, Warg J V., et al (2016) Genome sequence analysis of CsRV1: a pathogenic reovirus that infects the blue crab Callinectes sapidus across its trans-hemispheric range. Front Microbiol 7https://doi.org/10.3389/fmicb.2016.00126
Galavíz-Silva L, Molina-Garza ZJ, Alcocer-González JM et al (2004) White spot syndrome virus genetic variants detected in Mexico by a new multiplex PCR method. Aquaculture 242:53–68. https://doi.org/10.1016/j.aquaculture.2004.09.006
Gao Y, Liu S, Huang J, et al (2019) Cryo-electron microscopy structures of novel viruses from mud crab Scylla paramamosain with multiple infections. J Virol 93.https://doi.org/10.1128/jvi.02255-18
Gunasekaran T, Gopalakrishnan A, Deivasigamani B et al (2018) Spontaneous white spot syndrome virus (WSSV) infection in mud crab (Scylla serrata Forskal 1775) fattening pens farm of south east coast of India. Comp Clin Path 27:413–419. https://doi.org/10.1007/s00580-017-2607-z
Guo ZX, He JG, Xu HD, Weng SP (2013) Pathogenicity and complete genome sequence analysis of the mud crab dicistrovirus-1. Virus Res 171:8–14. https://doi.org/10.1016/j.virusres.2012.10.002
Hoover KL (1977) The effect of a virus infection on the hemocyte population in Carcinus maenas. Johns Hopkins University
Huang Z, Deng X, Li Y et al (2012) Structural insights into the classification of Mud crab Reovirus. Virus Res 166:116–120. https://doi.org/10.1016/j.virusres.2012.02.025
Huang Y, Wang W, Xu Z et al (2018) Eriocheir sinensis microRNA-7 targets crab Myd88 to enhance white spot syndrome virus replication. Fish Shellfish Immunol 79:274–283. https://doi.org/10.1016/j.fsi.2018.05.028
Huang Y, Han K, Wang W, Ren Q (2017) Host MicroRNA-217 promotes white spot syndrome virus infection by targeting tube in the Chinese Mitten Crab (Eriocheir sinensis). Front Cell Infect Microbiol 7. https://doi.org/10.3389/fcimb.2017.00164
Hungria DB, dos Tavares CPS, Pereira LÂ et al (2017) Global status of production and commercialization of soft-shell crabs. Aquac Int 25:2213–2226. https://doi.org/10.1007/s10499-017-0183-5
Hyodo K, Kaido M (2021) Dianthovirus (Tombusviridae). In: Bamford DH, Zuckerman M (eds) Encyclopedia of Virology (Fourth Edition), Fourth Edi. Academic Press, Oxford, pp 383–387
Ika Maharani R (2005) Sensitivitas Berbagai Stadia Kepiting Bakau (Scylla paramamosain Estampador) terhadap White Spot Syndrome Virus. Bioteknologi 2:. https://doi.org/10.13057/biotek/c020105
Inouye K, Yamano K, Ikeda N et al (1996) The penaeid rod-shaped DNA virus (PRDV), which causes penaeid acute viremia (PAV). Fish Pathol 31:39–45. https://doi.org/10.3147/jsfp.31.39
Jahromi SS (1977) Occurrence of rhabdovirus like particles in the blue crab, Callinectes sapidus. J Gen Virol 36:485–493. https://doi.org/10.1099/0022-1317-36-3-485
Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E (1997) Why drinking green tea could prevent cancer. Nature 387:561. https://doi.org/10.1038/42381
Jithendran KP, Poornima M, Balasubramanian CP, Kulasekarapandian S (2009) Diseases of mud crabs in India. Inst Brackishwat Aquacult 20:1–28
Johnson PT (1977) A viral disease of the blue crab, Callinectes sapidus: histopathology and differential diagnosis. J Invertebr Pathol 29:201–209. https://doi.org/10.1016/0022-2011(77)90194-X
Johnson PT (1978) Viral diseases of the blue crab, Callinectes sapidus. Mar Fish Rev 40:13–15
Johnson PT (1984) Viral diseases of marine invertebrates. Helgoländer Meeresuntersuchungen 37:65–98. https://doi.org/10.1007/BF01989296
Johnson PT (1988) Rod-shaped nuclear viruses of crustaceans: hemocyte-infecting species. Dis Aquat Org 5:11–122
Johnson PT (1983) Diseases caused by viruses, Rickettsiae, Bacteria, and Fungi. In: The Biology of Crustacea. pp 1–78
Kennedy VS, Cronin LE (2007) The blue crab: Callinectes sapidus. University of Maryland, College Park, Md, Maryland Sea Grant College
Kiatpathomchai W, Jaroenram W, Arunrut N et al (2008) Experimental infections reveal that common Thai crustaceans are potential carriers for spread of exotic Taura syndrome virus. Dis Aquat Organ 79:183–190. https://doi.org/10.3354/dao01903
Kong T, Lin S, Gong Y, et al (2020) Sp-CBL inhibits white spot syndrome virus replication by enhancing apoptosis in mud crab (Scylla paramamosain). Dev Comp Immunol 105. https://doi.org/10.1016/j.dci.2019.103580
Kou G-H, Peng S-E, Chiu Y-L, Lo C-F (1998a) Tissue distribution of white spot syndrome virus (WSSV) in shrimp and crabs. Adv Shrimp Biotechnol 11:267–271
Kou GH, Peng SE, Chiu YL, Lo CF (1998b) Tissue distribution of white sport syndrome virus (WSSV) in shrimp and crabs. Flegl TW Adv shrimp Biotechnol Natl Cent Genet Eng Biotechnol Bangkok 267–271
Krol RM (2002) Pathobiology white spot virus (WSV) in diverse crustaceans from the United States. University of Southern Mississippi
Lavilla-Pitogo CR, de la Peña LD, R.C AEC and L-P (2004) Nutritional diseases, diseases in farmed fish and mud crabs, Scylla spp.: diagnosis, prevention, and control.
Lavilla-Pitogo CR, De La Peña LD, Catedral DD (2007) Enhancement of white spot syndrome virus load in hatchery-reared mud crab Scylla serrata (Forsskål, 1775) juveniles at a low temperature. In: Aquaculture Research. pp 1600–1603
Li DF (2012) Purification of Portunus trituberculatus reovirus. China Chinese Patent 201210576754(2):2012
Li DF, Peng J, Liu LG et al (2012) Chicken embryo culture method of Portunus trituberculatus reovirus. China Chinese Patent 201210019133:4
Liessmann L, Owens A/ L, Zeng C (2005) Investigation into the mortalities of larval mud crabs, Scylla serrata and methods of control. Dissertation, James Cook University
Lightner D V. (1996) A handbook of shrimp pathology and diagnostic procedures for disease of cultured penaeid shrimp., World Aqua. Baton Rouge, Louisiana, USA
Lightner DV (2011) Virus diseases of farmed shrimp in the Western Hemisphere (the Americas): a review. J Invertebr Pathol 106:110–130
Lin S, He Y, Gong Y, et al (2020) SpBOK inhibits WSSV infection by regulating the apoptotic pathway in mud crab (Scylla paramamosain). Dev Comp Immunol 106. https://doi.org/10.1016/j.dci.2019.103603
Liu W, Qian D, Yan X (2011a) Proteomic analysis of differentially expressed proteins in hemolymph of Scylla serrata response to white spot syndrome virus infection. Aquaculture 314:53–57. https://doi.org/10.1016/j.aquaculture.2011.02.021
Liu W, Qian D, Yan XJ (2011b) Studies on pathogenicity and prevalence of white spot syndrome virus in mud crab, Scylla serrata (Forskal), in Zhejiang Province, China. J Fish Dis 34:131–138. https://doi.org/10.1111/j.1365-2761.2010.01221.x
Lo CF, Ho CH, Peng SE et al (1996) White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis Aquat Org 27:215–225. https://doi.org/10.3354/dao027215
LucidChart (2020) Flowchart creation tool. https://www.lucidchart.com/pages/examples/flowchart-maker
Ma Y, Dai T, Serwadda A, Shen H (2016) Detecting a novel Eriocheir sinensis reovirus by reverse transcription loop-mediated isothermal amplification assay. Lett Appl Microbiol 63:363–368. https://doi.org/10.1111/lam.12630
Ma X, Tang X, Lin S et al (2019) SpBAG1 promotes the WSSV infection by inhibiting apoptosis in mud crab (Scylla paramamosain). Fish Shellfish Immunol 94:852–860. https://doi.org/10.1016/j.fsi.2019.10.013
Maes P, Adkins S, Alkhovsky SV et al (2019) Taxonomy of the order Bunyavirales: second update 2018. Arch Virol 164:927–941. https://doi.org/10.1007/s00705-018-04127-3
Mari J, Bonami J-R (1987) A reolike virus of the Mediterranean shore crab Carcinus mediterraneus. Dis Aquat Organ 3:107–112
Mari J, Bonami J-R (1988a) PC84, a parvo-like virus from the crab Carcinus mediterraneus: pathological aspects, ultrastructure of the agent, and first biochemical characterization. J Invertebr Pathol 51:145–156
Mari J, Bonami J-RR (1988b) W2 virus infection of the crustacean Carcinus mediterraneus: a reovirus disease. J Gen Virol 69:561–571. https://doi.org/10.1099/0022-1317-69-3-561
Melo GAS (1996) Manual de identificação dos Brachyura (caranguejos e siris) do litoral brasileiro/ Gustavo Augusto Schmidt de Melo; [ilustrações Jamil Tannus Neto]. Pleiade, FAPESP, São Paulo, SP, Brazil
Meng XH, Jang IK, Seo HC, Cho YR (2009) White spot syndrome virus quantification in blue crab Portunus trituberculatus hatchery-produced larvae and wild populations by TaqMan real-time PCR, with an emphasis on the relationship between viral infection and crab health. Aquaculture 291:18–22. https://doi.org/10.1016/j.aquaculture.2009.02.003
Mertens P (2004) The dsRNA viruses. Virus Res 101:3–13. https://doi.org/10.1016/j.virusres.2003.12.002
Messick GA (1998) Diseases, parasites, and symbionts of blue crabs (Callinectes sapidus) dredged from Chesapeake Bay. J Crustac Biol 18:533. https://doi.org/10.2307/1549418
Messick GA, Shields JD (2000) Epizootiology of the parasitic dinoflagellate Hematodinium sp. in the American blue crab Callinectes sapidus. Dis Aquat Organ 43:139–152. https://doi.org/10.3354/dao043139
Messick GA, Sindermann CJ (1992a) Synopsis of principal diseases of the blue crab, Callinectes sapidus. US Dep Commer NOAA Tech Memo NMFS-F/NEC-88 1–20
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Momoyama K, Hiraoka M, Venegas CA (1999) Pathogenicity of penaeid rod-shaped DNA virus (PRDV) to juveniles of six crustacean species. Fish Pathol 34:183–188. https://doi.org/10.3147/jsfp.34.183
Mullis K, Faloona F, Scharf S et al (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51:263–273. https://doi.org/10.1101/sqb.1986.051.01.032
Norizan N, Harrison FS, Hassan M et al (2019) First detection of white spot syndrome virus (Wssv) in wild mud crab Scylla spp. (de haan, 1883) from setiu wetlands, malaysia. Songklanakarin J Sci Technol 41:45–52. https://doi.org/10.14456/sjst-psu.2019.6
OIE (2018) Manual of diagnostic tests for aquatic animals. Chapter 2.2.8. Infection with white spot syndrome vírus. World Organ. Anim. Heal.
Owens L, Liessmann L, La Fauce K et al (2010) Intranuclear bacilliform virus and hepatopancreatic parvovirus (PmergDNV) in the mud crab Scylla serrata (Forskal) of Australia. Aquaculture 310:47–51. https://doi.org/10.1016/j.aquaculture.2010.10.028
Pappalardo R, Bonami JR (1979) Infection ds crustacés marins due à un virus de type nouveau apparenté aux baculovirus. Comptes Rendus Hebd Des Séances L’académie Des Sci D 288:535–537
Pappalardo R, Mari J, Bonami JR (1986) τ (tau) virus infection of Carcinus mediterraneus: Histology, cytopathology, and experimental transmission of the disease. J Invertebr Pathol 47:361–368. https://doi.org/10.1016/0022-2011(86)90107-2
Perazzolo LM, Rosa RD, Barracco MA (2012) Defesas antivirais em crustáceos: Estado da Arte. In: Maringá (ed) Patologia e Sanidade de Organismos Aquáticos. Massoni, Maringá - PR, pp 315–354
Powell JWBB, Browdy CL, Burge EJ (2015) Blue crabs Callinectes sapidus as potential biological reservoirs for white spot syndrome virus (WSSV). Dis Aquat Organ 113:163–167. https://doi.org/10.3354/dao02829
Raja RA, Jithendran K, Rajan J (2019) Mortality among farmed mud crab, Scylla serrata due to white spot syndrome virus (WSSV) in Andhra Pradesh, India. Int J Adv Res 5:2699–2708
Rajendran KV, Vijayan KK, Santiago TC, Krol RM (1999) Experimental host range and histopathology of white spot syndrome virus (WSSV) infection in shrimp, prawns, crabs and lobsters from India. J Fish Dis 22:183–191. https://doi.org/10.1046/j.1365-2761.1999.00162.x
Ren X, Lin S, Kong T et al (2020) The miRNAs profiling revealed by high-throughput sequencing upon WSSV infection in mud crab Scylla paramamosain. Fish Shellfish Immunol 100:427–435. https://doi.org/10.1016/j.fsi.2020.03.002
Rogers HA, Taylor SS, Hawke JP et al (2015a) Disease, parasite, and commensal prevalences for blue crab Callinectes sapidus at shedding facilities in Louisiana, USA. Dis Aquat Organ 112:207–217. https://doi.org/10.3354/dao02803
Rogers HA, Taylor SS, Hawke JP, Anderson Lively JA (2015b) Variations in prevalence of viral, bacterial, and rhizocephalan diseases and parasites of the blue crab (Callinectes sapidus). J Invertebr Pathol 127:54–62. https://doi.org/10.1016/j.jip.2015.03.002
Rowley AF, Pope EC (2012) Vaccines and crustacean aquaculture-a mechanistic exploration. Aquaculture 334–337:1–11
Shen H, Ma Y, Hu Y (2015) Near-full-length genome sequence of a novel reovirus from the Chinese mitten crab, Eriocheir sinensis. Genome Announc 3. https://doi.org/10.1128/genomeA.00447-15
Shields JD (2003) Research priorities for diseases of the blue crab Callinectes sapidus. In: Bulletin of Marine Science. pp 505–517
Shields JD, Overstreet RM (2003) The blue crab: diseases, parasites, and other symbionts. Blue Crab Dis Parasites Other Symbionts 119
Somboonna N, Mangkalanan S, Udompetcharaporn A, et al (2010) Mud crab susceptibility to disease from white spot syndrome virus is species-dependent. BMC Res Notes 3. https://doi.org/10.1186/1756-0500-3-315
Spitznagel MI, Small HJ, Lively JA et al (2019) Investigating risk factors for mortality and reovirus infection in aquaculture production of soft-shell blue crabs (Callinectes sapidus). Aquaculture 502:289–295. https://doi.org/10.1016/j.aquaculture.2018.12.051
Spitznagel MI (2019) Mortality and reovirus infection in soft-shell blue crab (Callinectes sapidus) aquaculture. Dissertation, University of Maryland.
Stentiford GD, Shields JD (2005) A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis Aquat Organ 66:47–70. https://doi.org/10.3354/dao066047
Stentiford GD, Bonami JR, Alday-Sanz V (2009) A critical review of susceptibility of crustaceans to Taura syndrome, yellowhead disease and White Spot Disease and implications of inclusion of these diseases in European legislation. Aquaculture 291:1–17
Stentiford GD, Oidtmann B, Scott A, Peeler EJ (2010) Crustacean diseases in European legislation: implications for importing and exporting nations. Aquaculture 306:27–34. https://doi.org/10.1016/j.aquaculture.2010.06.004
Supamattaya K, Hoffmann RW, Boonyaratpalin S, Kanchanaphum P (1998) Experimental transmission of white spot syndrome virus (WSSV) from black tiger shrimp Penaeus monodon to the sand crab Portunus pelagicus, mud crab Scylla serrata and krill Acetes sp. Dis Aquat Organ 32:79–85. https://doi.org/10.3354/dao032079
Supraptp H (2012) The evidence of virus-like particles in crabs (Scylla sp.) isolated from insideand outside pond shrimp infected with white spot disease. J Sain Vet 21:. https://doi.org/10.22146/jsv.485
Tang KFJJ, Messick GA, Pantoja CR et al (2011) Histopathological characterization and in situ detection of Callinectes sapidus reovirus. J Invertebr Pathol 108:226–228. https://doi.org/10.1016/j.jip.2011.08.010
Team RC (2020) R: a language and environment for statistical computing.
Thibodeaux LK, Burnett KG, Burnett LE (2009) Energy metabolism and metabolic depression during exercise in Callinectes sapidus, the Atlantic blue crab: effects of the bacterial pathogen Vibrio campbellii. J Exp Biol 212:3428–3439. https://doi.org/10.1242/jeb.033431
Vaseeharan B, Jayakumar R, Ramasamy P (2003) PCR-based detection of white spot syndrome virus in cultured and captured crustaceans in India. Lett Appl Microbiol 37:443–447. https://doi.org/10.1046/j.1472-765X.2003.01428.x
Vlak J., Bonami J., Flegel T., et al (2005) Nimaviridae. In: Fauquet CM., Mayo J., Maniloff J., et al. (eds) Virus Taxonomy. Eighth report of the International Committee on Taxonomy of Viruses, Elsevier. Amsterdam, The Netherlands, pp 187–192
Waikhom G, John KR, George MR, Jeyaseelan MJP (2006) Differential host passaging alters pathogenicity and induces genomic variation in white spot syndrome virus. Aquaculture 261:54–63. https://doi.org/10.1016/j.aquaculture.2006.07.031
Walker PJ, Blasdell KR, Calisher CH et al (2018) ICTV virus taxonomy profile: Rhabdoviridae. J Gen Virol 99:447–448. https://doi.org/10.1099/jgv.0.001020
Wang Z, Sun B, Zhu F (2017) Epigallocatechin-3-gallate inhibit replication of white spot syndrome virus in Scylla paramamosain. Fish Shellfish Immunol 67:612–619. https://doi.org/10.1016/j.fsi.2017.06.050
Wang Z, Sun B, Zhu F (2018) Molecular characterization of glutaminyl-peptide cyclotransferase (QPCT) in Scylla paramamosain and its role in Vibrio alginolyticus and white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol 78:299–309. https://doi.org/10.1016/j.fsi.2018.04.059
Wang HC, Hirono I, Maningas MBB et al (2019) ICTV virus taxonomy profile: Nimaviridae. J Gen Virol 100:1053–1054. https://doi.org/10.1099/jgv.0.001248
Wang J, Hong W, Zhu F (2020) The role of Astakine in Scylla paramamosain against Vibrio alginolyticus and white spot syndrome virus infection. Fish Shellfish Immunol 98:236–244. https://doi.org/10.1016/j.fsi.2020.01.024
Wang YC, Lo CF, Chang PS, Kou GH (1998) Experimental infection of white spot baculovirus in some cultured and wild decapods in Taiwan. In: Aquaculture. pp 221–231
Weng SP, Guo ZX, Sun JJ et al (2007) A reovirus disease in cultured mud crab, Scylla serrata, in southern China. J Fish Dis 30:133–139. https://doi.org/10.1111/j.1365-2761.2007.00794.x
Williams AB (1973) The swimming crabs of the genus Callinectes (Decapoda: Portunidae). Government Printing Office, Washington, U.S
Yago PC (1966) A virus disease in Crustacea. Nature 209:1290. https://doi.org/10.1038/2091290a0
Yudin AI, Clark WH (1979) A description of rhabdovirus-like particles in the mandibular gland of the blue crab, Callinectes sapidus. J Invertebr Pathol 33:133–147. https://doi.org/10.1016/0022-2011(79)90146-0
Zhang S, Bonami JR (2007) A roni-like virus associated with mortalities of the freshwater crab, Eriocheir sinensis Milne Edwards, cultured in China, exhibiting “sighs disease” and black gill syndrome. J Fish Dis 30:181–186. https://doi.org/10.1111/j.1365-2761.2007.00796.x
Zhang S, Bonami JR (2012) Isolation and partial characterization of a new reovirus in the Chinese mitten crab, Eriocheir sinensis H Milne Edwards. J Fish Dis 35:733–739. https://doi.org/10.1111/j.1365-2761.2012.01398.x
Zhang SY, Zhang JH, Huang CH et al (2002) Preliminary studies on two strains of reovirus from crab Eriocheir sinensis. Viirologia Sin 17:263–265
Zhang S, Shi Z, Zhang J, Bonami JR (2004) Purification and characterization of a new reovirus from the Chinese mitten crab, Eriocheir sinensis. J Fish Dis 27:687–692. https://doi.org/10.1111/j.1365-2761.2004.00587.x
Zhang R, He J, Su H et al (2011) Identification of the structural proteins of VP1 and VP2 of a novel mud crab dicistrovirus. J Virol Methods 171:323–328. https://doi.org/10.1016/j.jviromet.2010.09.010
Zhang LP, Li DF, Liu LG, Zhang G (2015) Rapid immunochromatographic test strip to detect swimming crab Portunus trituberculatus reovirus. Dis Aquat Organ 117:21–29. https://doi.org/10.3354/dao02921
Zhao M, Flowers EM, Schott EJ (2021a) Near-complete sequence of a highly divergent reovirus genome recovered from Callinectes sapidus. Microbiol Resour Announc 10:e01278-e1320. https://doi.org/10.1128/mra.01278-20
Zhao M, dos Tavares CPS, Schott EJ (2021b) Diversity and classification of reoviruses in crustaceans: a proposal. J Invertebr Pathol 182:107568. https://doi.org/10.1016/j.jip.2021.107568
Acknowledgements
We thank the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for a research grant to Dr. Antonio Ostrensky (process number 304633/2017-8), for funding this research (grant number 381091/2014-7), and for a Ph.D. scholarship to Camila P. S. Tavares (grant Nº 141022/2017-5). We also thank CAPES for a sandwich doctoral scholarship (grant number 88881.190487/2018-01). We thank Dr. Eric Schott (IMET-UMCES) and Mingli Zhao (IMET-UMCES) for valuable and insightful discussions.
Funding
The research was funded by the National Council for Scientific and Technological Development (CNPq—Grant Numbers 304633/2017–8, 381091/2014–7, and 141022/2017–5) and Coordination for the Improvement of Higher Education Personnel (CAPES—Grant Number 88881.190487/2018–01).
Author information
Authors and Affiliations
Contributions
C. P. S. Tavares is the main author and is responsible for the conception and design of the paper, data collection, data analysis, and interpretation and writing of the article. U. A. T. Silva and M. Pie contributed significantly to the acquisition of data and materials and was involved in the critical revision of the final manuscript. A. Ostrensky is the lead of the project and was involved in the planning of the work, data analysis and critical revision of the article, as well in the final approval of the version to be published.
Corresponding author
Ethics declarations
Ethical approval
Not applicable. The manuscript does not need ethical approval.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tavares, C.P.d., Silva, U.A.T., Pie, M. et al. A review of viral diseases in cultured brachyuran crustaceans. Aquacult Int 31, 627–655 (2023). https://doi.org/10.1007/s10499-022-00993-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00993-6