Abstract
The prawn Macrobrachium rosenbergii post larvae (10.01 ± 2.00 mg) were evaluated using a synbiotic system with different fermentation (anaerobic) and microbial respiration (aerobic) strategies after 35 days. Rice bran, a mix of probiotic microorganisms, alkalizing agents, and water were used in the preparation of the synbiotic. There were five treatments in quadruplicate, consisting of the following: T12|12 = 12 h anaerobic and 12 h aerobic; T12|24 = 12 h anaerobic and 24 h aerobic; T24|0 = 24 h anaerobic; T24|12 = 24 h anaerobic and 12 h aerobic; T24|24 = 24 h anaerobic and 24 h aerobic. The prawns were fed four times a day (40% crude protein) and the main variables of water quality and prawn growth were evaluated. The synbiotic preparation strategies used did not influence the stabilization time of nitrogen compounds in water. There were no differences in the survival and the water quality variables, which remained adequate for the species. For the variables final average weight (mg) and yield (gm−3), treatments T24|24 (221.3 ± 22.0 and 195.4 ± 14.6) and T12|24 (218.2 ± 27.6 and 196.2 ± 33.4) were higher than 24|00 (176.1 ± 24.5 and 151.3 ± 21.6). Thus, it is concluded that a longer preparation time of the fertilizer, especially contemplating the anaerobic and aerobic stages, can promote greater performance of the reared prawns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater prawn farming has Macrobrachium rosenbergii (giant freshwater prawn) as one of the main crustaceans species reared, reaching a world production of 294,000 tons in 2020. Of this total, Brazil contributed around 150 tons (FAO, 2022).
The productive potential of this species—high fecundity, rusticity, and rapid growth (New et al., 2010), has stimulated the development of research aimed at the intensification of crops, in order to make it possible to obtain greater productivity and profitability linked to the minimum use of water and environmental control (Crab et al. 2010; Perez-Fuentes et al. 2013; Ballester et al. 2017, 2018; Miao et al. 2017; Negrini et al. 2017; Hosain et al. 2021; Santos et al. 2022). As a result, heterotrophic or mixotrophic farming, such as biofloc technology (BFT) and synbiotics, have gained prominence.
In these types of reared, the source of organic carbon as a promoter of microbial growth has become one of the most researched parameters, and its application tries to associate efficiency, low cost, and availability in the local market (Dauda 2019; Romano et al. 2018; Abakari et al. 2020a).
Thus, depending on the region, the most commonly used sources of organic carbon are carbohydrates—(CH2O)n—from monosaccharides and disaccharides (glucose, molasses, sucrose, glycerol) to polysaccharides (rice bran, soy, wheat, starch, etc.) (De Schryver et al. 2008; Avnimelech et al. 2012; Crab et al. 2010; Emerenciano et al. 2017; Dauda et al. 2017; Kumar et al. 2017; Abakari et al. 2020a; Hosain et al. 2021; Santos et al. 2022).
In the case of polysaccharides, such as rice, wheat, and soybean bran, for example, it is possible to find some works adopting fermentation and/or microbial respiration with Bacillus spp. and other probiotic microorganisms (Lactobacillus, Saccharomyces, etc.) in order to achieve better results in terms of solubility; increase in soluble sugars, crude protein, and lipid content; and decrease in crude fiber and antinutritional factors (Romano et al. 2018; Dawood and Kashio 2019; Santos et al. 2022).
This process is known as “synbiotic,” as this terminology basically consists of a combination of a prebiotic source (e.g., bran, such as rice, wheat, or soybean, used as a substrate) and the action of probiotic microorganisms (Romano 2017; Huynhtg et al. 2017; Romano et al. 2018; Andrade et al. 2021; Deepak et al. 2020; Liñan-Vidriales et al. 2021; Silva et al. 2021a, b; Santos et al. 2022). It is, therefore, the formulation of a biofertilizer using agro-industrial residues and probiotic microorganisms submitted to semi-solid and/or liquid fermentation and/or microbial respiration. As a result, a product nutritionally improved and rich in microorganisms beneficial to the reared environment can be obtained (Romano et al. 2018; Silva et al. 2021b).
Bacteria and yeasts are able to use a variety of agroindustry residues, such as rice bran and wheat bran, through fermentation and/or microbial respiration. Both Bacillus licheniformis and B. subtilis, for example, represent an attractive source of proteases when used in these processes (Shim et al. 2010; Huynhtg et al. 2017; Cienfuegos-Martinez et al. 2020; Dawood and Kashio 2019).
These processes generate a transformation in carbohydrates (increase in soluble sugar, crude protein, and lipid content; decrease in crude fiber, etc.), which results from the production of hydrolytic enzymes and secondary metabolites such as liposaccharides and peptidoglycans (Romano et al. 2018; Huynh et al. 2017; Hofvendahl and Hagerdal 2000; Vassileva et al. 2021). Rice bran has shown advantages in a synbiotic system due to its low cost and low protein content (Romano et al., 2018), thus avoiding problems with nitrogen in the system and leading to beneficial effects on fish (Romano et al. 2018), shrimp (Andrade et al. 2021), and prawn (Santos et al. 2022).
Fermentation of polysaccharide carbohydrates using probiotic microorganisms in the food and feed industry has been performed for quite some time (Huynh et al. 2017; Dawood and Koshio 2019). However, the application of this technique in the fertilization of aquaculture ponds is relatively recent, dating from the mid-1990s in Thailand, where it later became known as aquamimicry (Romano 2017). In synbiotic systems, these fermentation and/or microbial respiration strategies have been useful to promote greater flexibility to aquaculture farmers in terms of choosing carbon sources in the preparation of fertilizers in systems with minimal water exchange.
In addition, this system can generate beneficial effects on the reared environment, such as metabolization of nitrogen compounds, increase in beneficial bacteria, growth performance, and use as food by reared aquatic animals (Romano et al. 2018; Dauda 2019; Andrade et al. 2021; Liñan-Vidriales et al. 2021; Lima et al. 2021; Silva et al. 2021a, b; Santos et al. 2022). It is known, however, that for these fermentation processes to occur efficiently, it is necessary to know the environmental conditions that favor the metabolism of microorganisms, since variables such as temperature, aeration, pH, and fermentation time have a great influence on microbial growth, production, and enzyme activity (Potumarthi et al. 2007; Shim et al. 2010; Aguilar et al. 2019; Dawood and Koshio 2019; Sugiharto and Ranjitkar 2019).
Sudden changes in pH or inappropriate values for the metabolism of microorganisms, for example, can lead to a reduction or even inhibition of the production of important metabolites, in addition to a decrease in microbial growth and enzymatic activity (Naidu and Devi 2005; Dawood and Kashio 2019). Most microorganisms prefer a pH between 4.0 and 7.0, especially lactic acid bacteria and yeasts (Hofvendahl and Hagerdal 2000; Peña et al. 2015; Vassileva et al. 2021). To assist in this control, it is necessary to use alkalizing agents (limestone, carbonates, calcium and magnesium oxides or hydroxides, etc.) in adequate amounts.
Other authors have also reported the influence of fermentation time and/or microbial respiration in improving the nutritional value of the synbiotic fertilizer and the floc formed in the culture environment (increase in lipids and carbohydrate protein used as a substrate for the preparation of the synbiotic) (Romano et al. 2018; Santos et al. 2022).
The popularization of these rearing systems around the world has been accompanied by protocol variations in the preparation of the synbiotic, generating different effects on the synbiotic proximate composition, on the microbial floc composition, and on the performance of cultured aquaculture animals (Romano 2017; Romano et al. 2018; Andrade et al. 2021; Hussain et al. 2021; Lima et al. 2021; Liñan-Vidriales et al. 2021; Silva et al. 2021b; Santos et al. 2022).
Therefore, there is a need to contribute with information that helps to consolidate a protocol for synbiotic preparation using rice bran. Thus, the objective of this study was to evaluate the quality of the synbiotic prepared with different fermentation and microbial respiration times, as well as its effect on the water quality and zootechnical performance of the freshwater prawn Macrobrachium rosenbergii reared at the nursery stage.
Material and methods
Experimental structure and facilities
The experimental tests were carried out at the Laboratório de Carcinicultura (LabCarci [Shrimp Culture Lab]), of the Universidade Federal de Alagoas (UFAL [Federal University of Alagoas]), Brazil. For this purpose, rectangular tanks with dimensions of 0.55 × 0.35 × 0.30 m were used, totaling a bottom area equivalent to 0.19 m2, a useful internal surface area of 0.64 m2, and a useful volume of 48 L. The aeration in the experimental units was provided by a centrifugal compressor (0.28 hp) and two porous stones (2.5 cm Ø). This structure was used both to carry out the water maturation procedure with the synbiotic and to test the animals’ growth.
Experiment 1: monitoring pH in the preparation of synbiotic with different alkalizing agents
This experiment aimed to evaluate the effect of three alkalizing agents on pH variation during the preparation of the synbiotic. For the preparation of the synbiotic, alkalizing agents, microbiological mix, rice bran, and water were used in the following proportion: for every 100 g of rice bran (sieved through a 300-µm mesh), 10 g of alkalizing agent (sodium bicarbonate, dolomitic limestone, or Lithothamnium) was added, along with 0.2 g of microbiological mix and 1.0 L of water (filtered at 50 µm). The alkalizing agents used were sodium bicarbonate—NaHCO3 27% Na, PRNT (real neutralizing power) 55%, and PN (neutralizing power) 59%; Lithothamnium—Lothar, a commercial product containing minimum 32% Ca and 2.0% Mg, PRNT 90%, and PN 90%, extracted from the seaweed Lithothamnium calcareum; and dolomitic limestone filler—20% Ca and 9.5% Mg, PRNT 90%, and PN 90%. The probiotic product (microbiological mix) had a minimum concentration of 1.0 × 109 CFU g−1 (colony forming unit per gram) (Epicin G2—Epicore Networks, Eastampton, USA—containing Bacillus subtilis, B. licheniformis, B. pumilus, Lactobacillus acidophilus, and Saccharomyces cerevisiae).
After mixing the components, these mixtures were placed in a cylindrical-conical container (2.0 L—experimental unit) and kept according to the time established for the processes of fermentation and microbial respiration to occur. During this procedure, the pH of the solution was monitored at the beginning and every 12 h using a pH meter (Instrutherm pH 2600—Instrutherm, Brazil). Six replicates were used with the following pH preparation and measurement strategies for each alkalizing agent: 24|24 treatment = initial pH measurement, after 12 h of fermentation, and after 24 h of fermentation (aeration was added to the replicates of the experimental units and the pH was measured for the respiration phase—aerobic, that is, pH 36 h aerobic and 48 h aerobic); treatment 12|24 = initial pH measurement, after 12 h of fermentation (aeration was added to the experimental units and the pH was measured for the respiration phase—aerobic, i.e., pH 24 h aerobic and 36 h aerobic). The tested alkalizers had the following nomenclature: sodium bicarbonate (SB), dolomitic limestone (CA), and Lithothamnium (LT).
Experiment 2: preparation of water from bioreactors with the synbiotic
After experiment 1 of monitoring the pH in the preparation of the synbiotic, sodium bicarbonate (SB) was chosen as an alkalizing agent for the maturation of the water that would later be used for the rearing of the prawns. This bioreactor water preparation process was monitored in order to assess the effect of different synbiotic preparation strategies (fermentation and/or microbial respiration times) on the dynamics of nitrogen compounds. To accomplish this, 15 tanks (dimensions of 0.55 × 0.35 × 0.30 m) were used, which were divided into five treatments and three replicates. Treatment 12|12 = 12 h anaerobic and 12 h aerobic; treatment 12|24 = 12 h anaerobic and 24 h aerobic; treatment 24|0 = 24 h of anaerobic; treatment 24|12 = 24 h anaerobic and 12 h aerobic; and 24|24 treatment = 24 h anaerobic and 24 h aerobic. The same proportions of the components of experiment 1 were maintained for the preparation of the synbiotic, that is, for every 100 g of rice bran, 10 g of alkalizing agent (SB), 0.2 g of microbiological mix, and 1 L of water were added.
Initially, the water used for the bioreactors was filtered (50 µm), chlorinated (10 mg L−1 of active chlorine), and dechlorinated (sodium thiosulfate—5.0 mg L−1 + aeration). Afterwards, there was the initial fertilization of this water with triple superphosphate (0.287 g P m−3) and ammonium sulfate (2.7 g N m−3). Then, the protocol of feed addition (40% of crude protein) was initiated with nitrogen input in the experimental units, and the rice bran (synbiotic) as source of organic carbon. Thus, twice a week, feed (equivalent to 1.0 mg N L−1 = 40% CP × 0.16 = 6.4% N) and synbiotic prepared with rice bran (43% C) (Santos et al., 2022) were added to obtain a C:N ratio equivalent to 15:1, as adapted by Crab et al. (2012) and Emerenciano et al. (2017).
This bioreactor preparation stage lasted 35 days, and during this period the following variables were monitored: total alkalinity (at the start and at the end), total ammonia nitrogen (TAN), N-NO2 and N-NO3 (weekly).
Synbiotic proximate composition
In order to understand the effects of fermentation, microbial respiration, or a combination of these two on the lipid and protein content of the synbiotic, three forms of preparation were evaluated: ANA (24 h of fermentation—anaerobic), AER (24 h of respiration—aerobic), and ANA + AER (48 h—combining 24 h anaerobic plus 24 h aerobic). Synbiotic samples were collected to determine their proximate composition using a triplicate of the 600 mL from the cylindrical-conical container (2.0 L) and after incubation time were oven‐dried at 105 °C until constant weight (315 SE model, Fanem). After this time, the samples were cooled at room temperature in a glass desiccator and weighed again. The dried product was transformed into flour and stored in plastic bags at room temperature before analysis. The difference in weight before and after sample drying was recorded and expressed in percentage. These analyses were performed in triplicate using standard methods (AOAC, 2016) at the Laboratory of Small Ruminants, Department of Animal Science, Federal Rural University of Pernambuco (UFRPE), Recife, Brazil. For protein content, nitrogen (N × 6.25) was measured using the Kjeldahl method in a nitrogen still (TE 0363/180L model; 256 Tecnal, São Paulo, Brazil). The total lipid content, in turn, was obtained by the Soxhlet extraction method using hexane (98%) as solvent (model TE 1881/6, Tecnal, São Paulo, Brazil).
Experiment 3: evaluation of the prawns growth in the synbiotic culture system
The prawns used in the experiment (M. rosenbergii) were acquired in a commercial hatchery (Acquamarão Laboratory, Goiana, PE, Brazil) and acclimated to local conditions for 5 days (environmental and feeding variables). During this time, the water matured in experiment 2 was used as inoculum (20% of the volume) to prepare the experimental units of treatment 3, which followed the same nomenclature and preparation strategy of the synbiotic biofertilizer: treatment 12|12 = 12 h anaerobic and 12 h aerobic; treatment 12|24 = 12 h anaerobic and 24 h aerobic; treatment 24|0 = 24 h of anaerobic; treatment 24|12 = 24 h anaerobic and 12 h aerobic; 24|24 treatment = 24 h anaerobic and 24 h aerobic. Thus, there were five treatments in quadruplicate. After acclimatization and initial biometry (10.01 ± 2.0 mg), 48 prawns were transferred (1.0 PL L−1) into the experimental units, composing a completely randomized design.
Fertilization of the experimental units was performed three times a week with the synbiotic. For this fertilization in the experimental units, a C:N ratio of 5:1 was used, as recommended by Ebeling et al. (2006), who suggest that 82% of the total ammonia nitrogen will be converted by heterotrophic bacteria and 18% by nitrifying bacteria, reducing problems with nitrite accumulation in the water. This adopted ratio, however, did not take into account the amount of organic carbon present in the feed.
Water quality analysis
Water quality variables such as pH, temperature (°C), DO—dissolved oxygen (mg L−1), TDS—total dissolved solids (mg L−1), and electrical conductivity (µS cm−1) were measured daily using a multiparameter probe (HANNA® Instruments, model 9828). The variables TAN—total ammonia nitrogen (mg L−1), N-NO2 (mg L−1), and N-NO3 (mg L−1) were measured weekly with the aid of a HANNA® Instruments spectrophotometer, model HI 83,399 (Belgium), using the Nessler methods (TAN), an adaptation of the diazotization method (N-NO2), and an adaptation of the cadmium reduction method (N-NO3).
Total alkalinity (mg CaCO3 L−1) and total hardness (mg CaCO3 L−1) were determined every 10 days by titration with sulfuric acid (H2SO4) and EDTA (Na2H2Y), respectively (APHA, 2012). Settleable solids (SS) were determined at days 1, 12, 24, and 35 of culture, using 1000 mL samples from each experimental unit transferred to Imhoff cones and kept at rest for sedimentation during 50 min.
Feeding and prawn zootechnical performance
The prawns were fed four times a day (8 am, 11 am, 2 pm, and 4 pm) with commercial feed for shrimp (Purina, Camaronina CR2, 40% crude protein and 8.0% lipids). Weekly biometrics were performed to adjust the daily feeding rate, starting at 40% of body weight and gradually decreasing to 12% in the last 7 days of the experiment.
At the end of the experimental period (35 days), all prawns from each experimental unit were counted and weighed individually, which made it possible to assess survival (S% = (number of surviving shrimp/number of shrimp stocked) × 100); yield (g m−3), average final weight, weekly weight gain (WWG = weight gain/number of weeks of reared), specific growth rate (SGR = (ln final weight − ln initial weight/number of days of reared) × 100), and feed conversion rate (FCR = feed supplied/increase in biomass).
Statistical analysis
Possible differences between treatments were verified after their assumptions of variances homogeneity and data distribution normality with the Cochran (p < 0.05) and Shapiro–Wilk (p < 0.05) tests, respectively. The analysis of variance (ANOVA one-way) was used for data on zootechnical performance and synbiotic proximate composition. When the means were significantly different between treatments (p < 0.05), the test of Duncan (p < 0.05) was applied. In the case of water quality variables, Friedman’s non-parametric test was implemented. When the medians were significantly different between treatments (p < 0.05), the multiple Conover test with Holm-Bonferroni correction (p < 0.05) was used. Statistical routines and graphic illustrations were performed in the R Project 3.1.0 software (Package Agricolae) and SigmaPlot 12.0 software, respectively.
Results
Monitoring pH in the preparation of synbiotic with different alkalizing agents
The results of pH monitoring tests during fermentation and microbial respiration in the preparation of the synbiotic with rice bran showed a greater variation between minimum and maximum pH values when using the alkalizing dolomitic limestone (CA) and Lithothamnium (LT). For LT, the lowest pH values were observed during the anaerobic phase at 24 h. As for CA, the lowest pH values were recorded during the aerobic phase at 36 h. In Fig. 1, it is possible to visualize this variation for treatments SB, CA, and LT. The SB treatment, in comparison with the others, presented higher pH value and lower variation between minimum and maximum in the fermentation and the microbial respiration processes.
Fluctuation of pH (mean ± standard deviation) during the synbiotic preparation process. abc*Different letters between columns represent significant differences (p < 0.05) between treatments by Conover’s multiple test with Holm-Bonferroni correction. T24|24 = 24 h anaerobic and 24 h aerobic; T12|24 = 12 h anaerobic and 24 h aerobic. SB = sodium bicarbonate; CA = dolomitic limestone; LT = Lithothamnium
Preparation of water from bioreactors with the synbiotic
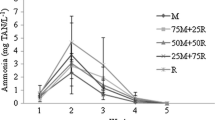
In the preparation of the bioreactors water (experiment 2), the variables total alkalinity (start 110.44 ± 9.80 and end 200.27 ± 7.80 mg CaCO3 L−1) and pH (start 7.90 ± 0.03 and end 8.20 ± 0.05) did not differ significantly (data not shown). Settleable solids also did not show significant differences (p > 0.05) between treatments. TAN peaked at the end of the first week of preparation for all treatments, with means varying between 1.25 ± 0.66 and 1.83 ± 0.28 mg L−1 (Fig. 2). N-NO2, in turn, had a different behavior, with peaks in the second week (T12|24 = 2.20 ± 1.03; 24|0 = 1.85 ± 0.90; T12|12 = 1.74 ± 0.04 mg L−1) and in the third maturation week (T24|24 = 2.03 ± 1.32; T24|12 = 2.20 ± 1.03 mg L−1) (Fig. 2). From the fourth week of maturation onwards, TAN and N-NO2 did not show significant differences (p > 0.05) between treatments. As for N-NO3, peaks occurred in the third week for all treatments, with a decrease in the subsequent week (Fig. 2).
Concentrations of nitrogen compounds during the preparation process of bioreactors with a synbiotic system over 5 weeks. T12|12 = 12 h anaerobic and 12 h aerobic; T12|24 = 12 h anaerobic and 24 h aerobic; T24|0 = 24 h anaerobic; T24|12 = 24 h anaerobic and 12 h aerobic; T24|24 = 24 h anaerobic and 24 h aerobic. TAN, total ammonia nitrogen
Water quality and prawns growth in the synbiotic culture system
The results of the prawns’ rearing environment water quality variables are shown in Table 1.
During the nursery phase in the experimental units, TAN fluctuated with maximum values in treatment 12|12 (0.58 ± 0.44 mg L−1) in the fourth week (Fig. 3A). Regarding N-NO2, variations were also observed during the reared cycle with maximum values (0.06 ± 0.08 mg L−1) in treatment 12|24 (Fig. 3B). N-NO3, in turn, showed a tendency to accumulate throughout the experimental period (Fig. 3C), with an average between 5.9 and 8.2 mg L−1 at the end of the reared. Settleable solids (SS) showed values above 15 mL L−1 at the end of the experiment for all treatments (Fig. 3D), with averages ranging from 17.25 ± 2.21 mL L−1 (24|12) and from 30.70 ± 13.32 mL L−1 (24|0) in the last week of reared.
Values of variables TAN (A), N-NO2 (B), N-NO3 (C), and settleable solids—SS (D) throughout the experimental period. Treatment 24|24 = 24 h anaerobic and 24 h aerobic; treatment 12|24 = 12 h anaerobic and 24 h aerobic; treatment 24|12 = 24 h anaerobic and 12 h aerobic; treatment 24|0 = 24 h anaerobic; treatment 12|12 = 12 h anaerobic and 12 h aerobic
The synbiotic proximate composition after the anaerobic and aerobic phases are presented in Table 2, and it is possible to identify a greater contribution of the aerobic phase in the increase of the lipid content.
Among the prawn zootechnical performance variables, only survival did not show significant differences (p > 0.05) between treatments. In addition, according to the results, the importance of the aerobic phase in the preparation of the synbiotic is highlighted, since the treatments with longer time in this phase (24|24 and 12|24) showed better results than those without the aerobic phase (24|00), especially for the variables final average weight and yield (Table 3).
Discussion
pH monitoring in synbiotic preparation
The SB treatment showed higher pH values than the LT and CA treatments for the different phases (anaerobic and aerobic). However, all pH values were within a range considered adequate for most of the probiotic microorganisms (4.5 and 6.5) (Hofvendahl and Hagerdal 2000; Vassileva et al. 2021). However, the smaller distance between the minimum and maximum values observed in the SB treatment represented better stability of this variable during the evaluation time. Sudden variations in pH can lead to a reduction or even inhibition of the production of important metabolites, microbial growth, and enzymatic activity by some microorganisms (Naidu and Devi 2005; Dawood and Kashio 2019).
Most microorganisms prefer neutral or slightly acidic pH, which enables a higher biomass production, in the range of 4.5 to 6.5 (Hofvendahl and Hagerdal 2000; Vassileva et al. 2021). Lactic acid bacteria, such as those of the genus Lactobacillus, are fermentable with oxygen tolerance, grow at temperatures ranging from 30 to 40 °C, and have an ideal pH for the production of organic acids between 5.0 and 7.0 (Hofvendahl and Hagerdal 2000). Yeasts, such as S. cerevisiae, show satisfactory growth in a pH range between 4.0 and 7.0, while pH values above 8.0 can affect the outcome of the fermentation process (production of hydrolytic enzymes and secondary metabolites) (Peña et al. 2015; Vassileva et al. 2021). Despite growing under extreme temperature and pH conditions, most Bacillus species express high growth at pH between 6.0 and 9.0 (Naidu and Devi 2005; Naraian and Kumari 2018).
These results, associated with a smaller effect on water hardness, when compared to other alkalizers, contributed to the choice of sodium bicarbonate (SB) in the preparation of synbiotics in the growth experiments of the prawn specie M. rosenbergii. However, it was possible to keep the pH within the appropriate range for the microorganisms used in the synbiotic with all the alkalizers evaluated, which increases the ability to select the product with better economic cost or greater availability in the local market.
Between the LT and CA alkalizers, there were no significant differences for most of the times measured during the preparation of the synbiotic, but the times 36 h and 48 h of the 24|24 strategy stood out, in which the CA treatment presented pH values close to 5.0. This value is tolerated for several Bacillus spp., but it is slightly below the range that optimizes their growth and protease production (Naidu and Devi 2005; Dawood and Kashio 2019).
During the anaerobic fermentation process, a decrease in the pH of the solution was observed, which is caused by the metabolism of microorganisms, especially the production of organic acids by lactic acid bacteria and CO2 by yeasts (Hofvendahl and Hagerdal 2000; Vassileva et al. 2021). Bacillus spp. present in the microbiological mix are preferentially aerobic and, given their bioremediation character, the introduction of aeration (aerobic phase) in the solution probably contributes to the increase of their microbial biomass.
In submerged microbial respiration (aerobic), as at this stage, dissolved oxygen is the main parameter for successful microbial growth, which directly affects metabolic activity and the type of end product (Vassileva et al. 2021). This aerobic phase may also have been responsible for changes in the downward trend of pH values, especially for the LT and CA treatments, which tended to increase or stabilize.
The oxygenation inside the container and the stirring of the mixture may have stimulated the solubility of these alkalizers (LT and CA), in addition to producing effects on alkalinity and pH, since they have a lower solubility and reaction time than SB, which is known for its efficiency in elevation of alkalinity and maintenance of pH stability (Loyless and Malone 1997). Furthermore, in the process of microbial respiration, the presence of CO2 contributes to the dissolution of CaCO3 and may have resulted in an increase in alkalinity and pH (Van Wyk and Scarpa 1999).
Preparation of bioreactors with synbiotic
The toxicity of nitrogen compounds, especially TAN and N-NO2 for intensive systems with minimal water exchange, can be considered critical and may cause animal mortality (New et al. 2010; De Schryver et al. 2008; Ebeling et al. 2006). Thus, some studies demonstrate the need for initial water preparation (maturation) to ensure the establishment of the nitrification process and reduce the risks of animal mortality (Crab et al. 2012; Emerenciano et al. 2017; Abakari et al. 2020b).
The results observed in experiment 2 showed that the strategies used in relation to the time of the anaerobic and aerobic phases were efficient in providing a suitable environment for the fixation of the bacterial community (heterotrophic and nitrifying) capable of metabolizing nitrogenous compounds in the bioreactors’ water (Ebeling et al. 2006; De Schryver et al. 2008; Romano et al. 2018; Santos et al. 2022).
The heterotrophic and ammonia-oxidizing bacteria started the nitrogen transformation processes from the first week of preparation for all treatments. This reduction in the concentration of ammonia and the appearance of nitrite is indicative of nitrification (Ebeling et al. 2006; De Schryver et al. 2008). The results of nitrite-oxidizing bacteria metabolism, in turn, began to be evidenced from the second week of preparation, when an increase in N-NO3 concentrations was observed.
For this water preparation process, it is common for N-NO3 concentrations to present higher values, since this is the end product of TAN metabolism within the system (Ebeling et al. 2006; Avnimelech et al. 2012; Emerenciano et al. 2017; Abakari et al. 2020b). Thus, it was noted that from the fourth week of preparation onwards, the water from all treatments was ready to be used as an inoculum in prawn farming units for the variables TAN (< 1.0 mg L−1) and N-NO2 (< 1.0 mg L−1) (New et al. 2010).
Rearing water quality
The variables temperature (> 25 °C), dissolved oxygen (> 5.0 mg L−1), and pH (7.0 to 8.5) were maintained as recommended for M. rosenbergii (New et al. 2002; 2010).
Total alkalinity presented averages above 120 mg CaCO3 L−1 in all treatments. These concentrations are in accordance with those recommended by Adhikari et al. (2007) (± 100 mg CaCO3 L−1) and Coyle et al. (2010) (> 50 mg CaCO3 L−1) for M. rosenbergii. Furthermore, it is worth noting that total alkalinity values above 100 mg CaCO3 L−1 are recommended for systems with minimal water exchange due to their role in controlling pH fluctuations and in the metabolism of nitrifying bacteria (Ebeling et al. 2006; Avnimelech et al. 2012; Emerenciano et al. 2017; Samocha et al. 2017).
Mean values of total hardness greater than 50 mg CaCO3 L−1 found in the present experiment are in agreement with the reports by New et al. (2010) (50 to 150 mg CaCO3 L−1) and the works by Vasquez et al. (1989) (20 to 200 mg CaCO3 L−1). High levels of water hardness can affect both growth and survival of M. rosenbergii, but the results of the present study and those presented by these authors demonstrate the wide tolerance of this species for this variable in the culture environment.
The different synbiotic preparation strategies, as well as the adoption of a C:N ratio of 5:1, were effective in controlling nitrogen compounds (TAN, N-NO2, and N-NO3) within the culture environment by colonizing heterotrophic and nitrifying bacteria (Ebeling et al. 2006; Abakari et al. 2020b) after water maturation.
This is evident when we observe that the concentrations of these variables found in the prawn growth experiment were in accordance with those recommended by several authors—TAN < 1.0 mg L−1, N-NO2 < 1.0 mg L−1, and N-NO3 < 10.0 mg L−1 (New 2002; New et al. 2010; Pérez-Fuentes et al. 2013; Dutra et al. 2020; Dong et al. 2020).
In addition, the efficiency in controlling these variables in a reared environment using carbohydrates with anaerobic and aerobic (synbiotic) processes has been reported by other authors (Romano et al. 2018; Abdel-Tawwab et al. 2020; Andrade et al. 2021; Hussain et al. 2021; Silva et al. 2021b; Santos et al. 2022).
The different preparation strategies of the synbiotic (anaerobic and aerobic) with rice bran had no effect on the TDS and electrical conductivity variables. Values above 270 mg L−1 and 444 µS cm−1 for these variables, respectively, recorded in the present experiment, indicate high availability of nutrients in the culture system (Sipauba-Tavares 1995; Samocha et al. 2017), which has been notably associated with synbiotic or heterotrophic systems (Crab et al. 2012; Romano 2017; Romano et al. 2018; Abdel-Tawwab et al. 2020; Deepak et al. 2020; Andrade et al. 2021; Hussain et al. 2021; Santos et al. 2022).
In the same way, settleable solids (SS) are another strong indication of the availability of nutrients and natural food within the system, although values above 15 mL L−1 (recorded in the second half of the experiment) may incur damage to the growth and survival of the shrimp (Emerenciano et al. 2017; Samocha et al. 2017).
Growth of prawn reared
The different synbiotic preparation strategies (anaerobic and aerobic) did not influence the prawn survival rates, which were all above 77% and similar to those found by other authors that reared the same species in a heterotrophic system (Crab et al. 2010; Ballester et al. 2017; Hosain et al. 2021; Santos et al. 2022).
According to New (2002), at the end of the nursery phase, a minimum survival of 75% is expected. Several factors may be associated with obtaining survivals similar to those found in the present experiment, such as maintenance of water quality, improvement of immune status due to probiotic supplementation, and adequate feeding and stocking density (Adhikari et al. 2007; New et al. 2010; Dutra et al. 2020; Dauda 2019; Dong et al. 2020; Miao et al. 2017).
Probiotic supplementation with yeasts and bacteria of the genus Bacillus spp. and Lactobacillus spp. using fermentation processes and microbial respiration (synbiotic) or bioflocs has shown efficiency in increasing the immunity of animals and reducing the count of pathogenic bacteria, whose growth was probably prevented due to competition for nutrients (Romano et al. 2018; Abdel-Tawwab et al. 2020; Miao et al. 2017; Andrade et al. 2021; Silva et al. 2021a). In this sense, the use of these microorganisms in the synbiotic preparation process, regardless of the strategy adopted, contributed to the maintenance of a culture environment favorable to the survival of the animals.
The treatments with longer preparation time with aerobic phase (24|24 and 12|24) showed better growth and yield results when compared to the treatment that did not include the aerobic phase (24|0). Despite evidence of beneficial effects of synbiotic preparation with only the anaerobic phase of rice bran for the growing environment (Romano et al. 2018; Abdel-Tawwab et al. 2020; Liñan-Vidriales et al. 2021), the results of the present experiment point to the aerobic step as an important component in the synbiotic preparation process.
Liñan-Vidriales et al. (2021) reared Penaeus vannamei in tanks fertilized with rice bran and fermented rice bran for 24 h (consortium of Bacillus and Lysinibacillus species or commercial probiotic containing B. subtilis, B. licheniformis, B. coagulans, L. acidophilus, and S. cerevisiae) and found no significant differences related to final mean weight, survival, SGR, and FCR. Abdel-Tawwab et al. (2020), however, reared P. vannamei using rice bran with B. subtilis (24 h anaerobic, pH 6–7, 28–30 °C) and observed greater growth compared to the control (commercial diet and water exchange). These differences may be linked to the forms of preparation and application of the synbiotic in water.
Within this context, the work carried out by Romano et al. (2018) provide important information on the effects of pre-treatment (24 h fermentation or 24 h respiration) of rice bran on its nutritional value and on the nutritional value of the flocs.
The results of these authors pointed to an increase in lipids (14.79%) and proteins (11.12%) resulting from pre-treatment, with the highest increase observed for microbial respiration (24 h aerobic). These results are compatible with those found in the present experiment, as the mean values of lipids in the AER and ANA + AER replicates were higher than those using only anaerobic (ANA).
The increase in microbial respiration time in the pre-treatment of rice bran stimulates the growth of bacteria of the genus Bacillus spp. (Vassileva et al. 2021), which play an important role not only in increasing the protein and lipid levels of the final product, but also in stimulating immunity and balance of the gastrointestinal microbiota and digestive enzyme activities in cultured animals (Romano et al. 2018; Cienfuegos-Martínez et al. 2020; Liñan-Vidriales et al. 2021; Andrade et al. 2021).
Most cultivable aquatic organisms satisfy the majority of their energetic needs through protein and lipid metabolism. Lipid metabolism produces several essential fatty acids to the growth and metabolic functions of these animals (Tseng and Hwang 2008; New et al. 2010; Li et al. 2017).
Furthermore, adequate levels of lipids in the diet of M. rosenbergii (< 10%) (New et al. 2010) and other aquatic organisms are associated with weight gain, reducing the need for dietary protein and their use for membrane synthesis and energy reserve (Santos et al. 2007; Tseng and Hwang 2008; New et al. 2010).
It can be seen, therefore, that a longer time dedicated to microbial metabolism with rice bran as a substrate (24|24 and 12|24) may have contributed to the production of a synbiotic that generated better nutritional value of the floc within the reared environment.
Even under similar conditions in terms of feeding, stocking density, and synbiotic preparation strategy (24 h anaerobic and 24 h aerobic), the results found for the variables final average weight, weekly weight gain, and yield were higher than those found by Santos et al. (2022) (122.85 ± 12.5 mg, 22.26 ± 2.97 mg, and 85.02 ± 2.15 g m−3, respectively).
This difference may be related to the composition of the microbiological mix used in the preparation of the synbiotic, denoting the importance of lactic acid bacteria (L. acidophilus) and yeasts (S. cerevisiae) in the preparation of rice bran. The FCR and SGR results obtained in this experiment were similar to those found by Hasain et al. (2021), who, reared M. rosenbergii with different carbon sources (wheat bran, rice flour, molasses, and corn starch) in a biofloc system, found averages of FCR between 2.21 and 4.47 and of SGR between 6.02 and 6.98% day−1.
Conclusion
The proportion of alkalizing agents used (10% in relation to the amount of rice bran) was efficient in maintaining the fermentation pH and microbial respiration adequate for most microorganisms (> 4.5). The different synbiotic preparation strategies did not influence the water preparation time of the bioreactors. The results of this research indicate that a longer preparation time of the synbiotic including the anaerobic and aerobic stages can promote better performance of the prawn reared, since the treatments T24|24 and T12|24 presented results of growth and yield superior to those of the treatment T24|00.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Abakari G, Luo G, Kombat EO (2020b) Dynamics of nitrogenous compounds and their control in biofloc technology (BFT) systems: a review. Aquac Fish 6:441–447. https://doi.org/10.1016/j.aaf.2020.05.005
Adhikari S, Chaurasia VS, Naqvi AA, Pillai BR (2007) Survival and growth of Macrobrachium rosenbergii (de Man) juvenile in relation to calcium hardness and bicarbonate alkalinity. Turk J Fish Aquat Sci 7:23–26
Aguilar JGS, Castro RJS, Sato HH (2019) Alkaline protease production by Bacillus licheniformis LBA 46 in a bench reactor: effect of temperature and agitation. Braz J Chem Eng 6:615–625. https://doi.org/10.1590/0104-6632.20190362s20180014
Andrade RJV, Santos EP, Costa GKA, Campos CVFS, Silva SMBC, Galvez AO, Brito LO (2021) Effect of different frequencies of the addition of Brachionus plicatilis on the performance of Litopenaeus vannamei in a nursery biofloc system with rice bran (anaerobic and aerobic) as an organic carbon source. Aquac 540:1–10. https://doi.org/10.1016/j.aquaculture.2021.736669
APHA, AWWA, Wef, 2012. Standard methods for the examination of water and wastewater, 22nd ed. American Public Health Association
Association of Official Analytical Chemists (AOAC) (2016) Official Methods of Analysis of AOAC International. 20ª ed., William Horwitz (Editor), Gaithersburg, MD.
Ballester ELC, Marzarotto SA, Castro CS, Frozza A, Pastore I, Abreu PC (2017) Productive performance of juvenile freshwater prawns Macrobrachium rosenbergii in biofloc system. Aquac Res 48:1–8. https://doi.org/10.1111/are.13296
Ballester ELC, Maurente LPB, Heldt A, Dutra FM (2018) Vitamin and mineral supplementation for Macrobrachium rosenbergii in biofloc system. Lat Am J Aquat Res 46:855–859. https://doi.org/10.3856/vol46-issue4-fulltext-25
Cienfuegos-Martínez K, Monroy-Dosta MC, Hamdan-Partida A, Hernández-Vergara MP, Becerril-Cortés D, López-García E (2020) A review of the use of probiotics in freshwater prawn (Macrobrachium sp.) culture in biofloc systems. Lat Am J Aquat Res 48:518–528. https://doi.org/10.3856/vol48-issue4-fulltext-2464
Coyle SD, Alston ED, Sampaio CMS (2010) Nursery systems and management. In: Valenti WC, Tidwell JH, D’Abramo LR, Kutty MN (eds) New MB. Freshwater prawns. Biology and farming. Blackwell Science, Oxford, pp 108–126
Crab R, Chielens B, Wille M, Bossier P, Verstraete W (2010) The effect of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenbergii postlarvae. Aquac Res 41:559–567. https://doi.org/10.1111/j.1365-2109.2009.02353.x
Crab R, Defoirdt T, Bossier P, Verstraete W (2012) Biofloc technology in aquaculture: beneficial effects and future challenges. Aquac 356–357:351–356. https://doi.org/10.1016/j.aquaculture.2012.04.046
Dauda AB, Romano N, Ebrahimi M, Karim M, Natrah I, Kamarudin MS, Ekasari J (2017) Different carbon sources affected biofloc volume, water quality and the survival and physiology of African catfish Clarias gariepinus fingerlings reared in intensive biofloc technology system. Fish Sci 83:1037–1048. https://doi.org/10.1007/s12562-017-1144-7
De Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W (2008) The basics of bioflocs technology: the added value for aquaculture. Aquac 277:125–137. https://doi.org/10.1016/j.aquaculture.2008.02.019
Deepak AP, Vasava RJ, Elchelwar VR, Tandel DH, Vadher KH, Shrivastava V, Prabhakar P (2020) Aquamimicry: new an innovative apporoach for sustainable development of aquaculture. J Entomol Zool Stud 8:1029–1031
Dong X, Liu Q, Kan D, Zhao W, Gu H, Lv L (2020) Effects of ammonia-N exposure on the growth, metabolizing enzymes, and metabolome of Macrobrachium rosenbergii. Ecotoxicol Environ Saf 189:1–10. https://doi.org/10.1016/j.ecoenv.2019.110046
Dutra FM, Rio GS, Zadinelo IV, Ballester ELC (2020) Exposure of Macrobrachium rosenbergii (De Man, 1879) post-larvae to different nitrate concentrations: effect on performance and welfare. Aquac 520:1–8. https://doi.org/10.1016/j.aquaculture.2019.734674
Ebeling JM, Timmons MB, Bisogni JJ (2006) Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquac 257:346–358. https://doi.org/10.1016/j.aquaculture.2006.03.019
Hofvendahl K, Hahn-Hagerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Technol 26:87–107
Hosain ME, Amin SMN, Arshad A, Kamarudin MS, Karim M (2021) Effects of carbon sources on the culture of giant river prawn in biofloc system during nursery phase. Aquac Rep 19:1–9. https://doi.org/10.1016/j.aqrep.2021.100607
Hussain AS, Mohammad DA, Sallam WS, Shoukry NM, Davis DA (2021) Effects of culturing the Pacific white shrimp Penaeus vannamei in “biofloc” vs “synbiotic” systems on the growth and immune system. Aquac 542:1–8. https://doi.org/10.1016/j.aquaculture.2021.736905
Huynhtg YL, Shiu TP, Nguyen QP, Truong Q, Chen J, Liu C (2017) Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: a review. Fish Shellfish Immunol 64:367–382. https://doi.org/10.1016/j.fsi.2017.03.035
Kumar S, Anand PSS, De D, Deo AD, Ghoshal TK, Sundaray JK, Ponniah AG, Jithendran KP, Raja RA, Biswas G, Lalitha N (2017) Effects of biofloc under different carbon sources and protein levels on water quality, growth performance and immune responses in black tiger shrimp Penaeus monodon (Fabricius, 1978). Aquac Res 48:1–15. https://doi.org/10.1111/are.12958
Li E, Wang X, Chen K, Xu C, Qin JG, Chen L (2017) Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev Aquac 9:57–75. https://doi.org/10.1111/raq.12104
Lima PCM, Silva AEM, Silva DA, Silva SMBC, Brito LO, Gálvez AO (2021) Effect of stocking density of Crassostrea sp in a multitrophic biofloc system with Litopenaeus vannamei in nursery. Aquac 530:735913. https://doi.org/10.1016/j.aquaculture.2020.735913
Liñan-Vidriales MA, Peña-Rodríguez A, Tovar-Ramírez D, Elizondo-González R, Barajas-Sandoval DR, Ponce-Gracía EI, Rodríguez-Jaramillo C, Balcázar JL, Quiroz-Guzmán E (2021) Effect of rice bran fermented with Bacillus and Lysinibacillus species on dynamic microbial activity of Pacific white shrimp (Penaeus vannamei). Aquac 531:1–8. https://doi.org/10.1016/j.aquaculture.2020.735958
Loyless CL, Malone RF (1997) A sodium bicarbonate dosing methodology for pH management in freshwater-recirculating aquaculture systems. Prog Fish Cult 59:198–205
Miao S, Zhu J, Zhao C, Sun L, Zhang X, Chen G (2017) Effects of C/N ratio control combined with probiotics on the immune response, disease resistance, intestinal microbiota and morphology of giant freshwater prawn (Macrobrachium rosenbergii). Aquac 476:125–133. https://doi.org/10.1016/j.aquaculture.2017.04.027
Naidu KSB, Devi KL (2005) Optimization of thermostable alkaline protease production from species of Bacillus using rice bran. Afr J Biotech 4:724–726
Negrini C, Castro CS, Bittencourt-Guimarães AT, Frozza A, Ortiz-kracizy R, Ballester ELC (2017) Stocking density for freshwater prawn Macrobrachium rosenbergii (Decapoda, Palaemonidae) in biofloc system. Latim Am J Aqua Res 45:891–899. https://doi.org/10.3856/vol45-issue5-fulltext-3
Peña A, Sánchez NS, Álvarez H, Ramírez CM, J, (2015) Effects of high medium pH on growth, metabolism and transport in Saccharomyces cerevisiae. FEMS Yeast Res 15:1–13. https://doi.org/10.1093/femsyr/fou005
Perez-fuentes JA, Perez-rostro CI, Hernandez-vergara MP (2013) Pond-reared Malaysian prawn Macrobrachium rosenbergii with the biofloc system. Aquac 400–401:105–110. https://doi.org/10.1016/j.aquaculture.2013.02.028
Potumarthi R, Ch S, Jetty A (2007) Alkaline protease production by submerged fermentation in stirred tank reactor using Bacillus licheniformis NCIM-2042: effect of aeration and agitation regimes. Biochem Eng J 34:185–192. https://doi.org/10.1016/j.bej.2006.12.003
Romano N, Dauda AB, Ikhsan N, Karim M, Kamarudin MS (2018) Fermenting rice bran as a carbon source for biofloc technology improved the water quality, growth, feeding efficiencies, and biochemical composition of African catfish Clarias gariepinus juveniles. Aquac Res 49:3691–3701. https://doi.org/10.1111/are.13837
Samocha TM, Prangnell DI, Hanson TR, Treece GD, Morris TC, Castro LF, Staresinic N (2017) Design and operation of super intensive, biofloc-dominated systems for indoor production of the Pacific white shrimp, Litopenaeus vannamei – the Texas A&M AgriLife Research experience. The World Aquaculture Society, Baton Rouge, Louisiana, p 368p
Santos FL, Azeredo VB, Martins ASA (2007) Effect of supplying food complemented with linseed on the chemical composition of Malaysian shrimp (Macrobrachium rosenbergii). Ciênc Tecnol Aliment 27:851–855
Santos RB, Coelho Filhio PA, Golçalves AP, Santos RA, Rodrigues ML, Correia ES, Oliveira VQ, Brito LO (2022) Effects of organic carbon sources on water quality, microbial flocs protein and performance of Macrobrachium rosenbergii post-larvae reared in biofloc and synbiotic systems. Aquac Res 53:388–397. https://doi.org/10.1111/are.15580
Shim YH, Shinde PL, Choi JY, Kim JS, Seo DK, Pak JI, Chae BJ, Kwon IK (2010) Evaluation of multi-microbial probiotics produced by submerged liquid and solid substrate fermentation methods in broilers. Asian Australas J Anim Sci 23:521–529
Silva AEM, Brito LO, Silva DA, Lima PCM, Farias RS, Gálvez AO, Silva MBC (2021a) Effect of Brachionus plicatilis and Navicula sp. on Pacific white shrimp growth performance, Vibrio, immunological responses and resistance to white spot virus (WSSV) in nursery biofloc system. Aquaculture 535:1–9. https://doi.org/10.1016/j.aquaculture.2020.736335
Silva AS, Lima PCM, Silva AEM, Filho PRCO, Silva SMBC, Gálvez AO, Brito LO (2021b) Effects of adding rotifers on the water quality, plankton composition and growth of Pacific white shrimp, Litopenaeus vannamei juvenile, when cultured with biofloc technology. Aquac Res 00:1–14. https://doi.org/10.1111/are.15276
Sugiharto S, Ranjitkar S (2019) Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: a review. Anim Nutr 5:1–10. https://doi.org/10.1016/j.aninu.2018.11.001
Tseng Y, Hwang P (2008) Some insights into energy metabolism for osmoregulation in fish. Comp Biochem Physiol C Toxicol Pharmacol 148:419–429. https://doi.org/10.1016/j.cbpc.2008.04.009
Vasquez OE, Rouse DB, Rogers WA (1989) Growth response of Macrobrachium rosenbergii to different levels of hardness. J World Aquac Soc 20:90–92. https://doi.org/10.1111/j.1749-7345.1989.tb00528.x
Vassileva M, Malusa E, Sas-Paszt L, Trzcinski P, Galvez A, Flor-Peregrin E, Shilev S, Canfora L, Mocali S, Vassilev N (2021) Fermentation strategies to improve soil bio-inoculant production and quality. Microorganisms 9:1–18. https://doi.org/10.3390/microorganisms9061254
Abakari G, Luo G, Kombat EO, Alhassan EH (2020a) Supplemental carbon sources applied in biofloc technology aquaculture systems: types, effects and future research. Reviews in Aquac 1–30. https://doi.org/10.1111/raq.12520
Abdel-Tawwab M, Khalil RH, Nour AM, Elkhayat BK, Khalifa E, Abdel-Latif HMR (2020) Effects of Bacillus subtilis fermented rice bran on water quality, performance, antioxidants/oxidants, and immunity biomarkers of white leg shrimp (Litopenaeus vannamei) reared at different salinities with zero water exchange. J Appl Aquac 1-27. https://doi.org/10.1080/10454438.2020.1844110
Avnimelech Y, De-Schryver P, Emmereciano M, Kuhn D, Ray A, Taw N (2012) Biofloc technology - a practical guide book (2nd ed.). The World Aquaculture Society, Baton Rouge, LA, 283p.
Dauda AB (2019) Biofloc technology: a review on the microbial interactions, operational parameters and implications to disease and health management of cultured aquatic animals. Rev Aquac 1–18. https://doi.org/10.1111/raq.12379
Dawood MAO, Koshio S (2019) Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev Aquac 1–16. https://doi.org/10.1111/raq.12368
Emerenciano MGC, Martínez-córdova LR, Martínez-porchas M, Miranda-baeza A (2017) Biofloc technology (BFT): a tool for water quality management in aquaculture. IN: Tutu, Hlanganani. Water quality. InTech. January. 426p.
FAO (2022) FishStatJ v4.02.06 [Software]. Retrieved from https://www.fao.org/fishery/en/statistics/software/fishstatj/en. Accessed 27 July 2022.
Naraian R, Kumari S (2018) Microbial production of organic acids. In: Microbial functional foods and nutraceuticals, first edition. Edited by Vijai Kumar Gupta, Helen Treichel, Volha (Olga) Shapaval, Luiz Antonio de Oliveira, and Maria G. Tuohy. © 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
New MB (2002) Farming freshwater prawns: a manual for the culture of the giant freshwater prawn (Macrobrachium rosenbergii). FAO fisheries technical paper 428. FAO, Rome.
New MB, Valenti WC, Tidwell JH, D’abramo LR, Kutty MN (2010) Farming freshwater prawns: biology and farming (1st ed.) Wiley-Blackwell, United Kingdom, 570p.
Romano N (2017) Aquamimicry: a revolutionary concept for shrimp farming. Global Aquaculture Advocate. 10 January 2017.
Sipaúba-Tavares LH (1995) Limnologia Aplicada à Aquicultura. São Paulo: FUNEP/UNESP Ed. (Boletim Técnico nº 1), 72p.
Van Wyk P, Scarpa J (1999) Water quality and management. p.128–138. In: Farming marine shrimp in recirculating freshwater systems. Van Wyk, P. et al., eds. Florida Department of Agriculture and Consumer Services, Tallahassee, FL
Acknowledgements
The authors are grateful for the financial support provided by the Brazil’s national Council for Scientific and Technological Development (Universal CNPQ 429301/2018-9) and the Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarships granted (process numbers: 88882.436207/2019-01). CNPq also provided a grant to Luis Otavio Brito (PQ309669/2021-9).
Funding
The Brazil’s national Council for Scientific and Technological Development (Universal CNPQ 429301/2018–9 and PQ 309669/2021-9) and the Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarships granted (process numbers: 88887.480067/2020–00).
Author information
Authors and Affiliations
Contributions
Robson Batista dos Santos: research, conceptualization, methodology, development of experiments, tabulation, data analysis, and writing (original version); Tais Nunes dos Santos, Josefa Honorio da Silva, Chaiane Santos Assunção, and Gênison Carneiro Silva: experimental management, water quality analysis, and biometrics; Petrônio Alves Coelho Filho: methodology and textual review; Luis Otavio Brito: guidance, methodology, conceptualization, and textual review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research undertaken complies with the current animal welfare laws in Brazil. Macrobrachium rosenbergii used in this experimental work does not need approval from the Ethics Committee for Animal Use in Brazil. All the authors agree to participate in this experiment.
Human and animal ethics
The authors followed international and institutional animal management guidelines for the experiments.
Consent for publication
All the authors of this article agree to the publication.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
dos Santos, R.B., Coelho-Filho, P.A., Assunção, C.S. et al. The effect of different synbiotic preparation strategies on water fertilization and zootechnical performance of Macrobrachium rosenbergii reared in the nursery stage. Aquacult Int 30, 3159–3178 (2022). https://doi.org/10.1007/s10499-022-00955-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00955-y