Abstract
The utilization of sustainable and innovative raw materials to substitute for traditional fishmeal (FM) ingredients is required for the aquaculture sector. Sacha inchi meal (SIM), a by-product of sacha inchi oil, is one of the promising FM replacers. In the present study, four isonitrogenous and isolipidic (approximately 30 and 8%, respectively) diets containing 0, 270, 330, and 415 g/kg of SIM (SIM0, 60, 80, and 100, respectively) were prepared. Four replicate groups of red hybrid tilapia (15.87 ± 0.02 g/fish, 20 fish per replicate, 80 fish per diet) were randomly distributed into 16 glass tanks (100 L each) and manually fed one of the test diets until apparent satiation twice daily for 10 weeks. All fish were used to calculate growth parameters. Dietary SIM replacement increased growth performance and feed conversion ratio (P < 0.001 for most parameters). The SIM80 diet resulted in the highest final weight (56.43 g/fish), weight gain (40.53 g/fish), specific growth rate (1.81%/day), and protein productive value (37.30%). The apparent digestibility coefficient of protein was significantly increased (81.65 ‒ 87.65%) by replacement of FM with SIM (P < 0.001) with a significant negative effect observed in the fish fed SIM100 (78.30%). Hematology, total cholesterol, triglycerides, and low-density lipoprotein cholesterol were significantly decreased in all SIM-supplemented groups (P < 0.05). Aspartate aminotransferase and alanine transaminase levels were significantly increased with the highest levels in the SIM100 group (75.20 and 42.61 UL−1, respectively, P < 0.001). Among the histological differences observed in the liver, nuclei shifting and hepatocyte vacuolization were the main abnormalities in the fish fed the SIM80 and SIM100 diets. Intestinal villi height and thickness were increased by the dietary replacement (P < 0.05). These results demonstrate that SIM can replace FM by up to 330 g/kg (SIM80) improving growth, digestibility, blood parameters, and histological integrity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture is the fastest-growing segment among the farmed agricultural food sections due to an increase in fish and seafood consumption. Since 2010, total aquaculture production has grown at an average annual growth rate of roughly 10.3% over the last 20 years, accounting for 89% of the global total fisheries production (FAO 2020; NRC 2011). Because of the rising demand for high-quality protein, the decline in wild fish catches, and advances in fish farming technology, global aquaculture production is expected to double by 2050 (Stentiford 2017). In aquafeed industry, fish meal (FM) is widely used as an animal protein source due to its plenty of balanced essential amino acids, high protein content, and palatability (Gatlin et al. 2007; NRC 2011). However, the global FM supply can not keep up with the rising demand for aquafeed production, resulting in a continuous increase in the price (FAO 2020). In addition, during the last two decades, FM has significantly suffered from stagnation in global supply, resulting in periodic fluctuations of pricing up to 300% (Beal et al. 2018; Hua et al. 2019). The major advantages of FM include its high protein content, lack of anti-nutritional factors (ANFs), high digestibility, palatability, and well-balanced amino acid composition. Due to the scarcity of FM, fish nutritionists have been looking for alternative protein sources that have similar properties and are both cost-effective and sustainable (Gatlin et al. 2007; NRC 2011).
Alternative plant protein sources are one of the most extensively used FM replacers because of their consistent supply, low cost, and nutritional advantages. As a result, there is ongoing interest in researching what kind of plant protein and how much of them can be used to replace FM in aquafeed (FAO 2020; NRC 2011). Several plant proteins have been widely used to replace FM in part or completely: cotton seed, soybean, lupin, pea, corn, corn gluten, and combination of plant proteins (Webster et al. 1992; Rinchard et al. 2003a; Hernández et al. 2007, 2021; Zhang et al. 2012; Diógenes et al. 2018). However, there are still a number of drawbacks that limit the utilization of plant protein sources in aquafeed: e.g., poor palatability, imbalanced essential amino acid profile, and the presence of ANFs such as tannin, trypsin inhibitor, phytic acid, and saponin. Dietary inclusion of plant protein-based diets, particularly at a high level of replacement, can jeopardize the growth, feed utilization, nutrient bioavailability, and well-being of fish, leading to physiological anomalies (Samtiya et al. 2020; Khieokhajonkhet et al. 2021). This is due, at least in part, to ANFs and a lack of lysine and methionine in plant proteins (Gatlin et al. 2007; NRC 2011).
The wild oleaginous plant sacha inchi (Plukenetia volubilis L., family Euphorbiaceae) is native to the Amazonian tropical rainforest of South America (Chirinos et al. 2013). It is frequently referred to as a “superfood” because of its excellent nutritional profile and potential health advantages, and the market is rapidly growing (Gutiérrez et al. 2011, 2017; Wang et al. 2018). Sacha inchi meal (SIM) is a by-product of cold-pressed sacha inchi oil manufacture (Muangrat et al. 2018). The cold pressing method is traditional but still very common because other non-cold techniques often add unpleasant off-flavors to sacha inchi products by deteriorating polyunsaturated fatty acids (PUFAs). Aside from the nutritional and sensory benefits, the cold pressing method is simple, safe (i.e., uses less chemical), and low cost (Siregar et al. 2015); and thus the future spread of sacha inchi is anticipated to increase and stabilize SIM supply. Previous studies revealed that SIM has a relatively high crude protein content of 539.7 g/kg, fat (41.30 ‒148.8 g/kg), total n-3 fatty acids (26.5 g/kg), and total n-6 fatty acids (24.3 g/kg) (Rawdkuen et al. 2016; Khieokhajonkhet et al. 2021), while some ANFs have also been detected (Rawdkuen et al. 2016). These superior nutritional features make SIM a promising candidate of the alternative protein resource in aquafeed, but to the best of our knowledge, only three reports have been published on the use of SIM in fish diet: one showed that SIM supplementation increased the apparent digestibility coefficient (ADC) of protein in rainbow trout, Oncorhynchus mykiss (Ortiz-Chura et al. 2018); another showed that the dietary inclusion of air-dried SIM did not successfully enhance growth in Nile tilapia, Oreochromis niloticus (Muichanta et al. 2020); the latest study showed that SIM could totally replace soybean meal for red hybrid tilapia, O. niloticus × O. mossambicus (Khieokhajonkhet et al. 2021).
Tilapia is a valuable commercial fish species, with the second most popular and widely farmed freshwater fish culture after cyprinids accounting for about 10% of all fish produced in the aquaculture sector (FAO 2021). The global demand for commercial tilapia feeds is expected to increase as production volumes increase and farming becomes more intensive, and there is a need to identify protein sources that can contribute to sustain this growth. Although the previous studies successfully demonstrated the potential of SIM as an aquafeed ingredient, none of them has examined whether FM can be replaced with SIM with no adverse effects. Thus, the present study investigates the effect of replacing FM with SIM on dietary digestibility, growth metrics, hematology, and liver and intestinal histological changes in tilapia. We used red hybrid tilapia, which was developed as the genetically improved farm tilapia (GIFT) by crossing Nile tilapia with Mozambique tilapia O. mossambicus, because of the favorable characteristics such as good taste, attractiveness, strong capacity to environmental stresses and disease resistance, and quick growth and short generation times (Islam et al. 2006; Haque et al. 2016).
Materials and methods
Preparation of sacha inchi meal
SIM was kindly provided by The Ultimate Bangkok Co. Ltd., Bangkok, Thailand, and prepared according to our previous study (Khieokhajonkhet et al. 2021). Briefly, it was finely ground and homogenized using a kitchen blender, screened through a 30-mesh strainer (mesh size approximately 560 µm), and then subjected to the extrusion process using a CTE-D25L32 twin-screw extruder (Chareon Tut Co, Ltd., Samutprakan, Thailand). The pelleting temperature was at 80 °C, and the extruding temperature never reached 100 °C. SIM was extruded at a shaft speed of 200 rpm to obtain 0.5-mm diameter with a feeding rate of 120 kg/h. To achieve a barrel type shape, moisture was set at approximately 150 ‒ 180 mL/kg. Obtained SIM pellets were dried at 70 °C using hot air-oven overnight, ground, and kept at ‒20 °C. SIM was used to analyze the chemical composition as described in the chemical scrutiny section. The chemical composition of SIM was presented in our previous study (Khieokhajonkhet et al. 2021).
Diet preparation
Four isonitrogenous (approximately 30% of crude protein) and isolipidic (approximately 8% of crude lipid) diets were prepared in this study. A basal diet (SIM0 as control) was formulated by using FM as the major protein source (350 g/kg). The three other diets were prepared by replacing FM and rice flour with the extruded SIM added at 270, 330, and 415 g/kg; these diets were designated SIM60, SIM80, and SIM100, respectively (Table 1). Fish oil was added to the control diet to obtain a similar crude lipid content to all experimental diets. To ensure the essential amino acid requirements for tilapia, methionine and lysine were supplemented to all test diets (NRC 1993). All fine feed ingredients were mixed according to the feed formulation shown in Table 1 using a C-B20G kitchen blender (CKI Family Co, Ltd., Nonthaburi, Thailand). Thereafter, fish oil and distilled water were added, and the mixture was further blended for 10 min. The mixture was then pelleted with a diameter of approximately 3 mm using a meat mincer (ICK family Co. Ltd., Nonthaburi, Thailand). The pellets were air-dried, sealed in polyethylene bags, and kept at ‒20 °C until used for feeding trial and further analysis.
Feeding trial
A total of 500 juvenile red hybrid tilapia (2.5 ‒ 3.0 g/fish) were purchased from a local commercial hatchery farm in Phrompiram district (Dokdin hatchery, Phitsanulok, Thailand). The experimental fish were transferred and acclimatized to the experimental conditions in indoor recirculation systems (volume 500 L) for 4 weeks with a natural photoperiod (12-h light: 12-h dark) at the Faculty of Agriculture Natural Resources and Environment, Naresuan University, Phitsanulok, Thailand. They were fed daily at 08:30 and 16:30 using a commercial diet containing approximately 32, 4, and 4% of crude protein, crude lipid, and crude fiber, respectively (NB Distribution Co, Ltd., Ratchaburi, Thailand). One-third of the water was exchanged every 2 days with dechlorinated water.
A total of 320 red hybrid tilapia with similar size (average initial weight 15.87 ± 0.02 g/fish) were fasted for 24 h and anesthetized using 50 mg mL−1 of clove oil solution (clove oil:ethanol, 1:9). Twenty fish of each tank were bulk weighed and randomly allocated into 16 experimental glass tanks (0.45 × 0.45 × 0.90 m with approximately 100-L capacity; 4 treatments × 4 replicates). As the initial sample, 10 whole body fish were randomly collected per each treatment and euthanized using an overdose of clove oil solution (approximately 100 mg/mL−1). The fish samples were stored at ‒20 °C and used for initial whole body composition analysis. During the feeding trial, fish were hand fed to satiation at 08:30 and 16:30 daily. The experimental diets were given until no more fish at the water surface actively took the feed pellets. Total daily feed consumption and mortality were recorded, and the dead fish were removed from the calculation of the total feed intake and feed conversion ratio (FCR). Approximately 60% of water in each tank was replenished using dechlorinated water every day. Water parameters were determined in the afternoon daily: temperature 28 – 30 °C, dissolved oxygen (DO) 3.0 – 6.5 mg L−1, and pH 7.0 – 8.2.
Growth performance and morphological indexes
At the end of the feeding trial, all fish were starved for 24 h and anesthetized using 50 mg mL−1 of clove oil solution. Fish in each individual tank were counted and bulk weighed, and used to calculate growth parameters and survival. The following equations were used: Specific growth rate (SGR, %/day) = 100 × (Ln final body weight – Ln initial body weight)/number of days; FCR = feed intake (g)/weight gain (g); Protein efficiency ratio (PER, %) = wet weight gain (g)/protein intake (g); Protein productive value (PPV) = protein gain (g)/protein intake (g); Survival (%) = 100 × (final number of fish)/(initial number of fish).
To determine morphological indexes, two fish per replicate tank (N = 8 per treatment) were individually weighed and measured for total body length (cm) to calculate the condition factor (K value). Liver and visceral tissues were dissected and individually weighed to calculate the hepatosomatic index (HSI) and viscerosomatic index (VSI), respectively. Liver weight was included in the calculation of VSI. Morphological indexes were calculated using the following equations: K value (g/cm3) = 100 × (individual final body weight, g)/(individual final body length, cm3); HSI (%) = 100 × (individual liver weight, g)/(individual whole body weight, g); VSI (%) = 100 × (individual viscera weight, g) /(individual final body weight, g).
Chemical scrutiny and composition
All feed ingredients, experiment diets, initial- and final whole-body fish (2 fish per replicate tank were pooled, N = 4) were used to determine proximate composition. The proximate analyses were performed following the standard procedures (AOAC 1990) in quadruple. Moisture content was determined using a hot air oven at 105 °C (Memmert model UL50, Germany, method 930.15) by heating samples until a constant weight was obtained. Crude protein content was measured by the Kjeldahl method after acid digestion (N × 6.25) using a Kjeldatherm block heating system (semi-automatic Kjeldahl, Gerhardt Vapodest, 45 s, Germany, method 984.13). Crude lipid content was determined by the conventional lipid extraction using petroleum ether and a classical Soxhlet apparatus following the method 920.85, whereas ash content was measured by a combustion method 942.05 at 550 °C for 6 h using a Carbolite ELF 11/14 muffle furnace (Hope Valley, England). Crude fiber content was determined after acid (H2SO4) and base (NaOH) digestion. Digested samples were air-dried and incinerated for 3 h using a muffle furnace (Yasumaru and Lemos 2014), and gross energy was calculated using combustion values (NRC 2011).
To determine the amino acid profile, experimental diets were analyzed in duplicate at The Central Instrument Facility (CIF) Service, Faculty of Science, Mahidol University, Bangkok, Thailand (Table 2). Briefly, 0.3 mg of each sample was hydrolyzed using 1 mL of 6 N HCl at 110 °C for 22 h. The obtained sample was then diluted in 0.02 N HCl. After filtration with a 0.45-µm microfilter, the sample was injected into an automatic amino acid analyzer (Hitachi-L8800, Tokyo, Japan). Predominant essential amino acids in the experimental diets included isoleucine, leucine, and threonine, while predominant non-essential amino acids were aspartic acid, alanine, serine, and glycine (Table 2).
Fatty acid profiles were determined according to a previous report by Khieokhajonkhet et al. (2021). Briefly, total lipids were extracted in duplicate from experimental diets with a mixture of methanol and chloroform (1:2 v/v) according to Folch et al. (1957). Thereafter, fatty acid methyl esters (FAMEs) were obtained using anhydrous methanol and 2% sulfuric acid and quantified with a gas chromatograph-mass spectrometer (GC–MS, Agilent technologies 7890B – 5977A, USA) equipped with a flame ionization detector (FID) and HP-5 capillary column (30 m × 0.32 mm; 0.25 μm film thickness). The initial oven temperature at 120 °C held for 1 min was increased to 190 °C at the rate of 5 °C min−1 and then to 200 °C at 10 °C min−1. The final temperature was held at 200 °C for 5 min. The fatty acid content was calculated using the retention time and peak area of the reference standard (Sigma-Aldrich, MO, USA). Fatty acid profiles of experimental diets are shown in Table 3, in which the effect SIM inclusion was well reflected in the fatty acid contents and compositions. Dietary inclusion of SIM quadratically decreased total saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) contents. Total polysaturated fatty acids (PUFA), n-3 fatty acids, n-6 fatty acids, and the n-3/n-6 ratio were significantly increased by SIM inclusion (ANOVA, P < 0.001). The increase was quadratic for total PUFA, n-3, and the n-6 fatty acid content.
Apparent digestibility coefficients (ADCs)
The ADCs of dry matter and protein were determined using inert chromic oxide (Cr2O3) as a marker by the indirect digestibility method (NRC 2011). Fish were fed with diets containing 5 g/kg diet of Cr2O3. After feeding this diet for 30 min, all-glass tanks were cleaned to ensure removal of the uneaten feed, sediment, and debris, and freshwater was completely replenished. Fresh feces were gently collected by siphoning at 3 ‒ 4 h after feeding for 10 days from the 57th to 70th days of the feeding trial. Feces collected from each glass tank were briefly rinsed with distilled water, pooled for each tank (n = 4 per diet), and kept at ‒20 °C until used for composition analysis. The pooled feces containing Cr2O3 were oven-dried at 65 °C and quantitatively analyzed using the 70% nitric acid (HNO3) followed by 70% perchloric acid (HClO4) digestion method according to Furukawa and Tsukahara (1966) along with experimental diets containing Cr2O3. A UV spectrophotometer (UV-1800, Shimadzu, Japan) was used to measure the absorbance at 350 nm. In order to calculate ADC of protein, protein content of the fecal samples was determined following the protocol described in the chemical scrutiny and composition section. The ADCs of dry matter and protein were calculated as follows: ADC of dry matter (%) = 100 ‒ (100 × Cr2O3 of diet/Cr2O3 of feces); ADC of protein (%) = 100 ‒ (100 × % Cr2O3 in diet/Cr2O3 in feces × % crude protein in feces/crude protein in diet).
Blood sampling and determination of hematological and biochemical parameters
One milliliter of blood was collected from the caudal vein of four fish per tank after the 10-week feeding trial using heparinized syringes. The collected blood was used to determine hematological parameters including red blood cells (RBCs), white blood cells (WBCs), and hematocrit according to Hesser (1960) methods. Another set of blood samples was withdrawn using non-heparinized syringes. The blood samples were stored on ice for 1 h and subjected to centrifugation at 5000 × g at 4 °C for 10 min. The resulting plasma was immediately used for biochemical analyses. Total protein and albumin concentrations were determined by following the Biuret and Bromocressol-green methods, respectively (Drupt et al. 1974; Scoffone and Fontana 1975). Globulin, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were determined using colorimetric methods (Coz-Rakovac et al. 2008). All blood biochemical analyses were performed with the automated blood analyzers P400 and PC400 (Horiba, Japan).
Histological analyses
After the feeding trial, two fish in each tank were euthanized using an overdose of the clove oil solution. Liver was dissected out and 4 ‒ 5 pieces of approximately 0.2 ‒ 0.3 cm3 were cut out for each individual fish. Dissected tissues were immediately preserved in a 10% neutral formaldehyde solution. Similarly, anterior intestinal organs were cut into 2 – 3 pieces (approximately 0.5 – 0.8 cm in length) and fixed using the fixative reagent. Two fixed tissue samples of each organ were used for histological examinations according to standard histological procedures. Briefly, tissue samples were dehydrated, cleared, and embedded using paraffin wax. Slices with 5 – 6-µm thickness were serially cut, stained with hematoxylin and eosin (H&E), and sealed with a coverslip. Tissue sections were observed with a light microscopy BX-51, Olympus (Tokyo, Japan). Histomorphometric analysis was performed on villi height and thickness, as well as the thickness of submucous and muscular layers based on the measurement of 8 microvilli per intestinal Sect. (8 microvilli per section, 2 × 8 = 16 villi per replicate tank) using the Axio Vision software (Carl Zeiss, Jena, Germany).
Statistical analysis
All data were analyzed using the statistical program SPSS (Version 17, Chicago, IL USA) for Windows. Analysis of variance (ANOVA) was used to find out if the SIM inclusion significantly affected the observed parameters with a 5% probability (P < 0.05). A pragmatic approach was used for the regression analysis, in which linear or second-order polynomials regression was applied to each dataset based on the plot. The optimal SIM levels were calculated for several parameters based on the polynomial regression. All results were expressed as mean ± SEM values. To compare the significant differences between treatments, Tukey’s post hoc test was performed.
Results
Growth performance
The growth metrics of experimental fish are shown in Table 4. Overall, ANOVA detected significant effects of diets on the growth metrics and ADCs (P < 0.001), and fish fed with the SIM80 diet showed the highest final weight, weight gain (WG), SGR, PER, PPV, ADC of dry matter, and ADC of protein among all groups. Furthermore, the final weight of the SIM80 group was 3.5 times higher than the initial body weight, reaching 56 g in 70 days (Table 4), achieving a satisfactory growth level of a commercial standard. On the other hand, a complete replacement of FM with SIM (the SIM100 diet) decreased several growth metrics (final weight, WG, SGR, PER, and PPV) and ADCs (Table 4). The survival rates showed no significant differences between the experimental groups (ANOVA, P > 0.05). HSI and VSI showed a similar trend. Namely, fish fed SIM60 and SIM80 decreased these values compared to the control, but in the SIM100 group these values became comparable to those of the control group.
Considering the distribution, the second-polynomial regression was applied to above data (Fig. 1). Significant quadratic effects of SIM inclusion were observed for SGR. The optimum SIM levels for this parameters were calculated to be 42.9% (Fig. 1A). The significant quadratic effect of SIM inclusion was observed in FCR, PER, and PPV (Fig. 1B–D). The optimum SIM level for this parameter was calculated to be 43.0, 28.4, and 29.8%, respectively.
Whole-body proximate composition and morphological indexes
The SIM inclusion significantly affected whole-body crude protein and crude lipid levels (ANOVA, P < 0.001) (Table 5). The increase in crude protein and crude lipid contents was the most pronounced in fish fed the SIM80 diet. The ash content was significantly decreased by the SIM inclusion.
Hematological and biochemical parameters
Dietary inclusion of SIM exhibited no significant effect on RBC, WBC, hematocrit, total protein, and albumin (ANOVA, P > 0.05, Table 6). These was a significant effect of SIM inclusion on globulin levels, where fish fed SIM-containing diets had lower levels than the control fish, with the SIM60 group having the lowest value. The AST and ALT activities were significantly affected by the SIM inclusion, and the SIM100 group showed the highest activities. Insignificant changes were observed in the ALP activity among the experimental groups (ANOVA, P > 0.05).
Dietary SIM inclusion significantly affected total cholesterol, triglycerides, and LDL-C with significant quadratic decreasing trends (Table 6). HDL-C was also significantly affected by SIM inclusion, but we observed no significant quadratic response (ANOVA, P < 0.05; Table 6).
Histology and quantitative observations
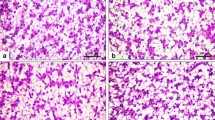
Hepatocytes of the fish fed the control and SIM60 diets showed a normal piscine presentation with abundant granules (Fig. 2A ‒ B). Enlargement of vacuolization at the cytoplasm periphery, nuclear atrophy, and nuclear displacement were observed in the hepatocytes of the SIM80 group with more density of these pathological differences found in SIM100 group (Fig. 2Da and Db).
Schematic representation of liver histology in red hybrid tilapia (Oreochromis niloticus × O. mossabicus) fed with different inclusion levels of sacha inchi meal for 10 weeks (original magnification × 40); A, control diet; B, SIM60; C, SIM80, and Da, SIM100. Panel Db depicted an area of the enlargement from the framed part in Da. The arrowheads show enlargement of vacuole. The arrows indicate nuclei displacement in hepatocyte cells
The effects of dietary SIM inclusions on the intestine morphology are depicted in Fig. 3. The intestine of fish fed control, SIM60, and SIM80 diets showed no morphological abnormalities (Fig. 3A – C), but gross microscopic examination revealed that the microvilli of fish fed the SIM100 group were thinner than those of other groups (Fig. 3D). In quantitative observations, SIM inclusion quadratically increased height and thickness of villi (Table 7) in which the SIM100 diet resulted in the lowest values. In addition, the submucous layer (SL) thickness was the highest and lowest in the SIM80 and SIM100 groups, respectively. In the SIM100 group, the muscular layer (ML) thickness had the lowest value (ANOVA, P < 0.05, Table 7).
Schematic representation of anterior intestine histology in red hybrid tilapia (Oreochromis niloticus × O. mossabicus) fed with different inclusion levels of sacha inchi meal for 10 weeks (original magnification × 40); A, control diet; B, SIM60; C, SIM80, and D, SIM100. Histometric measurements of microvilli are indicated in Fig. 3C. Abbreviations; VH, villi height; VT, villi thickness; SL, submucous layer thickness; ML, muscular layer thickness
Discussion
Partially or fully replacing FM with plant protein sources is an urgent challenge for developing sustainable aquaculture (FAO 2020). In the present study, SIM has been shown to be a suitable alternative plant protein source for red hybrid tilapia. SIM inclusion up to 330 g/kg (SIM80) increased final weight, WG, SGR, PER, PPV, and ADC of protein compared to the control group. While the optimum SIM inclusion levels were calculated to be 28.4 – 43.0% for SGR, FCR, PER, and PPV parameters, the SIM80 group generally showed the best growth performance. These findings are consistent with a study on rainbow trout, which showed that 296.1 g/kg of SIM significantly increased the ADC of protein more than those containing other plant feedstuffs (Ortiz-Chura et al. 2018). Furthermore, a recent study found that SIM could totally replace soybean meal in red hybrid tilapia, despite some negative impacts on growth and histological integrity of the liver and intestine were observed (Khieokhajonkhet et al. 2021). FM can be completely replaced in the diet of Nile tilapia by alternative plant protein sources, such as soy protein concentrate, soybean meal supplemented with lysine, cottonseed supplemented with iron, and a mixture of cottonseed, sunflower meal, linseed meal, and soybean meal (El-Saidy and Gaber 2002, 2003, 2004; Zhao et al. 2010). Red hybrid tilapia is projected to be able to accept plant protein sources, making it a useful aquaculture species. SIM is a novel promising plant protein source that provides an extra benefit (i.e., enhanced growth) to tilapia culture.
In the present study, the SIM100 diet had negative effects on growth, feed utilization, and ADCs. Three primary causes are commonly observed for the negative consequences of alternate plant protein sources including decreased palatability due to the lack of feed attractant, an imbalance in essential amino acids, and the presence of ANFs in plant feedstuffs (Dias et al. 2005; Espe et al. 2006). Pre-processing treatment can mitigate the detrimental effects of these elements. For example, previous studies showed that SIM treated with heat before use as the feedstuff for fish (Ortiz-Chura et al. 2018; Muichanta et al. 2020) resulted in better growth performance and feed utilization compared to the untreated plant ingredients, which often contain ANFs that could negatively affect intestinal digestion and absorption capacity, reducing feed utilization, digestibility, and growth performance in monogastric animals (Gatlin et al. 2007; Dawood et al. 2020; Muichanta et al. 2020). Although the current protocol, which allowed us to replace SIM80 of FM in the diet, is already useful in tilapia farming, future studies are needed to address the optimum pretreatment to enhance feed utilization of dietary SIM inclusion.
Fish fed experimental diets containing SIM60 and SIM80 displayed significant increases in whole-body moisture, crude protein content, and crude lipid content compared to the control group in this study (ANOVA, P < 0.001). Significant quadratic responses were found in these parameters. Fish fed the SIM100 diet had lower whole-body protein and lipid contents than those fed the other diets. These findings are in line with Dossou et al. (2018) and Kumar et al. (2020), who found that a high dietary inclusion of plants reduces protein utilization, carcass protein content, protein retention, and growth. Wang et al. (2020) attributed this to ANFs, such as gossypol in cottonseed.
Morphological indexes are rough indicators that can be used to determine nutritional states and physiological conditions (Dawood et al. 2016). The present study found that the K value is not affected by dietary SIM inclusions. On the other hand, HSI was significantly decreased as SIM levels increased. Because the HSI values in this study fell in the normal range of Nile tilapia HSI, 1.47 – 2.34 (Zhao et al. 2010; Khieokhajonkhet et al. 2021), these results suggest that SIM inclusion did not severely deteriorate health conditions in red hybrid tilapia. The effect of dietary inclusion of plant proteins on HSI has been plant- and fish-specific. It decreased HSI in pompano Trachinotus ovatus and gibel carp, Carassius auratus gibelio vas. CAS III (Ma et al. 2020; Zhang et al. 2020), but increased HSI in Senegalese sole, Solea senegalensis; hybrid grouper, Epinephelus lanceolatus × E. fuscoguttatus; and juvenile olive flounder, Paralichthys olivaceus (Cabral et al. 2013; Seong et al. 2018; Ye et al. 2019a). Plant-based diets often have higher levels of lipid and carbohydrate than FM-based diets, resulting in the increase of hepatic glycogen, liver weight, and HSI (Lee et al. 2002; Rueda-Jasso et al. 2004). The effect of SIM inclusion on HSI should be related to the species-specific lipid and carbohydrate metabolism of fish, which needs further investigation.
Blood parameters are frequently used as essential indicators to determine fish feeding regimes and physiological responses (NRC 2011). In the present study, hematological parameters (RBC, WBC, and hematocrit) were not significantly altered by the dietary SIM inclusion. Similarly, Nile tilapia fed with fermented sunflower meal inoculated with yeast and bacillus up to 75% (508 g/kg) of FM replacement did not show altered hematological parameters (Hassaan et al. 2018). Also, Jahanbakhshi et al. (2013) found that dietary plant protein had no effect on hematocrits or WBCs in great sturgeon, Huso huso. These findings imply that blood parameters are not very sensitive to the inclusion of plant protein sources, although RBC hemolysis has been reported in the presence of ANFs, such as saponin, in the diet (Dabrowski et al. 2001; Rinchard et al. 2003b).
In this study, total protein, albumin, and ALP activity were not altered by SIM’s inclusion diets. In comparison to fish fed the control diet, fish fed a dietary inclusion of SIM had higher AST and ALT activity as the level of SIM inclusion increased, which is consistent with many studies using dietary inclusion of plant protein sources for tilapia (Deng et al. 2017; Hassaan et al. 2017, 2018). The hepatic enzymes, AST and ALT, are involved in transamination; and thus, AST and ALT levels in the blood are linked to hepatocyte cell damage or failure. The fish fed the SIM100 diet exhibited the greatest levels of AST and ALT values, indicating that their hepatocytes had been damaged to some extent in this group.
Previous studies reported that dietary plant protein inclusion could interrupt lipid homeostasis by reducing lipid droplets, cholesterol, and LDL-C levels in the liver, which could reduce atherosclerosis in grouper and Nile tilapia (Deng et al. 2017; Ye et al. 2019b). These findings were in line with those of certain vertebrate studies (Gonzales and Gonzales 2014; Ambulay et al. 2020) and were likely attributed to the high ω – 3 fatty acid contents of SIM (Rawdkuen et al. 2016). Indeed, sacha inchi oil administered to humans resulted in a reduction of serum cholesterol levels (Gonzales and Gonzales 2014). A high level of 18:3n-3 in diets also reduced overall cholesterol levels in silver barb, Puntius gonionotus and hybrid sturgeon, Acipenser baeri Brandt × A. schrenckii Brandt (Liu et al. 2018; Nayak et al. 2020). Of note, 18:3n-3 is known to impede the biosynthesis pathway of fatty acids and cholesterol by inhibiting the transcription factor of sterol regulatory element-binding proteins (SREBPs) and fatty acid synthetase (FAS) mRNA expression (Fukumitsu et al. 2013; Ambulay et al. 2020), as well as boosting fatty acid oxidation which leads to improved health benefits (Fukumitsu et al. 2013).
In the present study, dietary SIM inclusion higher than 330 g/kg (SIM80) caused some histological abnormalities in the liver of red hybrid tilapia. Such alterations have been commonly observed in fish fed plant protein in the diet (Caballero et al. 2004). The histological differences of the liver could be attributed to high SIM inclusion level, which could have resulted in poor nutrient absorption and digestion (Khieokhajonkhet et al. 2021). Cytoplasmic vacuolization of hepatocytes has been observed in fish fed high replacement levels of FM by plant feed ingredients such as 75% of maize gluten in African catfish, Clarias gariepinus (Abdel-Warith et al. 2014), 75% of the mixture plant protein sources in hybrid grouper (Ye et al. 2019a), and up to 36% of cottonseed in hybrid grouper (Yin et al. 2018). The intestine is an organ that plays an important role in digestion and nutrient absorption, as well as acting as a mechanical defense (Siddik et al. 2018). The impairment of the intestinal barriers results in reduced digestion and absorption capacity of fish (Dawood et al. 2016). Villi morphology is used as an indicator of gut health that is associated with nutrient absorption (Chen et al. 2019) and body growth (Caspary 1992). In the present study, the microvilli height and thickness responded positively and quadratically to the dietary SIM inclusion, where the SIM100 diet had a detrimental effect on them. The growth reduction in the SIM100 group could be attributed to an altered digesting process or the low food intake caused by the presence of ANFs in the diet beyond the acceptability level. These results contradict the previous studies reporting a significant reduction in villi height and thickness, supranuclear vacuolization, brush border enzymatic activity, immune alternation, growth performance, and enteritis development after being fed plant-based protein inclusion in diets (Gu et al. 2016; Ismail et al. 2019; Krogdahl et al. 2020; Mohammadi et al. 2020). This may be a unique feature of SIM as an alternative plant protein source, which requires further validation.
Conclusion
In conclusion, the present study found that SIM has the potential to replace FM up to 330 g/kg (SIM80) without negatively affecting the growth performance of red hybrid tilapia. Moreover, the SIM80 diet was beneficial for improved growth, feed utilization, ADC of nutrients, gut health, and blood parameters. While the replacement beyond that level had some detrimental impacts on the fish, SIM is a promising alternative plant protein source in red hybrid tilapia.
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
Abdel-Warith AA, Younis EM, AL-Asgah NA, Allam HY (2014) Maize gluten meal as a protein source in the diets for African catfish Clarias gariepinus (Burchell, 1822) and its effect on liver glycogen and histology. Indian J Fish 6:74–82. https://doi.org/10.1016/j.aqrep.2021.100815
Ambulay JP, Rojas PA, Timoteo OS, Barreto TV, Colarossi A (2020) Effect of the emulsion of sacha inchi (Plukenetia huayabambana) oil on oxidative stress and inflammation in rats induced to obesity. J Funct Foods 64:103631. https://doi.org/10.1016/j.jff.2019.103631
AOAC (1990) Official methods of analysis of association of official analytical chemists. The association of official analytical chemists, Arlington, Virginia, 1298
Beal CM, Gerber LN, Thongrod S, Phromkunthong W, Kiron V, Granados J, Archibald I, Greene CH, Huntley ME (2018) Marine microalgae commercial production improves sustainability of global fisheries and aquaculture. Sci Rep 8:15064. https://doi.org/10.1038/s41598-018-33504-w
Caballero MJ, Izquierdo MS, Kjørsvik E, Fernández AJ, Rosenlund G (2004) Histological alterations in the liver of sea bream, Sparus aurata L., caused by short- or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. J Fish Dis 27:531–541. https://doi.org/10.1111/j.1365-2761.2004.00572.x
Cabral EM, Fernandes TJR, Campos SD, Castro-Cunha M, Oliveira MBPP, Cunha LM, Valente LMP (2013) Replacement of fish meal by plant protein sources up to 75% induces good growth performance without affecting flesh quality in ongrowing Senegalese sole. Aquaculture 380–383:130–138. https://doi.org/10.1016/j.aquaculture.2012.12.006
Caspary WF (1992) Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr 55:299–308. https://doi.org/10.1093/ajcn/55.1.299s
Chen XQ, Zhao W, Xie SW, Xie JJ, Zhang ZH, Tian LX, Liu YJ, Niu J (2019) Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 90:30–39. https://doi.org/10.1016/j.fsi.2019.03.068
Chirinos R, Zuloeta G, Pedreschi R, Mignolet E, Larondelle Y, Campos D (2013) Sacha inchi (Plukenetia volubilis): a seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem 141:1732–1739. https://doi.org/10.1016/j.foodchem.2013.04.078
Coz-Rakovac R, Smuc T, Topic Popovic N, Strunjak-Perovic I, Hacmanjek M, Jadan M (2008) Novel methods for assessing fish blood biochemical data. J Appl Ichthyol 24:77–80. https://doi.org/10.1111/j.1439-0426.2007.01041.x
Dabrowski K, Lee KJ, Rinchard J, Ciereszko A, Blom JH, Ottobre JS (2001) Gossypol isomers bind specifically to blood plasma proteins and spermatozoa of rainbow trout fed diets containing cottonseed meal. Biochim Biophys Acta 1525:37–42. https://doi.org/10.1016/s0304-4165(00)00168-9
Dawood MAO, Eweedah NM, Khalafalla MM, Khalid A (2020) Evaluation of fermented date palm seed meal with Aspergillus oryzae on the growth, digestion capacity and immune response of Nile tilapia (Oreochromis niloticus). Aquac Nutr 26:828–841. https://doi.org/10.1111/anu.13042
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S, El Basuini MF, Hossain MS, Nhu TH, Dossou S, Moss AS (2016) Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish Immunol 49:275–285. https://doi.org/10.1016/j.fsi.2015.12.047
Deng JM, Wang Y, Chen LQ, Mai KS, Wang Z, Zhang X (2017) Effects of replacing plant proteins with rubber seed meal on growth, nutrient utilization and blood biochemical parameters of tilapia (Oreochromis niloticus × O. aureus). Aquac Nutr 23:30–39. https://doi.org/10.1111/anu.12355
Dias J, Alvarez MJ, Arzel J, Corraze G, Diez A, Bautista JM, Kaushik SJ (2005) Dietary protein source affects lipid metabolism in the European seabass (Dicentrarchus labrax). Comp Biochem Physiol Part A Mol Integr Physiol 142:19–31. https://doi.org/10.1016/j.cbpb.2005.07.005
Diógenes AF, Castro C, Miranda AC, Oliva-Teles A, Peres H (2018) Dietary replacement of fishmeal by corn distillers dried grains with solubles (DDGS) in diets for turbot (Scophthalmus maximus, Linneaus, 1758) Juveniles. Aquaculture 492:113–122. https://doi.org/10.1016/j.aquaculture.2018.04.005
Dossou S, Koshio S, Ishikawa M, Yokoyama S, Dawood MAO, El Basuini MF, El-Hais AM, Olivier A (2018) Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture 490:228–235. https://doi.org/10.1016/j.aquaculture.2018.02.010
Drupt F, Paris M, Frydman A, Leclerc M (1974) Serum albumin assay by bromocresol green method: application to different automatic apparatus. Ann Pharma Fr 32:249–256
El-Saidy DMSD, Gaber MM (2004) Use of cottonseed meal supplemented with iron for detoxification of gossypol as a total replacement of fish meal in Nile tilapia, Oreochromis niloticus (L.) diets. Aquac Res 35:859–865. https://doi.org/10.1111/j.1365-2109.2004.01077.x
El-Saidy DMSD, Gaber MMA (2002) Complete replacement of fish meal by soybean meal with dietary L-lysine supplementation for Nile tilapia Oreochromis niloticus (L.) fingerlings. J World Aquac Soc 33:297–306. https://doi.org/10.1111/j.1749-7345.2002.tb00506.x
El-Saidy DMSD, Gaber MMA (2003) Replacement of fish meal with a mixture of different plant protein sources in juvenile Nile tilapia, Oreochromis niloticus (L.) diets. Aquac Res 34:1119–1127. https://doi.org/10.1046/j.1365-2109.2003.00914.x
Espe M, Lemme A, Petri A, El-Mowafi A (2006) Can Atlantic salmon (Salmo salar) grow on diets devoid of fish meal? Aquaculture 255:255–262. https://doi.org/10.1016/j.aquaculture.2005.12.030
FAO (2020) The state of world fisheries and aquaculture 2020. Sustainability in action, food and agriculture organization of the United Nations, Rome, Italy, pp 266
FAO (2021) Aquaculture feed and fertilized resources information system. In: FAO Fisheries Division, Rome, Italy. Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Fukumitsu S, Villareal MO, Onaga S, Aida K, Han J, Isoda H (2013) α-Linolenic acid suppresses cholesterol and triacylglycerol biosynthesis pathway by suppressing SREBP-2, SREBP-1a and -1c expression. Cytotechnology 65:899–907. https://doi.org/10.1007/s10616-012-9510-x
Furukawa A, Tsukahara H (1966) On the acid digestion method for the determination of chromic oxide as an index substrance in the study of digestibility of fish feed. Nippon Suisan Gakkaishi 32:502–506. https://doi.org/10.2331/suisan.32.502
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
Gonzales GF, Gonzales C (2014) A randomized, double-blind placebo-controlled study on acceptability, safety and efficacy of oral administration of sacha inchi oil (Plukenetia volubilis L.) in adult human subjects. Food Chem Toxicol 65:168–176. https://doi.org/10.1016/j.fct.2013.12.039
Gu M, Bai N, Zhang Y, Krogdahl Å (2016) Soybean meal induces enteritis in turbot Scophthalmus maximus at high supplementation levels. Aquaculture 464:286–295. https://doi.org/10.1016/j.aquaculture.2016.06.035
Gutiérrez LF, Quiñones-Segura Y, Sanchez-Reinoso Z, Díaz DL, Abril JI (2017) Physicochemical properties of oils extracted from γ-irradiated sacha inchi (Plukenetia volubilis L.) seeds. Food Chem 237:581–587. https://doi.org/10.1016/j.foodchem.2017.05.148
Gutiérrez LF, Rosada LM, Jiménez Á (2011) Chemical composition of sacha inchi (Plukenetia volubilis L.) seeds and characteristics of their lipid fraction. Grasas y Aceites 62:8. https://doi.org/10.3989/gya044510
Haque MR, Islam MA, Wehab MA, Hoq ME, Rahman MM, Azim ME (2016) Evaluation of production performance and profitability of hybrid red tilapia and genetically improved farmed tilapia (GIFT) strains in the carbon/nitrogen controlled periphyton-based (C/N-CP) on-farm prawn culture system in Bangladesh. Aquac Rep 4:101–111. https://doi.org/10.1016/j.aqrep.2016.07.004
Hassaan MS, Goda AMAS, Kumar V (2017) Evaluation of nutritive value of fermented de-oiled physic nut, Jatropha curcas, seed meal for Nile tilapia Oreochromis niloticus fingerlings. Aquac Nutr 23:571–584. https://doi.org/10.1111/anu.12424
Hassaan MS, Soltan MA, Mohammady EY, Elashry MA, El-Haroun ER, Davies SJ (2018) Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 495:592–601. https://doi.org/10.1016/j.aquaculture.2018.06.018
Hernández C, Lizárraga-Velázquez CE, Contreras-Rojas D, Sánchez-Gutiérrez EY, Martínez-Montaño E, Ibarra-Castro L, Peña-Marín ES (2021) Fish meal replacement by corn gluten in feeds for juvenile spotted rose snapper (Lutjanus guttatus): effect on growth performance, feed efficiency, hematological parameters, protease activity, body composition, and nutrient digestibility. Aquaculture 531:735896. https://doi.org/10.1016/j.aquaculture.2020.735896
Hernández MD, Martínez FJ, Jover M, García García B (2007) Effects of partial replacement of fish meal by soybean meal in sharpsnout seabream (Diplodus puntazzo) diet. Aquaculture 263:159–167. https://doi.org/10.1016/j.aquaculture.2006.07.040
Hesser EF (1960) Methods for routine fish hematology. Progress Fish-Culturist 22:164–171. https://doi.org/10.1577/1548-8659(1960)22[164:MFRFH]2.0.CO;2
Hua K, Cobcroft JM, Cole A, Condon K, Jerry DR, Mangott A, Praeger C, Vucko MJ, Zeng C, Zenger K, Strugnell JM (2019) The future of aquatic protein: implications for protein sources in aquaculture diets. One Earth 1:316–329. https://doi.org/10.1016/j.oneear.2019.10.018
Islam MA, Das DR, Khalequzzaman SM, Kamal D, Abdul Halim KM (2006) Extensive culture of red tilapia with four stocking densities at BeelKodalia, Bergerhat, Bangladesh. Pak J Biol Sci 9:1965–1969. https://doi.org/10.3923/pjbs.2006.1965.1969
Ismail T, Nassef ED, Hegazi E, Bakr A, Moustaf EM, Abdo W, Elbialy Z (2019) The modulatory effect of dietary betaine on intestinal absorptive capacity, lipogenesis and expression of lipid metabolism and growth related genes in Nile tilapia fed on soybean based diet. Slov Vet Res 56(22):25–38. https://doi.org/10.26873/SVR-741-2019
Jahanbakhshi A, Imanpoor MR, Taghizadeh V, Shabani A (2013) Hematological and serum biochemical indices changes induced by replacing fish meal with plant protein (sesame oil cake and corn gluten) in the Great sturgeon (Huso huso). Comp Clin Path 22:1087–1092. https://doi.org/10.1007/s00580-012-1532-4
Khieokhajonkhet A, Muichanta S, Aeksiri N, Ruttarattanamongkol K, Rojtinnakorn J, Kaneko G (2021) Evaluation of sacha inchi meal as a novel alternative plant protein ingredient for red hybrid tilapia (Oreochromis niloticus×O. mossambicus): growth performance, feed utilization, blood biochemistry, and histological changes. Anim Feed Sci Technol 278:115004. https://doi.org/10.1016/j.anifeedsci.2021.115004
Krogdahl Å, Kortner TM, Jaramillo-Torres A, Gamil AAA, Chikwati E, Li Y, Schmidt M, Herman E, Hymowitz T, Teimouri S, Storebakken T (2020) Removal of three proteinaceous antinutrients from soybean does not mitigate soybean-induced enteritis in Atlantic salmon (Salmo salar, L). Aquaculture 514:734495. https://doi.org/10.1016/j.aquaculture.2019.734495
Kumar V, Lee S, Cleveland BM, Romano N, Lalgudi RS, Benito MR, McGraw B, Hardy RW (2020) Comparative evaluation of processed soybean meal (EnzoMealTM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): effects on growth performance and growth-related genes. Aquaculture 516:734652. https://doi.org/10.1016/j.aquaculture.2019.734652
Lee SM, Jeon IG, Lee JY (2002) Effects of digestible protein and lipid levels in practical diets on growth, protein utilization and body composition of juvenile rockfish (Sebastes schlegeli). Aquaculture 211:227–239. https://doi.org/10.1016/S0044-8486(01)00880-8
Liu C, Wang J, Ma Z, Li T, Xing W, Jiang N, Li W, Li C, Luo L (2018) Effects of totally replacing dietary fish oil by linseed oil or soybean oil on juvenile hybrid sturgeon, Acipenser baeri Brandt♀ × A. schrenckii Brandt♂. Aquac Nutr 24:184–194. https://doi.org/10.1111/anu.12546
Ma Y, Li M, Xie D, Chen S, Dong Y, Wang M, Zhang G, Zhang M, Chen H, Ye R, Wang Y, Sun L, Wang S, Ning L, Hasan AKMM, Li Y (2020) Fishmeal can be replaced with a high proportion of terrestrial protein in the diet of the carnivorous marine teleost (Trachinotus ovatus). Aquaculture 519:734910. https://doi.org/10.1016/j.aquaculture.2019.734910
Mohammadi M, Imani A, Farhangi M, Gharaei A, Hafezieh M (2020) Replacement of fishmeal with processed canola meal in diets for juvenile Nile tilapia (Oreochromis niloticus): growth performance, mucosal innate immunity, hepatic oxidative status, liver and intestine histology. Aquaculture 518:734824. https://doi.org/10.1016/j.aquaculture.2019.734824
Muangrat R, Veeraphong P, Chantee N (2018) Screw press extraction of sacha inchi seeds: oil yield and its chemical composition and antioxidant properties. J Food Process Pres 42:e13635. https://doi.org/10.1111/jfpp.13635
Muichanta S, Khieokhajonkhet A, Wuthijaree K (2020) Effect of air dry with different heating temperature in sacha inchi on growth performance in Nile tilapia (Oreochromis niloticus). The 21st National Graduate Research Conference, Khon kaen University, Khon kaen, Thailand BMO5, pp. 1–11
Nayak M, Giri SS, Pradhan A, Samanta M, Saha A (2020) Effects of dietary α-linolenic acid/linoleic acid ratio on growth performance, tissue fatty acid profile, serum metabolites and Δ6 fad and elovl5 gene expression in silver barb (Puntius gonionotus). J Sci Food Agr 100:1643–1652. https://doi.org/10.1002/jsfa.10177
NRC (1993) Nutrient requirements of warmwater fishes and shellfishes. The National Academic Press, Washington DC, National Academic Press, 114
NRC (2011) Nutrient requirements of fish and shrimp. The National Academies Press. Washington DC, National academies press
Ortiz-Chura A, Pari-Puma RM, Rodríguez Huanca FH, Cerón-Cucchi ME, Araníbar Araníbar MJ (2018) Apparent digestibility of dry matter, organic matter, protein and energy of native Peruvian feedstuffs in juvenile rainbow trout (Oncorhynchus mykiss). Fish Aquatic Sci 21:32. https://doi.org/10.1186/s41240-018-0111-2
Rawdkuen S, Murdayanti D, Ketnawa S, Phongthai S (2016) Chemical properties and nutritional factors of pressed-cake from tea and sacha inchi seeds. Food Biosci 15:64–71. https://doi.org/10.1016/j.fbio.2016.05.004
Rinchard J, Lee KJ, Dabrowski K, Ciereszko A, Blom JH, Ottobre JS (2003a) Influence of gossypol from dietary cottonseed meal on haematology, reproductive steroids and tissue gossypol enantiomer concentrations in male rainbow trout (Oncorhynchus mykiss). Aquac Nutr 9:275–282. https://doi.org/10.1046/j.1365-2095.2003.00253.x
Rinchard J, Lee KJ, Czesny S, Ciereszko A, Dabrowski K (2003b) Effect of feeding cottonseed meal-containing diets to broodstock rainbow trout and their impact on the growth of their progenies. Aquaculture 227:77–87. https://doi.org/10.1016/S0044-8486(03)00496-4
Rueda-Jasso R, Conceição LEC, Dias J, De Coen W, Gomes E, Rees JF, Soares F, Dinis MT, Sorgeloos P (2004) Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 231:417–433. https://doi.org/10.1016/S0044-8486(03)00537-4
Samtiya M, Aluko RE, Dhewa T (2020) Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod Proc Nutr 2:6. https://doi.org/10.1186/s43014-020-0020-5
Scoffone E, Fontana A (1975) Proteins analysis. In: Needleman, S.B. (ed.), Protein sequence determination: a source book of methods and techniques springer-verlag, New York., pp. 162–203
Seong M, Lee S, Lee S, Song Y, Bae J, Chang K, Bai SC (2018) The effects of different levels of dietary fermented plant-based protein concentrate on growth, hematology and non-specific immune responses in juvenile olive flounder, Paralichthys olivaceus. Aquaculture 483:196–202. https://doi.org/10.1016/j.aquaculture.2017.10.023
Siddik MAB, Howieson J, Partridge GJ, Fotedar R, Gholipourkanani H (2018) Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi Lates Calcarifer. Sci Rep 8:15942. https://doi.org/10.1038/s41598-018-34182-4
Siregar AN, Ghani JA, Haron CHC, Rizal M, Yaakob Z, Kamarudin SK (2015) Comparison of oil press for jatropha oil – a review. Res Agric Eng 61:1–13. https://doi.org/10.17221/22/2013-RAE
Stentiford G (2017) Solving the $6 billion per year global aquaculture disease problem. Marine Science. February 2, 2017
Wang J, Clark G, Ju M, Castillo S, Gatlin DM (2020) Effects of replacing menhaden fishmeal with cottonseed flour on growth performance, feed utilization and body composition of juvenile red drum Sciaenops ocellatus. Aquaculture 523:735217. https://doi.org/10.1016/j.aquaculture.2020.735217
Wang S, Zhu F, Kakuda Y (2018) Sacha inchi (Plukenetia volubilis L.): nutritional composition, biological activity, and uses. Food Chem 265:316–328. https://doi.org/10.1016/j.foodchem.2018.05.055
Webster CD, Tidwell JH, Goodgame LS, Yancey DH, Mackey L (1992) Use of soybean meal and distillers grains with solubles as partial or total replacement of fish meal in diets for channel catfish, Ictalurus punctatus. Aquaculture 106:301–309. https://doi.org/10.1016/0044-8486(92)90262-J
Yasumaru F, Lemos D (2014) Species specific in vitro protein digestion (pH-stat) for fish: method development and application for juvenile rainbow trout (Oncorhynchus mykiss), cobia (Rachycentron canadum), and Nile tilapia (Oreochromis niloticus). Aquaculture 426–427:74–84. https://doi.org/10.1016/j.aquaculture.2014.01.012
Ye H, Xu M, Chen L, Tan X, Chen S, Zou C, Sun Z, Liu Q, Ye C, Wang A (2019a) Effects of dietary plant protein sources influencing hepatic lipid metabolism and hepatocyte apoptosis in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Aquaculture 506:437–444. https://doi.org/10.1016/j.aquaculture.2019.03.075
Ye H, Xu M, Liu Q, Sun Z, Zou C, Chen L, Su N, Ye C (2019b) Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile obscure puffer, Takifugu obscurus. Comp Biochem Physiol C Toxicol Pharmacol 216:75–81. https://doi.org/10.1016/j.cbpc.2018.11.00
Yin B, Liu H, Tan B, Dong X, Chi S, Yang Q, Zhang S, Chen L (2018) Cottonseed protein concentrate (CPC) suppresses immune function in different intestinal segments of hybrid grouper ♀Epinephelus fuscoguttatus×♂Epinephelus lanceolatu via TLR-2/MyD88 signaling pathways. Fish Shellfish Immunol 81:318–328. https://doi.org/10.1016/j.fsi.2018.07.038
Zhang X, Sun Z, Cai J, Wang J, Wang G, Zhu Z, Cao F (2020) Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish Immunol 102:430–439. https://doi.org/10.1016/j.fsi.2020.04.051
Zhang Y, Øverland M, Xie S, Dong Z, Lv Z, Xu J, Storebakken T (2012) Mixtures of lupin and pea protein concentrates can efficiently replace high-quality fish meal in extruded diets for juvenile black sea bream (Acanthopagrus schlegeli). Aquaculture 354–355:68–74. https://doi.org/10.1016/j.aquaculture.2012.03.038
Zhao H, Jiang R, Xue M, Xie S, Wu X, Guo L (2010) Fishmeal can be completely replaced by soy protein concentrate by increasing feeding frequency in Nile tilapia (Oreochromis niloticus GIFT strain) less than 2 g. Aquac Nutr 16:648–653. https://doi.org/10.1111/j.1365-2095.2009.00708.x
Acknowledgements
All the authors are grateful for all kinds of support. We express our sincere gratitude to Miss Mallika Supa-aksorn and Mr. Wasin Yaineum for their kindness in helping with the technical assistance and Mr. Julian Pieniazek for proof-reading the manuscript.
Funding
This study was supported by The Agricultural Research Development Agency (ARDA, Public organization), Bangkok, Thailand (grant number CRP6105020260).
Author information
Authors and Affiliations
Contributions
Anurak Khieokhajonkhet: project administration, conceptualization, methodology, funding acquisition, investigation, formal analysis, writing-original draft. Niran Aeksiri: blood analysis, funding acquisition. Jiraporn Rojtinnakorn: funding acquisition. Hien Van Doan and Gen Kaneko: writing-original draft, proofreading. All the authors have read and approved the final version of the article.
Corresponding author
Ethics declarations
Ethics approval
Experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Protocol to meet its animal welfare, which were reviewed and approved by the Naresuan University National Animal Care and Use Committee, Phitsanulok, Thailand (Protocol No: NU-AQ600603).
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khieokhajonkhet, A., Aeksiri, N., Rojtinnakorn, J. et al. Sacha inchi meal as a fish-meal replacer in red hybrid tilapia (Oreochromis niloticus × O. mossambicus) feeds: effects on dietary digestibility, growth metrics, hematology, and liver and intestinal histology. Aquacult Int 30, 677–698 (2022). https://doi.org/10.1007/s10499-022-00833-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00833-7