Abstract
Aquaculture is growing post-haste in recent years particularly in the fish and shrimp production. The rapid growth of aquaculture and increasing demand for fish have led to a rapid development of the fish and shrimp industry, resulting in increased production of both fish and shrimps. As a result, there is a greater risk of disease outbreaks. Mass mortalities in aquaculture are primarily due to infectious diseases caused by bacteria, viruses, and fungi. Among them, viral diseases are the most devastating, causing huge loss in the production of both cultured fish and shellfishes. There are several effective methods of treatment for these disease outbreaks. This review focuses on various methods of controlling the viral pathogens using various treatment methods like use of medicinal plants and seaweed extracts, bioactive compounds from actinomycetes, vaccines, probiotic microbes, chemicals, nanoparticles, and green synthesis of nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is considered as the major source for enhancing the fish supply (Reverter et al. 2014). It is considered as a major food-producing sector for a rapidly increasing population as it constitutes up to 45% of the total production of fish around the globe (Aseefa and Abunna 2018; Chauhan and Singh 2019). India ranks second in aquaculture and third in fishery production. Fisheries contribute to 1.07% of the total GDP of India. According to the National Fisheries Development Board, the fishery industry generates an export earnings of Rs 334.41 billion (Kushankar Dey 2020). Since it is now a global industry, the total production has exceeded 50 million tonnes annually with an estimated value of around 80 billion US$ (FAO 2009; Walker and Winton 2010). The rapid growth of aquaculture and increasing demand for fish has led to rapid growth of the fish industry, increasing exacerbation for fish and hence intensifying the risk of disease outbreaks (Reverter et al. 2014). At present, infectious disease outbreak in aquaculture is the major reason for the increasing economic loss to the fish industries. Carp, shrimps, and oysters are the major species produced in Asia accounting for 98%, 88%, and 95% respectively of the production. Countries like Norway, the US, Canada, and Chile account for 94% by value and 88% by volume of Atlantic Salmon produced (FAO 2008; Walker and Winton 2010). From the year 1970 to 2007, with an average growth of 6.9% annually, aquaculture has proved to be a rapidly growing food-producing sector, since it can soon overtake the capture fisheries as the main source of seafood (FAO 2008; Walker and Winton 2010). Excluding China, the share of Asian fish production in aquaculture has risen from 19.3% in the year 2000 to 42% in 2018 (FAO 2020).
Fish and shrimps are exposed to various viral pathogens in the environment and also within aquaculture sites (Scott et al. 2011). Viral diseases are reported to be the one of the main causes of mass mortalities in aquaculture (Muroga 2001).
As far as viral diseases in fish are concerned, a review by (Crane and Hyatt 2011) reported that viral pathogens which include aquabirnaviruses and infectious hematopoietic necrosis virus reported in fin fish are known since the first half of the twentieth century. In addition, betanodaviruses which have also emerged as pathogens in aquaculture have undergone dramatic expansion in the past few decades.
In 2001, a review paper regarding various viral infections in fish and shellfish in Japanese hatcheries was published. This paper gave a brief information of herpesvirus infections like viral epidermal hyperplasia occurring in Japanese flounder, nodavirus infections such as viral nervous necrosis in the Japanese parrotfish as well as in striped jack, birnavirus infections like viral ascites in yellowtail, baculoviral infections like baculoviral mid-gut gland necrosis, and unclassified virus infections like panaeid acute viremia in kuruma prawn (Muroga 2001).
In 2007, another review article which gave an insight into the viral pathogens was published. The authors covered several topical viruses causing problems in aquaculture as well as methods for their control and prevention (Austin and Austin 2007). Also, another review study done by (Munang’andu et al. 2016) reported the control and preventive measures for curing viral pathogens in aquaculture by reducing their prevalence.
Viral diseases in shrimp were reviewed in a paper on viral infections and their diagnosis as well as control measures in aquaculture was published in the year 2017. Some of the major viral pathogens like white spot syndrome virus (WSSV), hepatopancreatic parvovirus (HPV), white tail disease (WTD), monodon baculovirus (MBV), viral nervous necrosis (VNN), and infectious hypodermal and hematopoietic necrosis baculovirus (IHHNV) that predominately affected shrimp and fish aquaculture were reported (Ninawe et al. 2017). To deal with these pathogens which have been emerging, expanding, and responsible for disease outbreaks and threats of outbreaks, several efficacious treatments have been developed and reported.
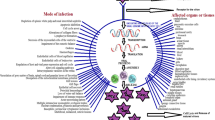
The aim of the present review is to highlight the methods of controlling several viral pathogens in aquaculture using various treatment methods. In this study, the control of these pathogens using treatments like medicinal plants, seaweeds, actinomycetes, vaccines, probiotic microbes, chemicals, nanoparticles, and green synthesis of nanoparticles against viral pathogens of fish and shrimps is reviewed.
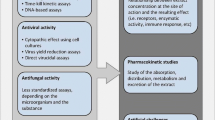
Prophylactic and treatment methods for viral diseases of fish and shellfish
Among the pathogenic diseases observed in the aquatic organisms, viral pathogens are mostly responsible and associated with half of them after bacterial pathogens followed by others (McLoughlin 2006). The treatment methods that can be used to cure infectious viral pathogens are mentioned below:
Prophylactic approach by administration of vaccines
Vaccination is one of the traditional methods of effectively preventing several infectious diseases. The different kinds of vaccines available for use in fishes includes attenuated vaccines, recombinant technology vaccines, killed vaccines, DNA vaccines, and synthetic peptide vaccines. The techniques and modes of administration of these vaccines in fish involves oral, immersion, and injection methods (Aseefa and Abunna 2018).
In the aquaculture industry, vaccines create a huge impact by reducing the use of certain antibiotics, thereby avoiding the possibility of drug resistance and protecting the fishes from these infectious diseases. Novel vaccine registration and its need for licensing is considered much simpler than antibiotics (Plant and LaPatra 2011).
Currently, killed vaccines are the most commonly used commercial vaccines in the aquaculture industry, since they can be designed effortlessly, are cheap, remain stable in storage, and have no problem of being virulent (Pridgeon and Klesius 2012). Several infectious diseases such as those caused by pathogens like infectious hematopoietic virus can be prevented using killed vaccines (Aseefa and Abunna 2018). On the contrary, few studies have also reported that inactivated/killed vaccines generate insufficient immunity as in the case of red sea bream iridovirus (RSIV) disease or salmon pancreas disease virus (SPDV) (Evensen and Leong 2013).
From laboratory studies, the efficacy of the live vaccines in fishes was observed, since it stimulated humoral, mucosal, and also cellular immunity (Shoemaker et al. 2009). The attenuated organisms were reported to replicate inside the targeted host without showing clinical signs (Lillehaug 2014).
DNA vaccines when injected intramuscularly give durable protection immediately from infections occurring in the farmed salmonids against certain infectious diseases like hematopoietic necrosis virus which have an economic impact (Ballesteros et al. 2015). It also acts against viral hemorrhagic septicemia virus (Cho et al. 2017). These vaccines have also been studied in infections involving viral hemorrhagic septicemia virus (VHSV), infectious hematopoietic necrosis virus (IHNV), and salmonid rhabdoviruses and were found to be very beneficial and effective against them for reducing the effect of these viruses. Moreover, these two DNA virus vaccines have been experimented on more viral diseases of fishes such as hirame rhabdovirus and spring viremia carp virus (Aseefa and Abunna 2018). It has been reported that nearly all DNA vaccines were developed for treatment of viral infections of fish (Dhar et al. 2014). For the protection of salmon from infectious diseases like salmon anemia and hematopoietic necrosis recombinant vector vaccines were developed in which viruses were used to express in the vectors (Dadar et al. 2016). Subunit vaccines are safer but since their immunogenicity is poor compared to whole organism, inactivated vaccine adjuvants are needed to improve their immunogenicity (Dadar et al 2016). Vaccines developed now use modern vaccine technology that targets particular pathogen components. Recombinant DNA or subunit vaccines that contain novel antigens produced with the aid of several expression systems are examples of this (Kelly and Rappuoli 2005; Cimica and Galarza 2017; Ma et al. 2019). Other expression systems that have been reported in the production of fish subunit vaccine experimentally include baculovirus for VHSV or IHNV proteins (Estepa et al. 1994; Lecocq-Xhonneux et al. 1994; Lorenzen and Olesen 1995; Cain et al. 1999; Biering et al. 2005; Crane and Hyatt 2011). Live attenuated vaccines can replicate to a lower titer value and can therefore stimulate both cellular and humoral immunity (Dadar et al. 2016).
Since there existed a lack of knowledge and understanding of the immune responses of fishes against various antigens which were also considered less potent, a carrier molecule was required. This is why many studies reported that vaccination of fish with the peptides is almost impracticable (Mweemba et al. 2014). Emmenegger et al. (1995) developed synthetic vaccines earlier in aquaculture in which the synthetic peptide antigen for IHNV was produced by coupling the antigens to bovine serum albumin (BSA). Later, Lin et al. (2002) made synthetic peptides to protect the freshwater shrimps against white spot disease by expressing the glycosylated phosphatidylinositol (GPI) anchoring membrane protein and immobilization antigen (I-antigen) using an assembly PCR technique to manufacture these peptides. Joshi et al. (2021) reported an in silico design of an epitope-based vaccine against seven banded grouper nervous necrosis virus affecting fish species. Recently, a study was reported by Kulkarni et al. (2021) on the immunoprotection trials and immune responses in the crustaceans focusing mainly on shrimps which highlighted potential development of effective immunoprophylactic strategies in shrimp. These vaccines have been used for a long time in the prevention of many infectious diseases including birnavirus, nodavirus, viral hemorrhagic septicemia, and rhabdovirus (Dadar et al. 2016).
Most of the viral vaccines for aquaculture available at present are mainly based on recombinant subunit proteins or inactivated/killed virus (Sommerset et al. 2005). Live viral vaccines have been found to show good results when tested in fishes (Benmansour and de Kinkelin 1997; Lopez-Doriga et al. 2001; Ronen et al. 2003). Kim et al. (2000) reported that DNA vaccines in fish induce an early, non-specific antiviral protection mediated by an alpha/beta interferon and, later, a specific immune response. Sommerset et al. (2005) reported that recombinant VP35 protein can induce immunity and protect grass carp against grass carp reovirus infection and that it could be used as a subunit vaccine.
Some diseases caused by pathogens like infectious pancreatic necrosis virus (IPNV), birnavirus, are reported to cause major problems in salmonids (Biering et al. 2005). Marine IPNV strains commonly known as aquatic birnaviruses are also reported to be pathogenic in a wide range of marine fish species that includes European sea bass, Japanese eel, halibut, yellowtail, and turbot, for which vaccines are unavailable (Sommerset et al. 2005). In addition, infectious hematopoietic necrosis virus (IHNV) (in North America and France), the rhabdoviruses, and viral hemorrhagic septicemia virus (VHSV) (in France and Denmark) were found to be predominantly affecting the salmonids and causing highly infectious diseases (Sommerset et al. 2005). Betanodaviruses causing viral nervous necrosis (VNN) have been affecting various farmed marine fish species such as Atlantic halibut, European sea bass, barramundi, and groupers (Munday et al. 2002) for which commercial vaccines are now available. Two formalin-inactivated commercial vaccines ICTHIOVAC® VNN (Hipra) and ALPHA JECT micro® 1Noda (Pharmaq) have been made available for vaccination in sea bass against red grouper nervous necrosis virus (RGNNV) genotype in the Mediterranean region (Valero et al. 2021).
Water temperature may be an important factor in deciding the time of vaccination. In addition, the size of fish is also responsible for the regulation of immune competence development (Vallejos-Vidal et al. 2014). Since oral feeding prevents stress in fish, oral vaccination can be easily administered by either incorporating it into the feed during its production or by encapsulating or coating with the pellets (Lillehaug 2014). Immersion method of vaccination is especially convenient in the case of smaller fishes and fingerlings in which other methods of vaccination are impractical. During immersion, vaccines are applied on the surface of the fish. The uptake of the antigen takes place through gills, skin, and also via the lateral line. Besides, this method causes minimal level of stress in fishes. Moreover, the vaccine solution can also be reused. Vaccination by injection gives the best protection in the case of adjuvant vaccines (Harikrishnan et al. 2011). This method of vaccination requires a relatively minimal amount of dose, since calculating the accurate dosage is simple as well as economical in the case of larger fishes. This method of injecting a vaccine is however not suitable for smaller fishes because of stress, adhesion formation, reduction in intake of feed, and damage occurring at the time of injection that may lead to massive deaths in fishes (Lillehaug 2014).
Recently, Zeng et al. (2021) reported a newly emerging pathogenic virus, tilapia lake virus (TiLV), that has created a huge impact on the tilapia industry globally for which currently no vaccines or antiviral drugs are available for its prevention and control. They developed a β-propiolactone inactivated TiLV vaccine that exhibited a higher protection efficacy against this virus compared to formaldehyde. β-Propiolactone inactivated vaccine combined with Montanide IMS 1312 VG adjuvant can result in higher level of protection from TiLV infection in tilapia fish (Zeng et al. 2021). In the year 2019, a study regarding fish immunization as an effective treatment method for the prevention of a wide range of viral and other pathogenic diseases was reported (Ma et al. 2019). Currently, there are many vaccines which have been approved for use in aquaculture in salmonids, catfish, rainbow trout, common carp (Cyprinus carpio), koi carp (Cyprinus rubrofuscus), Indian major carps, tilapia, grouper, lobster, and sea bass (Aseefa and Abunna 2018).
Treatment using chemicals
Therapeutic substances are often added into the water for controlling several infectious diseases affecting gills and body surface. In a few cases, therapeutic baths have also been used, since the active substances are absorbed via skin and thereby control these causative agents. Based on their exposure period, the therapeutic baths have been categorized as immersion baths (up to 5 min), short-term baths (from 5 min to 2 h and long-term baths (2 h to a few days). The chemical substances used in immersion bath of fishes include Lysol, KMnO4, lime milk, ammonia and trypaflavine, CuSO4·H2O. In the case of short-term baths, NaCl, formaldehyde, and KMnO4 are added. For long-term baths carried out in ponds as well as fish culture reservoirs, acriflavine, metronidazol, antibiotics, trichlorphon, formaldehyde KMnO4, and NaCl have been used (Tesarčík and Svobodová 1991). Shamsuzzaman and Biswas (2012) reported that chemicals like timsin and emsin are effective in destroying viruses by preventing viral infections. Rao (2020) reported that this is an eco-friendly solution for the treatment of viral diseases (currently, traditional preparations such as Wescodyne or Incodyne have been used on an increasingly large scale, since it helps in controlling viral diseases of the fish eggs (Tesarčík and Svobodová 1991)). Amend and Pietsch (1972) reported that two iodophors, Wescodyne® and Betadine® showed virucidal activity against salmonid viruses like IHNV, IPNV, and VHSV.
The therapeutic substances have been administered either in the form of granulated medicated feeds where the pellets have been incorporated with the drug or are amalgamated with the feeds. Poly(vinylpyrrolidone)–iodine (PVP-I), a broad-spectrum antiseptic was reported as one of the first antiviral compounds used in aquaculture that helps in inhibition of viral release as well as spread from the infected cells by blocking viral attachment to the cellular receptors (Sriwilaijaroen et al. 2009; Pereiro et al. 2020). There was a reduction observed in both morbidity and mortality in brook trout Salvelinus fontinalis caused by IPNV when fed with PVP-I (Pereiro et al. 2020; Economon 1963, 1973). Batts et al. (1991) reported that iodine was found to be effective in treating the IHNV infection in rainbow trout when added to water at lower concentrations. Moreover, several studies reported the ability of iodine complexes to destroy fish pathogenic viruses like IHNV, VHSV, and IPNV (Pereiro et al. 2020; Amend and Pietsch 1972; Elliott and Amend 1978). PVP-I is currently used to disinfect the surfaces of eggs in aquaculture (Cipriano et al. 2001). Boriskin et al. (2008) reported that arbidol hydrochloride is a fusion step of another compound that can block viral attachment to the target membranes. However, it was found to be ineffective against the iridovirus SGIV-infected grouper spleen (GS) cells at the particularly tested concentration (Jia et al. 2018; Pereiro et al. 2020).
Sushila et al. (2018) reported that lysosomotropic agent NH4Cl and clathrin-mediated endocytosis inhibitor chlorpromazine (CPZ) exhibited antiviral activities against nervous necrosis virus (NNV) infection in the Sahul Indian sea bass kidney cell line (SISK). Adachi et al. (2007) also demonstrated the efficacy of various lysosomotropic agents such as NH4Cl, monensin, bafilomycin A1, and chloroquine (CQ) against betanodavirus NNV infection in snakehead fish cell line E11. Huang et al. (2017) reported similar results in Asian sea bass cells against NNV virus-like particles (VLPs). In addition, both CQ and NH4Cl, chemical inhibitors of endosomal acidification, inhibited the entry of VLP (Huang et al. 2017; Pereiro et al. 2020). Guo et al. (2012) observed that NH4Cl, sucrose, CQ, and CPZ were inefficient in reducing the infection of fish iridovirus (ISKNV) in cell line of Mandarin fish fry (MFF-1), while phorbol 12-myristate 13-acetate (PMA) significantly reduced the virus entry. Vigant et al. (2013) reported that LJ001, a derivative of lipophilic thiazolidine, displayed its broad-spectrum antiviral activity against 3 different kinds of fish rhabdoviruses IHNV, SVCV, and VHSV infections (Balmer et al. 2017, 2018; Pereiro et al. 2020).
The efficacy of CQ against IHNV infection has been demonstrated in chinook salmon embryo (CHSE-214) cells by Hasobe and Saneyoshi (1985a, 1985b) but only an insignificant level of protection was observed when IHNV-infected rainbow trout fry was treated with CQ dissolved in water (Hasobe and Saneyoshi 1985b). De Las Heras et al. (2008) reported that CQ was efficient in reducing IHNV binding to bluegrill fry (BF-2) cell line but was found to be ineffective against VHSV and IPNV. Moreover, De Las Heras et al. (2008) observed the efficacy of tributylamine, another lysosomotropic compound against these three pathogenic fish viruses. Recently, Jaemwimol et al. (2019) reported that a buffered acid peroxygen solution called Virkon®, a multipurpose disinfectant, was effective against TiLV infection with greater than 5 log10 reduction condition.
Treatment using bioactive compounds from actinomycetes
Actinomycetes are the gram-positive bacteria which inhabit both terrestrial and marine environments. These species are known to be fastidious and hence are difficult to culture as well as isolate.
Marine actinomycetes are regarded as a beneficial source of several antimicrobial agents. In addition, it can also produce various antiviral drugs against DNA as well as RNA viruses. Since usage of these antiviral drugs in large amounts for treating viral diseases can stimulate mutagenicity as well as cross-resistance, marine actinomycetes are considered as a novel source that can be used as an antiviral drug (Moussa et al. 2015). Since they are important producers of mainly antibiotic compounds and vitamins, antitumor agents, enzymes, immunomodifying agents and are widespread, they have a major role in the production of pharmaceuticals (Lam 2006).
Sree Kumar et al. (2006) reported the antiviral property and effectiveness of marine actinomycetes in penaeid shrimps against WSSV. The isolated actinomycetes were used as feed additives to the WSSV-infected post-larvae of black tiger shrimp, Penaeus monodon, which displayed post-challenge survival ranging from 11 to 83%. A review paper by Jakubiec-Krzesniak et al. (2018) presented secondary metabolites of Actinobacteria, which possess antibacterial, antifungal, and antiviral activities.
Diseases transmitted by all kinds of microorganisms especially viruses are increasingly becoming a threat to society. Hence, to control the spread of these pathogens and also due to the increasing resistance to chemical drugs, there is an urgent need to develop novel antimicrobial agents extracted from these actinomycetes (Ganesan et al. 2017).
Use of probiotics
Probiotics usually consist of bacteria but can also include other microorganisms like microalgae, yeast, or fungi (Cordero et al. 2014). Use of probiotics is a positive alternative approach to prevent, control, and treat infectious diseases. Its effects include growth stimulation, improvement of digestion, immune response enhancement, and also recuperation of water quality. Generally, probiotics exhibit various antiviral properties which are beneficial to fishes, since they fight against these pathogens and thus improve their overall health. Currently, use of probiotics in aquaculture and its efficacy in aquatic environments needs to be explored (Chauhan and Singh 2019).
Probiotics that are used in the aquaculture are available in dry and liquid form. The former has a higher shelf life and is either mixed with the water or feed while the latter form of the probiotic is blended with the feed or is added into the tanks directly (Decamp and Moriarty 2007). Studies reported that due to lower density, the liquid form gives a much better and positive results compared to the dry form of probiotics (Nageswara and Babu 2006). Several aquatic probiotics have been reported to show activity against viral pathogens for the improvement of growth and even immunity of host (Chauhan and Singh 2019). The main search has always been for compounds that can be incorporated into the feed and hence orally delivered to the fish while others may be injected along with the vaccines (Chauhan and Singh 2019).
Based on their mode of administration, probiotics in liquid form are further classified into two categories which are used in shrimp and finfish aquaculture. The first class involves the probiotic bacteria being mixed with the feed supplement for enhancing the beneficial bacteria inside gut region. The second class deals with the probiotics added to the water directly so that available nutrients in the water can be consumed and pathogen proliferation can also be inhibited (Nageswara and Babu 2006; Sahu et al. 2008).
Methods of enhancing the natural defense mechanism of a fish have always been the main focus of research in aquaculture, since it has a lot of useful advantages (Defoirdt et al. 2011). Biological control of diseases in aquaculture has become the best approach for controlling the infectious diseases (Maqsood et al. 2011). Probiotics are considered non-pathogenic to fishes and other aquatic animals, since they are the bacterial cultures isolated from bacterial strains (Sharifuzzaman and Austin 2017). In other words, they can be described as a live organism that is administered to the hosts for developing a defensive immune system which after administration to the fishes later multiplies to inhabit the fish gut. Thus, they are useful in maintaining the normal microflora and also the microbial balance in hosts (Mastan 2015).
In aquaculture, among the many microorganisms, there are a few which have been reported as aquatic probiotics and are used in fishes and even in various cultured animals for disease prevention as well as weight gain promotion. They can either be incorporated to the feed or added directly to the water. The other method of administration is encapsulation which is useful for improving the nutritional value as well as delivering the microbe properly to the host (De et al. 2014). The microbes at high density are encapsulated in colloidal matrices by using alginate, pectin, carboxymethylcellulose, or chitosan for physical as well as chemical protection of the microorganisms (Hermosillo et al. 2012).
Currently, the most widely applied method of their administration is incorporation of the probiotics directly in to the feed pellets. In order to maintain the viability of the probiotics after their addition, they must be monitored continuously for confirming protective escalated immunity in fish. In addition, they can even be added as freeze-dried cultures which are later mixed with the lipids as dressings at the top of the feed (De et al. 2014).
Aquatic probiotics are popular because of their antiviral properties against various known pathogens. Although, some reports indicate that in aquaculture, virus inactivation can also take place by using different bacterial probiotic strain extracts, the mechanism by which probiotic bacteria show antiviral activity is unknown (Hasan and Banerjee 2020). However, in vitro analysis has revealed that viruses can be inhibited when bacteria such as Aeromonas, Pseudomonas, Corynebacterium, and Vibrio species secrete extracellular enzymes which are effective against infectious hematopoietic necrosis virus (IHNV) (Kamei et al. 1988; Zorriehzahra et al. 2016). It has been reported that probiotic strains like Bacillus megaterium increased the resistance against white spot syndrome virus (WSSV) in Litopenaeus vannamei. Vibrio species are also effective in protecting Litopenaeus vannamei against WSSV (Li et al. 2009; Balcazar 2003). Liu et al. (2012) reported that 50% higher survival rate and enhanced resistance was observed in some grouper fish infected by iridoviruses when the probiotic Bacillus subtilis E20 strain was used in comparison to the non-probiotic group. Another study by Son et al. (2009) reported higher rate of survival in grouper Epinephelus coioides against iridovirus by dietary administration of probiotic Lactobacillus plantarum. Moreover, Lactobacillus when used as a probiotic either as single strain or in combination with Sporolac gave better resistance against lymphocystivirus found in a marine fish, olive flounder Paraliichthys olivaceus (Harikrishnan et al. 2010a, b, c). Similarly, when yellow jack fish Carangoides bartholomaei was experimentally infected with Sima-aji neuro necrosis virus (SJNNV), the bacterial strain Pseudoalteromonas undina VKM-124 showed an inhibitory activity towards SJNNV (Maeda et al. 1997). Likewise, a review paper by Chiu et al. (2010) revealed the probiotic efficacy of dietary Saccharomyces cerevisiae which gave protection against iridoviruses (GIV) to grouper fishes at 5.3 × 107 CFU/kg.
Due to the occurrence as well as spread of various viral diseases such as white spot syndrome virus (WSSV), infectious hypodermal and hematopoietic necrosis virus (IHHNV), and lymphocystis disease virus (LCDV, shrimp aquaculture has faced huge economic loses compared to fish (Hoseinifar et al. 2018). Treatment of shellfishes like shrimp with probiotics has been reported by Lakshmi et al. (2013) as an efficient way for both control and prevention of these viral diseases. Most of studies conducted on the dietary administration of these probiotics revealed antiviral effect depending on the shrimp species (Hoseinifar et al. 2018). The antiviral activities of probiotics against the viruses are listed in Table 1.
A study conducted by Direkbusarakom et al. (1998a, b) on tiger shrimp infected with Vibrio spp. reported stronger antagonistic activity against both Oncorhynchus masou virus (OMV) and IHNV. Likewise, treatment of the whiteleg shrimp Litopenaeus vannamei by administration of 105 CFU/mL probiotic Vibrio alginolyticus showed enhanced resistance against WSSV in comparison to the shrimps that were not treated (Rodríguez et al. 2007). In another similar study, 1010 CFU/mL of probiotic Bacillus megaterium when administered against shrimp WSSV gave improved protection and higher survival rate (Li et al. 2009). A study by Leyva-Madrigal et al. (2011) reported a decrease in WSSV infection after treatment of the white shrimp with either Staphylococcus hemolyticus or Pediococcus pentosaceus. Dietary administration of 105 CFU/g multiple strains of lactic acid bacteria like BC1, BAL3, BAL7, and CIB1 were ineffective against WSSV infection in whiteleg shrimp Litopenaeus vannamei (Partida-Arangure et al. 2013).
Bacillus sp. LMG20363 administered as a feed in the diet of shrimp Litopenaeus vannnamei has shown decrease in the viral infection against WSSV (Balcázar et al. 2006). Chai et al. (2016), isolated Bacillus PC465 from the gut of the Chinese white shrimp Fenneropenaeus chinesis to evaluate its antiviral effects against WSSV infections. An enhancement of the gut microbial structures by promoting the immune system of the shrimp against WSSV was reported by them. Although the mechanisms by which the probiotics protect against WSSV infection remains unclear, some researchers have reported that the immunomodulatory nature of probiotics plays a key role in protection against WSSV infections (Merrifield et al. 2010).
Treatment using nanoparticles
Nanotechnology with the aid of its novel nanotools has a lot of potential in the aquaculture industry. However, very little data is available regarding the role of several nanoparticles in the prevention of fish diseases (Shah and Mraz 2019).
The use of antibiotics as well as chemical disinfectants against viral pathogens has created problems in the aquaculture industry (Huang et al. 2015). The use of nanoparticle-based vaccines against many viral pathogens is a developing field in fish medicine research (Shaalan et al. 2016). The efficacy of a construct DNA containing nodavirus’s extra small virus antisense gene encapsulated with the chitosan nanoparticles in giant freshwater prawn Macrobrachium rosenbergii has been reported to increase its survival rate (Ramya et al. 2014).
The production of the feed is one of the major applications of nanotechnology in aquaculture. An important application of nanotechnology in aquaculture is feed production. In this, the use of NPs has proven to be effective in many ways like (i) micronutrient delivery, (ii) amount of produced feed per unit time, and (iii) growth promotion (Khosravi-Katuli et al. 2017). The application of chitosan nanoparticles has been reported to increase the shelf life of vitamin C and its delivery significantly in the rainbow trout after feeding for 20 days (Alishahi et al. 2014). Similarly, chitosan nanoparticles were reported to have been applied in liver cell lines of zebra fish for delivering ascorbic acid and even for in vivo treatment in the case of the rotifer Brachionus plicatilis, since nanoparticles can easily penetrate the epithelium of fish intestine exhibiting a significant rise up to twofold in the level of ascorbic acid (Jiménez-Fernández et al. 2014).
Chitosan nanoparticles have been used in the encapsulation of nutrients which can be degraded easily while in contact with the water (Chatterjee and Judeh 2016; Ji et al. 2015). In addition, when coated chitosan nanoparticles were encapsulated with the calcium alginate, it was found to prevent the leakage of shark liver oil (Peniche et al. 2004). Another in vitro study reported that when these chitosan nanoparticles were encapsulated with oil droplets of tuna fish, their physical stability was enhanced and fatty acids liberated from emulsions were subsequently reduced (Klinkesorn and McClements 2009). Moreover, when SWCNTs (Fraser et al. 2011; Bisesi et al. 2015), C60 (Fraser et al. 2011), and nTiO2 (Ramsden et al. 2009) were added to rainbow trout, fathead minnows, and rainbow trout food, the physical properties of fish pellet were changed making them more compact than usual, thereby reducing the leaching of nutrients’ and their resultant waste in fishpond.
A study reported that polyamine carbon quantum dots (CQDs), carbonaceous nanoparticles in Litopenaeus vannamei, act against WSSV virus by attachment to the virus envelope which later resulted in inhibition of the viral infection. They described some antiviral strategies and therapeutic treatment methods using polyamine CQDs in aquaculture (Huang et al. 2020). The antiviral activities of NPs against the respective viruses are listed in Table 2.
Supplemental selenium nanoparticles due to its defensive antioxidant and bioavailability properties (Sonkusre et al. 2014) were reported to enhance the end weight and activity of glutathione peroxidase (GSH-Px) level in the blood plasma and liver and protein content in the muscle as well as reduced FCR activity in the crucian carp fish Carassius auratus gibelio (Zhou et al. 2009). In addition, selenium nanoparticles were shown to cause LDH enhancement and also increase in SOD, protein cellular contents, GSH-Px, and K+/Na+-ATPase in the crucian carp; the effect of this being both dose dependent and also dependent on the size of the nanoparticles (Wang et al. 2013). Moreover, Se nanoparticles at both moderate and high doses were reported to have a significant effect on Nile tilapia fish Oreochromis niloticus growth due to the spiked feed resulting in weight gain (Deng and Cheng 2003).
Zinc oxide nanoparticles was reported as a dietary zinc source that was found to show immune response as well as growth improvement in the grass carp fish Ctenopharyngodon idella (Faiz et al. 2015). Additionally, after feeding with zinc oxide nanoparticles feed for 90 days, there was also a significant rise in the protein content level, activity of the antioxidant enzymes, and increase in the weight of the freshwater prawn Macrobrachium rosenbergii (Muralisankar et al. 2014). Titanium oxide nanoparticles were used for improving the growth performance in Oncorhynchus mykiss rainbow trout fish (Ramsden et al. 2009).
In vivo studies using silver nanoparticles (AgNPs) highlighted their diverse characteristics, along with their antimicrobial effects in controlling shrimp diseases like white spot disease (WSD) caused by WSSV (Camacho-Jiménez et al. 2020) that can result in complete devastation of the shrimp culture (Sánchez-Martínez et al. 2007). The patented formulations of both Agrovit-4® (Romo-Quiñonez et al. 2020) and Agrovit® (Juarez-Moreno et al. 2017; Ochoa-Meza et al. 2019) have confirmed the antiviral effects of AgNPs against WSSV infections. Moreover, WSSV-infected shrimp Litopenaeus vannamei when treated with Agrovit® showed no signs or symptoms of white spot disease. Enhanced survival rate ranging from 70 to 80% was reported (Juarez-Moreno et al. 2017). Likewise, shrimp fed Argovit-4-supplemented feed did not show signs of toxicity for the assayed doses over the 192-h experiment period. The third and fourth bioassays showed that shrimp challenged with WSSV at 1000 µg/g feed exhibited reduced mortality (Romo-Quiñonez et al. 2020).
Nano-encapsulated vaccines have been developed against WSSV, i.e., white spot syndrome virus, IMNV, i.e., infectious myoncronis virus in shrimp farming (Rajeshkumar et al. 2009; Chalamcheria 2015), and Listonella anguillarum in the Asian carp fish (Rajeshkumar et al. 2009). Polyanhydride nanoparticles were used for encapsulation as well as liberation of the vaccine antigens for determining the immunization route, i.e., via feed or immersion (Ross et al. 2014). Nanoparticle-based carriers such as PLGA, i.e., polylactic-co-glycolide acid, alginates, and chitosan used for the vaccine antigens along with mild inflammatory inducers orally showed a higher level of protection to fishes and shellfishes with a relative survival rate up to 85% in the cultured shrimps (Rajeshkumar et al. 2009). Additionally, silica-based NPs can be used for drug (i.e., pharmaceuticals or other therapeutics) administration due to its porous structure and ability to incorporate high dose of administration (Strømme et al. 2009).
Treatment using medicinal plant extracts
Medicinal plants, herbal medicines, and plant extracts are being widely used in the aquaculture industry as a sustainable and effective alternative treatment to vaccines or chemicals. Since they consist of multiple bioactive compounds, there has been an enormous increase in the use of these natural plant products to control disease outbreaks in aquaculture (Reverter et al. 2014).
Due to the presence of bioactive molecules like alkaloids, flavonoids, terpenoids, phenolics, tannins, glycoside, steroids, saponins, and essential oils, these plant products are reported to enhance appetite and weight gain, toxicity enhancement, and culture species maturation. They also act as immunostimulant, growth promoters, and aphrodisiac and also have antipathogenic properties in shellfish and other fish in aquaculture (Citarasu 2010; Chakraborty and Hancz 2011). However, to convince the aquaculturists about their efficacy in aquaculture, there is a huge need to publicize the advantages of using these natural products.
In a review paper, it was reported that leaves of Azadirachta indica (neem) contain azadirachtin, nimbin, and meliantroil that possess several antiviral properties (Pandey and Sharma 2012). Similarly, a paper published by Balasubramanian et al. (2008a, b) reported that Panaeus monodon (black tiger shrimps) infected with white spot syndrome virus (WSSV), when treated with the extract of Bermuda grass Cynodon dactylon showed no symptoms of disease and zero mortality in comparison to the controls. Additionally, various plant extracts have exhibited strong antiviral property against shrimp and fish pathogenic viruses. Aqueous and ethanolic extract of guava (Psidium guajava) were found to show antiviral activity against infectious hematopoietic necrosis virus (IHNV) and Oncorhynchus masou virus (OMV) by reducing 65–100% and 21–100% plaques respectively in CHSE214 cell lines (Direkbusarakom et al. 1996). Moreover, extracts of Phyllanthus acidus, Psidium guajava, Phyllanthus amarus, and Cassia alata exhibited reduction in plaques on virus adsorption by 100% for IHNV, while P. acidus and C. alata showed 92–100% for infectious pancreatic necrosis virus (IPNV). On the other hand, effects of Ostreopsis siamensis and P. acidus extracts on viral replication displayed plaque reduction by 100% for IPNV and OMV (Direkbusarakom et al. 1996). P. amarus has been reported to show high antiviral efficacy against IHNV and OMV like fish viruses and the shrimp virus yellowhead virus (YHV) (Direkbusarakom 2004). Several antiviral tests were also carried out against the yellowhead virus (YHV) of shrimp via injection route by Direkbusarakom et al. (1997). Moreover, the extract of the herb, Clinacanthus nutans, showed efficacy against YHV of shrimp and also controlled the YHV infection effectively in shrimp (Direkbusarakom et al. 1998a, b).
A major compound, oleuropein (Ole), derived from the leaves of olive tree Olea europaea showed potent antiviral activity against RNA and DNA viruses (Fredrickson 2000) and controlled several fish viruses like viral hemorrhagic septicaemia virus (VHSV) and salmonid rhabdovirus (Micol et al. 2005). Another compound gymnemagenol (C30H50O4) extracted from leaves of miracle fruit plant, Gymnema sylvestre, when tested against grouper nervous necrosis virus (GNNV) and fish nodavirus under in vitro conditions using Sahul Indian Grouper Eye (SIGE) cell lines inhibited the GNNV proliferation up to 53% (Khanna et al. 2011). Under similar in vitro conditions, dasyscyphin C (C28H40O8) derived from the extract of false daisy plant Eclipta prostrata exhibited antiviral property against GNNV and fish nodavirus when tested in SIGE cell lines (Harikrishnan et al. 2010a, b, c). A recent study by Sun et al. (2021) reported inhibitory activity of almost 13 medicinal herbs in Procambarus clarkii crayfish against WSSV. Naringenin (NAR), an active compound from the extract of the plant Typha angustifolia, displayed high potency to inhibit the WSSV infection by 92.85%. The antiviral activities of medicinal plants against the respective viruses are listed in Table 3.
In the year 2015, Sivasankar et al. reported the significance of plant herbal extracts as a treatment method against viral diseases in fish and shrimp aquaculture. Several herbal extracts that showed antiviral activity in fish include Calophyllum inophyllum (tamanu), Clinacanthus nutans (snake grass), Cassia alata (candle bush), Glinus oppositifolius, Momordica charantia (bitter gourd), Ochrocarpus siamensis, Hura crepitans (sandbox tree), Ocimum sanctum (white) (basil/tulsi), Ocimun sanctum (red) (tulsi), Phyllanthus acidus (gooseberry), Phyllanthus debilis, Phyllanthus urinaria (chamber bitter), Phyllanthus amarus (gale of the wind), Phyllanthus reticulatus, Tinaspora cordifolia (heart-leaved moonseed), Tinaspora crispa, and Psidium guajava (guava) (Direkbusarakom 2004). Likewise, in shrimp aquaculture, various medicinal plants such as Allium sativum (garlic), Azadirachta indica (neem), Aegle marmelos (Indian bael), Aristolochia indica, Solanum nigrum (black nightshade), Curcuma longa (turmeric), Cassia fistula (golden shower), Lantana camara (West Indian lantana), Catharanthus roseus (Madagascar periwinkle), Cynodon dactylon (Scutch grass), Morus alba (mulberry), Psidium guajava (guava), Tylophora indica, Phyllanthus emblica (Indian gooseberry), Phyllanthus amarus (gale of the wind), Mimosa pudica, Ocimun Americanum (American basil), Melia azedarach (chinaberry), Momordica charantia (bitter melon), and Tridax procumban were used (Balasubramanian et al. 2007). Among these plants, the aqueous extract of C. dactylon showed strong antiviral activity at the concentration of 100 mg/kg of animal body weight. The methanol extract of M. charantia showed significant antiviral activity at the concentration of 150 mg/kg of animal body weight. The aqueous extract of L. camara and P. amarus and the methanol extract of A. marmelos showed partial antiviral activity at the concentration of 150 mg/kg of animal body weight.
There is a huge advantage of the use of extracts of medicinal plants in aquaculture, since they cannot only cause reduction in the treatment costs but are also more eco-friendly. This is because they are biodegradable more easily when compared to the synthetic molecules and also due to the increasing diversity of the plant extract-based molecules, they are less likely to develop drug resistance in parasites (Logambal et al. 2000; Blumenthal et al. 2000; Olusola et al. 2013). However, there is still very little information regarding the long-term efficacy of these plant extracts on fish physiology. There is also a lack of sufficient information on the standardization of herbal extract administration. To try and homogenize the main aspects such as method of extraction, dose and method of administration of extract, and effect of herbal extracts in different fish species in aquaculture, more studies are needed.
Treatment using seaweed extracts
Seaweeds are aquatic plants which are considered to be a major part of the marine food web which plays a significant role in the human diet. They have high nutritional value and also exhibit antioxidant, antimicrobial, and immunostimulatory activities. In the last two decades, there has been a vast increase in work on several seaweed extracts to study their antimicrobial or therapeutic application in the aquaculture industry. Among all the genera of seaweeds that manifest a broad and remarkable range of antimicrobial activities against various infectious diseases of marine fishes and shrimps are the brown seaweed Sargassum spp. and red seaweed Asparagopsis spp. that are outstanding (Vatsos and Rebours 2014).
Seaweeds in general contain highly bioactive components such as fatty acids, phenolics, polysaccharides, proteins, and terpenes that display significant antiviral, antitumor, and also antioxidative activities (Balboa et al. 2013). Moreover, polysaccharides that are isolated from seaweeds help in the inhibition of viral replication by preventing viral attachment to the target cells, its antiviral activity being totally dependent on the degree of polysaccharide’s sulfation (Ghosh et al. 2009; Plouguerné et al. 2013; Shi et al. 2017).
Similarly, there are several extracts which are water-soluble and are derived from the three red, green, and brown genera of seaweeds and contain sulfated polysaccharides that display various antiviral factors against most pathogenic viruses, like the Japanese encephalitis virus (flavivirus) (Chiu et al. 2012), the herpes simplex virus (Saha et al. 2012; Son et al. 2013), and the influenza virus (Jiao et al. 2012). Studies also disclose how the seaweed extracts regulate the virus replication as well as delay the exhibition of symptoms of these diseases by enhancing the rate of survival of the infected organisms. Beside these bioactive compounds, carrageenans, sulfoglycolipids, and fucoidans are the other substances reported in the seaweed extracts (Mohamed et al. 2012). Most of the sulfated polysaccharide’s function is by binding either to the surface of particularly enveloped viruses or to their receptors on the surface of host cells, thus disrupting their attachment as well as adsorption to host cells (Wang et al. 2012). A few carrageenans also display post-binding impeding effects that affect the stages of cellular activity within the cell of the infective agents (Buck et al. 2006), specifically replication and transcription of the virus (Wang et al. 2012). Apart from polysaccharides, antiviral fatty acids, terpenes, and alkaloids have also been isolated from algae (Soares et al. 2012; Pinto et al. 2012).
In the year 1992, the WSSV virus that was first isolated in Taiwan had been reported to cause deaths extensively in crustaceans like crabs and shrimps and also other aquatic animal species (Lo et al. 1996; Hossain et al. 2001). Recently, several studies have reported the activity of various seaweed species against WSSV virus (Lin et al. 2011; Sirirustananun et al. 2011). The extracts of red seaweed Gracilaria tenuistipitata have been found to increase significantly the immunological activities of the whiteleg shrimp Litopenaeus vannamei against WSSV, and hence, the shrimp mortality was reduced (Sirirustananun et al. 2011). Similarly, significant antiviral activity has also been reported in freshwater crab Paratelphusa hydrodomous against WSSV infection when treated with the methanolic extract of red macroalga Hypnea spinella (Dinesh et al. 2014).
In a review study by Mori et al. (2005), a protein named griffithsin was reported to be isolated from the aqueous extract of red alga Griffithsia sp. for the first time. It is the only compound so far isolated from microalgae that has reached the stage of clinical trials (Riccio et al. 2020) revealing both its in vivo as well as in vitro antiviral activities against different varieties of clinically enveloped viruses like HIV with minimum level of host toxicity (Lee 2019). In the year 2016, another study was published on the red algae Graciliaria chilensis that showed a clear antiviral activity against infectious salmon anemia (ISA) virus when added to the fish diet at a minimum concentration of 10% (Lozano et al. 2016).
Recently, a study conducted by Klongklaew et al. (2020) for determining the antiviral efficacy of hot water crude extracts (HWCEs) of 3 species of local Thai green macroalgae, namely, Ulva rigida (Ur), Ulva intestinalis (Ui), and Caulopa lentillifera (Cl) and even a commercial ulvan, Ulva armoricana (Ua), in Pacific white shrimp Litopenaeus vannamei against WSSV and YHV infections reported stronger antiviral efficacy of HWCEs from the alga U. intestinalis to inhibit both WSSV and YHV infections. Studies need to be carried out on several other emerging virulent viruses like Taura syndrome virus (TSV) and shrimp hemocyte iridescent virus (SHIV) that lead to mass mortalities in marine shrimp (Klongklaew et al. 2021). The antiviral activities of seaweeds against the viruses are listed in Table 4.
Administration of active seaweed compounds can be done either through seaweed water released from the sea weed directly or by adding the compounds in to it after their extraction from the water and also through medicated feed after extraction of sea weed compounds. (Vatsos and Rebours 2014). Another method, namely, incorporation of these extracts into feeds is reported to be the safest method of delivery, since the extract dose per organism is known to be more accurately calculated which is applicable to most farms and hence can reduce the bacterial load in the cells or tissues (Immanuel et al. 2004). These extracts when incorporated into the dry or live feeds function either by stimulating the immune system or by acting against the pathogens directly. Hence, this can be the most effective and easily applicable method for treatment and also containment of several infectious pathogens (Vatsos and Rebours 2014).
Treatment using green synthesis of nanoparticles
The synthesis of nanoparticles and its formation by using the plant extracts had a head start in terms of its association with the surroundings, since it is considered completely eco-friendly and hence even its waste is not harmful. Extracts of several plants like aloe vera, tea leaves, coriander, lemongrass, neem, ginger, cinnamon, eucalyptus, gooseberry, seaweeds, hibiscus, mint, extracts of mangrove plants, tulsi, lotus, latex, and banana have been used for the synthesis of green nanoparticles (Kurcheti et al. 2020). Sources of green syntheses of nanoparticles are mainly bacteria, plants, and fungi. In the aquaculture industry, nanoparticles have many uses like drug delivery, improvement of the water quality, and diagnosis and management of diseases. However, there are very few studies regarding the green synthesis of nanoparticles, since it is a relatively new field of aquaculture (Kurcheti et al. 2020).
Conclusion
Aquaculture is a rapidly growing industry worldwide. Since it is a rapidly growing industry, there are many major challenges and limitations which the industry is currently facing. One of the major challenges the industry is facing is the outbreak of infectious diseases, since it causes massive loss in the aquaculture output. Hence, based on principles that have been globally accepted as well as the strategies that are applicable locally, various approaches regarding controlling and preventing these problems are recommended. These approaches must first focus on preventing and curtailing the growth and development of these infections. Although combinational use of biological methods, legally approved drugs like antibiotics, and even immunoprophylaxis may result in a huge improvement in fish health, treatment methods like the use of extracts of medicinal plants, seaweeds, probiotics, actinomycetes, and green synthesis of nanoparticles have proved to be a great alternative treatment to vaccines or chemicals in aquaculture.
Among the pathogenic diseases observed in the aquatic organisms, about 50% of them are mostly associated with bacterial pathogens followed by viral, parasitic, and fungal diseases. Viral pathogens have either been around for decades or have emerged as new diseases and are also the indicators of some major serious disease outbreak problems which pose a threat to the aquaculture industry.
Actinomycetes, particularly Streptomyces species can be considered as the most significant group that is responsible for the production of antimicrobial agents, antibiotics, and several other antiviral drugs for treating viral diseases in fishes. Nanoparticles are currently of specific interest as a sensitive tool for diagnosing the viral diseases in aquaculture and hence have enormous potential for improving aquaculture with several novel nanotools.
The use of green synthesis of nanoparticles in aquaculture has become a simple, eco-friendly, and efficient way of using the antimicrobial activities of nanoparticles against various marine fish pathogens. Use of synthetic antibiotics often results in the development of antibiotic resistance in bacteria. Thus, biogenic nanoparticles can be used as an effective alternative for controlling several aquatic pathogens. Similarly, use of probiotics as a biological control agent has greatly improved the performance of fishes, disease prevention, immune response enhancement, and quality of the water in recent years. A few probiotics that have been tested in vitro may become effective probiotics which can be produced commercially. However, their use in controlling viral diseases needs to be explored thoroughly.
There are number of emerging viral diseases that affect both fishes and shrimps. Vaccination is one of the methods for treating these viral infections. However, vaccinating each and every fish and shrimp is not a feasible method. Nowadays, natural methods of treatments such as use of extracts of medicinal plants and seaweeds are being used for treating the infections, in addition to the use of probiotics and nanoparticles. For controlling fish diseases, adjuvants and immunostimulants that are being used in the case of fish vaccines may be capable of acting as a substitute to antimicrobial agents. Extracts of medicinal plants can be used as an immunostimulant not only against several infectious diseases but also as infection preventers, growth promoters, and stress-resistant boosters. Plants being rich in a wide variety of phytochemical constituents and secondary metabolites like alkaloids, flavonoids, terpenoids, phenolics, tannins, glycoside, steroids, saponins, polysaccharides, volatile oils, proteoglycans, and essential oils act against various types of diseases. Similarly, some seaweeds have high nutritional value and also exhibit antioxidant, antimicrobial, and immunostimulatory activities in fishes. Antibiotics are being used for treatment but they cause resistance. In order to prevent this, natural methods of prevention and treatment can be adopted.
Our take on this review by anchoring on currently available data is that the key challenge which exists is the significant increase in the number of emerging fish and shellfish viral diseases and the limited options available for preventing these infections. The solution to this specific problem includes the use of antiviral drugs, actinomycetes, nanoparticles, probiotics, and medicinal plants to combat the emerging viral infections. Therefore, the use of a multifaceted approach that involves several preventive as well as control strategies may provide much more effective and long-lasting solutions to reduce these viral diseases in marine aquaculture as compared to using certain single disease-controlling approaches like antibiotics and vaccination. Further studies however are necessary to gain more information regarding the treatment methods used for different types of infectious diseases as it has become a key issue for the rapidly growing aquaculture industry.
Treatment using plant extracts with their intriguing applications in aquaculture have been very useful. Very little information is available on the mode of action of most medicinal plants as well the safest and suitable form for effective administration which remains a grey area. Moreover, in order to develop a proper treatment strategy, it is necessary to obtain traditional knowledge from the fish farmers who handle plants regularly. Similarly, use of algae and seaweeds, and their bioactive compounds can also be considered as an alternative to improve fitness of fish and shellfish. For the development of novel therapeutics or enhancement of the mode of action of therapeutics currently available, incorporation of nanoparticles can be highly effective. In addition, evolution in technology specifically exploiting the diverse and dynamic nature of nanomedicine is necessary for combating the infectious pathogens effectively. Bioactive compounds extracted from actinomycetes function as potential antiviral antibiotics and hence when incorporated in feed can lower viral infections in fish and shrimps. We can now foresee a future where various non-synthetic antiviral agents effective against viral infections in aquaculture will be developed.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Adachi K, Ichinose T, Takizawa N, Watanabe K, Kitazato K, Kobayashi N (2007) Inhibition OF Betanodavirus infection by inhibitors of endosomal acidification. Arch Virol 152:2217–2224
Alishahi A, Proulx J, Aider M (2014) Chitosan as biobased nanocomposite in seafood industry and aquaculture. Seafood science: advances in chemistry, technology & applications, pp 211–231.
Amend DF, Pietsch JP (1972) Virucidal activity of two iodophors to salmonid viruses. J Fish Res Board Can 29(1):61–65
Aseefa A, Abunna F (2018) Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet Med Int 1–10
Austin B, Austin D (2007) Bacterial fish pathogens: diseases of farmed and wild fish, 4th edn. Springer-Praxis Publishing Ltd., Weilheim
Balasubramanian G, Sudhakaran R, Syed Musthaq S, Sarathi M, Sahul Hameed AS (2006) Studies on the inactivation of white spot syndrome virus of shrimp by physical and chemical treatments, and seaweed extracts tested in marine and freshwater animal models. J Fish Dis 29:569–572
Balasubramanian G, Sarathi M, Rajesh Kumar S, Sahul Hameed AS (2007) Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture 263(1–4):15–19
Balasubramanian G, Sarathi M, Venkatesan C, Thomas J, Sahul Hameed AS (2008a) Oral administration of antiviral plant extract of Cynodon dactylon on a large scale production against white spot syndrome virus (WSSV) in Penaeus monodon. Aquaculture 279(1):2–5
Balasubramanian G, Sarathi M, Venkatesan C, Tomas J, Sahul Hameed AS (2008b) Studies on the immunomodulatory effect of extract of Cynodon dactylon in shrimp, Penaeus monodon, and its efficacy to protect the shrimp from white spot syndrome virus (WSSV). Fish Shellfish Immunol 25(6):820–828
Balboa EM, Conde E, Moure A, Falqué E, Domínguez H (2013) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138(2–3):1764–1785
Balcazar JL (2003) Evaluation of probiotic bacterial strains in Litopenaeus vannamei. Final report. National Center for Marine and Aquaculture Research. Guayaquil, Ecuador
Balcázar JL, de Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Mứzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114(3–4):173–186
Ballesteros NA, Alonso M, Saint-Jean SR, Perez-Prieto SI (2015) An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish Shellfish Immunol 45(2):877–888
Balmer BF, Powers RL, Zhang TH, Lee J, Vigant F, Lee B et al (2017) Inhibition of an aquatic rhabdovirus demonstrates promise of a broad-spectrum antiviral for use in aquaculture. J Virol 91:e02181-e2216
Balmer BF, Getchell RG, Powers RL, Lee J, Zhang T, Jung ME et al (2018) Broad-spectrum antiviral JL122 blocks infection and inhibits transmission of aquatic rhabdoviruses. Virology 525:143–149
Batts WN, Landolt ML, Winton JR (1991) Inactivation of infectious hematopoietic necrosis virus by low levels of iodine. Appl Environ Microbiol 57:1379–1385
Benmansour A, de Kinkelin P (1997) Live fish vaccines: history and perspectives. In: Fish vaccinology, developments in biological standardization. In: Gudding R, Lillehaug A, Midtlyng PJ, Brown F (eds). Karger, Basel, 90:279–289
Biering E, Villoing S, Sommerset I, Christie KE (2005) Update on viral vaccines for fish. Dev Biol 121:97–113
Bisesi JH, Ngo T, Ponnavolu S, Liu K, Lavelle CM, Nabiul-Afrooz ARM, Saleh NB, Ferguson PL, Denslow ND, Sabo-Attwood T (2015) Examination of single-walled carbon nanotubes uptake and toxicity from dietary exposure: tracking movement and impacts in the gastrointestinal system. Nanomaterials 5(2):1066–1086
Blumenthal M, Goldberg A, Brinckmann J, Foster S, Tyler VE (2000) Herbal medicine. Integrative Medicine Communications, Newton (Mass.)
Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ (2008) Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 15:997–1005
Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT (2006) Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog 2(7):e69
Cain KD, Byrne KM, Brassfield AL, LaPatra SE, Ristow SS (1999) Temperature dependent characteristics of a recombinant infectious hematopoietic necrosis virus glycoprotein produced in insect cells. Dis Aquat Organ 36(1):1–10
Camacho-Jiménez L, Álvarez-Sánchez AR, Mejía-Ruíz CH (2020) Silver nanoparticles (AgNPs) as antimicrobials in marine shrimp farming: a review. Aquacult Rep 18:100512
Chai PC, Song XL, Chen GF, Xu H, Huang J (2016) Dietary supplementation of probiotic Bacillus PC465 isolated from the gut of Fenneropenaeus chinensis improves the health status and resistance of Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish Immunol 54:602–611
Chakraborty SB, Hancz C (2011) Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev Aquac 3(3):103–119
Chalamcheria V (2015) Nano vaccines: new paradigm in aqua health sector. J Aquac Mar Biol 3(2):00061
Chatterjee S, Judeh ZM (2016) Impact of encapsulation on the physico-chemical properties and gastrointestinal stability of fish oil. LWT-Food Sci Technol 65:206–213
Chauhan A, Singh R (2019) Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis 77(23):99–113
Chavalittumrong PA, Rugsamon Attawish P, Chuntapet P (1995) Toxicological study of Clinacanthus nutans (Burm. F.) Lindau. Bull Dept Med Sci 37:323–338
Chen X, Hu Y, Shan L, Yu X, Hao K, Wang G (2017) Magnolol and honokiol from Magnolia officinalis enhanced antiviral immune responses against grass carp reovirus in Ctenopharyngodon idella kidney cells. Fish Shellfish Immunol 63:245–254
Chen WC, Hu Y, Liu L, Shen YF, Wang GX, Zhu B (2018a) Synthesis and in vitro activities evaluation of arctigenin derivatives against spring viraemia of carp virus. Fish Shellfish Immunol 82:17–26
Chen WC, Liu L, Shen YF, Hu Y, Ling F, Wang GX et al (2018b) A new coumarin derivative plays a role in rhabdoviral clearance by interfering glycoprotein function during the early stage of viral infection. Cell Signal 51:199–210
Chithambaran S, David S (2011) Red hogweed fights WSSV, promotes growth in tiger shrimp. Global Aquaculture Advocate, pp 1–5
Chiu CH, Cheng CH, Gua WR, Guu YK, Cheng W (2010) Dietary administration of the probiotic Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and diseases resistance of the grouper (Epinephelus coioides). Fish Shellfish Immunol 29(6):1053–1059
Chiu YH, Chan YL, Li TL, Wu CJ (2012) Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar Biotechnol 14(4):468–478
Cho SY, Kim HJ, Lan NT et al (2017) Oral vaccination through voluntary consumption of the convict grouper Epinephelus septemfasciatus with yeast producing the capsid protein of red-spotted grouper nervous necrosis virus. Vet Microbiol 204:159–164
Chotigeat W, Tongsupa S, Supamataya K, Phongdara A (2004) Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture 233:23–30
Cimica V, Galarza JM (2017) Adjuvant formulations for virus-like particle (VLP) based vaccines. J Clin Immunol 183:99–108
Cipriano RC, Novak BM, Flint DE, Cutting DC (2001) Reap-praisal of the federal fish health recommendation for disinfecting eggs of Atlantic salmon in iodophor. J Aquat Anim Health 13:320–327
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int 18(3):403–414
Citarasu T, Babu MM, Punitha SMJ, Venket RK, Marian MP (2001) Control of pathogenic bacteria using herbal biomedicinal products in the larviculture system of Penaeus monodon. International Conference on Advanced Technologies in Fisheries and Marine Sciences, MS University, India
Cordero H, Esteban MA, Cuesta A (2014) Use of probiotic bacteria against bacterial and viral infections in shellfish and fish aquaculture. Sustain Aquac Tech 239–255
Crane M, Hyatt A (2011) Viruses of fish: an overview of significant pathogens. Viruses 3(11):2025–2046
Dadar M, Dhama K, Vakharia VN et al (2016) Advances in aquaculture vaccines against fish pathogens: global status and current trends. Rev Fish Sci Aquac 25(3):184–217
De BC, Meena DK, Behera BK, Das P, Das Mohapatra PK, Sharma AP (2014) Probiotics in fish and shellfish culture: immunomodulatory and ecophysiological responses. Fish Physiol Biochem 40(3):921–971
de las Heras AI, Rodríguez Saint-Jean S, Pérez-Prieto SI (2008) Salmonid fish viruses and cell interactions at early steps of the infective cycle. J Fish Dis 31(7):535–546
Decamp O, Moriarty D (2007) Aquaculture species profit from probiotics. Feed Mix 15(1):20
Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14(3):251–258
Deng Y, Cheng Q (2003) Effects of nano-selenium on the growth of Nile tilapia (Oreochromis niloticus). Inland Aquat Prodd 6:28–30
Dhar AK, Manna SK, Allnutt FCT (2014) Viral vaccines for farmed finfsh. Virus Dis 25(1):1–17
Dinesh S, Manasi K, Vinodhini S, Vidhya G, Hemalatha K, Sudhakaran R (2014) Confirmation of anti-WSSV activity from red algae Hypnae spinella in freshwater crab Paratelphusa hydrodomous. Int J Chem Tech Res 8:4022–4026
Direkbusarakom S (1998a) Effect of Thai traditional herbs extracts against fish and shrimp pathogenic bacteria. J Fish Pathol 33:431–441
Direkbusarakom S (1998b) Studies on the antiviral and antibacterial activity of Thai traditional herbs and application to prevent the viral and bacterial diseases in aquatic animals. PhD thesis, Faculty of Fisheries, Hokkaido University, Japan, pp 176
Direkbusarakom S (2004) Application of medicinal herbs to aquaculture in Asia. Walailak J Sci Technol 1(1):7–14
Direkbusarakom S, Herunsalee A (1993). Investigation on the bioactive Thai medicinal plants to virus in tiger prawns. Conference on Marine Biotechnology in the Asian Pacific Region, Bangkok, 16 - 20 November, pp. 104- 6
Direkbusarakom S, Herunsalee A, Boonyaratpalin S, Danayadol Y, Aekpanithanpong U (1995) Effect of Phyllanthus spp against yellow-head baculovirus infection in black tiger shrimp, Penaeus monodon. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian aquaculture II. Fish Health Section. Asian Fisheries Society, Manila, pp 81–8
Direkbusarakom S, Herunsalee A, Yoshimizu M, Ezura Y (1996) Antiviral activity of several Thai traditional herbs extracts against fish pathogenic viruses. Fish Pathol 31(4):209e13
Direkbusarakom S, Herunsalee A, Yoshimizu M, Ezura Y, Kimura T (1997) Efficacy of guava (Psidium guajava) extract against some fish and shrimp pathogenic agents. Diseases in Asian Aquaculture III
Direkbusarakom S, Ruangpan L, Ezura Y, Yoshimizu M (1998a) Protective efficacy of Clinacanthus nutans on yellow-head disease in black tiger shrimp (Penaeus monodon). Fish Pathol 33(4):401–404
Direkbusarakom S, Yoshimizu M, Ezura Y, Ruangpan L, Danayadol Y (1998b) Vibrio spp. the dominant flora in shrimp hatchery against some fish pathogenic viruses. J Mar Biotechnol 6(4):266–267
Economon PP (1963) Experimental treatment of infectious pancreatic necrosis of brook trout with polyvinylpyrrolidone-iodine. Trans Am Fish Soc 92:180–182
Economon PP (1973) Polyvinylpyrrolidone-iodine as a control for infectious pancreatic necrosis of brook trout. Verhandlun-Gen Des Internationalen Verein Limnologie 18:1661–1665
Elliott DG, Amend DF (1978) Efficacy of certain disinfectants against infectious pancreatic necrosis virus. J Fish Biol 12:277–286
Emmenegger E, Huang C, Landolt M et al (1995) Immune response to synthetic peptides representing antigenic sites on the glycoprotein of infectious hematopoietic necrosis virus. Vet Res 26:374–8
Estepa A, Thiry M, Coll JM (1994) Recombinant protein fragments from haemorrhagic septicaemia rhabdovirus stimulate trout leukocyte anamnestic responses in vitro. J Gen Virol 75(6):1329–1338
Evensen Ø, Leong JAC (2013) DNA vaccines against viral diseases of farmed fish. Fish Shellfish Immunol 35(6):1751–1758
Faiz H, Zuberi A, Nazir S, Rauf M, Younus N (2015) Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int J Agric Biol 17(3):568–574
FAO (2008) State of World Fisheries and Aquaculture (SOFIA). Food and Agricultural Organisation of the United Nations, Rome
FAO (2009) Fishstat Plus. Food and Agricultural Organisation of the United Nations, Rome
FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action, Rome. https://doi.org/10.4060/ca9229en
Fraser TW, Reinardy HC, Shaw BJ, Henry TB, Handy RD (2011) Dietary toxicity of single-walled carbon nanotubes and fullerenes (C60) in rainbow trout (Oncorhynchus mykiss)”. Nanotoxicology 5(1):98–108
Fredrickson WR (2000) Method and composition for antiviral therapy with olive leaves. U.S. patent no 6,117,844
Ganesan P, Reegan AD, David RHA, Gandhi MR, Paulraj MG, Al-Dhabi NA, Ignacimuthu S (2017) Antimicrobial activity of some actinomycetes from Western Ghats of Tamil Nadu, India. Alex J Med 53(2):101–110
Gao Q, Yang M, Zuo Z (2018) Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol Sin 39:787–801
Ghosh T, Pujol CA, Damonte EB, Sinha S, Ray B (2009) Sulfated xylomannans from the red seaweed Sebdenia polydactyla: structural features, chemical modification and antiviral activity. Antivir Chem Chemother 19(6):235–242
Guo CJ, Wu YY, Yang LS, Yang XB, He J, Mi S et al (2012) Infectious spleen and kidney necrosis virus (a fish iridovirus) enters Mandarin fish fry cells via caveola-dependent endocytosis. J Virol 86:2621–2631
Harikrishnan R, Balasundaram C, Heo MS (2011) Fish health aspects in grouper aquaculture. Aquaculture 320(1):1–21
Harikrishnan R, Balasundaram C, Heo MS (2010a) Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol 29(5):868–874
Harikrishnan R, Balasundaram C, Heo MS (2010b) Herbel supplemention diet on haematology and innate immunity in goalfish against Aeromonas hydrophila. Fish Shellfish Immunol 28(2):354–361
Harikrishnan R, Heo J, Balasundaram C, Kim MC, Kim JS, Han YJ, Heo MS (2010c) Effect of Punica granatum solvent extracts on immune system and disease resistance in Paralichthys olivaceus against lymphocystis disease virus (LDV). Fish Shellfish Immunol 29:668–673
Hasan KN, Banerjee G (2020) Recent studies on probiotics as beneficial mediator in aquaculture: a review. J Basic Appl Zool 81(1):1–16
Hasobe M, Saneyoshi M (1985a) A new method for the evaluation of antiviral agents against infectious hematopoietic necrosis virus (IHNV) on microtiter plates: CPE spot reduction method. Bull Jpn Soc Sci Fish 51(7):1079–1084
Hasobe M, Saneyoshi M (1985b) On the approach to the viral chemotherapy against infectious hematopoietic necrosis virus (IHNV) in vitro and in vivo on salmonid fishes. Fish Pathol 20(2/3):343–351
Hermosillo OAM, Mart P, Ib AL, Ram HC (2012) Use of probiotics in aquaculture. Int Sch Res Notices 2012:13 Article ID 916845
Hoseinifar SH, Sun Y-Z, Wang A, Zhou Z (2018) Probiotics as means of disease control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol 9:2429
Hossain MS, Chakraborty A, Joseph B, Otta SK, Karunasagar I, Karunasagar I (2001) Detection of new hosts for white spot syndrome virus of shrimp using nested polymerase chain reaction. Aquaculture 198:1–11
Hu Y, Liu L, Li B, Shen Y, Wang GX, Zhu B (2019a) Synthesis of arctigenin derivatives against infectious hematopoietic necrosis virus. Eur J Med Chem 163:183–194
Hu Y, Chen WC, Shen YF, Zhu B, Wang GX (2019b) Synthesis and antiviral activity of a new arctigenin derivative against IHNV in vitro and in vivo. Fish Shellfish Immunol 92:736–745
Huang S, Wang L, Liu L, Hou Y, Li L (2015) Nanotechnology in agriculture, livestock, and aquaculture in China. A Rev Agron Sustain Dev 35:369–400
Huang R, Zhu G, Zhang J, Lai Y, Xu Y, He J et al (2017) Betanodavirus-like particles enter host cells via clathrin-mediated endocytosis in a cholesterol-, pH- and cytoskeleton-dependent manner. Vet Res 48:8
Huang H-T, Lin H-J, Huang H-J, Huang C-C, Lin JH-Y, Chen L-L (2020) Synthesis and evaluation of polyamine carbon quantum dots (CQDs) in Litopenaeus vannamei as a therapeutic agent against WSSV. Sci Rep 10:7343
Ichinose T, Musyoka TM, Watanabe K, Kobayashi N (2013) Evaluation of antiviral activity of oligonol, an extract of Litchi chinensis, against betanodavirus. Drug Discov Ther 7:254–326
Immanuel G, Vincybai VC, Sivaram V, Palavesam A, Marian MP (2004) Effect of butanolic extracts from terrestrial herbs and seaweeds on the survival, growth and pathogen (Vibrio parahaemolyticus) load on shrimp Penaeus indicus juveniles. Aquaculture 236(1):53–65
Immanuel G, Sivagnanavelmurugan M, Balasubramanian V, Palavesam A (2010) Effect of hot water extracts of brown seaweeds Sargassum spp. on growth and resistance to white spot syndrome virus in shrimp Penaeus monodon postlarvae. Aquac Res 41:e545–e553
Immanuel G, Sivagnanavelmurugan M, Balasubramanian V, Palavesam A (2012) Sodium alginate from Sargassum wightii retards mortalities in Penaeus monodon postlarvae challenged with white spot syndrome virus. Dis Aquat Org 99:187–196
Jaemwimol P, Sirikanchana K, Tattiyapong P, Mongkolsuk S, Surachetpong W (2019) Virucidal effects of common disinfectants against tilapia lake virus. J Fish Dis 42(10):1383–1389
Jakubiec-Krzesniak K, Rajnisz-Mateusiak A, Guspiel A, Ziemska J, Solecka J (2018) secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Pol J Microbiol 67(3):259–272
Jeong EH, Vaidya B, Cho SY, Park MA, Kaewintajuk K, Kim SR et al (2015) Identification of regulators of the early stage of viral hemorrhagic septicemia virus infection during curcumin treatment. Fish Shellfish Immunol 45:184–193
Jia K, Yuan Y, Liu W, Liu L, Qin Q, Yi M (2018) Identification of inhibitory compounds against Singapore grouper iridovirus infection by cell viability-based screening assay and droplet digital PCR. Mar Biotechnol 20:35–44
Jiao G, Yu G, Wang W, Zhao X, Zhang J, Ewart SH (2012) Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J Ocean Univ China 11(2):205–212
Ji J, Torrealba D, Ruyra À, Roher N (2015) Nanodelivery systems as new tools for immunostimulant or vaccine administration: targeting the fish immune system. Biology 4(4):664–696
Jiménez-Fernández E, Ruyra A, Roher N, Zuasti E, Infante C, Fernández-Díaz C (2014) Nanoparticles as a novel delivery system for vitamin C administration in aquaculture. Aquaculture 432:426–433
Joshi A, Pathak DC, Mannan MA, Kaushik V (2021) In-silico designing of epitope-based vaccine against the seven banded grouper nervous necrosis virus affecting fish species. Netw Model Anal Health Inform Bioinform 10:37. https://doi.org/10.1007/s13721-021-00315-5
Juarez-Moreno K, Mejía-Ruiz CH, Díaz F, Reyna-Verdugo H, Denisse A, Vazquez-Felix EF, Bogdanchikova N (2017) Effect of silver nanoparticles on the metabolic rate, hematological response, and survival of juvenile white shrimp Litopenaeus vannamei. Chemosphere 169:716–724
Kamei Y, Aoki M (2007) A chlorophyll c2 analogue from the marine brown alga Eisenia bicyclis inactivates the infectious hematopoietic necrosis virus, a fish rhabdovirus. Arch Virol 152:861–869
Kamei Y, Yoshimizu M, Ezura Y, Kimura T (1988) Screening of bacteria with antiviral activity from fresh water salmonid hatcheries. Microbiol Immunol 32(1):67–73
Kang SY, Kang JY, Oh MJ (2012) Antiviral activities of flavonoids isolated from the bark of Rhus verniciflua stokes against fish pathogenic viruses in vitro. J Microbiol 50(2):293–300
Kang SY, Kim SR, Oh MJ (2008) In vitro antiviral activities of Korean marine algae extracts against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. Food Sci Biotechnol 17:1074–1078
Kelly DF, Rappuoli R (2005) Reverse vaccinology and vaccines for serogroup B Neisseria meningitidis. In: Pollard AJ, Finn A (eds) Hot topics in infection and immunity in children, 2nd edn. Springer, Boston, pp 217–223
Khanna GV, Kannabiran K, Babu VS, Hameed ASS (2011) Inhibition of fish nodavirus by gymnemagenol extracted from Gymnema sylvestre. J Ocean Univ China 10(4):402–408
Khosravi-Katuli K, Prato E, Lofrano G, Guida M, Vale G, Libralato G (2017) Effects of nanoparticles in species of aquaculture interest. Environ Sci Pollut Res 24:17326–17346
Kim C, Johnson M, Drennan J et al (2000) DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J Virol 74(15):7048–7054
Kim SY, Kim SR, Oh MJ, Jung SJ, Kang SY (2011) In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J Microbiol 49(1082):102–106
Klinkesorn U, McClements DJ (2009) Influence of chitosan on stability and lipase digestibility of lecithin-stabilized tuna oil-in-water emulsions. Food Chem 114(4):1308–1315
Klongklaew N, Praiboon J, Tamtin M, Srisapoome P (2020) Antibacterial and antiviral activities of local Thai Green macroalgae crude extracts in Pacific white shrimp (Litopenaeus vannamei). Mar Drugs 18(3):140
Klongklaew N, Praiboon J, Tamtin M, Srisapoome P (2021) Chemical composition of a hot water crude extract (HWCE) from Ulva intestinalis and its potential effects on growth performance, immune responses, and resistance to white spot syndrome virus and yellowhead virus in Pacific white shrimp (litopenaeus vannamei). Fish Shellfish Immunol 112:8–22
Kolkovski S, Kolkovski J (2011) Herbal medicine: herbal medicine in aquaculture. International aquafeed, Nutrakol Pty Ltd
Kulkarni A, Krishnan S, Anand D, Uthaman SK, Otta SK, Karunasagar I et al (2021) Immune responses and immunoprotection in crustaceans with special reference to shrimp. Rev Aquac 13:431–459
Kurcheti PP, Dhayanath D, Mary AJ, Jeena J, Alim H (2020) Biosynthesis of nanoparticles - a new horizon in fish biomedicine. J Aquac Fisheries 4(2):1–3
Kushankar Dey (2020) India’s Blue Economy net getting bigger. Financial Express. 14 February 2020. Retrieved 18 July 2020
Lakshmi B, Viswanath B, Sai Gopal DVR (2013) Probiotics as antiviral agents in shrimp aquaculture. J Pathog 2013:424123
Lam KS (2006) Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol 9(3):245–251
Lecocq-Xhonneux F, Thiry M, Dheur I, Rossius M, Vanderheijden N, Martial J, De Kinkelin P (1994) A recombinant viral haemorrhagic septicaemia virus glycoprotein expressed in insect cells induces protective immunity in rainbow trout. J Gen Virol 75(7):1579–1587
Lee C (2019) Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: from discovery to clinical application. Mar Drugs 17(10):567
Levya-Madrigal KY, Luna-González A, Escobedo-Bonilla CM, Fierro-Coronado JA, Maldonado-Mendoza IE (2011) Screening for potential probiotic bacteria to reduce prevalence of WSSV and IHHNV in whiteleg shrimp (Litopenaeus vannamei) under experimental conditions. Aquaculture 322–323(1):16–22
Li J, Tan B, Mai K (2009) Dietary probiotic bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp (Litopenaeus vannamei). Aquaculture 291(1):35–40
Li Y, Sun S, Pu X, Yang Y, Zhu F, Zhang S, Xu N (2018) Evaluation of antimicrobial activities of seaweed resources from Zhejiang coast, China. Sustainability 10(7):2158
Lillehaug A (2014) Vaccination strategies and procedures. In: Gudding R, Lillehaug A, Evensen Ø (eds) Fish vaccination, 1st edn. Wiley, Oxford, pp 141–150
Lin Y, Cheng G, Wang X, Clark TG (2002) The use of synthetic genes for the expression of ciliate proteins in heterologous systems. Gene 288:85–94
Lin Y, Yeh S, Li C, Chen L, Cheng A, Chen J (2011) An immersion of Gracilaria tenuistipitata extract improves the immunity and survival of white shrimp Litopenaeus vannamei challenged with white spot syndrome virus. Fish Shellfish Immunol 31(6):1239–1246
Liu CH, Chiu CH, Wang SW, Cheng W (2012) Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, epinephelus coioides. Fish Shellfish Immunol 33(4):699–706
Liu L, Hu Y, Shen YF, Wang GX, Zhu B (2017) Evaluation on antiviral activity of coumarin derivatives against spring viraemia of carp virus in Epithelioma papulosum cyprini cells. Antivir Res 144:173–185
Liu L, Shen YF, Hu Y, Lu JF (2018) Antiviral effect of 7-(4-benzimidazole-butoxy)-coumarin on rhabdoviral clearance via Nrf2 activation regulated by PKCa/b phosphorylation. Fish Shellfish Immunol 83:386–396
Liu L, Hu Y, Lu J, Wang G (2019) An imidazole coumarin derivative enhances the antiviral response to spring viremia of carp virus infection in zebrafish. Virus Res 263:112–118
Liu L, Qiu TX, Song DW, Shan LP, Chen J (2020) Inhibition of a novel coumarin on an aquatic rhabdovirus by targeting the early stage of viral infection demonstrates potential application in aquaculture. Antivir Res 174:104672
Lo CF, Ho CH, Peng SE, Chen CH, Hsu HC, Chiu YL, Chang CF, Liu KF, Su MS, Wang CH et al (1996) White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis Aquat Org 27:215–225
Logambal SM, Venkatalakshmi S, Michael RD (2000) Immunostimulatory effect of leaf extract of Ocimum sanctum Linn. in Oreochromis mossambicus (Peters). Hydrobiologia 430(1):113–120
Lopez-Doriga MV, Smail DA, Smith RJ et al (2001) Isolation of salmon pancreas disease virus (SPDV) in cell culture and its ability to protect against infection by the wild-type agent. Fish Shellfish Immunol 11(6):505–522
Lorenzen N, Olesen NJ (1995) Multiplication of VHS virus in insect cells. Vet Res 26(5–6):428–432
Lozano I, Wacyk JM, Carrasco J, Cortez-San Martín MA (2016) Red macroalgae Pyropia columbina and Gracilaria chilensis: sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. J Appl Phycol 28:1343–1351
Ma J, Bruce TJ, Jones EM, Cain KD (2019) A review of fish vaccine development strategies: conventional methods and modern biotechnological approaches. Microorganisms 7(11):569
Maeda M, Nogami K, Kanematsu M, Hirayama K (1997) The concept of biological control methods in aquaculture. Hydrobiologia 358:285–290
Manilal A, Sujith S, Selvin J, Seghal Kiran G, Shakir C (2009) In vivo antiviral activity of polysaccharide from the Indian green alga, Acrosiphonia orientalis (J. Agardh): potential implication in shrimp disease management. World J Fish Mar Sci 1:278–282
Maqsood S, Singh P, Samoon MH, Munir K (2011) International Aquatic Research emerging role of immunostimulants in combating the disease outbreak in aquaculture. Int Aquat Res 3:147–163
Mastan SA (2015) Use of immunostimulants in aquaculture disease management. Int J Fish Aquat Stud 2(4):277–280
McLoughlin M (2006) Fish vaccination—a brief overview. http://www.imb.ie/images/uploaded/documents/Fish%20Vaccine%20Overview.pdf. Accessed 14 May 2014
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RT, Bøgwald J et al (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302(1–2):1–18
Micol V, Caturla N, Pérez-Fons L, Más V, Pérez L, Estepa A (2005) The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV). Antivir Res 66(2–3):129–136
Modak B, Sandino AM, Arata L, Cardenas-Jiron G, Torres R (2010) Inhibitory effect of aromatic geranyl derivatives isolated from Heliotropium filifolium on infectious pancreatic necrosis virus replication. Vet Microbiol 141:53–58
Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K (2014) A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int 2014:186864
Mohamed S, Hashim SN, Rahman HA (2012) Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci Technol 23(2):83–96
Mori T, O’Keefe BR, Sowder RC, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, McMahon JB et al (2005) Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem 280(10):9345–9353
Moussa HS, Ibrahem ABB, El-Sayed AFM, Mohammed FA (2015) In vitro evaluation of anti-microbial activities of marine Streptomyces against viral models, bacterial and fungal strains. Int J Virol 11(1):20–31
Munang’andu HM, Mutoloki S, Evensen Ø (2016) Prevention and control of viral diseases in aquaculture. Aquac Virol 77–93
Munday BL, Kwang J, Moody N (2002) Betanodavirus infections of teleost fish: a review. J Fish Dis 25(3):127–142
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Manickam N, Srinivasan V (2014) Dietary supplementation of zinc nanoparticles and its influence on biology, physiology and immune responses of the freshwater prawn, Macrobrachium rosenbergii. Biol Trace Elem Res 160(1):56–66
Muroga K (2001) Viral and bacterial diseases of marine fish and shellfish in Japanese hatcheries. Aquaculture 202(1):23–44
Mweemba H, Mutoloki S, Øystein E (2014) Non replicating vaccines. In: Gudding R, Lillehaug A, Evensen Ø (eds) Fish vaccination, 1st edn. Wiley, Oxford, pp 22–29
Nageswara PV, Babu DE (2006) Probiotics as an alternative therapy to minimize or avoid antibiotics use in aquaculture. Fish Chimes 26(1):112–114
Ninawe AS, Hameed ASS, Selvin J (2017) Advancements in diagnosis and control measures of viral pathogens in aquaculture: an Indian perspective. Aquac Int 25(1):251–264
Ochoa-Meza AR, Alvarez-Sánchez AR, Romo-Quiñonez CR, Barraza A, Toledano-Magaña Y, Bogdanchikova N, Pestryakov A (2019) Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol 84:1083–1089
Olusola SE, Emikpe BO, Olaifa FE (2013) The potentials of medicinal plants extracts as bioantimicrobial in aquaculture. Int J Med Aromat Plants 3(3):404–412
Pandey G, Sharma M (2012) Medicinal plants useful in fish diseases. Plant Arch 12(1):1–4
Park YJ, Moon C, Kang JH, Choi TJ (2017) Antiviral effects of extracts from Celosia cristata and Raphanus sativus roots against viral hemorrhagic septicemia virus. Arch Virol 162:1711–1716
Partida-Arangure BO, Luna-González A, Fierro-Coronado JA, Flores-Miranda C, González-Ocampo HA (2013) Effect of inulin and probiotic bacteria on growth, survival, immune response, and prevalence of white spot syndrome virus (WSSV) in Litopenaeus vannamei cultured under laboratory conditions. Afr J Bio 12(21):3366–3375
Peniche C, Howland I, Carrillo O, Zaldıvar C, Argüelles-Monal W (2004) Formation and stability of shark liver oil loaded chitosan/calcium alginate capsules. Food Hydrocoll 18:865–871
Pereiro P, Figueras A, Novoa B (2020) Compilation of antiviral treatments and strategies to fight fish viruses. Rev Aquac 13(3):1–32
Pinto AMV, Leite JPG, Ferreira WJ, Cavalcanti DN, Villaça RC, Giongo V, Teixeira VL, Paixão ICND (2012) Marine natural seaweed products as potential antiviral drugs against Bovine viral diarrhea virus. Rev Bras Farmacogn 22(4):813–817
Plant KP, LaPatra SE (2011) Advances in fish vaccine delivery. Dev Comp Immunol 35(12):1256–1262
Plouguerné E, de Souza LM, Sassaki GL, Cavalcanti JF, Villela Romanos MT, da Gama BA, Pereira RC, Barreto-Bergter E (2013) Antiviral sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar Drugs 11(11):4628–4640
Pridgeon JW, Klesius PH (2012) Major bacterial diseases in aquaculture and their vaccine development CAB Reviews: Perspectives in agriculture veterinary science. Nutr Nat Resour 7(48):1–16
Qiu TX, Song DW, Shan LP, Liu GL, Liu L (2020) Potential prospect of a therapeutic agent against spring viraemia of carp virus in aquaculture. Aquaculture 515:734558
Rajeshkumar S, Venkatesan C, Sarathi M, Sarathbabu V, Thomas J, Basha KA, Hameed AS (2009) Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish Shellfish Immunol 26(3):429–437
Rameshthangam P, Ramasamy P (2007) Antiviral activity of bis(2-methylheptyl)phthalate isolated from Pongamia pinnata leaves against white spot syndrome virus of Penaeus monodon Fabricius. Virus Res 126(1–2):38–44
Ramsden CS, Smith TJ, Shaw BJ, Handy RD (2009) Dietary exposure to titanium dioxide nanoparticles in rainbow trout (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology 18(7):939–951
Ramya VL, Sharma R, Gireesh-Babu P, Patchala SR, Rather A, Nandanpawar PC, Eswaran S (2014) Development of chitosan conjugated DNA vaccine against nodavirus in Macrobrachium rosenbergii (De Man, 1879). J Fish Dis 37(9):815–824
Rao BM (2020) Chemicals and drugs used in shrimp aquaculture. https://www.researchgate.net/publication/340266772_CHEMICALS_AND_DRUGS_USED_IN_SHRIMP_AQUACULTURE. Accessed March 2020
Reichert M, Bergmann SM, Hwang J, Buchholz R, Lindenberger C (2017) Antiviral activity of exopolysaccharides from Arthrospira platensis against koi herpesvirus. J Fish Dis 40:1441–1450
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433:50–61
Riccio G, Ruocco N, Mutalipassi M, Costantini M, Zupo V, Coppola D, de Pascale D, Lauritano C (2020) Ten-year research update review: antiviral activities from marine organisms. Biomolecules 10(7):1007
Rodríguez J, Espinosa Y, Echeverria F, Cárdenas G, Román R, Stern S (2007) Exposure to probiotics and β-1,3/1,6-glucans in larviculture modifies the immune response of Penaeus vannamei juveniles and both the survival to white spot syndrome virus challenge and pond culture. Aquaculture 273(4):405–415
Romo-Quiñonez CR, Alvarez-Sánchez AR, Álvarez-Ruiz P, Chávez-Sánchez MC, Bogdanchikova N, Pestryakov A (2020) Evaluation of a new Argovit as an antiviral agent included in feed to protect the shrimp Litopenaeus vannamei against white spot syndrome virus infection. PeerJ 8:1–22
Ronen A, Perelberg A, Abramowitz J et al (2003) Efficient vaccine against the virus causing a lethal disease in cultured Cyprinus carpio. Vaccine 21(32):4677–4684
Ross KA, Loyd H, Wu W, Huntimer L, Wannemuehler MJ, Carpenter S, Narasimhan B (2014) Structural and antigenic stability of H5N1 hemagglutinin trimer upon release from polyanhydride nanoparticles. J Biomed Mater Res A 102(11):4161–4168
Saha S, Navid MH, Bandyopadhyay SS, Schnitzler P, Ray B (2012) Sulfated polysaccharides from Laminaria angustata: structural features and in vitro antiviral activities. Carbohydr Polym 87(1):123–130
Sahu MK, Swarnakumar NS, Sivakumar K, Thangaradjou T, Kannan L (2008) Probiotics in aquaculture: importance and future perspectives. Indian J Microbiol 48(3):299–308
Sánchez-Martínez JG, Aguirre-Guzmán G, Mejía-Ruíz CH (2007) White spot syndrome virus in cultured shrimp: a review. Aquac Res 38:1339–1354
Scott CJW, Morris PC, Austin B (2011) Cellular, molecular, genomics, and biomedical approaches. Mol Fish Pathol 3:2032–2045
Shaalan M, Saleh M, El-Mahdy M, El-Matbouli M (2016) Recent progress in applications of nanoparticles in fish medicine: a review. Nanomed Nanotechnol Biol Med 12(3):701–710
Shah BR, Mraz J (2019) Advances in nanotechnology for sustainable aquaculture and fisheries. Rev Aquac 12(2):1–18
Shamsuzzaman MM, Biswas TK (2012) Aqua chemicals in shrimp farm: a study from south-west coast of Bangladesh. Egypt J Aquat Res 38(4):275–285
Sharifuzzaman SM, Austin B (2017) Probiotics for disease control in aquaculture shellfish diagnosis and control of diseases of fish and shellfish, 1st edn. Wiley, Oxford, pp 189–222
Shen YF, Liu L, Feng CZ, Hu Y, Chen C, Wang GX et al (2018a) Synthesis and antiviral activity of a new coumarin derivative against spring viraemia of carp virus. Fish Shellfish Immunol 81:57–66
Shen YF, Liu L, Chen WC, Hu Y, Zhu B, Wang GX (2018b) Evaluation on the antiviral activity of arctigenin against spring viraemia of carp virus. Aquaculture 483:252–262
Shi Q, Wang A, Lu Z, Qin C, Hu J, Yin J (2017) Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr Res 453–454:1–9
Shoemaker CA, Klesius PH, Evans JJ, Arias CR (2009) Use of modified live vaccines in aquaculture. J World Aquac Soc 40(5):573–585
Sirirustananun N, Chen J, Lin Y, Yeh S, Liou C, Chen L, Sim SS, Chiew SL (2011) Dietary administration of a Gracilaria tenuistipitata extract enhances the immune response and resistance against Vibrio alginolyticus and white spot syndrome virus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 31(6):848–855
Sivagnanavelmurugan M, Marudhupandi T, Palavesam A, Immanuel G (2012) Antiviral effect of fucoidan extracted from the brown seaweed, Sargassum wightii, on shrimp Penaeus monodon postlarvae against white spot syndrome virus. J World Aquac Soc 43:697–706
Sivasankar P, Anix Vivek Santhiya A, Kanaga V (2015) A review on plants and herbal extracts against viral diseases in aquaculture. J Med Plants Stud 3(2):75–79
Soares AR, Robaina MCS, Mendes GS, Silva TSL, Gestinari LMS, Pamplona OS, Yoneshigue-Valentin Y, Kaiser CR, Romanos MTV (2012) Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Rev Bras Farmacogn 22(4):714–723
Sommerset A, Krossøy B, Biering E, Frost P (2005) Vaccines for fish aquaculture. Expert Rev Vaccines 4(1):89–101
Son VM, Chang CC, Wu MC, Guu YK, Chiu CH, Cheng W (2009) Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol 26(5):691–698
Son M, Lee M, Sung GH, Lee T, Shin YS, Cho H, Lieberman PM, Kang H (2013) Bioactive activities of natural products against herpesvirus infection. J Microbiol 51(5):545–551
Sonkusre P, Nanduri R, Gupta P, Cameotra SS (2014) Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention. J Nanomed Nanotechnol 5(2):1–9
Sree Kumar SS, Philip R, Achuthankutty CT (2006) Antiviral property of marine actinomycetes against white spot syndrome virus in penaeid shrimps. Curr Sci 91(6):807–811
Sriwilaijaroen N, Wilairat P, Hiramatsu H, Takahashi T, Suzuki T, Ito M et al (2009) Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities. Virol J 6:124
Strømme M, Brohede U, Atluri R, Garcia-Bennett AE (2009) Mesoporous silica-based nanomaterials for drug delivery: evaluation of structural properties associated with release rate. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1(1):140–148
Sun Z-C, Chen C, Xu F-F, Li B-K, Shen J-L, Wang T, Jiang H-F, Wang G-X (2021) Evaluation of the antiviral activity of naringenin, a major constituent of Typha angustifolia, against white spot syndrome virus in crayfish Procambarus clarkia. J Fish Dis. https://doi.org/10.1111/jfd.13472?af=R
Sushila N, Hameed ASS, Prasad KP, Majeed SA, Tripathi G (2018) In vitro screening of selected antiviral drugs against betanodavirus. J Virol Methods 259:66–73
Takahashi Y, Uehara K, Watanabe R, Okumura T, Yamashita T, Omura H, Yomo T, Kawano T, Kanemitsu A, Narasaka H, Suzuki N, Itami T (1998) Efficacy of oral administration of fucoidan, a sulfated polysaccharide, in controlling white spot syndrome in kuruma shrimp in Japan. In: Flegel TW (ed) Advances in shrimp biotechnology. National Center for Genetic Engineering and Biotechnology, Bangkok, pp 171–173
Tesarčík J, Svobodová Z (1991) Diagnostics, prevention and therapy of fish diseases and intoxications, manual for international training course on fresh-water fish diseases and intoxications: diagnostics, prophylaxis and therapy
Valenzuela B, Rodrıguez FE, Modak B, Imarai M (2018) Alpinone exhibited immunomodulatory and antiviral activities in Atlantic salmon. Fish Shellfish Immunol 74:76–83
Valero Y, Olveira JG, Vázquez CL, Dopazo CP, Bandin I (2021) BEI inactivated vaccine induces innate and adaptive responses and elicits partial protection upon reassortant betanodavirus infection in Senegalese sole. Vaccines 9(5):458
Vallejos-Vidal E, Reyes-Lopez F, MacKenzie S (2014) Immunostimulant diets and oral vaccination in fish. Diagnosis and control of diseases of fish and shellfish. Austin B, Newaj-Fyzul A (eds), Wiley, Oxford, pp. 77–89
Vatsos IN, Rebours C (2014) Seaweed extracts as antimicrobial agents in aquaculture. J Appl Phycol 27(5):2017–2035
Velmurugan S, Babu MS, Punitha SMJ, Viji VT, Citarasu T (2012) Screening and characterization of antiviral compounds from Psidium guajava Linn. Root bark against white spot syndrome virus. Indian J Nat Prod Resour 3(2):208–214
Vigant F, Lee J, Hollmann A, Tanner LB, Ataman ZA, Yun T et al (2013) A mechanistic paradigm for broad-spectrum antivirals that target virus-cell fusion. PLoS Pathog 9:e1003297
Walker PJ, Winton JR (2010) Emerging viral diseases of fish and shrimp. Vet Res 41(6):51
Wang W, Wang SX, Guan HS (2012) The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs 10(12):2795–2816
Wang Y, Yan X, Fu L (2013) Effect of selenium nanoparticles with different sizes in primary cultured intestinal epithelial cells of crucian carp, Carassius auratus gibelio. Int J Nanomed 8(1):4007–4013
Yang HK, Jung MH, Avunje S, Nikapitiya C, Kang SY, Ryu YB et al (2018) Efficacy of algal Ecklonia cava extract against viral hemorrhagic septicemia virus (VHSV). Fish Shellfish Immunol 72:273–281
Yeh S-P, Chang C-A, Chang C-Y, Liu C-H, Cheng W (2008) Dietary sodium alginate administration affects fingerling growth and resistance to Streptococcus sp. and iridovirus, and juvenile non-specific immune responses of the orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol 25(1–2):19–27
Yu XB, Liu GL, Zhu B, Hao K, Ling F, Wang GX (2014) In vitro immunocompetence of two compounds isolated from Polygala tenuifolia and development of resistance against grass carp reovirus (GCRV) and Dactylogyrus intermedius in respective host. Fish Shellfish Immunol 41:541–548
Yu Q, Liu M, Xiao H, Wu S, Qin X, Lu Z et al (2019) The inhibitory activities and antiviral mechanism of Viola philippica aqueous extracts against grouper iridovirus infection in vitro and in vivo. J Fish Dis 42:859–868
Zeng W, Wang Y, Hu H, Wang Q, Bergmann SM, Wang Y, Li B, Lv Y, Li H, Yin J, Li Y (2021) Cell culture-derived tilapia lake virus-inactivated vaccine containing Montanide adjuvant provides high protection against viral challenge for tilapia. Vaccines 9:86
Zhou X, Wang Y, Gu Q, Li W (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291(1):78–81
Zorriehzahra MJ, Delshad ST, Adel M, Tiwari R, Karthik K, Dhama K, Lazado CC (2016) Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review. Vet Q 36(4):228–241
Acknowledgements
The authors are thankful to VIT, Vellore, for providing the required facilities to carry out this work.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mondal, H., Chandrasekaran, N., Mukherjee, A. et al. Viral infections in cultured fish and shrimps: current status and treatment methods. Aquacult Int 30, 227–262 (2022). https://doi.org/10.1007/s10499-021-00795-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00795-2