Abstract
Growth of fed aquaculture is dependent on finding sustainable and inexpensive ingredients that would satisfy the nutritional requirements of fish. The present work assessed restaurant post-consumer food waste as an ingredient in feeds for small (ca 6.5 g) rainbow trout, Oncorhynchus mykiss. Food waste from Lebanese-food restaurants was used to prepare seven iso-nitrogenous feeds (ca 42.5% protein, 18% lipid) by replacing soybean meal (SBM), soy oil, and whole wheat in a control diet. Seven diets containing 0, 5, 10, 15, 20, 25, and 30% food waste were made. Fish were stocked in triplicate 52-L tanks (15 fish per tank) and offered the feeds at 4% body weight daily for 8 weeks. Growth, hematology, and proximate chemical composition of the fish were assessed. Results show that food waste can be incorporated as 25% of the diet without affecting growth of O. mykiss. The best growth (ca 317% from initial stocking weight) was observed in fish offered feed with 20% food waste. Feed treatment did not significantly affect hematology, but significantly affected body composition. Total fish body protein proportion was greater in the treatment offered 30% food waste feed than in the control. There seems to be a potential for using food waste as an ingredient in trout feed, and this could mitigate the environmental consequences of disposal of food waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean meal (SBM) is the most prominent plant-based protein source used in aquaculture, livestock, and poultry production. In aquaculture, SBM is often used to partially replace fishmeal (Refstie et al. 1997; Choi et al. 2004; Voorhees et al. 2019) because soybeans are rich in proteins and have a sufficiently balanced amino acid profile (Dozier and Hess 2011) although deficient in methionine. However, because of the competitive and increasing demand on SBM, prices have been increasing. In fact, in the past 20 years, prices of SBM have increased by 90.49% (Indexmundi 2020), rendering it less attractive for fish farmers, especially in developing countries. Therefore, it is important to find sustainable and cost-effective protein sources that could supplement SBM in aquafeed. Researchers have investigated replacing SBM by other plant-based products such as cottonseed meal (Robinson and Li 1994; El-Saidy and Saad 2011), peanut meal (da Silva et al. 2017), and canola meal (Lim et al. 1998; Zhou and Yue 2010). However, oilseeds often contain antinutritional factors and lack some essential amino acids (Li and Robinson 2015). Moreover, oilseeds are not necessarily produced in countries with aquaculture and thus need to be imported. A dietary ingredient that could be used in fish feed without many of the drawbacks associated with oil seeds is restaurant plate food waste.
Food waste (FW) is human food that is lost or discarded at the retailers’ or consumers’ levels (FAO 2011). In 2011, FAO estimated that one-third of the food destined for human consumption is lost or wasted every year. Wasted food accounts for 6% of the global greenhouse gas (GHGs) emissions (Ritchie 2020). Therefore, FAO (2014 a) suggested several strategies to mitigate the environmental effects of FW. One of the most environmentally friendly strategies is to reuse FW either in food banks or in animal feed (FAO 2014 a). Several studies investigated the use of FW in poultry (Chen et al. 2007), swine (Westendorf et al. 1998), or cattle feed (Angulo et al. 2012), but little attention was given to aquafeed. The use of food waste in aquafeed was previously tested on herbivorous fish (Nasser et al. 2019) and omnivorous fish (Nasser et al. 2018). However, Lebanese restaurant food waste is rich in fats and oils and therefore more amenable to inclusion in trout feed than in Nile tilapia (Oreochromis niloticus) feed.
Rainbow trout, Oncorhynchus mykiss, is a carnivorous fish native to the Pacific coast of North America (Cowx 2005). Rainbow trout has been introduced to many countries either for farming or as a sports fish. In 2016, rainbow trout accounted for 2% of the total finfish species produced by aquaculture worldwide and is an important species in European aquaculture (FAO 2018). Rainbow trout is a hardy fish, considered to be a healthy source of protein and essential fatty acids. In the wild, rainbow trout feeds on fresh water shrimp, mollusks, insects, small fish, etc. (Cowx 2005). In aquaculture rainbow trout is offered high-energy manufactured feeds, rich in protein (35–45%) and lipids (16–22%) (Cowx 2005). Between 1995 and 2015, trout consumed 2% of aquafeed produced (FAO 2018). It would be interesting to find alternatives to traditional aquafeed ingredients in order to decrease trout production costs without compromising fish health or nutritional benefits. Several studies have investigated the use of alternate protein sources to SBM in rainbow trout aquaculture (Bılgın et al. 2007; Dernekbaşi and Karayücel 2017; Hardy et al. 2018; Pach and Nagel 2018; Yadollahi et al. 2018) but all these use ingredients which can be used as feed for alternate animal production and are not destined for landfills or compost production. In the present study, we assessed the feasibility of incorporating post-consumer restaurant FW as a partial replacement of SBM in the diets of Oncorhynchus mykiss. The effects of the food waste on survival, growth, proximate composition, and hematology of rainbow trout were assessed.
Materials and methods
Fish acquisition and maintenance
Rainbow trout O. mykiss juveniles used in the present work were obtained from an aquaculture farm on the Assi River, Hermel, Lebanon. Fish were offered a 40% crude protein and 14% lipid commercial feed (EXTR 400, Rangen Inc., Buhl, ID) daily to apparent satiation. Water temperature was 15 °C and pH at time of collection was 7.8. Trout juveniles were randomly caught in flow-through raceways and transported live in an insulated transport container with mechanical aeration and ice packs to the aquaculture research laboratory at the American University of Beirut (AUB).
At AUB, the fish were maintained in 52-L glass tanks connected to a biological filter and a chiller in a recirculation system. Fish were offered the commercial diet and allowed to acclimate to their new environment for 7 days.
Treatment/feed preparation
Post-consumer food waste was collected from Lebanese restaurants, sorted to remove plastic, paper, metal, and glass material then dried at 60 °C in a forced-air oven. The food waste was composed mainly of plant origin material such as bread, rice, pasta, and vegetables, but contained shrimp and fish heads and body remains, chicken meat and bones, and some meat. All the dried food waste was then ground into fine powder and stored in a freezer until used in the present experiment. Five samples of FW were randomly collected and proximate composition determined (Table 1). To determine protein content, 1 g of each sample was weighed and analyzed using the Kjeltec™ 8400 FOSS analyzer (FOSS A/S, Hillerød, Denmark). Aspartic acid and glycine were used as standards. To determine lipid content, 1 g of each sample was weighed and analyzed using the reflux extractor ANKOM XT15 Fat Analyzer (ANKOM Technology Corporation, Macedon, NY, USA). The solvent used consisted of a 9:1 mixture of petroleum ether:diethyl ether. Ash content was quantified by placing the samples in a muffle furnace at 520 °C for 8 h. To determine fiber content, fat-extracted samples were analyzed using the ANKOM200 fiber analyzer (ANKOM Technology Corporation). Nitrogen-free extract (NFE) was calculated as:
Seven iso-nitrogenous and iso-caloric diets (Table 2) were prepared such that diet 1 contained 0% FW; diet 2: 5% FW; diet 3: 10% FW; diet 4: 15% FW; diet 5: 20% FW; diet 6: 25% FW; and diet 7: 30% FW. Soybean meal, soy oil, and whole-wheat quantities were reduced as FW was increased in order to maintain similar amounts of crude protein and lipid in the diets. Gross energy content was calculated by using factors of 23.6, 17.6, and 39.5 kJ/g of protein, carbohydrate, and lipid, respectively (NRC 2011). Every feed was randomly assigned to three replicate tanks. Proximate analysis of the diets (protein and lipid contents) was performed as described for FW.
Fish growth
The experiment was performed in 21 glass tanks (52 L) connected to a biological filter, a sand filter, and a chiller. Water in the tanks was aerated using a regenerative blower and submerged air diffusers. Oncorhynchus mykiss juveniles (ca 6.5 g) were size-sorted by hand, and 15 fish were group-weighed (94.6 ± 1.5 g; mean ± SD) and stocked in each tank to give an initial tank biomass of 94.6 g. Initially, fish were offered diets at 4% body mass. The daily feed allowance was divided into four equal portions (at 7:00, 11:00, 15:00, and 19:00 h), 6 days a week for 8 weeks. If fish in any tank finished all the feed on any given day, feed quantity was increased by 5% for all tanks the next day.
Water quality parameters were maintained at conditions conducive to optimal trout growth. Temperature was set at 14.7–15.7 °C. Salinity and dissolved oxygen were measured daily using a YSI Pro 2030 (Yellow Springs Inc., OH, USA) and were maintained at 0.8–1.5 ppt and 7 mg/L and greater, respectively. pH was measured daily using a commercial hand-held pH meter and ranged between 7.5 and 8.5. Total ammonia nitrogen and nitrite nitrogen were assessed weekly using a HACH Aquaculture Test Kit Model FF-3 and remained less than 2 ppm and 0.1 ppm, respectively. Photoperiod was set at 14:10 (light:dark). Tanks were siphoned daily to remove uneaten feed and fish feces that settled at the bottom of the tanks.
Harvest
Fish were starved for 24 h before harvest. At harvest, fish in every tank were group-weighed; then weight (g) and total length (cm) of every fish were measured. Weight and length data were used to calculate Fulton’s condition index (K) as:
Specific growth rate (SGR; % per day) was calculated as:
Hematological parameters
Four fish were randomly removed from each tank, anesthetized, and used to determine hematological parameters: (hemoglobin (Hb), hematocrit (Hct), and total plasma protein (TPP). Blood was collected from the caudal arch of the fish using heparinized 1-mL syringe and a 25-gauge needle. Hemoglobin (g/dL) was determined using Cyanmethemoglobin procedure (Larsen and Snieszko 1961). Hematocrit (%) was determined using the indirect method for hematocrit measurement (Klontz 1994). Total plasma protein (g/dL) was determined by using a veterinary refractometer (Alexander and Ingram 1980).
Following blood withdrawal, the fish were euthanized using an overdose of tricaine methane sulfonate (MS222; Pharmaq, Fordingbridge, UK) followed by destruction of the brain. Their liver and viscera were then extracted and weighed. Data were used to calculate hepatosomatic (HSI) and viscerosomatic (VSI) indices:
Proximate analysis
Four fish were randomly collected from each tank to determine proximate composition. The fish were pooled, and macerated using a food processor; then each sample was spread onto three aluminum plates. Samples were dried in the oven at 105 °C, and moisture content of the samples was determined as the difference in weight before and after drying. Dried samples were then ground into fine powder and used to determine protein, lipid, and ash content as described for the food waste samples.
Statistical analysis
Statistical analysis was performed using SAS (V.9.2, SAS Institute Inc., Cary, NC, USA). All data were reported as mean values ± standard error of the mean and compared using one-way ANOVA. Significant differences among means were analyzed using the Student-Newman-Keuls (SNK) mean separation test. Differences among treatment means were considered significant at p < 0.05.
Results
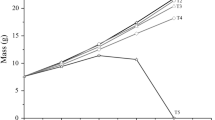
There were no significant differences in survival of rainbow trout offered diets with various proportions of food waste (Table 3). Final body weight (FBW) did not vary among fish offered control diet (21.42 ± 1.15 g; mean ± SE), 5% FW 24.04 ± 1.14 g) or 30% FW (23.97 ± 1.22 g). However, the FBW of fish offered 10, 15, 20, and 25% FW was significantly greater than the FBW of control fish (p value < 0.05). Final total length (TL) was not significantly different among fish offered control diet, 5, 10, and 30% FW (p > 0.05), but the length of fish offered 15, 20, and 25% FW was significantly greater than length of control fish. Fish offered 20% FW in their diets had the greatest FBW (26.25 ± 1.34 g; 317% growth), while fish offered 25% FW had the greatest length (15.11 ± 0.16 cm). Fulton’s condition index (K) was significantly different among treatments, and fish offered 10% FW had the greatest condition index (0.76 ± 0.01).
Specific growth rate did not vary significantly among fish offered 5% and 30% FW and control fish but was significantly greater in fish offered 10, 15, 20, and 25% FW than that in the control fish. Fish offered 15% FW had the greatest SGR (2.50 ± 0.06% per day).
Hemoglobin was significantly greater in fish offered 30% FW (5.41 ± 0.49 g/dL) than in fish offered 10% FW (3.70 ± 0.33 g/dL). Otherwise, hematocrit and total plasma protein did not vary significantly among treatments. Hematocrit ranged from 18.91 ± 0.73% (10% FW) to 21.50 ± 0.61% (5% FW), while TPP ranged between 3.68 ± 0.09 g/dL (25% FW) and 4.03 ± 0.13 g/dL (15% FW). Moreover, there were no significant differences in the hepatosomatic and viscerosomatic indices of fish among treatments. HSI ranged between 1.29 ± 0.07% (20% FW) and 1.42 ± 0.04% (15% FW), while VSI ranged from 12.21 ± 0.30% (30% FW) to 13.31 ± 0.45% (5% FW) (Table 4).
Proximate composition of fish offered various percentages of FW is summarized in Table 5. Moisture content of the fish offered 10% FW (71.89 ± 0.35%) was significantly less than moisture content of fish offered all the other treatments. Protein, lipid, and ash content were significantly different among treatments. Fish offered 30% FW had the greatest protein and ash content (15.68 ± 0.60% and 2.34 ± 0.13% respectively), while fish offered 10% FW had the greatest lipid content (10.49 ± 1.00%).
Discussion
Results of the present experiment suggest that food waste can be incorporated into trout diets at up to 25% by weight without having a negative effect on growth of the fish. Similar results were observed by Nasser et al. (2018, 2019) working with Nile tilapia and with marbled rabbitfish (Siganus rivulatus). At 30% FW, the rate of growth of the fish declined, suggesting that the nutritional value of the diet decreased. Interestingly, the protein content of the fish increased as the percentage of food waste in the diets increased. Rainbow trout seem to process and assimilate food waste better than soybean meal. Inclusion levels of SBM in the diets of carnivorous fish, such as O. mykiss, are usually limited because of the inability of these fish to process plant-based products. In fact, 30% of the carbohydrates in SBM are indigestible by fish (Storebakken et al. 2000) and could be deleterious for carnivorous fish growth (NRC 2011). Additionally, SBM is known to induce injurious changes in the distal intestines of Salmonids as well as modify their intestinal microbiota (Refstie et al. 2000; Merrifield et al. 2009; Bruce et al. 2018) which increases fish susceptibility to diseases (Krogdahl et al. 2000). Present results suggest that uneaten cooked human food such as restaurant food waste is more digestible by trout than is SBM.
Another result of using FW as partial replacement of SBM and whole wheat was a significant improvement in growth rate. This suggests that FW-based diets are more efficient than SBM-based diets. Additionally, using FW in rainbow trout diets decreases the cost of feed. Accordingly, we strongly encourage replacing part of the dietary SBM with restaurant plate waste in trout feed.
Soybean meal contains several antinutritional factors (ANFs) such as lectins, saponins, soy antigens, proteinase inhibitors, and sterols (Van Eys et al. 2004; Yasothai 2016) that could be mitigated by processing. However, processing would increase the price of the SBM. Restaurant food waste appears to be an inexpensive yet nutritive substitute for SBM in carnivorous fish diets.
Replacing soybean meal by food waste did not affect total plasma protein or hematocrit of the fish (Table 4). However, hemoglobin levels varied significantly among treatments, but because there was no trend in hemoglobin correlated to food waste in diet, we refrain from discussing hemoglobin variation among treatments.
Lipid content in fish is usually inversely related to moisture content. Fish offered 10% FW had the highest body lipid content. However, this diet (10% FW) had a slightly greater lipid content (19.34 ± 0.08%; mean ± SD) and a lower protein content (41.15 ± 0.21%) than the other experimental diets. The difference in proximate content of the diets seems to be reflected in the proximate composition of the fish. Moreover, the relatively low protein content in diet 3 seems to be reflected in hemoglobin levels of fish offered this diet (3.7 ± 1.05 g/dL; mean ± SD). Nevertheless, despite the significant differences in fish body lipid content among treatments, there were no differences in HSI and VSI among treatments, probably because rainbow trout tend to store lipids in the muscles rather than the viscera and the liver (Weil et al. 2013).
Results of the present work strongly suggest that food waste is an acceptable feed ingredient in rainbow trout diets, and its incorporation would be positive financially, environmentally, and ethically. The value of discarded food exceeds 936 billion dollars yearly (FAO 2014 b), and reusing it would greatly reduce water usage and carbon footprint. Additionally, food waste can be sourced locally near feed production plants, thus reducing shipping and storage costs and associated feed production expenses. From an environmental perspective, food waste is considered a major environmental problem. Wasted food ends up in landfills, where it decomposes releasing several toxic gases including methane (Crowley et al. 2003), a greenhouse gas 25 times more potent than CO2. Food waste can also result in heavily polluted leachate that can contaminate underground aquifers (Crowley et al. 2003). Landfilling food waste can attract insects and vermin, as well as generate wind-blown litter. Finally, using food waste in fish feed could help increase food security especially in developing countries. In fact, more than 820 million people in the world are undernourished (FAO, IFAD, UNICEF, WFP, and WHO 2019), while industrialized countries waste almost 222 million tons of food every year (FAO 2011). In conclusion, if we incorporate FW in fish feed, fish would act as bio-processors (Dou et al. 2018), converting human food waste into affordable high-quality protein easily accessible to people in developing countries.
References
Alexander JB, Ingram GA (1980) A comparison of five of the methods commonly used to measure protein concentrations in fish sera. J Fish Biol 16(2):115–122. https://doi.org/10.1111/j.1095-8649.1980.tb03691.x
Angulo J, Mahecha L, Yepes SA, Yepes AM, Bustamante G, Jaramillo H, Valencia E, Villamil T, Gallo J (2012) Nutritional evaluation of fruit and vegetable waste as feedstuff for diets of lactating Holstein cows. J Environ Manag 95:S210–S214. https://doi.org/10.1016/j.jenvman.2011.06.050
Bılgın Ö, Türker A, Tekinay AA (2007) The use of hazelnut meal as a substitute for soybean meal in the diets of rainbow trout (Oncorhynchus mykiss). Turk J Vet Anim Sci 31(3):145–151. http://journals.tubitak.gov.tr/veterinary/issues/vet-07-31-3/vet-31-3-1-0509-44.pdf
Bruce TJ, Neiger RD, Brown ML (2018) Gut histology, immunology and the intestinal microbiota of rainbow trout, Oncorhynchus mykiss (Walbaum), fed process variants of soybean meal. Aquac Res 49(1):492–504. https://doi.org/10.1111/are.13480
Chen K-L, Chang H-J, Yang C-K, You S-H, Jenq H-D, Yu B (2007) Effect of dietary inclusion of dehydrated food waste products on Taiwan native chicken (Taishi No. 13). Asian Australas J Anim Sci 20(5):754–760. https://doi.org/10.5713/ajas.2007.754
Choi SM, Wang X, Park GJ, Lim SR, Kim KW, Bai SC, Shin IS (2004) Dietary dehulled soybean meal as a replacement for fish meal in fingerling and growing olive flounder Paralichthys olivaceus (Temminck et Schlegel). Aquac Res 35(4):410–418. https://doi.org/10.1111/j.1365-2109.2004.01046.x
Cowx IG (2005) Oncorhynchus mykiss (Walbaum, 1792). In Cultured Aquatic Species Information Programme. Food and Agriculture Organization of the United Nations (FAO) . In Cultured Aquatic Species Information Programme. Food and Agriculture Organization of the United Nations (FAO). http://www.fao.org/fishery/culturedspecies/Oncorhynchus_mykiss/en
Crowley D, Staines A, Collins C, Bracken J, Bruen M, Fry J, Hrymak V, Malone D, Magette B, Ryan M, Thunhurst C (2003) Health and environmental effects of landfilling and incineration of waste - a literature review. Reports. Paper 3. http://arrow.dit.ie/schfsehrep/3
da Silva RL, Damasceno FM, Rocha MKHR, Sartori MMP, Barros MM, Pezzato LE (2017) Replacement of soybean meal by peanut meal in diets for juvenile Nile tilapia, Oreochromis niloticus. Lat Am J Aquat Res 45(5):1044-1053. https:// doi.org/10.3856/vol45-issue5-fulltext-19
Dernekbaşi S, Karayücel I (2017) Partial replacement of soybean meal by peanut and sesame seed meals in practical diets for rainbow trout, Oncorhynchus mykiss. J Aquac Mar Biol 6(1):00146. https://doi.org/10.15406/jamb.2017.06.00146
Dou Z, Toth JD, Westendorf ML (2018) Food waste for livestock feeding: feasibility, safety, and sustainability implications. Glob Food Secur 17:154–161. https://doi.org/10.1016/j.gfs.2017.12.003
Dozier WA III, Hess JB (2011) Soybean meal quality and analytical techniques. In: El-Shamy H (ed) Soybean and nutrition. InTech, Rijeka, pp 111–124
El-Saidy DMSD, Saad AS (2011) Effects of partial and complete replacement of soybean meal with cottonseed meal on growth, feed utilization and haematological indexes for mono-sex male Nile tilapia, Oreochromis niloticus (L.) fingerlings. Aquac Res 42:351–359. https://doi.org/10.1111/j.1365-2109.2010.02629.x
FAO, IFAD, UNICEF, WFP and WHO (2019) The state of food security and nutrition in the world 2019: safeguarding against economic slowdowns and downturns. Rome, FAO. http://www.fao.org/3/ca5162en/ca5162en.pdf
Food and Agriculture Organization of the United Nations (FAO) (2011) Global food losses and food waste. Extent, causes and prevention. Study conducted for the International Congress. SAVE FOOD! at Interpack2011 Düsseldorf, Germany
Food and Agriculture Organization of the United Nations (FAO) (2014a) Mitigation of food wastage. Societal costs and benefits. Rome, Italy. http://www.fao.org/3/a-i3989e.pdf
Food and Agriculture Organization of the United Nations (FAO) (2014b) Food wastage footprint full-cost accounting: final report. Rome. http://www.fao.org/3/a-i3991e.pdf
Food and Agriculture Organization of the United Nations (FAO) (2018) The state of world fisheries and aquaculture. Meeting the sustainable development goals. Rome. http://www.fao.org/3/i9540en/i9540en.pdf
Hardy RW, Patro B, Pujol-Baxley C, Marx CJ, Feinberg L (2018) Partial replacement of soybean meal with Methylobacterium extorquens single-cell protein in feeds for rainbow trout (Oncorhynchus mykiss Walbaum). Aquac Res 49(6):2218–2224. https://doi.org/10.1111/are.13678
Indexmundi 2020. Commodity prices. Soybean Meal. https://www.indexmundi.com/commodities/?commodity=soybean-meal&months=240. .
Klontz GW (1994) Fish hematology. In: Stolen JS, Fletcher TC, Rowley AF, Zelikoff JT, Kaattari SL, Smith SA (eds) Techniques in fish immunology 3. SOS Publications, Fair Haven, pp 121–130
Krogdahl A, Bakke-McKellep AM, Roed KH, Baeverfjord G (2000) Feeding Atlantic salmon Salmo salar L. soybean products: effects on disease resistance (furunculosis), and lysozyme and IgM levels in the intestinal mucosa. Aquac Nutr 6(2):77–84
Larsen HN, Snieszko SF (1961) Comparison of various methods of determination of hemoglobin in trout blood. Progress Fish Cult 23(1):8–17. https://doi.org/10.1577/1548-8659(1961)23[8:COVMOD]2.0.CO;2
Li MH, Robinson EH (2015) Complete feeds – intensive systems. In: Davis DA (ed) Feed and feeding practices in aquaculture. Sawston Waltham Maryland, Woodhead, pp 111–126
Lim C, Klesius PH, Higgs DA (1998) Substitution of canola meal for soybean meal in diets for channel catfish Ictalurus punctatus. J World Aquacult Soc 29(2):161–168. https://doi.org/10.1111/j.1749-7345.1998.tb00975.x
Merrifield DL, Dimitroglou A, Bradley G, Baker RTM, Davies SJ (2009) Soybean meal alters autochthonous microbial populations, microvilli morphology and compromises intestinal enterocyte integrity of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32(9):755–766. https://doi.org/10.1111/j.1365-2761.2009.01052.x
Nasser N, Abiad MG, Babikian J, Monzer S, Saoud IP (2018) Using restaurant food waste as feed for Nile tilapia production. Aquac Res 49(9):3142–3150. https://doi.org/10.1111/are.13777
Nasser N, Babikian J, Hatem MG, Saoud IP, Abiad MG (2019) Evaluation of post-consumer food waste as partial replacement of commercial feed in marbled rabbitfish, Siganus rivulatus aquaculture. Int J Environ Sci Technol 16:4059–4068. https://doi.org/10.1007/s13762-018-2051-x
National Research Council (NRC) (2011) Nutrient requirements of fish and shrimp. Washington D. C: National Academy. 17226(/13039):10
Pach F, Nagel F (2018) Replacing the substitute—guar meal as an alternative for non-genetically modified soybean meal in the nutrition of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792). Aquac Nutr 24(1):666–672. https://doi.org/10.1111/anu.12557
Refstie S, Helland SJ, Storebakken T (1997) Adaptation to soybean meal in diets for rainbow trout, Oncorhynchus mykiss. Aquaculture 153:263–272. https://doi.org/10.1016/S0044-8486(97)00025-2
Refstie S, Korsøen ØJ, Storebakken T, Baeverfjord G, Lein I, Roem AJ (2000) Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture 190(1–2):49–63. https://doi.org/10.1016/S0044-8486(00)00382-3
Ritchie H (2020) Food waste is responsible for 6% of global greenhouse gas emissions. Our World in Data. https://ourworldindata.org/food-waste-emissions. Accessed on 22 June 2020.
Robinson EH, Li MH (1994) Use of plant proteins in catfish feeds: replacement of soybean meal with cottonseed meal and replacement of fish meal with soybean meal and cottonseed meal. J World Aquacult Soc 25(2):271–276. https://doi.org/10.1111/j.1749-7345.1994.tb00190.x
Storebakken T, Refstie S, Ruyter B (2000) Soy products as fat and protein sources in fish feeds for intensive aquaculture. In: Drackley JK (ed) Soy in animal nutrition. Federation of Animal Science Societies. IL, USA, pp 127–170
Van Eys JE, Offner A, Bach A (2004) Manual of quality analyses for soybean products in the feed industry, 2nd edn. American Soybean Association, USA
Voorhees JM, Barnes ME, Chipps SR, Brown ML (2019) Bioprocessed soybean meal replacement of fish meal in rainbow trout (Oncorhynchus mykiss) diets. Cogent Food Agric 5(1):1579482. https://doi.org/10.1080/23311932.2019.1579482
Weil C, Lefèvre F, Bugeon J (2013) Characteristics and metabolism of different adipose tissues in fish. Rev. Fish Biol Fish 23:157–173. https://doi.org/10.1007/s11160-012-9288-0
Westendorf ML, Dong ZC, Schoknecht PA (1998) Recycled cafeteria food waste as a feed for swine: nutrient content, digestibility, growth, and meat quality. J Anim Sci 76:2976–2983. https://doi.org/10.2527/1998.76122976x
Yadollahi V, Shamsaie M, Baboli MJ, Yadollahi F, Hajivar EN, Moëzzi F (2018) Replacement of soybean meal with guar meal in the diet of rainbow trout, Oncorhynchus mykiss (Walbaum 1792): biological parameters and flesh quality. Asian Fish Sci 31:181–190
Yasothai R (2016) Antinutritional factors in soybean meal and its deactivation. Int J Environ Sci Technol 5(6):3793–3797
Zhou Q-C, Yue Y-R (2010) Effect of replacing soybean meal with canola meal on growth, feed utilization and hematological indices of juvenile hybrid tilapia, Oreochromis niloticus × Oreochromis aureus. Aquac Res 41:982–990. https://doi.org/10.1111/j.1365-2109.2009.02381.x
Funding
The present work was funded by the University Research Board of the American University of Beirut under the WEFRAH grant program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable national and institutional guidelines for the care and use of animals were followed by the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
ZeinEddine, R., Ireland, B., Monzer, S. et al. Preliminary assessment of restaurant food waste as a feed ingredient for small juvenile rainbow trout (Oncorhynchus mykiss). Aquacult Int 29, 669–679 (2021). https://doi.org/10.1007/s10499-021-00650-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00650-4