Abstract
In this study, Dolichospermum affine was cultivated under starvation and limitation conditions, using six different concentrations of nitrate (NaNO3) and phosphate (K2HPO4) in BG-11 medium. The biomass, pigments, proteins, carbohydrates, lipids, triacylglycerol-TAG, and fatty acid responses to different modified media were examined in terms of cell density, dry biomass, optical density, growth rate, chlorophyll-a, carotene content, and lipid productivity. Molecular characterization of D. affine isolates was performed using Fourier transform–infrared spectroscopy (FT-IR) and polymerase chain reaction (PCR). In the 20-day batch culture period, one of the modified media, %50 N and P limitation (N-PLimitation), exhibited higher values than the others in terms of cell density (1.41 × 107 cells/mL), dry biomass (1.68 gL−1), optical density (1.26), growth rate (0.48 day−1), chlorophyll-a (3.76 μg L−1), and total carotenoid (12.99 μg L−1). The maximum percentage lipid content of D. affine was obtained in the control group at 10.67%. Fatty acid profiles have been investigated in six conditions and consisted of 10 fatty acids. The basic saturated fatty acid palmitic acid was (C16:0) for all conditions, and its rate was 40.61% for starvation of N and P (N-PDeprivation) conditions. Oleic acid (C18:1) rate was detected in all groups, but the highest rate was found in the control group with 33.72%. In all conditions, TAG values were found similar, but the highest value was determined at 50% P and 100% N (PLimitation-NControl) (27.5%) modified media. Carbohydrate content (20.37%) was found to be high under N and P starvation (N-PDeprivation), while protein content (66.92%) was high under 50% N and 100% P (NLimitation-PControl) conditions. The results obtained from this study showed that nitrogen and phosphorus starvation and limitation affecting the biomass, valuable secondary metabolites and lipid accumulation and affected the fatty acid composition of D. affine cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are unicellular, filamentous, prokaryotic, and oxygenic phototrophs with the ability to fix atmospheric nitrogen and are present in all habitats (Castenholz 2001; Garcia-Pichel and Pringault 2001). The morphology of cyanobacteria can vary depending on the environment or culture conditions, and they can be classified based on cellular differentiation, biochemical, physiological, and genetic criteria (Abed et al. 2009). The information gathered from studies on the diversity and physiology of cyanobacteria has provided a basis for investigating their applications in biotechnology. Because of these investigations, cyanobacteria have drawn considerable interest as a rich source of bioactive compounds and are one of the most promising groups of organisms to produce these compounds (Dahms et al. 2006).
Today, both the effects of climate change and the depletion of fossil fuels caused humanity to search for an environmentally friendly and renewable alternative energy source. Cyanobacteria were used in initial biofuel production studies, but accumulation levels of lipids were relatively low. However, recently, cyanobacteria emerged as a potential source of biofuel because of the growing abilities of some species in extreme environments and the potential of their rapid reproduction (Hannon et al. 2010). Features like rapid growth rates, ability to sequester CO2, high lipid yield in extraction, and ability to grow in areas not suitable for agriculture made cyanobacteria a potential candidate for producing biofuel. Cyanobacteria have unique properties that can turn into a promising model for transforming all C sources into valuable fuels (Quintana et al. 2011; Dutta et al. 2005). Cyanobacteria strains such as Anabaena, Calothrix, Oscillatoria, Cyanothece, Nostoc, Synechococcus, Microcystis, Gloeobacter, Aphanocapsa, Chroococidiopsis, and Microcoleus can produce hydrogen gas under various culture conditions (Abed et al. 2009).
The growth of algae and also cyanobacteria depends on several environmental factors, such as temperature, light intensity, nutrients, and pH of the surrounding environment (Zhu et al. 1997; Hu and Gao 2005; Tang et al. 2010). These factors also influence the composition of the biomass produced by altering the metabolism (Hu et al. 2008; Ilavarasi et al. 2011). Nitrogen and phosphorus play an important role in cyanobacteria growth. The most significant element limiting nutrients on cyanobacteria growth has always been identified as N sources such as NH4+ and NO3−. N limitation is related to cellular fatty acids and cellular growth; in other words, it is effective for enriching fatty acids (Uslu 2011; Mujtaba et al. 2012; Wong et al. 2017; Yeesang and Cheirsilp 2011; Gouveia and Oliveira 2009). On the other hand, phosphorus is one of the most important nutrients for normal growth and development of algae, and it plays a critical role in the regulation of cellular metabolic processes (Bhamawat 2010; Wong et al. 2017).
When it is needed to increase the amount of valuable intracellular biochemical, it is crucial to choose the species with short doubling period and, in particular, those yield a high amount of biochemical substance (Pruvost et al. 2011). The biochemical composition of the algae can be increased by various environmental stresses, such as nitrogen, phosphate, temperature, salinity, pH, and heavy metals, as well as light stress (Sharma et al. 2012; Battah et al. 2014). Because of such stresses, lipid metabolism can have an adaptive response for the survival of the algae (Guschina and Harwood 2006). Algae, under stress conditions, alter the biosynthetic lipid pathways and accumulate neutral lipids, mostly in the form of triacylglycerols (TAGs). This allows the algae to withstand these adverse conditions (Schuhmann et al. 2011). TAGs serve as energy storage in algae and can be easily converted into biodiesel through transesterification reactions (Chisti 2008).
Many studies have been conducted on different algae groups to increase the proportion of valuable intracellular compounds, in particular, lipid and fatty acids. To increase the performance of the algal intracellular production, it is important to optimize environmental conditions and nutrient media. Recently, altered environmental conditions (light, pH, etc.) and nutrient components (especially nitrogen and phosphorus concentrations) were shown to improve the production performance of algal intracellular secondary metabolites. Although such studies have started to focus on cyanobacteria due to the abovementioned properties, no such studies were encountered in the literature with the Dolichospermum affine species. This study aims to investigate the effects of modified nitrogen and phosphorus ratios in nutrient media BG-11 on the growth and lipid productivity response of D. affine. Especially, nutritional stress (starvation and limitation) of nitrogen and phosphorus and their combined effects on the D. affine’s biochemical composition and amount were investigated.
Materials and methods
Cyanobacteria culture
In our previous studies, D. affine was isolated in samples collected from various freshwater resources in Ankara, Turkey. One-cell growth technique was used for the isolation of strains (Parvin et al. 2007). Taxonomic identification of the isolate was based on morphological features and identified with the use of species keys (Prescott 1973; John et al. 2002; Guiry and Guiry 2018). Molecular characterization of D. affine isolates was performed using Fourier transform–infrared spectroscopy (FTIR) and polymerase chain reaction (PCR). D. affine species is preserved in the Kırşehir Ahi Evran University (AEU-CCA) catalog of culture collection with code number CCA01Ana01. The temperature was kept at 24 ± 3 °C in the laboratory where the experimentation took place. Light was provided by cool white fluorescent lamps (Philips, 50 μmol photons m−2 s−1) at a vertical distance of 22 cm from the cultures. The light was applied to cultures in a 16 L: 8D period. Figure 1 presents the taxonomic classification (left) of D. affine (Guiry and Guiry 2018) and its appearance under a microscope (middle) and FTIR image (right).
Molecular characterization of D. affine
Infrared analysis was carried out at Bilkent University Institute of Materials Science and Nanotechnology (UNAM), Ankara, Turkey, using a Vertex 70 with a Hyperion microscope fitted with a Bruker Tensor 37 FTIR spectrometer. FTIR measurements were done in the range of 400 and 5000 cm−1, with 4 cm−1 resolution and a 20 × 20 μm square aperture. One hundred twenty-eight scans were taken for 1 spectra (Duygu Yalcin et al. 2012).
The first stage analysis of DNA was extracted from the algal cells using a DNA extraction kit from Mo Bio Laboratories. The DNA was then cleaned using a Qiagen QIAquick PCR purification kit and run on a 1% gel for visualization. PCR was carried out using a Roche Expand High Fidelity PCR kit. 16S rRNA gene amplicons were amplified by using the universal primers 27F and 1492R (~ 1.5 kb amplicon size). The 16S PCR product was then cleaned using a Qiagen QIAquick PCR purification kit to remove salt/enzymes from the PCR reaction. The DNA was measured using a nanodrop (http://www.nanodrop.com/Productnd2000overview.aspx), and the data are presented in the following table.
Cyanobacteria | 260/280 | 260/230 | ng/μl DNA |
D. affine | 1.80 | 3.15 | 20.0 |
As the algae sample gave a concentration close to the required range, it was sent to GATC-Biotech for single-read sequencing (http://www.gatc-biotech.com/en/index.html). Subsequent sequence analysis was performed using BLAST.
Culture media
BG 11 (blue-green medium) nutrient media (Stanier et al. 1971; Rippka et al. 1979) was modified to observe the effect of limiting nutrients on the cell growth and biochemical composition of D. affine (CCA01Ana01). BG 11 medium contained (in g/L) NaNO3, 1.5; K2HPO4, 0.04; MgSO4·7H2O, 0.075; CaCl2·2H2O, 0.036; citric acid, 0.006; ferric ammonium citrate, 0.006; EDTA, 0.001; and Na2CO3, 0.02. This medium was amended with 1 mL trace solution of composition (in g/L) H3BO3, 2.86; MnCl2, 1.81; ZnSO4·7H2O, 0.222; Na2MoO4·2 H2O, 0.39; CuSO4·5 H2O, 0.079; and Co(NO3)2·6H2O, 0.0494. All the chemicals were obtained from Merck, Germany. Modified media used in this study were as follows: (1) control; (2) NLimitation-PControl 50%N(−)/100%P; (3) PLimitation-NControl 50%P(−)/100%N; (4) N-PLimitation 50%N/P(−); (5) NDeprivation-PControl 100%N(−)/100%P; and (6) N-PDeprivation 100%N/P(−). The pH of nutrient media was adjusted to 6.5–7.
The individual compositions of nitrogen and phosphorus at six-culture media were listed in Table 1. The nitrogen source used for the BG 11 nutrient media includes NaNO3, ferric ammonium citrate (C6H8FeNO7), and EDTA (C10H16N2O8), while the phosphorus source was K2HPO4. For nitrogen and phosphorus limitation conditions (50%), the amount of NaNO3 and K2HPO4 used in BG 11 was halved. Since ammonium citrate and EDTA were already used in low concentrations, their concentration was not changed. Although sulfur could significantly affect the production of biomass and biochemical, the sulfur content was ignored in this study, and the effect of nitrogen and phosphorus was investigated.
The experiment was carried out in 5 L glass jars containing 2250 mL nutrient media and 250 mL suspended culture. D. affine cultures were inoculated in the 6 different modified culture media and allowed to grow for 20 days. The initial cell concentration of D. affine was counted as 1.65 × 106 cells/mL. Illumination was provided by cool white fluorescent lamps (Philips, 50 μmol photons m−2 s−1) at a vertical distance and light/dark cycle (16:8 h) at 24 ± 3 °C. All the glassware and media were always sterilized prior to inoculation. Sterilized air was supplied, and all tests were carried out in triplicate. The optical density, cell density, and chlorophyll-a content of the cultures used for inoculation were measured at the beginning of the study (Table 2).
Analytical methods
The algal sample (10 mL) was centrifuged at 5000 rpm for 5 min (Nüve NF 200); the supernatant was discarded and then washed with 20 mL of distilled water. The samples were filtered using vacuum filtration with glass-fiber filters (Whattman, GF/C). Concentrated biomass was dried in an oven under 105° for 24 h. The dry biomass content was calculated by subtracting the initial mass of the filter paper before filtration from its final mass with dried microalgae (Chia et al. 2013).
Total cell counts were microscopically determined by drop count method using the Sedgewick-Rafter counting cell slide. In the course of cell counting, 0.03 mL sample was dropped on the slide and covered later. When the microscope objective lens was at 10 × 40, 36 views were identified. The calculation was made by counting at least six views and extrapolating into 36 transect views.
The maximum absorbance was inspected by scanning the sample cultures between 550 and 800 nm using a UV-Vis spectrophotometer (Biochrom Libra, S22). The maximum absorbance value for microalgae was used to obtain the growth curve by optical density (OD) (Santos-Ballardo et al. 2015). Optical density was recorded at 681 nm for D. affine. A linear regression equation was derived in order to describe the relationship between optical density and cell density. Using the growth kinetics, specific growth rate and duplication time were calculated. The specific growth rate (μ) at the exponential phase was calculated according to μ = ln(× 2 − × 1)/(t2 − t1), where × 2 and × 1 are the cell density at t2 and t1, respectively (Godoy-Hernández and Vázquez-Flota 2006). Duplication time was calculated using the formula DT = ln2/μ (Godoy-Hernández and Vázquez-Flota 2006).
Volumetric biomass productivity PBiomass was calculated using PBiomass (gL−1 day−1) = (×2 − × 1) × (t2 − t1)−1, where × 1 and × 2 are the biomass DW concentrations (g L−1) on days t1 (start point of cultivation) and t2 (endpoint of cultivation), respectively, given as an average productivity (Hempel et al. 2012).
Pigment extraction of the cyanobacterium biomass was performed using acetone to quantify chlorophyll-a and carotenoid content (Parsons and Strickland 1963). Absorbance at 630, 645, 665, and 750 nm wavelengths was measured with UV-Vis spectrophotometry. To determine the total carotenoid, absorbance at 480 nm was measured using UV-Vis spectrophotometry and Eq. 3 was used to reach the total carotenoid content (Parsons and Strickland 1963). The chlorophyll-a concentrations were calculated using the equations below (Eqs. 1 and 2):
- C:
-
chlorophyll-a content from the first equation
- V :
-
filtered water (L)
- v :
-
volume of acetone (mL)
- 1:
-
path length of the cuvette (cm)
D. affine samples (1800 mL) were centrifuged with a high-speed centrifuge (Rotor R10A5) at 10,000 rpm and 4 °C. Approximately, 5 mL samples were transferred to Falcon tubes and lyophilized for 48 h under − 85 °C and 0.002 mbar vacuum. Freeze-dried samples were used for nutrient analysis. Nutrient analysis was carried out at Ankara University Faculty of Veterinary Medicine, Ankara, Turkey. Nutrients (crude protein, total lipid, and total carbohydrate), dry matter, and ash of samples were determined according to the Association of Analytical Communities Manuel (AOAC 1990). Triacylglycerol (TAG) determination of cultures was performed using a commercial kit in a special laboratory. The fatty acid methyl ester was prepared according to the method of Metcalf et al. (1966). Fatty acid methyl esters were determined through gas chromatography.
Statistical analysis
All analyses were performed in triplicate (n = 3). All data are presented as mean values ± and standard deviation (SD). The data, cell density, DW, chlorophyll-a, and total carotenoid collected from the modified culture media were analyzed statistically using the ANOVA. The statistical analysis was carried out using the SPSS software. The significance level was set at p < 0.05. Linear regression was used to examine relationships among the optical density and cell density.
Results
Cell density
The initial cell number was kept constant (1.65 × 106 cells/mL) across the six culture conditions. In Fig. 2a, the total increase of cell density in nutrient media throughout the 20-day study period is illustrated. On the 20th day of the study, the modified nutrient media of N-PLimitation had a high cell density of (1.41 × 107 ± 3.92 × 106 cells/mL). The lowest cell density (9.83 × 106 ± 8.95 × 105 cells/mL) was detected in N-PDeprivation–modified nutrient media (p > 0.05) (Table 2).
Dry biomass
Dry biomass of the cultures was measured in parallel with cell counts, and the results are given in Table 2. Figure 2b shows the total increase in biomass productivity (as concentration) through the 20-day study period. On the 20th day, N-PLimitation–modified nutrient media had highest biomass concentration of (1.68 ± 0.01 gL−1), while N-PDeprivation–modified nutrient media had the lowest concentration (0.97 ± 0.01 gL−1). As seen from Table 2, the conditions in the culture media considerably impacted the final dry biomass (p > 0.05).
Pigment formation
The highest concentrations of chlorophyll-a were found in N-PLimitation cultures (3.76 μg L−1). N-PDeprivation cultures had a low concentration of chlorophyll-a (3.19 μg L−1, Fig. 3a). Total carotenoid accumulation in the culture media was highest in N-PLimitation culture media (12.99 μg L−1, Fig. 3b) and lowest in N-PDeprivation culture media (9.24 μg L−1) (p > 0.05).
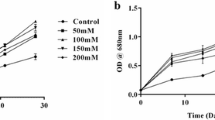
Optical density
At the end of the 20-day, the optical density absorbance at 681 nm was highest in N-PLimitation–modified nutrient media (1.26 ± 0.004) and lowest in N-PDeprivation–modified nutrient media (0.92 ± 0.012).
The calibrated data for the relationship between the absorbance and cell concentrations of the cultures are shown in Fig. 4a. The correlation (Pearson) between optical density (nm) and cell concentration (cells/mL) were found to be linear (0.95).
Growth rate
Growth curves were drawn from D. affine in both control and modified nutrient media. The algal growth rate was found to be highest in N-PLimitation–modified nutrient media on the 7th day (0.48 day−1), and doubling time was calculated as 1.45 day−1. The lowest rate was in N-PDeprivation–modified nutrient media on the 10th day (0.18 day−1), and doubling time was determined as 4.20 day−1 (Fig. 4b and Table 2).
Table 2 shows the average biomass productivity PBiomass (gL−1 day−1) for the six different modified media compositions. The maximum PBiomass of 0.072 gL−1 day−1 and 0.069 gL−1 day−1 were recorded for N-PLimitation and NDeprivation-PControl growth media compositions, respectively. It is clear that the maximum biomass productivity (cell number) in mL/L/day was obtained in N-PLimitation at 8.59 × 106 cells/mL/L/day, and the lowest in N-PDeprivation at 6.44 × 106 cells/mL/L/day (Table 2).
Fourier transform–infrared spectroscopy analysis and phylogenetic analysis
The absorption spectra of the cultures had 11 clear bands over the wavenumber range of 400 to 5000 cm−1 (Fig. 5). Each peak was assigned a functional group. The bands were tentatively identified on the basis of published data for phytoplankton and bacteria and on other biological materials and reference standards (Giordano et al. 2001; Sigee et al. 2002; Benning et al. 2004; Dean et al. 2007). Protein spectra were characterized by two strong features at 1654 cm−1 (amide I) and 1544 cm−1 (amide II). These bands were due primarily to C=O stretching vibration and a combination of N–H and C–N stretching vibrations in amide complexes (Ponnuswamy et al. 2013). Lipid spectra were characterized by two sets of strong vibrations: C–H at 2957 cm−1 and the C=O mode of the side chain from the ester carbonyl group at 1736 cm−1, while carbohydrate absorption bands due to C–O–C of polysaccharides were observed at 1148 cm−1, 1077 cm−1, and 1043 cm−1, respectively (Duygu Yalcin et al. 2012). Several other classes of compounds, such as nucleic acids, have functional groups with absorption bands in the same region of the spectrum (Table 3).
The 16-s sequencing results came back in the following fasta format. The results confirmed 99% of D. affine’ molecular expression, consistent with other Anabaena species.
> D. affine
TTAGTGGCGGACGGGTGAGTAACGCGTGAGAATTTGGCTTCAGGTCGGGGACAACCACTGGAAACGGTGGCTAATACCGGATATGCCGGGAGGTGAAAGATTTATTGCCTGAAGATAAGCTCGCGTCTGATTAGCTAGTTGGTGGGGTAAGAGCCTACCAAGGCGACGATCAGTAGCTGGTCTGAGAGGATGATCAGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTGGGGAATTTTCCGCAATGGGCGAAAGCCTGACGGAGCAATACCGCGTGAGGGAGGAAGGCTCTTGGGTCGTAAACCTCTTTTCTCAGGGAAGAAAAAAATGACGGTACCTGAGGAATAAGCATCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGATGCAAGCGTTATCCGGAATGATTGGGCGTAAAGGGTCCGCAGGTGGTACTGTAAGTCTGCTGTTAAAGAGCAAGGCTCAACCTTGTAAAAGCGGTGGAAACTACAGAACTAGAGTGCGTTCGGGGCAGAAGGAATTCCTGGTGTAGCGGTGAAATGCGTAGATATCAGGAAGAACACCGGTGGCGAAAGCGTTCTGCTAGGCCGCAACTGACACTGAGGGACGAAAGCTAGGGGAGCGAATGGGATTAGATACCCCAGTAGTCCTAGCCGTAAACGATGGATACTAGGCGTGGCTTGTATCGACCCGAGCCGTGCCGGAGCTAACGCGTTAAGTATCCCGCCTGGGGAGTACGCACGCAAGTGTGAAACTCANAGGAATTGACGGGGGCCCGCACAAGCGGTGGAGTATGTGGTTTAATTCGATGCAACGCGAAGAACCTTACCAAGACTTGACATGTCGCGAATTTCGGTGAAAGCTGAAAGTGCCTTCGGGAGCGCGAACACAGGTGGTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTCGTTTTTAGTTGCCAGCATTAAGTTGGGCACTCTAGAG |
Biochemical analysis
The highest total lipid content obtained during the 21-day batch culture period was found in the control group at 10.67%. The highest level of TAG was found at 27.5 mg/dL in culture condition PLimitation-NControl. The highest protein content obtained was 66.92% in NLimitation-PControl culture. The highest amount of total carbohydrate was determined as 20.37% in N-PDeprivation culture condition. Fatty acid profile of all conditions was tabulated in Table 4.
The highest lipid level of D. affine was found in the control group (10.67%); the biomass concentration in the control group was 0.99 ± 0.01 gL−1. The culture media with the highest biomass (1.68 ± 0.01 gL−1) was N-PLimitation, and it contained 8.10% lipid (Fig. 6).
Discussion
The composition of nutrient media has an important effect on both the growth rate and the final biomass concentration of the microalgae. A large number of macro and micronutrients are essential for algal growth. Elements such as N, P, K, Mg, Ca, S, Fe, Cu, Mn, and Zn are essential for algae development, and in general, the growth and intracellular biochemical cycles depend on the availability of N and P in the environment (Kaplan et al. 1986; Yalçın Duygu et al. 2018).
In this study, BG 11 nutrient medium was modified in six different ways, and the effect on D. affine was investigated in both growth, biochemical compositions and fatty acids profile. Results indicated that N-PLimitation–modified nutrient media had the highest cell density at the end of the 20-day (1.41 × 107 ± 3.92 × 106 cells/mL) compared with the other nutrient media. The N-PLimitation and NDeprivation-PControl cultures showed a higher cell density compared with the control group. This result indicated that low nitrogen concentration does not inhibit but rather enhances the reproduction of the cultures. No lag phase was observed in the cultures at end of the 20-day. During this period, the cultures were determined to be in the exponential phase, and the stationary phase was not seen either. N-PDeprivation condition results in the lowest dry biomass concentration (0.97 ± 0.01 gL−1) and average biomass productivity (0.035 ± 0.001 gL−1 day−1) (Table 2). Dry biomass concentration in the control (0.99 ± 0.01 g/L−1), NLimitation-PControl (0.98 ± 0.01 g/L−1), and N-PDeprivation (0.97 ± 0.01 g/L−1) were not significantly different. Results indicated that the N-PLimitation condition with higher dry biomass concentration and cell concentration was better than the control group. The absence of phosphorus and nitrogen N-PDeprivation resulted in significantly lower dry biomass concentration (0.97 ± 0.01 gL−1) throughout the 20-day period. In the modified BG 11 nutrient media optimization study for Anabaena spiroides and Nostoc punctiformae, Das and Sarma (2015) increased the amount of NaNO3 and K2HPO4, and on the 28th day, Anabaena spiroides and Nostoc punctiformae had 59 mg/100 mL (DW) 61 mg/100 mL (DW) respectively. Nalewajko and Murphy (2001) stated that there are differences in the growth response of Anabaena sp. during phosphorus and nitrogen limitation. Anabaena sp. could not fix N2 when the amount of nitrogen was very limited; the maximum phosphorus intake ratio was very low if phosphorus was limited and this affected the development of the strain. As can be seen from the abovementioned literatures, researches on the genus Anabaena are quite limited, and studies have been reported on Anabaena sp. or different species of Anabaena. In this study, it is thought that the difference between the obtained cell density and the amount of biomass results with the other studies is due to the species used, the purpose of the experiment, and the methods. In this study, it is considered that the findings detected for the cell density, DW, and pigment amount of D. affine species can be accepted as definitive, and these findings may give path to the basis for further studies.
Chlorophyll and total carotenoid concentrations affect the biomass of the microalgae (Wong et al. 2017). In our study, a positive correlation was found between the pigment content and the growth and photosynthesis rate of D. affine. N-PLimitation had the highest amount of chlorophyll-a (3.76 μg L−1) and total carotenoid (12.99 μg L−1) (day 21). N-PDeprivation displayed relatively a slow growth in both chlorophyll-a (3.19 μg L−1) and total carotenoid (9.24 μg L−1) (day 21). The result of N-PDeprivation, when compared with that of Control group, shows that the absence of N and P caused a reduction in chlorophyll-a and carotenoid content (Fig. 3). Bojović and Stojanović (2005) reported that the limitation/absence of N and P in the environment can affect the composition of the pigment and alter the concentration of chlorophyll and carotenoid, and consequently change the rate of photosynthesis. Das and Sarma (2015) doubled the concentration of N and P in Anabaena spiroides nutrient media BG 11 and found higher chlorophyll concentration compared with the other seven modified culture medium conditions. Nalewajko and Murphy (2001), using Anabaena sp., diluted Chu 10 media and examined the effect of N:P ratios on the growth. They found that the growth of Anabaena sp. did not change under N or P limitation. In a study by Solovchenko et al. (2008), the amount of nitrogen in the nutrient medium was increased, and a significant increase in carotenoid and chlorophyll content was recorded for Parietochloris incise.
The detection of changes in biochemical compositions of D. affine under different modified nutrient media was performed via standard methods (AOAC 1990), and the results are tabulated in Table 4. The modification of N and P in the nutrient media led to the differentiation of results in both cell growth and intracellular biochemical composition. The maximum percentage lipid content of D. affine on day 21 was obtained in control at 10.67%. The lowest lipid yield was obtained in N-PDeprivation–modified culture medium at 8.05%. On the other hand, TAG concentration was highest in PLimitation-NControl at 27.5% and in N-PLimitation and NDeprivation-PControl at 27%. Microalgae assimilate carbohydrates, lipids, and proteins via photosynthesis for storage. Lipids are high-value compounds and compared with other biochemical, they have the highest energy content (Quintana et al. 2011). In the logarithmic growth phase, microalgae do not produce many lipids for storage. The lipid accumulation in microalgae is affected by nutrient levels and culture condition–related stresses. Nitrogen is the most critical nutrient for microalgae and affects lipid metabolism (Alonso et al. 2000; Bhamawat 2010). Some species essentially accumulate neutral lipids, such as triacylglycerols (TAG), under N limiting conditions. TAG accumulation occurs as a way to create a ready-to-use energy store (Radakovits et al. 2010; Hsieh and Wu 2009). In green algae, metabolism supports starch accumulation, and these organisms have been shown to be resistant to lipid accumulation. However, under stress, they start to accumulate TAG, similar to seed oils (Johnson and Alric 2013; Liu et al. 2011). Alonso et al. (2000) reported a small increase in TAG levels (69 to 75% of total lipids) and phospholipids (6 to 8%) from the microalgae Phaeodactylum tricornutum because of low nitrogen concentrations. Nigam et al. (2011) produced microalgae Chlorella pyrenoidosa at different nitrogen concentrations, to investigate its effect on growth and lipid content, and reported that as the nitrate concentration decreased, the lipid content increased but biomass production decreased. In this study, the higher TAG content of cultures under these conditions than in the control group was attributed to the N and P limitation.
Highest total carbohydrate content was found in N-PDeprivation at 20.37% and in NDeprivation-PControl at 20.22%. While these conditions have higher carbohydrate contents, their protein concentrations were found to be lowest for NDeprivation-PControl at 42.47% and N-PDeprivation at 57.89%, which show that D. affine accumulates carbohydrates as a storage compound. Highest protein content was found in NLimitation-PControl condition (66.92%). The obtained results illustrate the importance of N and P in the nutrient media for the synthesis of biochemical components of the cells. In literature, the intracellular composition of Anabaena sp. was reported as 43–56% protein, 4–7% lipid, and 25–30% carbohydrate (Becker 2007; Klinthong et al. 2015). When comparing the results obtained from our study with these values, the protein (66.92%) and lipid (10.67%) values detected in D. affine have been found higher than the protein and lipid content detected in Anabaena sp., but the content of carbohydrate (20%) has been found at a less level.
The fatty acid profiles obtained in the study are presented in Table 4. Palmitic acid in N-PDeprivation (40.61%), stearic acid in N-PLimitation and NDeprivation-PControl (64.01%), and cis-9-oleic acid in control (33.72%) were measured as the highest. Linoleic acid was highest in PLimitation-NControl (7.30%) and was not observed in N-PLimitation, NDeprivation-PControl, and N-PDeprivation groups. Cis-11-eicosanoic acid (gondoic) was highest in NLimitation-PControl (21.78%), was 14.09% in control group, and was not detected in other conditions. ALA was only found in N-PDeprivation with 29.75% ratio. In PLimitation-NControl condition, capric acid (2.28%), lauric acid (2.12%), and myristic acid (6.78%) were detected, and these fatty acids were undetected in other conditions. Řezanka et al. (2003) described fatty acids in freshwater cyanobacteria (Chroococcus minutus, Lyngbya ceylanica, Merismopedia glauca, Nodularia sphaerocarpa, Nostoc linckia, and Synechococcus aeruginosus). They found hydroxy and dioic fatty acids as minor components and hexadecenoic, 9-hexadecenoic, hexadecadienoic, octadecanoic, and 9-octadecenoic fatty acids were also identified. In this study, the essential fatty acids from D. affine cultures (Table 4) were not significantly different from the previously studied cyanobacteria (Sallal et al. 1990; Sharathchandra and Rajashekhar 2011). This study also showed that D. affine cultures under N and P limitation/deprivation might have differences in the composition and amount of fatty acids.
Research on cyanobacteria has focused largely on ecology, morphology, physiology, and 16S rRNA-based phylogeny in recent years (Abed et al. 2009). Photosynthetic prokaryotes, cyanobacteria and prochlorophytes are genetically related based on 16S rRNA sequences and are commonly used to generate a gene-based phylogeny of cyanobacteria (Woese 1987). Cyanobacterial strains such as Anabaena, Nostoc, Phormidium, Microcystis, Synechococcus, and Synechocystis have been analyzed using molecular techniques such as DNA sequencing (Neilan et al. 1997). This study carried out genetic research in the light of the literature. The results confirmed 99% of D. affine’ molecular expression consistent with other Anabaena species.
Under the class Cyanophyceae, as in other filamentous species similar to D. affine, the death of cells in the middle causes the filament to be fragmented into parts comprising a few cells. These are named “hormogonium”. They develop again and form new filaments. Hormogonium was formed under abnormal conditions; they transform into new plants in normal conditions (John et al. 2002). As Fig. 1 indicates (middle), under N and P limitation and absence, cells of D. affine generated many filaments and were unable to reach the required cell size. As a result of the increase in the number of hormogonia of D. affine, rather than storing lipid, protein, and carbohydrate in their cells, it was observed that they preferred reproduction.
Conclusion
Today, microalgae have drawn the attention of researchers on themselves due to their specifications such as their photosynthetic transposition, their rapid growth, and their capacity of producing various kinds of biofuel sources. Along with their usage in the food and pharmaceutics sectors, microalgae have lately become proper candidates for solving the energy problems especially because of their potential in the renewable energy use. Microalgae species are able to give reaction under different stress conditions by means of producing various fatty acids or changing the compositions of these fatty acids. Among the lipid increasing techniques in microalgae, nitrogen starvation is used as the most widespread; also the changes in temperature, pH, salinity, and heavy metals may increase the lipid production in an effective way. The clearest way to comprehend how the environment can change the microalgae metabolism and how to increase the lipid production is obtained by examining the species under the controlled laboratory conditions. In this study, it has been aimed also to detect under the controlled laboratory conditions the changes brought by the effect of N and P limitations and absences in the nutrition medium over the production rate and biochemical composition of cyanobacteria D. affine. The results obtained have shown that these practices applied in the nutrition medium affected the concentration of the seconder metabolites of D. affine and the amount of biomass. So, in the algal production, ensuring the industrially usable seconder metabolites (lipid, protein, carbohydrate, pigment etc.) and the effect of this method used in its production have been observed. In the light of the results obtained, through optimizing its growth medium, algae production can be ensured at lower costs and at large-scale productions. But, it is considered that developing hybrid production systems being low-costed but with high productivity rate is needed by taking into consideration the various environmental factors at large-scale productions, and there is a need to carry out explorations on this issue.
References
Abed RMM, Dobretsov S, Sudesh K (2009) Applications of cyanobacteria in biotechnology. J Appl Microbiol 106:1–12
Alonso DL, Belarbi EH, Fernández-Sevilla JM, Rodríguez-Ruiz J, Grima EM (2000) Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 54:461–471
AOAC (1990) Official methods of analysis of the association of official analytical chemists. Arlington
Battah MG, El-Ayoty YM, Esmael AE, Abd El-Ghany SE (2014) Effect of different concentrations of sodium nitrate, sodium chloride, and ferrous sulphate on the growth and lipid content of Chlorella vulgaris. J Agric Technol 10(2):339–353
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Benning LG, Phoenix VR, Yee N, Tobin MJ (2004) Molecular characterization of cyanobacterial silification using synchrotron infrared micro-spectroscopy. Geocim Cosmochim Acta 68:729–741
Bhamawat PM (2010) Growth of Chlamydomonas reinhardtii under nutrient-limited conditions in steady-state bioreactors. Dissertation, Faculty of the Graduate School, of Cornell University.
Bojović BM, Stojanović J (2005) Chlorophyll and carotenoid content in wheat cultivars as a function of mineral nutrition. Arch Biol Sci 57:283–290
Castenholz RW (2001) Phylum BX. Cyanobacteria. Oxygenic photosynthetic bacteria. In: Garrity G, Boone DR, Castenholz RW (eds) Bergey’s manual of systematic bacteriology. Springer-Verlag, New York, pp 474–487
Chia MA, Lombardi AT, Melao MGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. Ann Braz Acad Sci 85(4):1427–1438
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotech 26:126–131
Dahms HU, Xu Y, Pfeiffer C (2006) Antifouling potential of cyanobacteria: a mini-review. Biofouling 22:317–327
Das K, Sarma GC (2015) Optimization of culture media for the growth of Anabaena spiroides and Nostoc punctiformae of Jorhat district, Assam. IOSR J Pharm Biol Sci 10(2):37–41
Dean AP, Martin MC, Sigee DC (2007) Resolution of codominant phytoplankton species in a eutrophic lake using synchrotron-based Fourier transform infrared spectroscopy. Phycologia 46(2):151–159
Dutta D, De D, Chaudhuri S, Bhattacharya SK (2005) Hydrogen production by cyanobacteria. Microb Cell Factories. https://doi.org/10.1186/1475-2859-4-36
Duygu DY, Udoh AU, Ozer TB, Akbulut A, Erkaya IA, Yildiz K, Guler D (2012) Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr J Biotechnol 11(16):3817–3824
Garcia-Pichel F, Pringault O (2001) Cyanobacteria track water in desert soils. Nature 413:380–381
Giordano M, Kansiz M, Heraud P, Beardall J, Wood B, McNaughton D (2001) Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine alga Chaetoceros muellerii (Bacillariphyceae). J Phycol 37:271–279
Godoy-Hernández G, Vázquez-Flota FA (2006) Growth measurements: estimation of cell division and cell expansion. In: Loyola-Vargas VM, Vázquez-Flota F (eds) Plant cell culture protocols. Humana Press Inc, New Jersey, pp 51–58
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Guiry MD, Guiry GM (2018) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Cited 21 October 2018
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S (2010) Biofuels from algae: challenges and potential. Biofuels 1(5):763–784
Hempel N, Petric I, Behrendt F (2012) Biomass productivity and productivity of fatty acids and amino acids of microalgae strains as key characteristics of suitability for biodiesel production. J Appl Phycol 24:1407–1418
Hsieh CH, Wu WT (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Hu H, Gao K (2005) Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol Lett 28:987–992
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Ilavarasi A, Mubarakali D, Praveenkumar R, Baldev E, Thajuddin N (2011) Optimization of various growth media to freshwater microalgae for biomass production. Biotechnology 10(6):540–545
John DM, Witton BA, Brook AJ (2002) The freshwater algal Flora of the British Isles. Cambridge
Johnson X, Alric J (2013) Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: metabolic constraints for carbon partitioning between oil and starch. Eukaryot Cell 12:776–793
Kaplan D, Richman A, Boussiba S, Vonshak A, Cohen Z, Abeliovich A (1986) Development of an outdoor system for production of lipid-rich halotolerant microalgae: effect of light on biomass production in two halotolerant microalgae Nannochloropsis salina and Isochrysis galbana. Solar Energy Research Institute, Golden
Klinthong W, Yang YH, Huang CH, Tan CH (2015) A review: microalgae and their applications in CO2 capture and renewable energy. Aerosol Air Qual Res 15:712–742
Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 102:106–110
Metcalf LD, Schmitz AA, Pelka JR (1966) Rapid preparation of fatty acid esters from lipids for gas chromatographic. Anal Chem 38:514–515
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283
Nalewajko C, Murphy TP (2001) Effects of temperature, and availability of nitrogen and phosphorus on the abundance of Anabaena and Microcystis in Lake Biwa, Japan: an experimental approach. Limnology 2:45–48
Neilan BA, Jacobs D, del Dot T, Blackall LL, Hawkins PR, Cox PT, Goodman AE (1997) rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int J Syst Bacteriol 47:693–697
Nigam S, Rai MP, Sharma R (2011) Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Am J Biochem Biotechnol 7(3):124–129
Parsons TR, Strickland JDH (1963) Discussion of spectrophotometric determination of marine plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. J Mar Res 21(3):115–163
Parvin M, Zannat MN, Habib MAB (2007) Two important technique for isolation of microalgae. Asian Fish Sci 20:117–124
Ponnuswamy I, Madhavan S, Shabudeen S (2013) Isolation and characterization of green microalgae for carbon sequestration, waste water treatment and bio-fuel production. Int J Bio Sci Bio Technol 5(2):17–26
Prescott GW (1973) Algae of the western great lakes area. C. Brown Company Publishers, Michigan
Pruvost J, Vooren G, Gouic B, Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102:150–158
Quintana N, Van der Kooy F, Van de Rhee MD, Voshol GP, Verpoorte R (2011) Renewable energy from cyanobacteria: energy production optimization by metabolic pathway engineering. Appl Microbiol Biotechnol 91:471–490
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Řezanka T, Dor I, Prell A, Dembitsky VM (2003) Fatty acid composition of six freshwater wild cyanobacterial species. Folia Microbiol 48(1):71–75
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stainer RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Sallal AK, Nimer NA, Radwan SS (1990) Lipid and fatty acid composition of freshwater cyanobacteria. J Gen Microbiol 136:2043–2048
Santos-Ballardo DU, Rossi S, Hernández V, Gómez RV, Rendón-Unceta MC, Caro-Corrales J, Valdez-Ortiz A (2015) A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture 448:87–92
Schuhmann H, Lim DKY, Schenk PM (2011) Perspectives on metabolic engineering for increased lipid contents in microalgae. Biofuels 3:71–86
Sharathchandra K, Rajashekhar M (2011) Total lipid and fatty acid composition in some freshwater cyanobacteria. J Algal Biomass Utln 2(2):83–97
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Sigee DC, Dean A, Levado E, Tobin MJ (2002) Fourier-transform infrared spectroscopy of Pediastrum duplex: characterization of a micro-population isolated from a eutrophic lake. Eur J Phycol 37:19–26
Solovchenko A, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak M (2008) Effects of light and nitrogen starvation on the content and composition of carotenoids of the green microalga Parietochloris incisa. Russ J Plant Physiol 55:455–462
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Tang H, Abunasser N et al (2010) Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl Energy 88:3324–3330
Uslu L (2011) The effects of the changes in the composition of nutrient on lipid content of microalgae. Dissertation, Çukurova University Institute of Natural and Applied Sciences Basic Science Department of Fisheries
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Wong YK, Ho YH, Ho KC, Leung HM, Yung KKL (2017) Maximization of cell growth and lipid production of freshwater microalga Chlorella vulgaris by enrichment technique for biodiesel production. Environ Sci Pollut Res 24:9089–9101
Yalçın Duygu D, Erkaya Açıkgöz I, Özer T (2018) Investigating the effect of different growth media on biomass production of Pseudopediastrum boryanum (Turpin) E. Hegewald isolates. J Limnol Freshw Fish Res 4(1):6–12
Yeesang C, Cheirsilp B (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102:3034–3040
Zhu CJ, Lee YK, Chao TM (1997) Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J Appl Phycol 9:451–457
Acknowledgments
I would like to thank Andrew P. Dean from the University of Manchester for the PCR analysis and Ankara University Faculty of Veterinary Medicine for the nutrient analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she no conflict of interest.
Ethical approval
This article does not contain any studies with animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yalcin, D. Growth, lipid content, and fatty acid profile of freshwater cyanobacteria Dolichospermum affine (Lemmermann) Wacklin, Hoffmann, & Komárek by using modified nutrient media. Aquacult Int 28, 1371–1388 (2020). https://doi.org/10.1007/s10499-020-00531-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00531-2