Abstract
In this research, dietary effect of garlic powder on growth parameters, carcass composition, some blood indices, and disease resistance against Yersinia ruckeri was studied in brown trout (Salmo caspius). Two hundred forty juvenile brown trout with mean initial weight of 19.94 ± 1.10 g were divided into 12 tanks (20 juveniles per each tank). Treatments prepared based on different levels of garlic led to four experimental diets including 0 (control), 10, 20, and 30 g/kg garlic powder and were used for 6 weeks. Fish fed 20 g/kg garlic had higher value (P < 0.05) of body weight increasing and specific growth rate than those with other experimental diets. Addition of 30 g/kg garlic led to significant increase in body protein while body fat decreased numerically in 30 g/kg garlic when compared to control diet. Results of blood factors showed that Hb, Hct, WBC, RBC, MCV, MCH, and MCHC did not change by addition of garlic (P > 0.05). Lysozyme activity was improved by increasing the level of garlic in diet (30 g/kg); meanwhile, the use of 20 g/kg garlic caused the highest total protein value. Fish fed diet containing garlic showed higher survival rate against Y. ruckeri than that of control fish. Garlic powder is severely advised according to these results of improving growth, serum factors, and resistance against Y. ruckeri.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicine and antibiotics which are used to eliminate disease in aquatic animal have destructive impact on environment and ecosystems considering aquaculture development. Moreover, antibiotic utilization cause survival and increase the resistance of pathogen and eventually inactivate the antibiotic effect on pathogen (Diab et al. 2002). Using some pharmaceutical plants as a replacement for antibiotics have some advantages than that of chemical material including reduced costs and infection and more immunological effect due to having plant nature (Khodadadi et al. 2013). Garlic (Allium sativum) is a plant with antibiotic effect (Pour et al. 2014) that increases macrophage and the other immune cell in animal body (Khodadadi et al. 2013). Garlic acts as an immune stimulant and disease control in aquatic organism and improves feeding indices and body chemical composition (Diab et al. 2008; Janz et al. 2007; Shalaby et al. 2006; Demir et al. 2003). Garlic caused enhancement of lysozyme activity in hybrid tilapia (Oreochromis niloticus x Oreochromis aureus) (Ndong and Fall 2011). Allicin is a sulfur compound which has an important role as antibacterial, antifungal, and antioxidant material. Furthermore, amino acids, minerals, vitamins, and flavonoids are the other compounds in garlic (Lanzotti 2006; Crozo-Martinez et al. 2007). Brown trout (Salmo caspius) belongs to the salmonid family which is important because of pleasurable meat, economic value, and stock preservation. Salmo caspius is an anadromous species which migrates up to the river for spawning but lives in the Caspian Sea (Askarifar et al. 2015). Yersinia ruckeri is a common bacterium that infects the salmonid family, and antibiotics are used to control the disease. The antibacterial property of 1 mg allicin has been reported equal to that of 15 IU penicillin (Khodadadi et al. 2013). On the other hand, garlic improves growth and food conversion ratio by stimulating appetite (Abd-El Allatif and Ebraheem 1996; Metwally 2009). A study was conducted to clear the garlic performance from the immune system and influence of garlic powder in S. caspius considering the positive effect of garlic on feed utilization and immunological improvement.
Materials and methods
Fish preparation and treatment
Juvenile brown trout with initial mean weight of 19.94 ± 1.10 g were obtained from an institute rearing brown trout in Tonekabon (Iran) and transferred to Sari Agriculture Science and Natural Resources University, Iran. The fish were fed with commercial feed (GFT1, MANAQUA, Mazandaran, Iran) for 2 weeks for adaptation. Physical observations and bacteriology were used to ensure that healthy fish were used in this experiment (Adel et al. 2016). Afterward, 240 of the brown trout were distributed into 12 polyethylene tanks (20 juveniles in each tank) with 250 L of water preservation volume. Different treatments were based on garlic level in diet that included four diets, 0, 10, 20, and 30 g/kg garlic (Farahi et al. 2010). The fish were fed manually to apparent satiation level two times daily at 09.00 and 18.00 h during 6 weeks of trial. Photoperiod was the same for all treatments, 12 h light/12 h darkness. The water parameters were measured weekly to control the water quality which was DO 7.66 ± 0.25 mg L−1, NO2 + 0.15 mg L−1, pH 7.42 ± 0.1, salinity 0.4 ppt, electrical conductivity 598.13 ± 12.8 μs cm−1, and water temperature 14.15 ± 0.27 °C during the experiment. Continuous aeration was provided to each tank through an air stone connected to a central air compressor.
Experimental diet
Garlic bulbs were obtained from local market crushed and well-dried in an oven at a maximum temperature of 50 °C and were turned to garlic powder. Commercial food of rainbow trout (GFT1) were purchased from the aquatic food company of Sari (MANAQUA, Iran) and crunched in grinder (MULINEX 30214). The commercial food was mixed with garlic powder in doses of 0, 1, 2, and 3 g/kg feed and then pelletized using a meat grinder with a 2-mm mesh size (Cerezuela et al. 2008). Pellets were air-dried at 50 °C and stored at −20 °C until use. The proximate composition of experimental diets is given in Table 1.
Experimental procedure
On the last day of the experiment, all fish from each tank were weighed individually. Growth parameters including body weight increasing (BWI), specific growth rate (SGR), feed conversion ratio (FCR), conditional factor (CF), and survival rate were measured using these formulas (Ai et al. 2004; Kestemont et al. 2007).
where W f, W i = final and initial weight of fish (g), t = time (days), F = feeding intake (g), W = fish weight, and L = total length.
Analyses of carcass and experimental food were according to AOAC 2005. For this intention, three fish were randomly selected from each tank and sacrificed using overdosed clove essence solution and integrated by grinder. Feed and carcass were analyzed for dry matter by drying all samples for 24 h at 105 °C until constant weight (ISO 1983). Ash content was determined by incineration in a muffle furnace at 550 °C for 4 h (ISO 1978). Crude protein (N 9 6.25) was measured by the Kjeldahl method after acid digestion according to ISO 1979. Fat was extracted by petroleum ether in a Soxhlet apparatus.
Hematological and immunological parameters
Five fish were randomly taken from each tank and anesthetized using 120 mg/L clove oil solution after they were starved for 24 h (Anderson et al. 1997). Blood samples were collected thoroughly by 1.5-mL syringes and transferred into the tube containing heparin to prevent clotting. Blood factors, like white blood cells (WBC), red blood cells (RBC) according to Hoston (1990), hemoglobin (Hb), and hematocrit (Hct) as described by Drobkin (1945), were measured. Blood serum was separated by centrifuging blood in 4600 rpm for 10 min. Total protein, glucose, and albumin were determined via the method of Olesen and Jorgensen (1986). Lysozyme activity was calculated by photo absorbance of samples in a biophotometer (Sartorius, Germany) according to the methods of Ellis (1990).
Preparation of bacteria
After 6 weeks of trial, fish resistance against Y. ruckeri (ATCC ،KC291153) had been examined for 2 weeks. The strain of bacteria was prepared and lyophilized in Persian Type Culture Collection (PTCC), Tehran, Iran, cultivated in TBS plate using a standard procedure in the Institute of Caspian Sea Ecology in Sari, Iran, and then transferred into a Muller-Hinton broth plate in sterile condition. A final concentration of 1.5 × 106 was obtained and injected to fish intraperitoneally (Goudarzi et al. 2011). Mortality induced by Yersinia infection was determined by counting the number of brown trout died showing signs of disease during 2 weeks after injection.
Statistical analysis
Data are presented as means of each treatment measurement with standard deviation. All data were verified for the normality distribution of data by Kolmogorov-Smirnov test. One-way ANOVA was used to determine the effects of the garlic on fish. Duncan test was employed to compare differences between the means at 0.05% probability. SPSS version 22 for Windows software program and Excel was used for analysis and drawing chart, respectively.
Results
Growth performance and feed efficiency
Fish were gradually adopted to get the experimental diet during the first days. According to Table 2, final weight, body weight increasing, and specific growth rate were significantly (P < 0.05) increased in fish fed diet containing 20 g/kg garlic over control diet, while visceral somatic index and hepatosomatic index were significantly increased in diet with 10 g/kg garlic (P < 0.05). There were no significant differences in conditional factor, food conversion ratio, and survival rate between groups fed garlic and control diet (P > 0.05). Survival rate was 100% in all groups.
Body composition
Analysis of carcass composition of brown trout is shown in Table 3. Addition of 30 g/kg garlic to diet led to significant increase in body protein (P < 0.05). Moisture and ash content were higher in diet containing 10 g and 20 g/kg garlic, respectively (P < 0.05). However, no significant differences were observed in body fat (P > 0.05).
Hematological parameters
No considerable changes was evident in blood parameters including hematocrit, hemoglobin, white blood cells, red blood cells, MCV, MCH, and MCHC among groups as shown in Table 4 (P > 0.05).
Immunological factors
Serum indices
Glucose and albumin showed no significant differences between fish fed garlic and control group (P > 0.05), while total protein had significant higher value in diet containing 20 g/kg garlic than that in the other diets (Table 5). The lower value of total protein was observed in fish fed control diet (P < 0.05).
Lysozyme activity
Lysozyme activity was increased significantly by addition of garlic (P < 0.05; Table 5). The value of lysozyme activity was higher in garlic supplementation at 30, 20, and 10 g/kg, respectively, over control group without garlic (P < 0.05).
Bacterial challenge
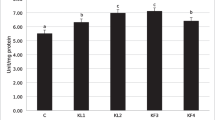
Survival percentage of fish fed garlic supplementation and control diet against Y. ruckeri for 14 days of challenge after 6 weeks of feeding period are shown in Fig. 1. The results showed that the survival rate of bacteria challenge in fish fed garlic is more than that of the control group. The survival percentage of fish were 80, 70, and 50 in garlic addition of 30, 20, and 10 g/kg to the diet, respectively. The survival rate was higher in garlic administration at 30 g/kg and it was 80%. Furthermore, mortality began on the sixth day of challenge in fish fed 20 and 30 g/kg garlic and on the third day in fish fed diet containing 10 g/kg garlic. While in the control group, mortality commenced from the first day of experiment and it was 100% after 6 days of challenge. All of the fish died showed sign of infection.
Discussion
In this study, the effect of garlic supplementation in brown trout diet was investigated. Results of growth performance represented that the addition of 20 g/kg garlic to diet led to higher BWI and SGR than in those of the control diet. Since the control diet which was without garlic supplementation had lower value of BWI and SGR, it could say that garlic led to enhanced growth performance. The bioactive compound of garlic, allicin, enhanced growth by stimulating the digestive enzyme and balancing the enteric microbial flora (Talpur and Ikhwanuddin 2012; Khalil et al. 2001). In many other works, dietary garlic had positive effect on BWI and SGR that are similar to this work (Diab et al. 2002; Shalaby et al. 2006; Nya and Austin 2009; Farahi et al. 2010). Moreover, dietary garlic in Asian sea bass (Lates calcarifer) resulted in the increase of growth and survival rate (Talpur and Ikhwanuddin 2012). In the present research, survival rate before bacterial challenge was 100% in all treatments. Positive effect of garlic supplementation in cichlid (Tilapia wesafu) (Megbowon 2013) and sterlets (Acipenser ruthenus) (Lee et al. 2012) has been studied in the recent years. On the other hand, high levels of dietary garlic in diet have an opposite effect because of pungent smell (Platel and Srinivasan 2004; Aly et al. 2008). In the present study, 30 g/kg garlic in diet had lower growth than that in 20 g/kg garlic which could be because of garlic’s pungent smell. Higher visceral index in 10 g/kg garlic addition seems to be because of more feed intake induced by increasing appetite. It is possible that decreasing the value of visceral index in higher level of dietary garlic may be due to improved digestion and high performance of garlic in higher value. Analysis of the body composition of brown trout fed treatments containing garlic powder showed body protein increased by increasing the levels of garlic supplement while body fat decreased numerically in 30 g/kg of garlic than that of the other diets. These results are comparable to those reported in Nile tilapia (Oreochromis niloticus), Asian sea bass (L. calcarifer), and benni fish (Mesopotamichthys sharpeyi) (Khattab et al. 2005; Shalaby et al. 2006; Talpur and Ikhwanuddin 2012; Maniat et al. 2014). Garlic compounds lead to increase bile acid in hamster according to some researches (Yaoling et al. 1998). Allicin existent in garlic prevents the accumulation of fat in fish body possibly by the effect of bile acid which facilitates fat digestion (Elkayam et al. 2003). Body fat was not significantly different between treatments but numerically decreased by garlic addition. This diversity in results between studies could be because of the differences in the experimental period. Bioactive compounds act differently in long term (Bricknell and Dalmo 2005; Fazlolahzadeh et al. 2011). The hematological parameters were measured to clarify the health status of fish. Blood factors including Hb, Hct, WBC, RBC, MCV, MCH, and MCHC did not show significant differences between groups. In the study of Talpur and Ikhwanuddin (2012), Fazlolahzadeh et al. (2011), and Nya and Austin (2009), it has been found that addition of garlic enhances the hematological parameters. The reason of this contradiction may be the experimental condition and fish species. Hematological parameters are affected by stressful condition (Talpur and Ikhwanuddin 2012). Furthermore, the immune system has been suppressed by immune stimulator during long term, so results are influenced by the feeding period (Bricknell and Dalmo 2005). Glucose and albumin were lower numerically in the fish fed diet containing garlic compared to those in the control fish. This result was similar to that of authors (Shalaby et al. 2006; Sahu et al. 2007; Talpur and Ikhwanuddin 2012) in which glucose and albumin value decreased when garlic added to the diet. Total protein was significantly higher in fish fed dietary garlic that was considered as the bioindicator of immune stimulation (Jha et al. 2007; Nya and Austin 2009). In the study of Nya and Austin (2009), garlic addition to diet increased antiprotease activity in rainbow trout. Increasing total protein may be due to antiprotease activity induced by garlic which caused total protein enhancement. The result of lysozyme activity represents that the immune system was improved by garlic addition which could be explained by the role of lysozyme in humoral immunity. Similarly, in the study of Talpur and Ikhwanuddin (2012) in Asian sea bass (L. calcarifer), lysozyme activity increased by the inclusion of 10, 15, and 20 g/kg garlic to the diet. Moreover, 5 and 10 g/kg of garlic supplementation to diet led to increase lysozyme activity in rainbow trout (Nya and Austin, 2009). Lysozyme restrains infection by preventing pathogen connectivity and reproduction (Mirsa et al. 2004; Mirsa et al. 2006). Enhancement of lysozyme activity indicates antibacterial property of garlic. Garlic acts as an immunological bioactive stimulator and multiplies lymphocyte in the time of disease resistance (Eggset et al. 1997). Bacterial resistance in brown trout showed higher survival rate in fish fed diet containing garlic than that of control diet during 2 weeks of challenge against Y. ruckeri, while all of the control fish perished in day 6 of challenge with signs of Yersinia disease. Also, more survival rates were obtained by increasing garlic level in diet. This could be due to garlic antibacterial and immune stimulant properties. Similarly, administration of 5 and 10 g/kg garlic led to increase bacterial resistance and protection against Aeromonas hydrophila (Nya and Austin 2009). Similar results have been obtained in the work of Sahu et al. (2007) on Labeo rohita. Also, dietary garlic had positive effects on protection of Asian sea bass against Vibrio harveyi (Talpur and Ikhwanuddin 2012).
In conclusion, the present study supports the idea of garlic stimulant effect on the immune system in fish, considering positive effect of garlic on growth, increasing the lysozyme activity and also brown trout protection against Y. ruckeri. Twenty grams per kilogram garlic is advised to promote growth and immunological enhancement.
References
Abd-Elallatif A, Ebraheem K (1996) Studies on the effects of Hibiscus subdariffa, Allium sativum and Negella sativa on some bacterial isolates of chickens. Fac Vet Med Assute University Egypt 17:245–251
Adel M, Safari R, Yeganeh S et al (2016) Effect of GroBiotic®-A supplementation as a prebiotic on the intestinal microflora, growth performance, haemato-serological parameters, survival rate and body composition in juvenile beluga (Huso huso Linnaeus 1754). Aquaculture Nutrition 1–8
Ai Q, Mai K, Zhang C et al (2004) Effects of dietary vitamin C on growth and immune response of Japanese seabass, Lateolabrax japonicus. Aquaculture 242:489–500
Aly SM, Atti NMA, Mohamed MF (2008) Effect of garlic on the survival, growth, resistance and quality of Oreochromis niloticus. Paper presented at 8th International Symposium on Tilapia in Aquaculture, The Central Laboratory for Aquaculture Research, Cairo, Egypt, 12–14 October 2008, 277–296 P
Anderson WG, McKinley RS, Colavecchia M (1997) The use of clove oil as an anaesthetic for rainbow trout and its effect on swimming performance. North Am J Fish Mana 17:301–307
AOAC (2005) Official Methods of Analysis, 16th edn. Association of Official Analytical Chemists, Washington DC, USA
Askarifar S, Hedayatifard M, Khara H (2015) Improvement of growth properties, survival, hematological-humoral immunity factors and intestinal bacterial density of brown trout (Salmo trutta caspius Kessler, 1870) affected by different levels of Beta-Plus as probiotic. Aquat Physiol Biotechnol 3(3):1–28
Bricknell I, Dalmo RA (2005) The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol 19:457–472
Cerezuela R, Cuesta A, Meseguer J et al (2008) Effects of inulin on gilthead seabream (Sparus aurata L.) innate immune parameters. Fish Shellfish Immunol 24(5):663–668
Corzo-Martinez M, Corzo N, Villamiel M (2007) Biological properties of onions and garlic. Trends Food Sci Tech 18:609–625
Demir E, Sarica S, Özcan MA et al (2003) The use of natural feed additives as alternatives for an antibiotic growth promoter in broiler diets. Br Poult Sci 44:44–45
Diab AS, El-Nagar GO, Abd-El-Hady YM (2002) Evaluation of Nigella sativa L (black seeds; baraka), Allium sativum (garlic) and BIOGEN as feed additives on growth performance and immunostimulants of Oreochromis niloticus fingerlings. Suez Canal Vet Med J 745–775
Diab AS, Aly SM, John G et al (2008) Effect of garlic, black seed and Biogen as immunostimulants on the growth and survival of Nile tilapia, Oreochromis niloticus (Teleostei: Cichlidae), and their response to artificial infection with Pseudomonas fluorescens. Afr Aqua Sci 33(1):63–68
Drobkin DR (1945) Crystallographic and optical properties of human hemoglobin: proposal for standardization of hemoglobin. Am J Med Sci 209:268–270
Eggset G, Mikkelsen H, Killie JA (1997) Immunocompetence and duration of immunity against Vibrio salmonicida and Aeromonas salmonicida after vaccination of Atlantic salmon (Salmo salar L.) at low and high temperatures. Fish Shellfish Immunol 7:247–260
Elkayam A, Mirelman D, Peleg E et al (2003) The effects of allicin on weight in fructose-induced hyperinsulinemic, hyperlipidemic, hypertensive rats. Am J Hypertens 16(12):1053–1056
Ellis AE (1990) Lysozyme assay, techniques in fish immunology, 2nd edn. Fair Haven, USA, pp 100–102
Farahi A, Kasiri M, Sudagar M et al (2010) Effect of garlic (Allium sativum) on growth factors, some hematological parameters and body compositions in rainbow trout (Oncorhynchus mykiss). AACL BIOFLUX 3(4):317–323
Fazlolahzadeh F, Keramati K, Nazifi S et al (2011) Effect of garlic (Allium sativum) on hematological parameters and plasma activities of ALT and AST of rainbow trout in temperature stress. Aust J Basic Appl Sci 5(9):84–90
Goudarzi MA, Hamedi B, Malekpoor F et al (2011) Sensitivity of Lactococcus garvieae isolated from rainbow trout to some Iranian medicinal herbs. J med Plant res 5:3067–3073
Hoston AH (1990) Blood and circulation. In: Shreck CB, Moyle PB. Methods in fish biology. Bethesda, Maryland: American Fisheries society. P: 273-335
ISO (1978) Animal feeding stuffs. Determination of crude ash. ISO 5984. International Organization for Standardization, Geneva, Switzerland
ISO (1979) Animal feeding stuffs. Determination of nitrogen content and calculation of crude protein content. ISO 5983. International Organization for Standardization, Geneva, Switzerland
ISO (1983) Animal feeding stuffs. Determination of moisture content. ISO 6496. International Organization for Standardization. Geneva, Switzerland
Janz JAM, Morel PCH, Wilkinson BHP et al (2007) Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci 75(2):350–355
Jha AK, Pal AK, Sahu NP et al (2007) Haemato-immunological responses to dietary yeast RNA, w-3fatty acid and beta-carotene in Catla catla juveniles. Fish Shellfish Immunol 23:917–927
Kestemont P, Xueliang X, Hamza N et al (2007) Effect of weaning age and diet on pikeperch larviculture. Aquaculture 264:197–204
Khalil RH, Nadia BM, Soliman MK (2001) Effects of Biogen and Levamisol Hcl on the immune response of cultured Oreochromis niloticus to Aeromonas hydrophila vaccine. Beni-Suef Vet Med J Egypt XI(2):381–392
Khattab YAE, Shalaby AME, Abdel-Rhman AA (2005) Use of probiotic bacteria as growth promoters, anti-bacterial and their effects on physiological parameters of Oreochromis niloticus. Aquaculture 28:74–81
Khodadadi M, Peyghan R, Hamidavi A (2013) The evaluation of garlic powder feed additive and its effect on growth rate of common carp, Cyprinus carpio. J Clin Vet Sci Iran 6(2):17–26
Lanzotti V (2006) The analysis of onion and garlic. J Chromatogr a 1112:3–22
Lee DH, Ra CS, Song YH et al (2012) Effect of dietary garlic extracts on growth, feed utilization and whole body composition of juvenile sterlet sturgeon (Acipenser ruthenus). Asian Australas J Anim Sci 25(4):577–583
Maniat M, Ghotbeddin N, Rajabzadeh-Ghatrami E (2014) Effect of garlic on growth performance and body composition of benni fish (Mesopotamichthys sharpeyi). Int J Biosci 5:269–277
Megbowon I, Adejonwo OA, Adeyemi YB et al (2013) Effect of garlic on growth performance, nutrient utilization and survival of an ecotype cichlid, ‘Wesafu’. IOSR J Agr Vet Sci 6(3):10–13
Metwally MAA (2009) Effects of garlic on some antioxidant activities in Tilapia nilotica. World J Fish Mar Sci 1:56–64
Misra CK, Das J, Pradhan P et al (2004) Changes in lysozymal enzyme activity and protection against Vibrio infection in Macrobrachium rosenbergii (De man) post larvae after bath immuno-stimulantion with β-glucan. Fish Shellfish Immunol 17:389–395
Misra CK, Das BK, Mukherjee SC et al (2006) Effect of multiple injections of beta-glucan on non-specific immune response and disease resistance in Labeo rohita fingerlings. Fish Shellfish Immunol 20:305–319
Ndong D, Fall J (2011) The effect of garlic (Allium sativum) on growth and immune responses of hybrid tilapia (Oreochromis niloticus x Oreochromis aureus). J Clin Immunol Immunopathol Res 3(1):1–9
Nya EJ, Austin B (2009) Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish dis 32:963–970
Olesen NJ, Jorgensen PV (1986) Quantification of serum immunoglobulin in rainbow trout (Salmo gairdneri) under various environmental conditions. Dis Aquat org 1:183–189
Platel K, Srinivasan K (2004) Digestive stimulant action of spices: a myth or reality? Indian J med res 119:167–179
Pour F, Maniat M, Vahedasl A et al (2014) Enhancement of growth performance and body composition in molly fish (Poecilia sphenops) associated with dietary intake of garlic (Allium sativum). Int J Biosci 5(8):115–121
Sahu S, Das BK, Mishra BK et al (2007) Effects of Allium sativum on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. J Appl Ichthyol 23:80–86
Shalaby AM, Khattab YA, Abdel Rahman AM (2006) Effects of (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). J Venom Anim Toxins 12:172–201
Talpur AD, Ikhwanuddin M (2012) Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 364:6–12
Yaoling L, Jiunrong C, Mengsyh S et al (1998) The effects of garlic powder on the hypolipidemic function and antioxidative status in hamsters. J Sci Nut 23:171–187
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaefarian, A., Yeganeh, S. & Adhami, B. Dietary effects of garlic powder (Allium sativum) on growth, blood indices, carcass composition, and lysozyme activity in brown trout (Salmo caspius) and resistance against Yersinia ruckeri infection. Aquacult Int 25, 1987–1996 (2017). https://doi.org/10.1007/s10499-017-0169-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0169-3