Abstract
Soybean meal was subjected to autoclaving for different durations (0, 5, 10, 15, and 20 min) to alter its protein solubility index (PSI). As a result of autoclaving, the PSI of soybean meal was reduced from 85–64% but the protein quantity was not affected. Among amino acids, methionine and cystine were reduced significantly (P < 0.05) beyond autoclaving for 15 min. Trypsin inhibitor was below detectable level after 20 min of autoclave. Saponin and phytic acid were reduced by 0.3–8 and 1–24%, respectively, in treated soybean meal. Five iso-nitrogenous diets were formulated by replacing untreated soybean meal using processed soybean meal, and its effects on growth performance, feed efficiency, and digestibility parameters were assessed in Penaeus vannamei. The results revealed that the growth rate was not affected (P > 0.05) in shrimp fed with diets having soybean meal autoclaved up to 10 min (PSI 72%). The similar trend was noticed in feed efficiency parameters. The apparent dry matter and crude protein digestibility parameters were reduced (P < 0.05) in shrimp fed diets having soybean meal autoclaved for 15 and 20 min (PSI 68 and 64%). The inclusion of processed soybean meal has not influenced the shrimp carcass composition. The present study showed that though anti-nutritional factors were reduced in prolonged heat treatment, the declined protein solubility has resulted in the reduction of growth parameters and digestibility in those treatments. Hence, the present preliminary results suggest to scrutinize the quality of protein whenever heat is being applied during the processing of soybean meal and also other protein sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nutritional quality of any ingredient is assessed by its gross chemical composition. The protein digestibility and its amino acid composition generally, determine the protein quality. Protein solubility index (PSI) is most widely used to determine the overprocessing of protein sources especially for soybean meal (Araba and Dale 1990) as this influences its utilization. Searching for alternative ingredients for fishmeal is a priority area of research in aquaculture nutrition. The plant protein sources are widely used in multi feed formulations but their usage was limited in shrimp feeds because of the challenging problems of digestibility due to higher fiber fraction, anti-nutritional factors, and imbalanced profiles of certain essential amino acids (methionine, lysine, and tryptophan). In order to increase the utilization of plant protein sources in shrimp feeds, various pre-digestive processes were employed nowadays (Shi et al. 2015), but the preference was given to solid state fermentation by the researchers due to less drawbacks of all the invented processing methods (Wee 1991).
The microbial fermentation has improved the nutritional quality but reduced the PSI of the soybean meal by 10% (Chen et al. 2010). The reduced PSI was attributed to the temperature and duration employed during autoclave (Chen et al. 2010), because fermentation with specific inoculum needs sterilization by autoclaving to prevent the cross contaminations. The process of autoclaving denatures the protein and results in decreased protein hydrolysis and thereby reduced protein availability to the cultured species (Chen et al. 2010). The effect of protein solubility has been studied in terrestrial animals like pig and poultry in an earlier study (Parsons et al. 1991), but so far, there were no reports available in aquatic species. Fermented soybean meal currently represents as a predominant choice as alternative to fishmeal in shrimp feed (Imelda Joseph et al. 2008; Shiu et al. 2015; Sharawy et al. 2016); there is need to address the quality of protein when heat is being applied during the processing. Despite nutrient enrichment, the reduced protein solubility during microbial fermentation (Chen et al. 2010) spurred to study the minimum required quality of protein in the processed samples. Hence, in the present study, soybean meal was selected and its chemical indicators viz., proximate constituents, amino acid composition, anti-nutritional factors and protein solubility, and the biological indicators, viz. growth, digestibility, and feed efficiency, were investigated to determine the threshold of heat treatment with minimum damage to protein solubility of soybean meal in the diet of Penaeus vannamei.
Materials and methods
Sample preparation and experimental diets
The solvent extracted commercial soybean meal having 480 g kg−1 of crude protein (540 g kg−1 dry matter basis) was sourced (n = 6) from local markets in and around Chennai, India, and was ground to a particle size of <500 μm using a micropulverizer. The ground soybean meal was hydrated with water to obtain approximately 60 to 65% moisture with pH of 6.5 to 7.0 and then autoclaved at 121 °C (105 kPa) for 0, 5, 10, 15, and 20 min to produce a range of protein solubility indices. The autoclaved soybean meal was dried at room temperature and was analyzed for chemical compositions. Five iso-nitrogenous diets were prepared by using processed soybean meal (Table 1). All the dry ingredients were mixed with oil sources and water in a domestic mixer and manually kneaded into dough. The homogenized dough was steamed for 5 min at atmospheric pressure and pelleted in a table top pelletizer with a 2-mm diameter die (Dayal et al. 2003). 5 g kg−1 of chromic oxide was used in all the diets as an inert marker to study the apparent digestibility parameters. The pellets were dried in a forced-air oven at 60 °C for 12 h and stored in a refrigerator until being used.
Biochemical analysis
The protein solubility index (PSI) was analyzed according to Araba and Dale (1990). Briefly, 1 g of sample was incubated with 50 ml of 0.2% KOH in a shaking incubator at room temperature for 20 min. The content was centrifuged at 6000 rpm for 10 min and the known volume of supernatant was analyzed for protein by Kjeldhal method. The proximate composition of ingredients, feed, and shrimp carcass in terms of moisture, crude protein, ether extract, crude fiber, and total ash was analyzed by AOAC (1997) method. Briefly, the moisture content was determined by drying the samples at 105 °C in a hot air oven overnight. Nitrogen content was analyzed by micro Kjeldahl method (Kjeltec™-8100, Tecator™ Line), and the analyzed nitrogen was converted into crude protein by multiplying with common empirical factor of 6.25. The ether extract was estimated using petroleum ether (60 to 80 °C) in Soxhlet extraction unit (Scocs Plus-SCS 6). The samples were digested using 1.25% sulphuric acid (30 min) followed by 1.25% sodium hydroxide (30 min) using Fiber cap method (FOSS-2022, Tecator™) for determining the crude fiber. The total ash content was measured by incinerating samples at 540 °C in muffle furnace for 6 h. Nitrogen-free extract was calculated by difference.
Amino acid profiles were analyzed using a pre column HPLC gradient system (Shimadzu Corp, LC-30 AD) after hydrolyzing the samples with 6 N hydrochloric acid in a sealed tube for 22 h at 110 °C in a vacuum oven (Finlayson 1964). The excess acid was evaporated using a vacuum rotary evaporator and the residue was placed into a diluent (0.1 N hydrochloric acid) and then filtered using a 0.2-μm membrane syringe filter. Separation of amino acids was done in a column (YMC-Triart C18, RRHD 1.8 μm, 2.1 × 100 mm dimension) under gradient elution using phosphate buffer (20 mmol) as mobile phase A and combination of acetonitrile:methanol:water (45:40:15) as mobile phase B at the flow rate of 0.3 ml min−1. The gradient was changed by increasing mobile phase B concentration at the rate of 11 to 13% for 3 min, 31% for 5 min, 37% for 15 min, 70% for 20 min, and 100% for 25 min. Mercaptopropionic acid, O-pthaladehyde, and fluorenylmethoxycarbonyl chloride were used as derivatizing agents. Amino acids were qualified and quantified by fluorescent detector (RF-20AXS) using amino acid mixer as an external standard (Sigma Aldrich, Cat. No: AAS18-5ML). Tryptophan, being labile to acid hydrolysis, was measured after alkali hydrolysis by spectrophotometric method at 500 nm (Sastry and Tammuru 1985). The partial oxidation of other sulfur containing amino acids (cystine and methionine) during acid digestion was prevented by adding 0.1% phenol (Jajic et al. 2013).
Trypsin inhibitor was assayed by determining the residual trypsin activity using Kakade et al. (1974) method with slight modifications. Briefly, trypsin inhibitor was extracted by 0.01 N sodium hydroxide for 3 h. The reaction mixture contained 2 ml of extract, 2 ml of trypsin, and 5 ml of BAPA (trypsin substrate) were incubated at 37 °C for 10 min. The reaction was immediately stopped after incubation period by adding 1 ml of acetic acid (30%). A blank and control were also run simultaneously. The absorbance was read at 410 nm in UV-spectrophotometer (Shimadzu, UV-1800). Decrease in 0.019 of ∆A indicates the presence of 1 μg of Trypsin inhibitor in the sample.
Saponin was extracted from the acetone extracted residual matter for 3 h using methanol (AOAC 1997). To 1 ml of methanolic extract, water and organic solvents (chloroform and methanol) were added at the ratio of 1:2 and allowed to separate after mixing thoroughly. The upper aqueous layer (1 ml) was kept at 110 °C in a hot air oven till complete evaporation of the solvent. To which 0.1 and 0.4 ml of vannilin reagent and perchloric acid were added, respectively, and kept at 70 °C for 10 min. The intensity of color development was read at 540 nm in a UV spectrophotometer (Shimadzu, UV-1800) after adding 2.5 ml acetic acid. Diosgenin was used as a standard at different concentration to calculate the saponin content.
The estimation of phytic acid was carried out by Davies and Reid (1979) method after extracting the samples with 0.5 N nitric acid for 3 h. To the known volume of extract, 1 ml of ferric ammonium sulfate was added and placed in a boiling water bath for 20 min followed by 5 ml of amyl alcohol. The test tubes were shaken well and then centrifuged at 3000 rpm for 10 min. Finally, the intensity of color was read at 465 nm in a UV spectrophotometer (Shimadzu, UV-1800) against amyl alcohol blank exactly after 15 min with addition of ammonium thiocyanate.
Chromium content in feeds and feces was analyzed after digestion using nitric acid and perchloric acid (Furukawa 1966). To the known volume of digested sample, 1 ml of diphenyl carbozide reagent was added and kept at room temperature for 10 min. The intensity of the bright pink color was read at 350 nm against reagent blank in a UV spectrophotometer (Shimadzu, UV-1800).
Shrimp husbandry and experimental conditions
The juveniles of Penaeus vannamei with an average weight of 4.0 g procured from local farm near Chennai, India. The shrimps (10 shrimps/tank) were randomly stocked in experimental tanks (500 l) in triplicates after acclimatization for 2 weeks. Shrimps were fed a basal diet thrice daily at the rate of 6% to the total biomass, and the amount of diet in relation to survival, body weight, and intake was adjusted. During the experimental period (30 days), shrimp fed with the respective diet in a static mode to prevent the leaching of feces. The uneaten feed pellets and other particles were removed after an hour of feeding. Feces were gently siphoned off from the tanks into a bolting silk cloth after 2 h of each feeding from the second week onwards. It was then gently rinsed in distilled water, dried on filter paper, and frozen immediately at −20 °C. The apparent digestibility coefficient of dry matter and crude protein of the experimental diets were calculated according to Smith and Tabrett (2004) as follows,
Water quality parameters were measured once in 10 days. The water temperature, dissolved oxygen, and total ammonia-nitrogen were maintained in the optimum range of 26 to 29 °C, 5.5 to 7.5 mg l−1, and <0.1 ppm, respectively. At the end of the experiment, the growth performance in terms of specific growth rate (SGR), feed conversion ratio (FCR), protein efficiency ratio (PER), apparent protein utilization (APU), and survival was determined as follows,
Statistical analysis
The data collected from the present work was statistically evaluated by one-way ANOVA using SPSS version 17.0 to find significance among the treatments. Prior to statistical evaluation, the data was checked for determining the homogeneity of variance after ascertaining the normal distribution. The percent survival data was arc sin transformed before testing for the ANOVA. Comparison of means was carried out by least significance differences at the 5% level (P < 0.05).
Results
The PSI (Fig. 1) was gradually decreased (P < 0.05) from 85% (at 0 min of autoclaving time) to 79, 72, 68, and 64% at 5, 10, 15, and 20 min, respectively. The proximate composition of raw and treated soybean meal is presented in Table 2. As a result of heat treatment, the crude protein quantity was significantly (P > 0.05) unaltered whereas the crude fiber and total ash contents were significantly (P < 0.05) reduced. The amino acid composition of soybean meal was not affected by autoclaving except sulfur containing amino acids viz., methionine and cystine (Table 2). Both the amino acids were reduced significantly (P < 0.05) after autoclaving for 15 and 20 min. The significant (P < 0.05) changes in anti-nutritional factors (Table 2) were noticed in processed soybean meal, and their quantities were inversely proportional to the duration of autoclaving. Trypsin inhibitor was reduced (P < 0.05) due to heat treatment and was below the detectable level after 20 min of autoclaving. The ranges of reduction in saponin and phytic acid were 0.3 to 8% and 1 to 24%, respectively, in treated soybean meal compared to unprocessed soybean meal.
The growth performance and feed utilization of P. vannamei fed with experimental diets containing processed soybean meal is represented in Table 3. The final weight and SGR were significantly affected (P < 0.05) in shrimp fed diets having soybean meal autoclaved for 15 and 20 min (PSI 68 and 64%) compared to the control and other experimental diets. Significant increase (P < 0.05) in FCR and significant decrease in PER and APU were observed in shrimp fed diets having soybean meal autoclaved beyond 15 min (PSI <68%) than those autoclaved for 0, 5, and 10 min (PSI 85, 79, 72%). There was no significant difference in survival among the dietary treatments.
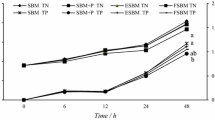
The apparent dry matter (ADMD) and crude protein (ACPD) digestibility of experimental diets are given in Fig. 2, and they were in the ranges of 58.64 to 74.31% and 62.43 to 80.25%, respectively. No significant difference was found in both ADMD and ACPD in shrimp fed diets having soybean meal autoclaved for 0, 5, and 10 min (PSI 85, 79 and 72%, respectively). However ADMD was significantly (P < 0.05) reduced by 7.23 and 9.86 in shrimp fed diets having soybean meal autoclaved for 15 and 20 min (PSI 68 and 64%, respectively) compared to the control group (PSI 85%), whereas the reduction was slightly higher for ACPD (13.77 and 17.82%) in such treatments. The body composition (Table 4) of P. vannamei fed different experimental diets having unprocessed and heat-treated soybean meal did not differ significantly.
Discussion
Effect of autoclave on PSI
The KOH-PSI is an important index, most widely used to assess the effect of overprocessed soybean meal (Araba and Dale 1990). The reduction of PSI in the treated soybean meal was attributed to denatured protein due the application of heat during autoclave (Chen et al. 2010), which would have resulted in low solubility by altering the hydrophobicity/hydrophilicity balance (Odjo et al. 2012). Akkerman (2014) stated that the heat treatment denatured the protein by modifying structural integrity especially secondary and tertiary structure by weakening hydrogen bonds and functional groups. It twists, rotates, and bends the proteins and converts the folded protein into unfolded shape which leads to loss of its functional activity. For an example, if the protein is functioned as an enzyme, denaturation causes to lose its enzymatic activity. In addition, the unfolded proteins fail to expose the binding site to the hydrolyzing medium and the respective enzymes resulted in decreased protein hydrolysis and thereby reduced digestibility and availability of protein to the animals. The marked decrease in protein solubility in the present study was corroborated with the findings of Parsons et al. (1991) and Batal et al. (2000). But the rate of reduction was inconsistent with earlier reports (84 to 52% by Parsons et al. 1991 and 89 to 76% by Batal et al. 2000) compared to the present result (85 to 64%). In addition to the duration of autoclaving, other factors like pH (6.5 to 7.0), temperature (121 °C), solvents (water), moisture, and particle size (500 μm) are also responsible for reduction of PSI (Ries-kautt and Ducruix 1997). All the abovementioned factors were maintained almost constant among the studies except the hydration volume during the process of autoclaving, which might be a reason for such differences observed among the studies.
Nutrients and anti-nutrients in raw and treated soybean meal
The analyzed results revealed no significant (P > 0.05) difference in crude protein content between raw and treated soybean meal, though heat treatment resulted in declined protein quality. This result was in agreement with Mutia and Uchida (1993) in autoclaved soybean for 0 to 90 min. The higher lipid content in autoclaved soybean meal than control could be due to the dissociation of lipid from the complex materials as previously documented by Akpanum and Achinewhu (1985); Ragab et al. (2010) in cowpea and legume seeds, respectively. The crude fiber value was higher (P < 0.05) in raw soybean meal (6.95% dry mater basis) than autoclaved soybean meal (6.42 to 6.50%), which indicates that the steam pressure could destroy the part of crude fiber (Mahata et al. 2012). The present result was in accordance with Mirzah (1990) and Mahata et al. (2012) who found the reduction of crude fiber from 14.40 to 11.52% and 13.36 to 12.02% in autoclaved shrimp waste and juice waste, respectively. The autoclaving has shown an appreciable increase in NFE content probably due to the hydrolysis of structural polysaccharides (fibers) into soluble sugars. Earlier studies have been reported that the process of autoclaving has significantly (P < 0.05) decreased total ash contents when the materials are autoclaved with higher quantity of water (sample and water ratio at 1:10). It was attributed to leaching of minerals to the autoclaving medium (Udensi et al. 2010; Idoko et al. 2014; Alagbaoso et al. 2015). But in the present study, soybean meal was hydrated with 60 to 65% of water and kneaded into dough during the process of autoclaving. It clearly indicates that there was no possibility of leaching for minerals as there was no excess of water. However, total ash content was reduced in autoclaved soybean meal than the untreated material in the present investigation. It would be due to the proportionate increase of other nutrients (Table 2) like ether extract and nitrogen-free extract (Rozan et al. 1996). The decreased ash content was supported by Onyeike and Omubo-Dede (2002) who reported that the ash content was decreased from 38.6 to 27.7 g kg−1 in African yam bean flour after autoclaving such.
The quality of protein source is mainly assessed by its amino acid composition. In the present study, amino acid composition was not affected by the process of autoclaving, although sulfur containing amino acids like methionine and cystine were reduced significantly (P < 0.05) in soybean meal autoclaved beyond 15 min. The similar reduction of cystine was reported in autoclaved soybean meal (Parsons et al. 1992; Mutia and Uchida 1993) and soy protein concentrate (Kim and Barbeau 1991). The partial destruction of cystine due to the heating process forms cysteic acid (Kim and Barbeau 1991) which would be a possible reason for the reduction observed in cystine content. Methionine is the first limiting amino acid in soybean meal, and its level was reduced significantly (P < 0.05) form 7.58 g kg−1 (AT O) to 6.86 and 6.28 g kg−1 after 15 and 20 min of autoclaving, respectively. The result was in agreement with findings of Sibbald (1980) in autoclaved soybean flakes. McNaughton and Reece (1980) reported that lysine tends to be more sensitive to heat than methionine, but in contrast, lysine content was not significantly affected in the present study after heat treatment. The result of Mutia and Uchida (1993) has shown an increase in the availability of lysine from 3.63 to 4.59, 3.99, 3.94, and 3.93 mg/16 g N in winged bean after autoclaving for 0, 15, 30, 45, and 60 min, respectively.
The trypsin inhibitor was gradually decreased (P < 0.05) with increasing the duration of autoclave (Table 2). The splitting of covalent bonds or hydrolysis of peptide bonds and interchange or destruction of disulfide bonds might be a reason for the inactivation of trypsin inhibitor during heat treatment (Alonso et al. 1998). Roychaudhuri et al. (2004) reported that the trypsin inhibitor in soybean meal belongs to the family of anti-parallel β-sheet proteins, which may renature after processing, and thus it might not be possible to completely remove it by processing methods, but in the present study, it was below the detectable level after autoclaving for 20 min. The complete removal of trypsin inhibitor after heat treatment has been previously reported by Mubarak (2005) and Embaby (2011) in green gram and peanut seed, respectively.
The phytic acid was reduced to a maximum of 24% in a 20-min autoclaved sample compared to that of unprocessed soybean meal. The formation of phytate-protein and phytate-protein-mineral complexes or the hydrolysis of inositol hexaphosphate present in phytic acid to penta and tetra phosphate during heat treatment might be a reason for the apparent reduction of phytic acid in the present study (Siddhuraju and Becker 2001). The reduction of saponin was not appreciable with autoclaving, which might be due to heat stable properties of saponins (Oenning et al. 1994). The boiling of soybean flour in water (1:10 ratio) reduces significant quantities of saponin, while the substantial increase could be noticed during the process of roasting (Chaturvedi et al. 2012). It clearly indicates that the removal of saponin content varies with the type of heat treatment employed during the processing.
Growth indices
In general, the growth response and feed utilization was efficient in relation to feed quality. The reduction of anti-nutritional factors was more in soybean meal autoclaved for 15 and 20 min (PSI 68 and 64%) than other treatments, but the poor growth performance observed in these treatments might be attributed to the reduced hydrolysis of denatured protein due to the prolonged heat treatment (Hong et al. 2004). The similar reduction was evidenced by Parsons et al. (1991) during the incorporation of heat-treated soybean meal as a sole source of protein in the diet of pig and chick. Mateo and Conejos (2009) have evaluated the quality of soybean meal obtained from different countries on chicks. The percentage of weight gain (113 to 139%) was differed significantly (P < 0.05) despite it had similar protein levels (46.8 to 48.0%) due to overprocessing. The reduction in the quality of soybean meal diminishes the protein hydrolysis which in turn reduces the availability of protein to the shrimp (Lemos et al. 2000). In addition, the alteration in amino acid composition in heat-treated soybean meal (Mutia and Uchida 1993) might have resulted in reduced growth performance. Though the amino acid contents were not much affected in the present study, the heat treatment significantly (P < 0.05) reduced the limiting amino acid, methionine, in soybean meal after autoclaving for 15 and 20 min (Table 2). It would also be a reason for the retarded growth rate in such treatments.
The feed intake (8.5 to 9.1 g/shrimp) which was almost comparable among the treatments indicates no palatability problems due to the inclusion of heat-treated soybean meal in the diet of P. vannamei, which might be due to the reduction of anti-nutritional factors (Table 2). But the increase in FCR and decrease in PER and APU were observed in shrimp fed diets having soybean meal autoclaved for 15 and 20 min (PSI 68 and 64%) compared to other diets. It confirms the unfavorability of prolonged heat-treated soybean meal, and the same was reflected on the growth performance. The shrimp survival was in the range of 86.67 to 93.33%, and no significant difference was evidenced between the experimental groups. This attributes to the overall quality of rearing conditions that were maintained within the optimal ranges in the present study. It also confirms that there was no negative dietary effect of heat-treated soybean meal on shrimp survival. The present preliminary results showed that soybean meal can be autoclaved for 10 min (PSI 72%) which is optimum for heat treatment.
Apparent digestibility coefficients
The nutritional composition and nutritive value of feed varies with its ingredient composition, and all the components within the feed were not equally digested. Hence, the apparent digestibility coefficients were measured to assess the total quantity of feed that were absorbed and digested. The digestibility values were gradually declined with decrease of protein solubility, which was attributed to the denaturation of protein pool during heat treatment (Hong et al. 2004). The other factors that are responsible for decreased digestibility may be due the formation of secondary crosslink which altered the structure of protein, blocking of the active site of enzymes, or inducing the compounds which inhibit the proteolytic enzyme activity resulting in the reduction of protein hydrolysis (Akkerman 2014). All the above said factors might have resulted in reduced digestibility parameters in the shrimp fed diets having soybean meal autoclaved beyond 15 min (PSI <68%).
Of all the treatments, the apparent digestibility of dry matter and crude protein for the diet having soybean meal autoclaved for 5 min (PSI 79%) were found to be high. This is partly due to the reduction of anti-nutritional factors especially trypsin inhibitor (reduced by 37% than the control). In addition, the lesser reduction of protein quality (79%) compared to other treated soybean meal (64 to 72%) could also be a possible reason for the better result observed with 5 min autoclaved soybean meal. Though both ADMD and ACPD were reduced with decreasing protein solubility, the reduction found to be high in protein digestibility (9.9 to 17.8%) than dry matter digestibility (7.2 to 13.8%) in diets having prolonged autoclaved soybean meal (PSI 68 and 64%). The reasons attributed to this reduction are the changes in chain flexibility and accessibility of protein to their respective enzymes during heat treatments (Akkerman 2014) and resulted in the poor protein availability to the cultured species due to their insolubilization. The similar result was also corroborated by Odjo et al. (2012), who reported that the non-covalent hydrophobic interaction and intermolecular disulfide crosslink denature the corn protein during heat treatment. The present result clearly indicates that the solubility of protein is mainly determining the protein digestibility even it has a similar protein level.
Conclusion
The result of the present study revealed that the growth rate of shrimp was retarded due to the inclusion of overprocessed soybean meal. Hence from the present preliminary investigation, it can be concluded that though heat treatment is beneficial in destroying anti-nutritional factors, it in turn affects certain heat-liable essential nutrients. Therefore, assessing the quality of heat-liable nutrients, especially protein in addition to quantity, would be more beneficial to formulate the high quality feed rather fulfilling dietary quantitative requirements in the diet of shrimp. In the present study, growth performance and feed utilization was not affected up to the treatment having soybean meal with 72% PSI (autoclaved at 10 min). Reduction of PSI below this level due to increased duration of autoclaving showed poor growth performance in P. vannamei. It indicates that the processing of soybean meal with heat at 121 °C for 10 min is acceptable beyond such duration is not advisable. The results of the present study generate a baseline data to support the feed nutritionist for ascertaining the information about the effect of overprocessed ingredients in the diet of P. vannamei.
References
Akkerman M (2014) The effect of heating process on milk whey protein denaturation and rennet coagulation properties. Dissertation, Aarhus university, Denmark
Akpanum MA, Achinewhu SC (1985) Effect of cooking, germination and fermentation on the chemical composition of Nigerian cowpea (Vigna unguiculata). Plant Food Hum Nutr 35:353–358
Alagbaoso SO, Nwosu JN, Njoku NE, Umelo MC, Eluchie C, Agunwa IM (2015) Effect of processing on the nutritional and anti nutritional properties of Canavalia plagiosperma piper seeds. Eur J Food Sci Technol 3:45–69
Alonso R, Orice E, Marzo F (1998) Effects of extrusion and conventional processing methods on protein and anti nutritional factor contents in pea seeds. Food Chem 63:505–512
AOAC (1997) Official methods of analysis of Association of Analytical Chemists, 18th edn. Association of Official Analytical Chemists, Benjamin Franklin Station, Washington DC
Araba M, Dale NM (1990) Evaluation of protein solubility as an indicator of over processing soybean meal. Poult Sci 69:76–83
Batal AB, Douglas MW, Engram AE, Parsins CM (2000) Protein dispersibility index as an indicator of adequately processed soybean meal. Poult Sci 79:1592–1596
Chaturvedi S, Hemamalini R, Khare SK (2012) Effect of processing conditions on saponin content and antioxidant activity of Indian varieties of soybean (Glycine max Linn) annals of. Phytomedicine 1:62–68
Chen CC, Shih YC, Choiu PWS, Yu B (2010) Evaluating nutritional quality of single stage and two stage fermented soybean meal. Asian Aust J Ani Sci 23:598–606
Davies N, Reid H (1979) An evaluation of the phytate, zinc, copper, iron and manganese contents of, and Zn availability from, soya-based textured-vegetable-protein meat-substitutes or meat-extenders. Br J Nutr 41:579–589
Dayal JS, Ahamad Ali S, Ambasankar K, Singh P (2003) Effect of dietary protein level on its in vitro and in vivo digestibility in the tiger shrimp Penaeus monodon (Crustacea: Penaeidae). Indian J Mar Sci 32:151–155
Embaby HE (2011) Effect of heat treatment on certain anti-nutrients and in vitro protein digestion of peanut sesame seeds. Food Sci Techonol Res 17:31–38
Finlayson AJ (1964) Amino acid recovering in the analysis of some feed samples. Can J Plant Sci 45:184–188
Furukawa T (1966) On the acid digestion method for determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull Jpn Soc Sci Fish 32:207–217
Hong KJ, Lee CH, Kim SW (2004) Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food 7:430–436
Idoko AS, Oladiji AT, Yakubu MT, Aska AS (2014) Effect of heat treatment on nutrient and anti-nutrient components of melon (Citrullus colocynthis) husks. Res J Chem Sci 4:28–32
Imelda Joseph R, Raj P, Bhatnagar D (2008) Effect of solid state fermentation on nutrient composition of selected feed ingredients. Indian J Fish 55:327–332
Jajic I, Krstovic S, Glamocis D, Jaksis S, Abramovic B (2013) Validation of an HPLC method for the determination of amino acids in feed. J Serb Chem Soc 78:839–850
Kakade ML, Rackis JJ, McGhee JE, Puski G (1974) Determination of trypsin inhibitor activity of soy products: a collaborative analysis of an improved procedure. Cereal Chem 51:376–382
Kim YA, Barbeau WE (1991) Changes in the nutritive value of soy protein concentrate during autoclaving. Plants Foods Hum Nutr 41:179–192
Lemos D, Ezquerra JM, Garcia-Carreno FL (2000) Protein digestion in penaeid shrimps: digestive proteinases, proteinase inhibitors and feed digestibility. Aquaculture 186:89–105
Mahata ME, Rizal Y, Wu G (2012) Improving the nutrient quality of juice waste mixture by steam pressure for poultry diet. Pakistan J Nutr 11:172–175
Mateo CD, Conejos JR (2009) Evaluation of the protein quality of soybean meal from different sources in broiler chicks fed with semi-purified diets. Philippine J Sci 138:153–159
McNaughton JL, Reece FN (1980) Effect of moisture content and cooking time on soybean meal urease index, trypsin inhibitor content and broiler growth. Poult Sci 59:2300–2306
Mirzah (1990) Effect of the use of flour limbah udang diolahdan without processed in against performance of broiler rations. Dissertation, Graduate University of Padjadaran, Bandung, Indonesia
Mubarak AE (2005) Nutritional composition and anti-nutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem 89:489–495
Mutia R, Uchida S (1993) Effect of heat treatment on nutritional value of winged bean (Psophocarpus tetragonobus) as compared to soybean. Chemical characteristics of heat treatment winged bean. Asian-Australian J Ani Sci 6:19–26
Odjo S, Malumba P, Dossou J, Janas S, Béra F (2012) Influence of drying and hydrothermal treatment of corn on the denaturation of salt-soluble proteins and color parameters. J Food Eng 109:561–570
Oenning G, Jullerat M, Fay L, Asp N (1994) Degradation of oat saponins during heat processing effect of pH, stainless steel and iron at different temperatures. J Agric Food Chem 42:2578–2582
Onyeike EN, Omubo-Dede TT (2002) Effect of heat treatment on the proximate composition, energy values and level of some toxicants in African yam bean (Sphenostylis stenocarpa) seed varieties. Plant Foods Hum Nutr 57:223–231
Parsons CM, Hashimoto K, Wedekind KJ, Baker DH (1991) Soybean KOH solubility in potassium hydroxide: an in vitro test of in vivo protein quality. J Ani Sci 69:2918–2924
Parsons CM, Hashimoto K, Wedekind KJ, Han Y, Baker DH (1992) Effect of over processing on availability of amino acids and energy in soybean meal. Poult Sci 71:133–140
Ragab HI, Kijora C, Abdelalti KF, Danier J (2010) Effect of traditional processing on the nutritive value of some legumes seeds produced in Sudan for poultry feeding. Int J Poult Sci 9:198–204
Ries-Kautt M, Ducruix A (1997) Inferences drawn from physicochemical studies of crystallogenesis and precrystalline state. In: methods in enzymology. Academic Press, New York, pp 23–59
Roychaudhuri R, Sarath G, Zeece M, Markwell J (2004) Stability of the allergenic soybean Kunitz trypsin inhibitor. Biochem Biophys Acta 16:207–212
Rozan P, Villaum C, Bau HM, Schwertz A, Nicolas JP, Mejean L (1996) Detoxication of rapeseed meal by Rhizopus oligosporus sp-T3: a first step towards rapeseed protein concentrate. Int J Food Sci Technol 31:85–90
Sastry CSP, Tammuru MK (1985) Spectrophotometric determination of tryptophan in protein. J Food Sci Technol 22:146–147
Sharawy Z, Goda AMAS, Hassaan MS (2016) Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, post larvae. Anim Feed Sci Technol 212:90–99
Shi C, He J, Yu J, Yu B, Huang Z, Mao X, Zheng P, Chen P (2015) Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J Anim Sci Biotechnol 6:13–19
Shiu YL, Wong SL, Guei WC, Shin YC, Liu CH (2015) Increase in the plant protein ratio in the diet of Litopenaeus vannamei (Boone), using Bacillus subtilis E20-fermented soybean meal as a replacement. Aquaculture 46:382–394
Sibbald IR (1980) The effect of heat on the clearance time, true metabolizable energy, and true available amino acids of raw soybean flakes. Poult Sci 59:2358–2360
Siddhuraju P, Becker K (2001) Effect of various domestic processing methods on antinutrients and in vitro-protein and starch digestibility of two indigenous varieties of Indian pulses, Mucuna pruries var utilis. J Agric Food Chem 49:3058–3067
Smith DM, Tabrett SJ (2004) Accurate measurement of in vivo digestibility of shrimp feeds. Aquaculture 232:563–580
Sun H, Tang J, Yao X, Wu Y, Wang X, Liu Y (2016) Effects of replacement of fish meal with fermented cottonseed meal on growth performance, body composition and haemolymph indexes of Pacific white shrimp, Litopenaeus vannamei Boone, 1931. Aquac Res 47:2623–2632
Udensi EA, Arisa NU, Ikpa E (2010) Effects of soaking and boiling and autoclaving on nutritional quality of Mucuna flagellipes (Ukpo). Afr J Biochem Res 4:47–50
Wee KL (1991) Use of non conventional feed stuffs of plant origin as fish feeds - is it practical and economically feasible? In: De Silva SS (ed) Fish Nutrition Research in Asia. Proceedings. 4th Asian Fish Nutrition Workshop, Asian Fisheries Society, Manila, The Philippines, p 13–32
Acknowledgements
The authors are grateful to Dr. K. K. Vijayan, Director, ICAR-Central Institute of Brackishwater Aquaculture (CIBA), Chennai, for providing all the necessary facilities. The authors acknowledge the Indian Council of Agricultural Research (ICAR) for the financial support through the project of National Innovation on Climate Resilient Agriculture (NICRA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jannathulla, R., Dayal, J.S., Ambasankar, K. et al. Effect of protein solubility of soybean meal on growth, digestibility and nutrient utilization in Penaeus vannamei . Aquacult Int 25, 1693–1706 (2017). https://doi.org/10.1007/s10499-017-0147-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0147-9