Abstract
The present work deals with the growth efficiency of Picochlorum maculatum and Oithona rigida in shrimp-cultured wastewater. In addition, the effects of wastewater (WW)-cultured P. maculatum and O. rigida on the growth and survival of Litopenaeus vannamei post-larvae (PLs) was studied and the results were compared with artificial culture media (ACM)-cultured P. maculatum and natural seawater (NSW)-cultured O. rigida. The results revealed that the high density obtained in microalgae and low in copepod using wastewater as a medium. Further, shrimp PLs fed with WW-cultured microalgae, and NSW-cultured copepod had specific growth rate and higher survival, but it was not significantly different (p > 0.05) from PL fed on ACM-cultured microalgae and WW-cultured copepod, indicate that P. maculatum has potential to be used as live feed for the hatchery rearing of L. vannamei PLs, in replacing microdiet. Further study is needed on optimization of wastewater-cultured copepod as a live feed to yield maximum growth and survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plankton is the natural food item of many marine and freshwater fishes and crustaceans. They have been used extensively to rear larvae and fry (Kibria et al. 1999). Both live (Ananthi et al. 2011) and frozen live feed (Sargent et al. 1997) have been used to rear fish and crustaceans. Earlier studies have shown that the larvae performed better when fed with plankton than when fed with artificial dry or commercial feed (Dabrowski 1984; Dave 1989). As microalgae and copepod protein is of good quality, with amino acid profiles comparable to that of other reference food proteins (Becker 2007), it could be a possible alternative to commercial feeds. In addition, microalgae, which are the source of all photosynthetically fixed carbon in the food web of aquatic animals (Kwak and Zedler 1997), may be an ideal replacement for commercial diet. Some of the workers have successfully reared the fish and shrimp by using microalgal species such as C. mulleri and I. galbana (Sangha et al. 2000), Spirulina extraction (Palmegiano et al. 2005), Nanofrustulum sp., Tetraselmis sp. (Kiron et al. 2012), and I. galbana (Rohani-Ghadikolaei et al. 2013).
Similarly, marine copepods are considered to be “nutritionally superior” for marine fish and prawn larvae, and they are valuable sources of proteins, lipids, carbohydrates, amino acids, fatty acids and enzymes (amylase, protease, exonuclease and esterase), which are essential for larval digestion and metamorphosis (Yurkowski and Tabachek 1979; Fluchter and Rembold 1986; Munilla-Moran et al. 1990; Pillay 1990; Stottrup 2000). Ananthi et al. (2011) have suggested that the marine copepod (Oithona rigida, M. gracilis and Pseudodiaptomus sp.) enhances the growth, survival and astaxanthin of tiger shrimp Penaeus monodon when compared to Artemia.
It is well known that the plankton (microalgae and copepod) grow abundantly in the nutrient-rich wastewaters such as shrimp- and fish-cultured wastewater (SCWW and FCWW), but the resource remains unutilized. However, to date, there is no research on the utilization of this resource for secondary aquaculture in Indian context except Rajthilak et al. (2013). On the other hand, commercially available artificial culture media (ACM) is widely utilized for cultivating algae. There are a number of predictable media, such as Walne’s or Conway, F/2 media, Miquel’s, and Scheiber’s, being used for the culture and maintenance of microalgae in research laboratories as well as in fish and shrimp hatcheries (Guillard 1975; Ip et al. 2004). The production of ACM is tedious and often too costly. Therefore, it is essential to evaluate other source as alternative culture medium for culturing microalgae and copepod. This study examined the nutritional composition of wastewater-grown microalgae (Picochlorum maculatum) and copepod (O. rigida) and evaluated their suitability as live feed for white leg shrimp Litopenaeus vannamei. As a first step, in this direction, we have cultured marine microalga Picochlorum maculatum and marine copepod O. rigida in 90-day-old shrimp-cultured wastewater. Then, the PLs 10 of L. vannamei were fed with ACM- and WW-cultured microalgae and NSW- and WW-cultured copepod for 21 days. The length, weight and survival were analyzed every 7 days, and nutritional characters were analyzed initial and final day of the experiment.
Materials and methods
Wastewater collection
The 90-day-old L. vannamei-cultured wastewater was collected from Parangipettai, Tamil Nadu, India (11°28′4.18′′N; 79°43′45.12′′E). The collected wastewater was transported to laboratory using temperature-controlled ice box and kept undisturbed for the suspended particles to settle down.
Collection and culture of microalgae
Marine microalgae Picochlorum maculatum (PSDK01) (Accession number: KJ754560) was isolated from Palk Bay region of Muthukuda coast (9°51′48′′N; 79°7′15′′E), Tamil Nadu, India and isolated by using agar plating technique (Robert 2005). Indoor algal stock culture was maintained in a special air-conditioned room, and algal culture was made according to Anderson (2005). Stock cultures were kept in 250-ml culture flasks. Seawater was filtered using filter bag (1 µm) and sterilized using autoclave. After cooling, water was transferred to the culture flask plugged with cotton. Vessels used for algal culture was sterilized properly and dried in an oven before use. The Walne medium (Walne 1970) was used for indoor culture. About 10 ml of inoculum in the growing phase was transferred to the culture flasks, and the culture was maintained with 12:12 h light and dark cycle provided with 200 µmol m−2 s−1 using fluorescent bulbs. After 5–8 days, the maximum exponential phase was obtained. Temperature and salinity was maintained in the range between 23 and 25 °C and 28 and 30 ppt., respectively, for entire culture period. The same light conditions and temperature were maintained for culturing microalgae in wastewater. The wastewater was used as a culture medium instead of Walne medium.
Copepod culture
The copepod O. rigida were used from our culture collection. From these, the healthy gravid female of O. rigida was picked up by using a fine capillary tube. The isolated copepods were kept overnight in 250-ml beakers containing filtered seawater (1 µm) of ambient salinity (34 psu) with vigorous aeration for starving prior to the experiment, and the same conditions were maintained for culturing copepod in wastewater. NSW and WW copepod were fed one time per day with ACM and WW-grown microalgae, respectively, with average cell concentration (20,000 cells ml−1).
Feeding experiment
In this experiment, twelve rectangular Fiberglass Reinforced Plastics (FRP) tanks (70 cm × 50 cm × 30 cm size and 6 mm thick; outside blue and inner white tanks, 100 L capacity) each containing 50 L clean, filtered (5 µm filter bag) seawater were used. Four treatments were maintained and were fed with (1) artificial culture medium (ACM)-cultured microalgae (2) wastewater (WW)-cultured microalgae (3) natural seawater (NSW)-cultured copepod and (4) wastewater (WW)-cultured copepod with all treatment in triplicates. Litopenaeus vannamei PL stage 10 (PL10) were obtained from the Rank Marine Hatchery (Marakkanam, Tamil Nadu, India) and were stocked at a density of 50 PL L−1. An air compressor was used to provide constant aeration to each FRP tanks. The FRP tanks were maintained under a 12- h:12-h light and dark cycle. Shrimp PLs were regularly fed every 8-h interval daily with ACM microalgae (Treatment 1), WW microalgae (Treatment 2), NSW copepod (Treatment 3) and WW copepod (Treatment 4) with densities of microalgae (approximately 1,00,000 cells ml−1) and copepod (8–10 individual’s ml−1). NSW and WW copepod O. rigida were cultured in 5-L-round-bottom plastic container and fed with ACM and WW microalgae P. maculatum (PSDK01), respectively, at 18,000–20,000 cells ml−1. The experiment lasted for 21 days.
Water quality analyses
Temperature, salinity, pH and dissolved oxygen were recorded daily in culture tanks. Temperature was estimated using standard centigrade thermometer. Salinity was estimated by using Hand Refractometer (Erma, Japan). pH was estimated using Elico grip pH meter. Dissolved oxygen, total dissolved phosphorous (TP) and total dissolved nitrogen (TN) were estimated according to Strickland and Parsons (1972). TP and TN were estimated on the final day of the experiment. Daily water exchange was carried out at 30 %, and uneaten food and fecal matter were siphoned out. Ahead of larval rearing, heavy metal (Zn, Cr, Cu, Pb and Cd) concentration in wastewater was estimated by APDC (ammonium pyrolidine dithiocarbamate) and MIBK (methyl isobutyl ketone) extraction method with help of atomic absorption spectrophotometer (1983- 400 HGA 900/AS 800 PerkinElmer) (Brooks et al. 1967).
Morphometric analyses
Shrimp sampling was made every 7 days once for morphometric analyses (Length, weight and survival). Shrimp growth rate (dry weight) was calculated from the body weight (mg) based on the formula derived from Ricker (1979): G = (W 2 − W 1)/(T 2 − T 1), where W 2 and W 1 represent the final and initial weight of the shrimp, respectively, and (T 2 − T 1), the duration of the experimental period. For length (L) analyses, initial length (I L ) of shrimp was subtracted from final length (F L ) with the help of following formula (L = F L − I L ). The survival of the PL was also determined at each sampling. Survival was calculated as per the percentage of shrimp remaining in each tank from the estimated number stocked initially.
Nutritional composition analyses
ACM- and WW-cultured microalgae (P. maculatum) were harvested at exponential phase, washed with double distilled water for removing salts from the algae then freeze-dried and used for further analyses. NSW- and WW-cultured copepod (O. rigida) were harvested on day 20 based on the life span, freeze-dried and used for analysis. In case of L. vannamei, the initial and final concentrations of nutritional compounds of shrimp PLs were analyzed. The moisture, protein, carbohydrate, lipid and ash contents in live feeds and shrimp larvae were estimated following standard methods (Rajendran 1973; Raymont et al. 1964; Dubois et al. 1956; Folch et al. 1956; AOAC 1995). Amino acids composition of live feed and shrimp larvae were analyzed according to Yamamoto et al. (1994) using high-performance liquid chromatography (HPLC). For analyses of fatty acid composition in live feeds and shrimp larvae, 0.4 g of dried samples was homogenized with 2:1 (v/v) combination of chloroform and methanol mixture and they were extracted using the modified method of Bligh and Dyer (1959). Further, extracted samples were esterified with 1 % H2SO4, and fatty acid methyl esters (FAME) were estimated according to AOAC (1995) using gas chromatography–mass spectrometry (GC–MS).
Statistical analyses
Data were analyzed using one-way analysis of variance (ANOVA). Significant differences among the different treatments were determined using Duncan multiple range test at 0.05 level of probability. Statistical analyses were accomplished using the Graph Pad Prism (Version 5.0).
Results
Growth of microalgae and copepod in wastewater
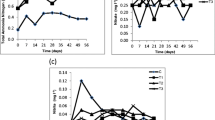
Figure 1a illustrates the cell growth rate of P. maculatum (PSDK01) in artificial culture medium (ACM) and shrimp-cultured wastewater (WW). The maximum cell growth (0.88 abs) was obtained on the 12th day in wastewater compared to artificial culture medium (0.66 abs). For the entire culture period (2 months), the total mean production of copepod O. rigida in NSW was 2,18,835 nos./l. comprising 1,12,345 nauplii, 71,234 copepodids and 35,256 adults (Fig. 1b), whereas in wastewater medium consisting 65,735 nauplii, 49,128 copepodids and 29,982 nos./l adults with a total of 1,44,845 nos./l was found.
Nutritional composition of live feeds
Picochlorum maculatum and O. rigida were cultured in normal seawater and wastewater. Slight variation was observed in live feed fed with different culture medium and results are as shown in Fig. 2a–e. The moisture, protein, carbohydrate, lipid and ash contents of ACM and WW-cultured P. maculatum were 30.29, 58.42, 12.27, 14.64, 4.98 and 31.85 %, 60.57, 10.34, 16.72 and 4.22 %, respectively. From the nutritional point of view, the normal seawater (NSW)- and wastewater (WW)-cultured O. rigida had moisture content (83.07 and 84.64 %), protein (69.34 and 68.21 %), carbohydrate (15.36 and 15.05 %), lipid (11.44 and 13.12 %) and ash (3.96 and 3.62 %), respectively. The total amino acid content of ACM and WW-cultured P. maculatum, NSW and WW-cultured O. rigida were 84.72, 85.36, 99.24 and 94.18 %, respectively. The total amino acid content from WW-cultured Picochlorum maculatum was 85.36 % which is comparatively higher than ACM-cultured P. maculatum (84.72 %) (Table 1). But while looking copepod, NSW-cultured copepod resulted in higher amino acid content (99.24 %) than WW-cultured copepod (94.18 %). Among these, serine, glycine, valine, threonine, phenylalanine, leucine were prevailing components and were recorded with the percentage of 6.08, 6.34, 5.23, 5.24, 5.77 and 7.5 in WW-cultured microalgae, whereas in NSW-cultured copepod, glutamic acid, aspartic acid, serine, lysine, leucine and arginine were found to be dominant with the percentage of 10.32, 8.79, 7.97, 12.97, 8.94 and 7.94.
The saturated fatty acid (SFA) content of ACM and WW-cultured microalgae and NSW and WW-cultured copepod was 35.72, 42.67, 44.21 and 45.46 %, respectively (Table 2). The recorded MUFA and PUFA contents in ACM algae, WW algae, NSW copepod and WW copepod were 27.85, 25.22, 22.43, 21.48 % and 14.77, 15.51, 31.84, 28.67 %, respectively. Fatty acids such as palmitoleic acid (8.79 and 8.57 %), stearic acid (12.24 and 13.17 %) and linoleic acid (6.64 and 7.48 %) were found dominant in ACM-cultured algae and WW-cultured algae, respectively. In the case of NSW- and WW-cultured copepod myristic acid (10.67 and 11.95 %), palmitic acid (7.41 and 6.0 %), stearic acid (10.96 and 10.21 %) and elaidic acid (7.94 and 8.6 %) were found to be predominant.
Feeding trial of white leg shrimp L. vannamei post-larvae with various live feeds
Microalgae
A rapid growth and maximum survival were noticed when L. vannamei post-larvae were fed on WW-cultured algae in comparison with ACM-cultured algae (Fig. 3a, c, e). The final average length, weight and survival of post-larvae fed on WW-cultured algae were found to be high as 1.8 ± 0.37 mm, 9.01 ± 0.63 mg and 70 ± 1.95 %, respectively, which was better than ACM-cultured algae (1.4 ± 0.48 mm, 8.45 ± 0.75 mg and 68 ± 2.01 %). The growth and survival of L. vannamei post-larvae fed with ACM and WW-cultured algae were significantly different (p < 0.05).
Copepod
The slender growth and survival of L. vannamei PL was found when feeding with NSW-cultured copepod instead of WW-cultured copepod (Fig. 3b, d, f). Finally, 2.6 ± 0.27 mm of length, 20.27 ± 0.85 mg of weight and 86 ± 2.7 % of survival were found in post-larvae fed on NSW-cultured copepod. The final mean length, weight and survival of post-larvae fed on WW-cultured copepod were 2.4 ± 0.14 mm, 18.68 ± 0.72 mg and 80 ± 3.5 %, respectively. The growth and survival of L. vannamei post-larvae fed with NSW and WW-cultured copepod were significantly different (p < 0.01).
Nutritional composition of shrimp larvae
P. maculatum-fed post-larvae
The nutritional composition of the L. vannamei larvae fed with ACM and WW-cultured P. maculatum is shown in Fig. 4a–e. All the nutritional compositions were found to be high in WW-cultured P. maculatum-fed larvae than in ACM-cultured P. maculatum-fed larvae. The percentage of moisture, protein, carbohydrate, lipid and ash contents in ACM-cultured algae-fed post-larvae were 83.49 ± 0.42, 62.33 ± 0.29, 9.36 ± 0.5, 12.34 ± 0.25 and 3.99 ± 0.27 which were lower than WW-cultured algae-fed post-larvae. and the values are 84.59 ± 0.62, 63.54 ± 0.01, 10.22 ± 0.57, 12.73 ± 0.21 and 4.38 ± 0.41. Among the two algae feed regimes, maximum amino acid content of 55.36 % in dry matter was obtained in ACM-cultured P. maculatum-fed larvae than in the WW-cultured P. maculatum (52.80 %) (Table 3). Among the amino acids, aspartic acid (7.71 %), valine (2.84 %), methionine (2.2 %), lysine (4.98 %), leucine (4.87 %), isoleucine (2.34 %), histidine (2.85 %) and arginine (3.18 %) were found to be dominant in ACM-cultured Picochlorum maculatum-fed larvae. The fatty acids such as myristic acid (3.81 %), heptadecanoic acid (7.85 %) and heneicosanoic acid (3.95 %) were high in ACM algae-fed larvae (Table 4). Myristic acid (8.36 %), palmitic acid (9.89 %), stearic acid (9.53 %), elaidic acid (10.72 %), linolelaidic acid (7.24 %) and eicosapentaenoic acid (4.68 %) were found high in WW algae-fed larvae. Higher concentration of fatty acids have been recorded in WW algae-fed larvae (83.44 %) compared to ACM-fed larvae (77.90 %)..
Oithona rigida-fed larvae
The nutritional compositions of O. rigida-fed larvae are shown in Fig. 4. The nutritional composition was found to be high in NSW copepod-fed larvae than in WW copepod-fed larvae except for moisture and lipid. The recorded level of protein, carbohydrate and ash content was found high as 69.34 ± 0.18 %, 15.36 ± 0.22 % and 3.96 ± 0.36, respectively, in NSW copepod-fed larvae. The moisture (84.64 ± 0.0.15 %) and lipid (13.12 ± 0.13 %) were found to be high in WW copepod-fed larvae. A total amino acid contents were found higher in NSW copepod-fed larvae (71.41 %) than WW copepod-fed larvae (66.35 %) (Table 3). Among these, aspartic acid (9.45 %), glycine (4.42 %) and serine (2.52 %) were found to be high in WW copepod-fed larvae. In NSW copepod-fed larvae, higher values of glutamic acid (11.46 %), alanine (9.63 %), valine (3.72 %), lysine (5.78 %), leucine (4.70 %) and arginine (3.34 %) were recorded. Totally, 97.43 % of fatty acids were recorded in NSW copepod-fed larvae consisting of 39.40 % saturated fatty acids, 30.32 % monounsaturated fatty acids and 27.71 % polyunsaturated fatty acids (Table 4). Among these, myristic acid (8.28 %), palmitoleic acid (5.33 %), stearic acid (9.13 %), elaidic acid (10.54 %), arachidonic acid (7.1 %), eicosapentaenoic acid (7.17 %) and docosahexaenoic acid (9.04) were found higher in NSW copepod-fed larvae. Saturated fatty acids (41.54 %), monounsaturated fatty acids (26.67 %) and polyunsaturated fatty acids (22.69 %) with total of 90.50 % fatty acids were recorded in WW copepod-fed larvae. Palmitic acid (21.66 %), arachidic acid (0.8 %) and behenic acid (0.23 %) were found dominant in WW copepod-fed larvae.
Analyses of water quality parameters in larval rearing tanks
No significant differences (p > 0.05) were detected in the shrimp larviculture water with regard to pH, salinity, dissolved oxygen, temperature, total phosphorus and total nitrogen among the four treatments (Table 5). The heavy metal concentrations (before experiment) of wastewater are summarized in Table 6.
Discussion
Kovalenko et al. (2002) suggested that the live feed has been treated to be a controlling factor in the commercial culture of shrimp and fish larvae. It is an important factor in the overall production cost as well. Competition in shrimp production in overseas market is rapidly increasing, and it is necessary to find more efficient ways to culture the best quality shrimp. Recently, there has been a great interest in wastewater treatment by using microalgae, after which their biomass can be use not only for biofuels, but also for products that could be processed for food, feed, pharmaceuticals and other high-value chemicals (Kiron et al. 2012; Dinesh Kumar et al. 2014, 2015). According to Wilson (2002) report, amino acid requirements of the crustacean species, the algae and algal products will be able to provide most of the essential amino acids.
Based on the above statements, the present studies were attempted on culture of microalgae (P. maculatum) and copepod (O. rigida) in shrimp culture wastewater and estimate the live feed suitability in L. vannamei post-larvae. A number of studies are available on use of microalgae (Sangha et al. 2000; Zelaya et al. 2007; González‐Davis et al. 2012; Kiron et al. 2012; Khatoon et al. 2009, 2013; Rohani‐Ghadikolaei et al. 2013) and copepod (Ananthi et al. 2011; Jothiraj 2012; Nandakumar 2015) as feed for shrimp larvae. But there is no study for wastewater-grown microalgae and copepod feeding by shrimp and fish or cultivable organisms especially P. maculatum and O. rigida. Santhanam (2002), Santhanam et al. (2004), Ananthi (2012), Raju (2012), Santhanam and Perumal (2012), Kathiresan (2013) and Santhanam and Perumal (2013) estimated the live feed suitability of O. rigida on Lates calcarifer, P. monodon and L. vannamei. In this concern, this study might be a first report for the live feed suitability of wastewater-grown P. maculatum and O. rigida on white leg shrimp L. vannamei.
As per the early reports, the heavy metal concentration of WW has been found as permissible for larval rearing (FAO 2007). In algal feeding, maximum growth and survival were recorded in WW-cultured algae-fed larvae. This could be attributed to the higher amount of protein, lipid and amino acid contents in the WW-cultured algae. Laurence (1977) advised that the larvae need more energy while culturing in tanks. Feeding ration might affect the larval growth. The length, weight and survival were all significantly lower in ACM-cultured algae-fed larvae when compared to WW-cultured algae-fed larvae. During copepod feeding, maximum growth and survival was found in NSW-cultured copepod-fed larvae compared to WW-cultured copepod-fed larvae. This might be due to culture density of copepod in wastewater and natural seawater. In wastewater, density of copepod was quietly low when compared to the natural seawater. In O. rigida, culture density consists of variety of stages such as nauplii, copepodite and adults. Rajkumar and Kumaraguru Vasagam (2006) suggested that the O. rigida size changes (Nauplii-I to Copepodite-V) directly ruled in the larval length and weight. The biochemical composition of live feed plays a vital role in their physiological functions, metabolism, nutritive value, energy transfer and secondary production. Mostly, prawn and fish depend on plankton at some stage during their life span, and some feed exclusively on plankton during their entire life cycle (Sumitha 2006).
In the present study, moisture, protein, lipid and total amino acid contents were high in both WW-cultured algae and WW-cultured algae-fed larvae. In the case of fatty acid contents, saturated fatty acids and polyunsaturated fatty acids were found to be high in WW-cultured algae, and monounsaturated fatty acids was high in WW-cultured algae-fed larvae. Moisture, lipid, non-essential amino acids and saturated fatty acids were found to be high in WW-cultured copepod and WW-cultured copepod-fed larvae. In the cultured microalgae and copepod, protein was identified as a major component which played a major role than compared to lipid and carbohydrate and these findings were supported by Santhanam and Perumal (2012).
In the present study, the higher protein content was found in microalgae cultured with WW compared to ACM algae. But the carbohydrate values were shown high in ACM algae than in WW algae. This result was comparable to Luis et al. (2010). They experienced the similar result with aquaculture fertilizer and agricultural fertilizer used for the culture of Chaetoceros mulleri. Changes in protein and carbohydrate levels attribute to the alteration in the media composition and nutrient changes in the WW (Gatenby et al. 2003). The level of lipid content was observed in ACM algae (%) than in WW algae (%). Brown et al. (1997) reported that culture condition and growth phase lead to changes in lipid composition of microalgae. Lipid content of microalgae increased with advanced growth as supported by Gatenby et al. (2003). The calorific content of the larvae fed with ACM algae and WW algae showed greater variations. The protein, carbohydrate and lipid values were higher in the larvae fed with WW algae. The higher level of biochemical composition noticed in the larvae might be a reflection of the feed supply (Evjemo et al. 2003; Payne et al. 2001).
Few differences were observed in the amino acid composition of WW algae (85.36 %) and ACM algae (84.72 %). The essential amino acid accounted to 44.17 %, and this level was comparatively higher than the earlier report (Derrien et al. 1998). In our study, glutamic acid was the dominant in all the 16 amino acids recorded in both ACM and WW algae as agreed by Martin-Jezequel et al. (1988), who found a high concentration of aspartic acid and glutamic acid in Skeletonema costatum. Depending on the culture conditions and microalgae cultured, the amino acid composition varies from species to species (Brown 1991; Brown et al. 1997; Carbajal Miranda et al. 2005; Moura Junior et al. 2007).
The stearic acid was found to be the most dominant in WW Algae followed by palmitic acid. In the case of ACM algae, palmitic acid was the most dominant and the second dominant was stearic acid as supported by Widianingsih et al. (2013). They found that palmitic acid were dominant in diatom Nitzchia sp. The exponential phase of the WW microalgae accumulates high amount of lipids than ACM algae. This might be due to the nitrogen limitation in the medium (Sánchez-Saavedra and Voltolina 2005). Growth and survival of L. vannamei larvae were superior with WW algae diet due to more amounts of 20:5 n-3 (EPA) and 22:6n-3 (DHA) as supported by Payne and Rippingale (2000) and Santhanam and Perumal (2013). Due to the low nutritional quality of ACM algae, low survival and growth were found in ACM algae-fed larvae. From this study, it is clearly understood that nutritional value of live feed, in particular the fatty acids such as EPA and DHA and amino acids (glutamic acid), is known to affect the growth and survival of larvae (Stottrup 2000; Evjemo et al. 2003). In the present study, the protein content of the NSW copepod and NSW copepod-fed L. vannemei larvae was low compared to WW copepod. The presently observed variation in the protein content might be due to the fact that it is utilized as a metabolic substrate as reported by Nageswara Rao and Krupanidhi (2001); Santhanam and Perumal (2013). But lipid content of the WW copepod showed small variation compared to NSW copepod, and it did not influence the larvae. The larvae fed with WW copepod showed lower lipid content than NSW copepod-fed larvae. The variations in the lipid content are attributed to its storage and utilization during starved period (Vengadeshperumal et al. 2010). Variations in the protein and lipid content of WW copepod observed in the present study is related to the type of feeds used (Rajkumar and Kumarguru Vasagam 2006; Santhanam and Perumal 2013).
The essential amino acids of the WW Copepod showed adequate level of the larvae (Rajkumar and Kumarguru Vasagam 2006; Ananth and Santhanam 2011), but lower than the NSW copepod. Among the amino acids recorded, isoleucine, threonine, alanine and glycine were dominant in the WW copepod. Rajkumar and Kumaraguru Vasagam (2006) reported dominance of lysine, alanine and glutamic acid in A. clausi. Santhanam and Perumal (2013), noticed glutamic acid, leucine, lysine and arginine as most prevailing amino acids in the same species of the present study. The larvae fed with WW copepod showed the dominance of methionine and histidine in L. vannamei larvae. The above result was different from Santhanam and Perumal (2013), who reported methionine and histidine as low in O. rigida-fed sea bass larvae.
The saturated fatty acids were found to be higher in WW copepod, and it is reflected in the larvae also when compared to the NSW copepod. In the present study, 16:0 (Palmitic acid), 18:0 (Stearic acid), 20:5 (EPA) and 22:6 (DHA) were dominant in both NSW copepod and WW copepod. Similar results were experienced by many authors in various copepods, Acartia clausi (Rajkumar and Kumaraguru Vasagam 2006), O. rigida (Santhanam and Perumal 2013) and Nanton and Castell (1998) in Tisbe sp. The above dominant fatty acids may be synthesized by the copepods or by the feed given. Nanton and Castell (1998) documented the influence of feed on the lipid and fatty acid composition of the copepod Tisbe sp. The live feed compatibility with copepods is transferred by several authors Rajkumar and Kumaraguru Vasagam (2006), Santhanam and Perumal (2013) in sea bass larvae, Ananthi et al. (2011) with Penaeus monodon larvae, Nandakumar (2014) with ornamental angel fish Monodactylus argenteus and Kathiresan (2013) with L. vannamei. However, the use of WW-cultured copepod is the first approach. Even though the growth and survival of larvae fed with NSW copepod showed higher values, they did not show much variation. This result showed that the adequate amount of nutrition especially HUFA is provided from WW copepod to the shrimp larvae. The small variation in growth of the larvae observed in this study is due to the nutritional quality of the food provided to the larvae (Payne et al. 2001).
Conclusion
The present study has helped us to understand the potential of the wastewater-cultured microalgae and copepod as replacements for commercial fish feeds of white leg shrimp L. vannamei. The two live feeds (P. maculatum and O. rigida) could be incorporated at levels tested in this study. Some significant effects were noticed on growth and survival as an effect of higher inclusions. Further studies are necessary to confirm the suitability of wastewater-grown microalgae and copepod on larval rearing of other shrimp and fish species.
References
Ananth S, Santhanam P (2011) Laboratory culture and biochemical profile of marine copepod, Macrosetella gracilis (Dana). Aquaculture 12:49–55
Ananthi P (2012) Laboratary culture and application of marine copepod Oithona rigida for larval rearing of Litopenaeus vannamei (Boone, 1931) with special reference to astaxanthin development, M.Phil. thesis, Bharathidasan University
Ananthi P, Santhanam P, Nandakumar R, Ananth S, Jothiraj K, Dinesh Kumar S, Balaji Prasath B, Jayalakshmi T (2011) Production and utilization of marine copepods as live feed for larval rearing of tiger shrimp Penaeus monodon with special emphasis on astaxanthin enhancement. Indian J Nat Sci 2:494–503
Anderson RA (2005) Algal culturing techniques. Elsevier Academic Press, Burlington, Massachusetts
AOAC (1995) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Washington, DC
Becker EW (2007) Microalgae as a source of protein. Biotechnol Adv 25:207–210
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brooks RR, Presley BJ, Kaplan IR (1967) APDC-MIBK extraction system for the determination of trace metals in saline waters by atomic adsorption spectroscopy. Talanta 14:809–816
Brown MR (1991) The amino acid and sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Biol Ecol 145:79–99
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Carbajal Miranda MJ, Sanchez Saavedray MDP, Simental Trinidad JA (2005) Effect of monospecific and mixed benthic diatom cultures on the growth of red abalone postlarvae Haliotis rufescens (Swainson 1822). J Shellfish Res 24:401–405
Dabrowski K (1984) The feeding of fish larvae: present ‘state of the art’ and perspective’s. Reprod Nutr Dev 24:807–823
Dave G (1989) Experiences with wastewater-cultured Daphnia in the start-feeding of rainbow trout (Salmo gairdneri). Aquaculture 79:337–343
Derrien A, Coiffard LJ, Coiffard C, De Roeck-Holtzhauer Y (1998) Free amino acid analysis of five microalgae. J Appl Phycol 10:131–134
Dinesh Kumar S, Santhanam P, Lewis-Oscar F, Thajuddin N (2014) A dual role of marine microalga Chlorella sp. (PSDK01) in aquaculture effluent with emphasis on initial population density. Arab J Sci Eng 40:29–35
Dinesh Kumar S, Santhanam P, Jayalakshmi T, Nandakumar R, Ananth S, Shenbaga Devi A, Balaji Prasath B (2015) Ex-situ studies on excessive nutrients and heavy metals removal efficacy of marine microalga Chlorella marina (Butcher) for wastewater treatment. Indian J Mar Sci 44:43–50
Dubois M, Gills KA, Hamilton JK, Robert PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Evjemo OJ, Reitan IK, Olsen Y (2003) Copepods as live food organisms in the larval rearing of halibut larvae (Hippoglossus hippoglossus L.) with special emphasis on the nutritional value. Aquaculture 227:191–210
FAO (2007) Improving Penaeus monodon hatchery practices: manual based on experience in India. No. 446. Food & Agriculture Organisation
Fluchter J, Rembold H (1986) Soluble factor essential for metamorphosis of coregonid larvae has been partially purified from Artemia salina. Arch Hydrobiol 22:197–202
Folch JM, Lees M, Sloane-Stanley GH (1956) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
González-Davis O, Ponce-Rivas E, Sánchez-Saavedra M, Muñoz-Márquez ME, Gerwick WH (2012) Bioprospection of microalgae and cyanobacteria as biocontrol agents against Vibrio campbellii and their use in white shrimp Litopenaeus vannamei culture. J World Aquac Soc 43:387–399
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Ip PF, Wong KH, Chen F (2004) Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem 39:1761–1766
Jothiraj K (2012) Studies on biology, culture and efficacy of marine copepod Nannocalanus minor for fish larviculture. Ph.D. thesis, Bharathidasan University
Kathiresan M (2013) Composition and community structure of plankton from Muthupet coastal waters and application of marine copepod Oithona rigida for larval rearing of Pacific white shrimp Litopenaeus vannamei. Ph.D. thesis, Bharathidasan University
Khatoon H, Banerjee S, Yusoff FM, Shariff M (2009) Evaluation of indigenous marine periphytic Amphora, Navicula and Cymbella grown on substrate as feed supplement in Penaeus monodon postlarval hatchery system. Aquac Nutr 15:186–193
Khatoon H, Banerjee S, Yusoff FM, Shariff M (2013) Use of microalgal-enriched Diaphanosoma celebensis Stingelin, 1900 for rearing Litopenaeus vannamei (Boone, 1931) postlarvae. Aquac Nutr 19:163–171
Kibria G, Nugegoda D, Fairclough R, Lam P, Bradley A (1999) Utilization of wastewater-grown zooplankton: nutritional quality of zooplankton and performance of silver perch Bidyanus bidyanus (Mitchell 1838) (Teraponidae) fed on wastewater-grown zooplankton. Aquac Nutr 5:221–227
Kiron V, Phromkunthong W, Huntley M, Archibald JA, Scheemaker G (2012) Marine microalgae from refinery as a potential feed protein source for Atlantic salmon, common carp and white leg shrimp. Aquac Nutr 18:521–531
Kovalenko EE, D’Abramo LR, Ohs CL, Buddington RK (2002) A successful microbound diet for the larval culture of freshwater prawn Macrobrachium rosenbergii. Aquaculture 210:385–395
Kwak TJ, Zedler JB (1997) Food web analysis of southern California coastal wetlands using multiple stable isotopes. Oecologia 110:262–277
Laurence GC (1977) A bioenergetic model for the analysis of feeding and survival potential of winter flounder, Pseudopleuronectes americanus, larvae during the period from hatching to metamorphosis. Fish B-NOAA 75:529–546
Luis E, Conceicao C, Yuera M, Makridis P, Morais S, Dinis TM (2010) Live feeds for early stages of fish rearing. Aquac Res 41:613–640
Martin-Jezequel V, Poulet SA, Harris RP, Moal J, Samain JF (1988) Inter specific and intra specific composition and variation of free amino acids in marine phytoplankton. Mar Ecol Prog Ser 44:303–313
Moura Junior AM, Bezerra Neto E, Koening ML, Leça EE (2007) Chemical composition of three microalgae species for possible use in mariculture. Braz Arch Biol Technol 50:461–467
Munilla-Moran R, Stark JR, Barbout A (1990) The role of exogenous enzymes in digestion in cultured turbot larvae (Scoph- thalamus maximus L.). Aquaculture 88:337–350
Nageswara Rao I, Krupanidhi G (2001) Biochemical composition of zooplankton from the Andaman Sea. J Mar Biol Assoc India 43:49–56
Nandakumar R (2012) Laboratory culture of Macrosetella gracilis (Dana) and comparison of astaxanthin enrichment proficiency of different live feeds (copepods and Artemia nauplii) in pacific white shrimp Litopenaeus vannamei (Boone, 1931) post larvae, M.Phil. thesis, Bharathidasan University
Nandakumar R (2015) Eco- biology, culture and live feed suitability of zooplankton for nursery rearing of ornamental fish Monodactylus argentus with special emphasis on marine copepod Nitocra affinis. Ph.D. thesis, Bharathidasan University
Nanton DA, Castell JD (1998) The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use as a live food for marine fish larvae. Aquaculture 163:251–261
Palmegiano GB, Agradi E, Forneris G, Gai F, Gasco L, Rigamonti E, Sicuro B, Zoccarato I (2005) Spirulina as a nutrient source in diets for growing sturgeon (Acipenser baeri). Aquac Res 36:188–195
Payne MF, Rippingale RJ (2000) Rearing west Australian seahorse, Hippocampus subelongatus, juveniles on copepod nauplii and enriched Artemia. Aquaculture 188:353–361
Payne MF, Rippingale RJ, Cleary JJ (2001) Cultured copepods as food for West Australian dhufish (Glaucosoma hebraicum) and pink snapper (Pagrus auratus) larvae. Aquaculture 194:137–150
Pillay TVR (1990) Aquaculture: principles and practices. Fishing New Books, England
Rajendran M (1973) A guide to the study of freshwater calanoids. J Madurai Uni India 1:1–86
Rajkumar M, Kumaraguru Vasagam KP (2006) Suitability of the copepod, Acartia clausi as a live feed for Seabass larvae (Lates calcarifer Bloch): compared to traditional live-food organisms with special emphasis on the nutritional value. Aquaculture 261:649–658
Rajthilak C, Santhanam P, Sivakumar J, Prem Kumar C, Perumal P (2013) Intensive culture of marine harpacticoid copepod Nitokra affinis (Gurney 1927) in aquaculture wastewater under laboratory condition-A new emphasis. Int J Res Sci Eng Technol 2:6158–6163
Raju P (2012) Comparative studies on growth, survival and fatty acid enrichment on Pacific white shrimp Litopenaeus vannamei (Boone 1931) using enriched and un-enriched Artemia nauplii and marine copepod Oithona rigida. M.Phil. thesis, Bharathidasan University
Raymont JEG, Austin A, Ligfold E (1964) Biochemical studies on marine zooplankton. The biochemical composition of Neomysis integer. ICES J Mar Sci 28:354–363
Ricker WE (1979) Growth rates and models. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology. Bio-energetics and Growth, vol VIII. Academic Press, New York, pp 677–743
Robert AA (2005) Algal culturing techniques: traditional microalgae isolation techniques. Elsevier, USA
Rohani-Ghadikolaei K, Abdolalian E, Hojatollah F, Masoud G, Ng WK (2013) The nutritional effect of Isochrysis galbana and Chaetoceros muelleri cultured with different seaweed extracts on the larval development, growth and survival of the marine shrimp, Penaeus indicus. Aquac Res 44:1444–1454
Sánchez-Saavedra MP, Voltolina D (2005) The growth rate, biomass production and composition of Chaetoceros sp. grown with different light sources. Aquac Eng 35:161–165
Sangha RS, Cruz AC, Chavez-Sanchez MC, Jones DA (2000) Survival and growth of Litopenaeus vannamei (Boone) larvae fed a single dose of live algae and artificial diets with supplements. Aquac Res 31:683–689
Santhanam P (2002) Studies on the ecology, experimental biology and live-food suitability of copepod, Oithona rigida Giesbrecht from Parangipettai coastal environments (India) Ph.D. thesis, Annamalai University
Santhanam P, Perumal P (2012) Feeding, survival, egg production and hatching rate of the marine copepod Oithona rigida Giesbrecht (Copepoda: Cyclopoida) under experimental conditions. J Mar Biol Assoc India 54:38–44
Santhanam P, Perumal P (2013) Developmental biology of brackishwater copepod Oithona rigida Giesbrecht: a laboratory investigation. Indian J Mar Sci 42:236–243
Santhanam P, Perumal P, Rajkumar M (2004) Effect of feeding Artemia on growth and survival of P. monodon larvae. J Appl Fisher Aquacult 4:42–46
Sargent JR, Mc-Evoy LA, Bell JG (1997) Requirements presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 155:117–127
Stottrup JG (2000) The elusive copepods: their production and suitability in marine aquaculture. Aquac Res 31:703–711
Strickland SC, Parsons TR (1972) A practical handbook of seawater analyses. Bulletin of Fisheries Research Board of Canada, Ottawa
Sumitha AT (2006) Studies on biology, population growth and biochemical composition of freshwater cladoceran, Moina micrura (Crustacea: Cladocera: Moinidae), Ph.D. thesis, Madras University
Vengadeshperumal N, Damotharan P, Rajkumar M, Perumal P, Vijalakshimi S, Balasubramanian T (2010) Laboratory culture and biochemical characterization of the calanoid copepod, Acartia southwelli Sewell, 1914 and Acartia centrura Giesbretch, 1889. Adv Biol Res 4:97–107
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilis. Fish Invest 26:1–62
Wilson RP (2002) Amino acids and proteins. In: Halver JE, Hardy RW (eds) Fish nutrition. Academic Press, California
Yamamoto T, Marcouli PA, Unuma T, Akiama T (1994) Utilization of malt protein flour in fingerling rainbow trout diets. J Fish Sci 60:455–460
Yurkowski M, Tabachek JL (1979) Proximate and amino acid composition of some natural fish foods. In: Halver JE, Tiews K (eds) Proceedings of the world symposium on finfish nutrition and fish feed technology, vol 2, Hamburg 20 ± 23 June 1978. Heenemann, Hamburg, pp 435–448
Zelaya O, Davis DA, Rouse DB (2007) The influence of Artemia and algal supplements during the nursery phase of rearing Pacific white shrimp, Litopenaeus vannamei. J World Aquac Soc 38:486–496
Acknowledgments
Authors are thankful to the Head, Department of Marine Science and authorities of Bharathidasan University, Tiruchirappalli, for the facilities provided. Authors are indebted to Department of Biotechnology, Government of India for Microalgae and Copepods culture facility provided through extramural project (BT/PR 5856/AAQ/3/598/2012). Authors (SDK & SA) thank the Department of Biotechnology, Government of India for fellowship provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinesh Kumar, S., Santhanam, P., Ananth, S. et al. Evaluation of suitability of wastewater-grown microalgae (Picochlorum maculatum) and copepod (Oithona rigida) as live feed for white leg shrimp Litopenaeus vannamei post-larvae. Aquacult Int 25, 393–411 (2017). https://doi.org/10.1007/s10499-016-0037-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0037-6