Abstract
The aim of the study was to investigate the feasibility of nutrient recycling from a marine recirculating aquaculture system (RAS) for fish (European sea bass, Dicentrarchus labrax L.) through three salt-tolerant, halophyte plant species, Tripolium pannonicum (Jacq.) Dobrocz., Plantago coronopus L., and Salicornia dolichostachya (Moss.). Halophytes, illuminated by sunlight and supplemented with artificial light, were maintained in hydroponic cultures integrated in a RAS water treatment system operating at 16 psu salinity. During a 35-day experiment, 248 fishes gained 5.6 kg of weight. Total plant biomass production reached 23 kg in 14 m2 hydroponic culture area. Gain of shoot biomass was 27, 18, and 60 g m−2 day−1 for T. pannonicum, P. coronopus, and S. dolichostachya, respectively. The plants retained 7 g phosphorus and 46 g nitrogen under the experimental conditions. This was equivalent to 9 % of the N and 10 % of the P introduced with the fish feed. The edible part of the harvested plant material was microbially safe and approved for human consumption. The coupling of production in a RAS–IMTA was tested as a feasible cascading production technology for sustainable aquaculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sustainable expansion of aquaculture worldwide requires technologies, which allow for the recycling of matter and energy (Jegatheesan et al. 2011). Presently, aquaculture is still operating in mono-species systems. The system-immanent loss of nutrients (Islam 2005) and organic matter (Franco-Nava et al. 2004; Herbeck et al. 2013) from open installations is a cause for concern. In the global view, also the nitrogen emissions from aquaculture fish production will affect natural cycles (Galloway et al. 2004). Kroeze et al. (2013) emphasize a potential increase in harmful algae blooms because of nutrient shifts in coastal waters. Thus, open coastal aquaculture affects its own environment and jeopardizes a production important for a growing human population. Besides the ecological impact, the resource aspect must be considered. Cooper et al. (2011) show much larger global phosphate rock reserves than previously assumed. They still emphasize that recycling of phosphorus from food production systems should be made a priority. The energy demand for the chemical synthesis of nitrogen fertilizer accounts for 1 % of the global energy consumption (Kitano et al. 2012). The reuse of nitrogen and phosphorous from waste streams, therefore, appears to be the logical way for sustainable resource management in aquaculture and other food production.
A possible way to improve sustainability of aquaculture is by reproducing natural nutrient cycles on small scale. Integrated multi-trophic aquaculture (IMTA) combines the production of fish with filter feeders and plants or algae (Chopin et al. 2008). Another trend in global aquaculture is toward land-based recirculation aquaculture systems (RAS) (Martins et al. 2010; Dalsgaard et al. 2013), which operate independently from natural environments. They allow the production of almost every aquaculture species regardless of their natural distributional range (Orellana et al. 2014). This publication reports about an effort to combine both technologies to address the requirements of future aquaculture.
In RAS, substantial amounts of organic and inorganic matter from non-retained feed accumulate within their system boundaries. Water quality needs to be controlled by bioprocess technology (Orellana et al. 2014). This allows to reduce the water consumption rate in zero-exchange RAS to <1 % of the system volume per day. The resulting inorganic nitrogen and phosphorous flux complies with the nutrient requirements of plants. The process technology allows directing the nutrients to an integrated recycling by plants.
Approaches to combining fish and plant production are dated back to 1978 and 1984, when Lewis et al. (1978) and Watten and Busch (1984) combined the production of tilapia and tomatoes. In marine aquaculture, examples of co-production of fish and plants are less common. Webb et al. (2012) investigated the feasibility of constructed wetlands to recycle nutrients from a commercial RAS operation. They grew Salicornia europaea successfully with effluents from a shrimp, sole, and turbot farming. The wetland technology as an end of pipe treatment removed a large amount of the nutrients.
More recently, Sikawa and Yakapitiyage (2010) published results from experiments combining hybrid catfish (Clarias macrocephalus × C. gariepinus) and lettuce (Lactuca sativa L.) in a pond/hydroponic system. Their results proved the feasibility but also showed constraints. The high particle load in the process water of the RAS turned out to be a drawback for integration. Modern marine RAS technology includes additional process steps to decrease turbidity and remove small particles including bacteria and other microorganisms (Orellana et al. 2014). The decay of organic substance through the ozonation process (Orellana et al. 2014) is another important step toward better process water quality (Schroeder et al. 2011).
In RAS, low dissolved phosphorus and nitrogen concentrations are maintained in the process in order to ensure welfare of cultured organisms. Typical concentrations are in the range of 100 mg l−1 for nitrate-N and between 1 and 15 mg l−1 for phosphate-P (Deviller et al. 2004; Tal et al. 2009; van Bussel et al. 2012; Orellana et al. 2014). These concentrations are far below those commonly used for hydroponic plant growth (Park et al. 2009). The integration of hydroponic plant production into RAS is possible if sufficient nutrients can be supplied with the recirculated process water. In this study, we examined the growth of the halophytic species S. dolichostachya, a high-value vegetable, and compared growth with that observed in wetland conditions. Tripolium pannonicum and P. coronopus were tested as herbs with special flavoring substances. The species were shown to grow under hydroponic conditions (Buhmann et al. 2015). The aim of this study was to investigate the growth and nutrient uptake in a cascading process and to define parameters that can later be used to scale up RAS–IMTA.
Materials and methods
RAS operation
An experimental RAS was employed as nutrient source for the hydroponic cultures. Fish were kept in an 8 m3 rectangular fish tank (5 × 2.5 × 0.7 m). Water quality was maintained through a number of water treatment processes (drum filtration, flotation, ozonation, biofiltration, degassing, and aeration) according to Orellana et al. 2014 (Fig. 1). The RAS was operated with artificial seawater (Seequasal GmbH, Münster, Germany) made up of five major ions (Cl−, Na+, SO4 −, Mg2+, and K+) according to Turekian (1968). Salinity was adjusted to 15–16 psu.
The RAS was stocked with 248 D. labrax juveniles obtained from a commercial marine inland fish farm (Meeresfischzucht Völklingen GmbH, Germany). The initial average weight of fish was 38.1 g. Fish were fed to satiation with commercial pelleted feed (1.2–1.5 mm pellet size Coppens International Marico Apex, Helmond, The Netherlands). The feed contained 9.3 % nitrogen (N) and 1.3 % phosphorous (P) according to the specification of the manufacturer.
Growth of fish is exponential (Ricker 1975) and can be expressed as \(w_{1} = w_{0} \cdot e^{G \cdot t}\) where w 0 and w t are the individual weights of animals at the beginning and the end of the growth period (t). G denotes the instantaneous growth rate which can be calculated from \(G = (\log_{e} ({w_{1} })-\log_{e} ({w_{0}}))/ {(t_{1} - t_{0} )}.\) The instantaneous growth rate corresponds to a specific growth rate in % body weight per day that is more commonly computed in aquaculture production research. The feed conversion rate (FCR) was calculated as a quotient of the feed given and the weight gained.
Hydroponic operation
The experimental system was combined of the RAS, a sand bed nursery, and three hydroponic culture tanks for experimental plant production (Fig. 1). The nursery system and the hydroponic tanks were set up in two greenhouses of 12 and 40 m2 base area.
The hydroponic tanks (1.9 m3) exhibited a length, width, and depth of 6.0, 0.8, and 0.5 m. They covered 30 m2 of the base area in the greenhouse. Water level within tanks was maintained at 0.35 m. The tanks were covered with a non-transparent black PE sheet. A metal grid served as support for the PE sheet as well as for the plants. The hypocotyl of plants was fixed with soft foam in square openings.
Each hydroponic culture tank was supplied with RAS process water at a flow rate of 0.15 m3 h−1 from 8 am to 8 pm (1.8 m3 day−1). This is much less than the flow rate through the nitrifying biofilter (15 m3 h−1, 24 h day−1), which converts ammonia excreted by fish to nitrate. Although hydroponic water temperature was linked to RAS process water temperature, the weather conditions influenced the process water temperature in hydroponic cultures. Air temperature and process water temperature were monitored with min–max thermometers. Process water was aerated to maintain a well-mixed water column and to equilibrate dissolved gas concentrations. The photosynthetic active radiation (PAR) was continuously monitored with a quantum sensor (Licor, Bad Homburg, Germany) 1 m above the water surface. T. pannonicum and P. coronopus were cultured under natural light conditions. The hydroponic culture tank used for S. dolichostachya was separated from the others by a curtain. Two high pressure sodium lamps were mounted above the S. dolichostachya hydroponic (Fig. 1). The natural light was supplemented for 18 h with artificial light to suppress flower induction (Ventura et al. 2011).
Trace elements were added to hydroponic tanks three times a week. Every addition included iron citrate (0.18 g Fe), zinc (2.05 × 10−5 g), molybdenum (7.54 × 10−7 g), cobalt (4.48 × 10−6 g), copper (4.73 × 10−8 g), and manganese (3.45 × 10−5 g).
Plant material
Seeds of T. pannonicum (Jacq.) Dobrocz. and S. dolichostachya Moss were collected at the North Sea Jade Bay, Germany (53°29′13 N; 8°03′16″O). P. coronopus L. seeds were obtained from Jelitto Staudensamen GmbH (Schwarmstedt, Germany). Plants were grown to seedling size in a greenhouse at the University of Hannover (Germany) at an average temperature of 22 °C. During daytime, the natural light was supplemented for 14 h with artificial light (sodium vapor lamps SONT Agro 400 Philips Amsterdam, The Netherlands). At 1–2 cm shoot length, seedlings were transplanted to pots filled with sand of 2 mm grain size.
During the nursery phase, seedlings were irrigated with tap water. Twice a week, Hoagland solution was supplied. One week before the start of hydroponic experiments, the plants were adapted to the experimental salinity by adding sodium chloride to the irrigation water. Salinity was increased by 5 psu every second day. Nursery periods were 4, 3, and 7 weeks in T. pannonicum, P. coronopus, and S. dolichostachya, respectively.
At the beginning of the experiment, 185 plants of each species were evenly distributed over the surface area of one hydroponic tank. Horticultural care was carried out throughout the experimental period. Non-vital plants were removed and counted. Pest control was carried out with beneficial insects (Aphidoletes aphidimyza, Aphidius colemani, Chrysoperla carnea, Hatto Welte, Reichenau, Germany). Plant biomass at the beginning was determined for pooled samples of 15 randomly selected plants. After 35 days, three samples of 15 plants were harvested in 1, 3, and 5 m distance from the head end of the hydroponic tank and analyzed.
Analytical methods
Samples for the determination of nutrient concentrations of process water influent and effluent to the hydroponic tanks were collected three times a week. Samples were passed through a filter (0.2 µm) and stored in sterile polypropylene centrifuge tubes at −20 °C until analysis. The concentrations of nitrate-N and phosphate-P were measured with an autoanalyzer (AA3 Seal Analytical GmbH, Norderstedt, Germany). Dissolved inorganic carbon (IC) and total carbon (TC) as well as total nitrogen (TN) were determined using an automated CN analyzer (multi N/C 3100 Analytik Jena, Jena, Germany). Total ammonium-N and nitrite-N concentrations in RAS process water were determined with photometric tests (Hach-Lange, Düsseldorf, Germany).
Fresh weight of shoots and roots was determined after removing adherent water. Dry weight was determined after drying to constant weight at 110 °C. Subsamples of dried material were grinded to fine powder (MM 400 Retsch GmbH, Haan, Germany) and analyzed in a CNS elemental analyzer (Vario EL III Elementar Analysensysteme, Hanau, Germany). For determination of the P content, aliquots of approximately 38 mg dry mass were reduced to ashes during 8 h (muffle furnace, M104 Thermo Fisher Scientific Corporation, Waltham, Mass., USA). The ash was cooled to room temperature, and 1.5 ml of 66 % nitric acid was added. After 10 min, 13.5 ml of ultrapure water was added. The solution was filtered (0.45 μm pore size, Carl Roth) and stored at −60 °C. Phosphorus concentration was determined in an inductively coupled plasma optical emission spectrometer (ICP-OES, iCAP 6000 ICP Spectrometer, Thermo Fisher Scientific Corporation). A blank value was obtained by treating an empty vial in the same way.
Chlorophyll and carotenoid content of shoots was determined in shock-frozen plant material. Pigments were extracted with ice-cold 80 % acetone (400 µl) added to aliquots of freeze-dried and ground leaf material (50 mg). The samples were kept on ice for 10 min and mixed every 2 min. Cell debris was separated by centrifugation (14,000g, 5 min, 0 °C). Final extraction of pigments from the precipitate was repeated three times using 200 µl ice-cold 80 % acetone. The supernatants were pooled and stored on ice for pigment determination. Extinction was measured at 470.0, 646.8, 663.2, and 750.0 nm (Uvikon XS spectrophotometer, Biotech Instruments, Germany). Total chlorophyll and carotenoid content was calculated according to Lichtenthaler (1987).
Microbiological quality
At the end of the hydroponic experiment, three shoots of every halophyte species were randomly harvested and pooled. The fresh material was processed in a licensed microbiological laboratory (MikroBiologie Krämer, Dillingen, Germany). The samples were inspected for relevant bacteria in vegetables and fish (Escherichia coli, Salmonella spp., Lysteria monocytogenes, Enterobacteriaceae, Pseudomonas spp., Vibrio spp.). The total counts of mesophilic bacteria were also determined (DGHM 2004, 2007).
Results
RAS operation
Fish individual weight increased during the 35-day experimental period from 32 to 54 g on average (Table 1). Stocking density in RAS was low throughout the experiment. The specific growth rate of fish was derived from the instantaneous relative growth rate which is based on an exponential growth model (Ricker 1975). During the 35-day experiment, fish grew at specific growth rate of 1.5 % day−1 and exhibited an FCR of 0.93.
Water quality in the RAS production tank was monitored regularly. The process water pH leveled around 8.1 ± 0.1. Salinity was 15.8 ± 0.5 psu. Total ammonium-N varied between 0 and 0.07 mg l−1. The average value amounted to 0.01 ± 0.02 mg l−1. Nitrite-N also remained low during the production period. The average amount was 0.025 ± 0.002 mg l−1. Nitrate-N and phosphate-P concentrations are reported in the section below.
Hydroponic plant growth
A major stress factor for the plants was extreme air temperatures in the greenhouse at the beginning of the experiment. During daytime, air temperature exceeded 40 °C (Fig. 1). P. coronopus looked slightly withered at temperatures above 40 °C, but recovered quickly when temperature decreased. During nighttime, air temperatures leveled between 25 and 20 °C. The process water temperature was less affected due to the large water volume of hydroponic tanks and the RAS water volume. Process water temperature never exceeded 27 °C (Fig. 1). The temperature slowly decreased from 24–27 °C to 20–22 °C in the time from August to September.
T. pannonicum and P. coronopus were grown under natural light. The photosynthetically active photon flux reached values above 900 µmol m−2 s−1 during daytime in August. At the end of the experiment, in September, a maximum of 500 µmol m−2 s−1 was monitored. Heavy rainfall or overcast sky reduced the maximum daytime photon flux to 250 µmol m−2 s−1, even under late summer conditions (Fig. 2).
The natural light level above the S. dolichostachya hydroponic tank was supplemented with two lamps. They generated a photon flux of 250 µmol m2 s−1 in the center of the light beams. At the periphery, the photon flux was <20 µmol m2 s−1 (Fig. 3). No plants bloomed during and after the experiment, indicating that even the lowest photon flux was sufficient to suppress flower induction.
Total ammonium-N and nitrite-N concentrations determined in the effluent of the hydroponic tanks remained below the detection level throughout the experiment. The TN concentrations agreed well with the nitrate-N concentrations in all measurements (Table 2). No obvious differences were found between TN and nitrate-N concentrations determined in RAS process water and the water leaving the T. pannonicum and P. coronopus hydroponics, but TN and nitrate-N concentrations were lower in the S. dolichostachya hydroponic outlet. Phosphate-P in process water leveled around 3 mg l−1 (Table 2). No differences were found between process water samples and water samples collected from the hydroponic outlets. Dissolved TC and IC were high in all samples (19–20 mg l−1). A clear difference between TC and IC, which would have accounted for dissolved organic carbon, was not observed. Most of the IC in the process water was hydrogen carbonate, which equilibrates with dissolved carbon dioxide produced by fish (Fig. 4).
More than 90 % of plants had survived the 35-day experimental period. The fresh weight per plant increased from 1 to 31 g in T. pannonicum. In P. coronopus, the fresh weight increased from 1 to 21 g (Table 3). The fresh weight of S. dolichostachya seedlings and harvested plants was two to three times higher than that of the other two species. The plant exhibited different daily rates of increase (27, 18, and 60 g m−2 day−1) due to different weights at the beginning of the hydroponic growth but grew at similar specific growth rates of 9.0–9.9 % day−1. In all plants species, 70–80 % of the total biomass production was generated by the shoots.
During the experiment, all plants received N and P exclusively from RAS process water. At the end, all plants exhibited a lower N content of biomass (Table 4). N content of T. pannonicum was 37 and 13 % lower in shoots and roots. In the whole plant, the initial N concentration decreased by 33 %. Nitrogen concentration in P. coronopus shoots decreased by 27 %. In the root system, the change was negligible (1 %). In the whole plant, the N concentration decreased by 23 %. S. dolichostachya showed a minor decrease in the N content of only 3 %.
The P content of T. pannonicum decreased by 33 % during the experiment. While a 42 % reduction was measured in the shoot, the P concentration increased by 10 % in the root system. In whole plants of P. coronopus and S. dolichostachya, the P concentration of biomass increased during the experiment by 43 and 37 %. Both species exhibited a higher accumulation of P in root biomass than in shoot biomass. A particular high value was observed in the root system of P. coronopus corresponding to a 300 % increase in P content (Table 4).
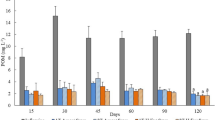
In all plants, the shoot tissue contained 81–83 % of total plant N and 62–71 % of total plant P (Table 4) because of the larger share of shoot tissue. The retention of nutrients in plants was calculated from the N and P concentrations of the shoot and root tissue. The amount of N and P at the beginning was subtracted from that at the end of the experiment, and the result was multiplied by the amount of gained biomass. S. dolichostachya incorporated 167 mg N and 23 mg P per plant during the 35-day experimental period. T. pannonicum assimilated 61 mg N and 9 mg P per plant. In P. coronopus, a retention of 34 mg N and 7 mg P was calculated (Fig. 5).
Gain of biomass and retention of nitrogen and phosphorus during hydroponic growth. The increase in fresh and dry weight and the uptake of nitrogen and phosphorus was determined for the halophyte species T. pannoniucum, P. coronopus, and S. dolichostachya. For each parameter, values for shoot, root, and total plant are shown
Pigment concentration of plants (chlorophyll, carotenoid) changed during the experiment. In S. dolichostachya, the initial chlorophyll concentration (301 mg g−1 dry weight) decreased to 246 ± 58 mg g−1 dry weight. The carotenoid concentration remained stable (61 ± 2 mg g−1 dry weight). In P. coronopus, the initial chlorophyll concentration of 672 mg g−1 dry weight declined to 419 ± 37 mg g−1 dry weight. The carotenoid concentration decreased from an initial concentration of 128 mg g−1 dry weight to 78 ± 7 mg g−1 dry weight after 35 days. However, these changes in pigment content did not show up as visible leaf discolorations. In contrast, leafs of T. pannonicum showed different shades of green at the end of the experiment. On average, the chlorophyll concentration decreased from 1093 ± mg g−1 dry weight to 262 ± 55 mg g−1 dry weight, the carotenoid concentration from 191 mg g−1 dry weight to 52 ± 12 mg g−1 dry weight.
Microbiological quality of harvested plants
Consumer protection must carefully be considered in IMTA using a trophic cascade (Blidariu and Grozea 2011). Table 5 summarizes the results of the microbial inspection of shoot tissue harvested at the end of the experiment. It also shows guidance and warning levels that are applied in Germany to fresh salads and marine fish (DGHM 2004 and 2007). The results show that bacteria counts of potential pathogenic bacteria were low. The numbers found remain at least one order of magnitude below guidance and warning levels. From this, it can be concluded that the plant material is not harmful for human nutrition.
Discussion
The experimental setup allowed to investigate the growth of three halophytic plant species in RAS process water under low steady state concentrations of nitrate (19–21 mg N l−1) and phosphate (3 mg P l−1). These concentrations were more than ten times lower than commonly used for tomato and cucumber hydroponic culture (Park et al. 2009). The results proof the feasibility of a cascading production and show a new perspective for an environmentally sound RAS aquaculture.
The RAS technology employed was similar to that described by Orellana et al. (2014). It is characterized by non-turbid, clear water. The microbial environment is controlled by low concentration of dissolved organic C. Apparently, such an environment is favorable for fish and halophytes. The calculated daily weight increase of the three species was within the ranges reported by Webb et al. (2012) and Shpigel et al. (2013) for Salicornia spp and small leafy plants grown in freshwater aquaponic systems (Trang et al. 2010; Sikawa and Yakupitiyage 2010).
After 35 days, T. pannonicum and S. dolichostachya had reached marketable size with average shoot weights of 25 and 60 g. High air temperatures in the greenhouse may have limited the growth of P. coronopus plants (17 g) at the beginning of the experiment. The high final weight of S. dolichostachya plants was not caused by a higher specific growth rate but mainly was due to a larger size of seedlings. The supplementation of natural light by artificial light was essential to avoid flower induction in S. dolichostachya (Ventura et al. 2011). However, very low light levels (photon flux < 20 µMol m−2 s−1) were sufficient to control the physiological state. Thus, the energy consuming lamps used in this study can certainly be replaced by efficient electric lighting solutions, such as LED (Poulet et al. 2014).
Low nutrient concentrations were a point of concern in the setup of IMTA with halophytes (Webb et al. 2012). In this study, changes of tissue nutrient contents were observed in almost all plant species. While the P content of T. pannonicum decreased, that of the two other species increased during the hydroponic growth. The high increase in P content of roots was likely caused by an adsorption of P to the root surface. The buildup indicates that these two species experienced no shortage in P during the experiment. The N content of S. dolichostachya plants remained unchanged during hydroponic growth. In contrast, the N content in the seedlings of T. pannonicum and P. coronopus was higher than that of the plants harvested at the end of the experiment. Dissolved nutrients were plenty in supply during the whole experimental period. Nitrate is taken up by plant root systems, even if the uptake rate is dependent from the ambient nitrate concentration and differs between plant species (Fang Yao et al. 2011). Thus, the observed reduction in tissue N content is not necessarily indicating a shortcoming of N due to low process water concentration. The diminished tissue nitrogen content was possibly linked to the physiological state of the seedlings and belonged to the maturation process of plants.
A side effect of the ozone treatment of RAS process water is the formation and precipitation of iron and manganese oxides. While fish receive trace elements through feed, the oxidative elimination of trace elements may limit plant growth in RAS–IMTA. The pigment content of T. pannonicum visibly decreased during hydroponic growth. Pigment loss can be an indicator of nutrient deficiency. However, the specific growth rate was higher than that observed in P. coronopus and S. dolichostachya. T. pannonicum is well known for a high iron demand (Ventura et al. 2013). The micronutrients supplied during the experiment were apparently not sufficient to support a balanced growth of this species. Hence, T. pannonicum may be less suited for hydroponic cultivation in a RAS–IMTA system.
All investigated halophyte species were capable to remove nutrients from process water and can be used as secondary biological nutrient filters. The concept of a zero-exchange RAS–IMTA was proofed. However, the possible areal plant production, i.e., the balance between nutrient release by fish and nutrient uptake by plants, was not yet met. At the time of harvest, even the largest plants in the experiment had ample space for further growth and expansion. Planting density in a commercial operation is adjusted to the expected harvesting size of plants or fruits. Much higher densities were reported for small leafy vegetables. Fallovo et al. (2009) reported 1857 plants per square meter of area. In contrast, tomato and zucchini are planted at 2–3 plants per square meter (Auerswald et al. 1999; Rouphael and Colla 2005; Incrocci et al. 2006). Leafy vegetables of intermediate size such as radish, spinach, and lettuce are cultivated at 30–100 plants per square meter (De Pinheiro Henriques and Marcelis 2000; Yorio et al. 2001; Frantz et al. 2004). Lennard and Leonard (2006) grew 38 heads of Lactuca per m2 to a marketable size of 132 g fresh weight. Thus, space is usually much better utilized than in our experiment. Webb et al. (2012) and Shpigel et al. (2013) planted 90–100 halophytes per square meter in constructed wetlands and reported similar growth rates compared to this study. Therefore, the productivity and nutrient uptake in RAS–IMTA can be enhanced by increasing plant density.
The potential of RAS–IMTA can be demonstrated using the results from this study. In this study, the juvenile sea bass, D. labrax, in co-culture with halophytes were fed with 5.2 kg commercial aquaculture feed. They gained 5.6 kg fresh weight during the 35-day experiment. The given feed contained 484 g nitrogen. After Robaina et al. (1999), 39 % of the nitrogen is excreted in D. labrax. The noxious ammonia (Randall and Tsui 2002) is oxidized to nitrate in the biofiltration process that is part of the water treatment in RAS. It can be assumed that 189 g nitrogen became immediately available as nitrate-N for hydroponic plant production. Usually, the nitrate-N in RAS is removed by denitrifying biofilters (Van Rijn et al. 2006). However, the hydroponic removal process complies better with the requirements of sustainable aquaculture; it synergistically increases total output of RAS.
The production of plant material in the experiment amounted to 6, 4, and 13 kg for T. pannonicum, P. coronopus, and S. dolichostachya, respectively. The plants incorporated in total 46 g N and 7 g P during the 35 days of the experiment, which was equivalent to 9 % of the N and 10 % of the P introduced with fish feed. In view of an amount of 189 g N estimated for the nitrogen excretion of fish, only 24 % of N was assimilated by plants. If S. dolichostachya would have been the only consumer of nutrients in the 14.4 m2 hydroponic area, 1128 plants would have been necessary to remove 189 g N excreted by fish. The areal density for S. dolichostachya would increase to 78 plants per square meter which is a feasible density. The 1128 plants would produce 84 kg biomass fresh weight, of which 64 kg is marketable leafy vegetable. Thus, the marketable plant biomass would exceed the fish production by factor 11. The P input by feed amounted to 66 g. The P retained in fish body mass was estimated to 37 g according to Ballestrazzi et al. 1998). Thus, 29 g phosphorous became available for plant production of which 26 g (91 %) would have been used in hydroponic plant production. Based on the results and calculations, an increased plant density in the hydroponics would have balanced nutrient input by feed and nutrient removal by plants in the RAS–IMTA.
Fish biomass in the experimental RAS was small compared with commercial RAS operation. The potential plant production in a commercial RAS–IMTA would excel the experimental plant production likely by orders of magnitude; the necessary hydroponic area would be tremendous, and the size of production would be a challenge for the marketing.
Conclusions
RAS production of fish provides plenty of nutrients for plant growth in hydroponic culture. The use of nutrients in a trophic cascade is potentially beneficial for animal welfare and the bioeconomy of RAS–IMTA aquaculture. The plant production requires a much larger production area than the fish production in RAS.
Biomass production and nutrient removal rates are comparable with those observed in saline wetland systems and freshwater aquaponics. However, the system investigated here reliably feeds nutrients into the hydroponic culture. Thus, all nutrients released from fish production can be captured by plants.
T. pannonicum appeared to be less suited for hydroponic culture. On the other hand, the growth and quality (appearance) of two plant species, S. dolichostachya and P. coronopus, were excellent. Food safety was confirmed by negligible counts of relevant pathogens on the marketable leafy plant biomass.
Abbreviations
- IMTA:
-
Integrated multi-trophic aquaculture
- RAS:
-
Recirculation aquaculture systems
References
Auerswald H, Schwarz D, Kornelson C, Krumbein A, Brückner B (1999) Sensory analysis sugar and acid content of tomato at different EC values of the nutrient solution. Sci Hortic 82:227–242
Ballestrazzi R, Lanari D, D’Agaro E (1998) Performance, nutrient retention efficiency, total ammonia and reactive phosphorus excretion of growing European sea-bass (Dicentrarchus labrax, L.) as affected by diet processing and feeding level. Aquaculture 161:55–65
Blidariu G, Grozea A (2011) Increasing the economical efficiency and sustainability of Indoor fish farming by means of aquaponics–review. Anim Sci Biotechnol 44:1–8
Buhmann AK, Waller U, Wecker B, Papenbrock J (2015) Optimization of culturing conditions and selection of species for the use of halophytes as biofilter for nutrient-rich saline water. Agric Water Manag 149:102–114
Chopin T, Robinson SMC, Troell M, Neori A, Buschmann AH, Fang J (2008) Multitrophic integration for sustainable marine aquaculture. In: Jørgensen SE, Fath BD (eds) The encyclopedia of ecology, ecological engineering, vol 3. Elsevier, Oxford, pp 2463–2475
Cooper J, Lombardi R, Boardman D, Carliell-Marquet C (2011) The future distribution and production of global phosphate rock reserves. Resour Conserv Recycl 57:78–86
Dalsgaard J, Lund I, Thorarinsdottir R, Drengstig A, Arvonen K, Bovbjerg Pedersen P (2013) Farming different species in RAS in Nordic countries: current status and future perspectives. Aquac Eng 53:2–13
De Pinheiro Henriques AR, Marcelis LFM (2000) Regulation of growth at steady-state nitrogen nutrition in lettuce (Lactuca sativa L.): interactive effects of nitrogen and irradiance. Ann Bot 86:1073–1080
DGHM, Deutsche Gesellschaft für Hygiene und Mikrobiologie (2004) Richt- und Warnwerte für Seefische. http://www.dghm-richt-warnwerte.de/, Accessed at 20 Nov 2013
DGHM, Deutsche Gesellschaft für Hygiene und Mikrobiologie (2007) Richt- und Warnwerte für Mischsalate. Mittelwerte für abgepackte Ware bei Abgabe an den Verbraucher. http://www.dghm-richt-warnwerte.de/, Accessed at 20 Nov 2013
Deviller G, Aliaume C, Nava MAF, Cesellas C, Blancheton JP (2004) High-rate algal pond treatment for water reuse in an integrated marine fish recirculating system: effect on water quality and sea bass growth. Aquaculture 235:331–344
Fallovo C, Rouphael Y, Rea E, Battistelli A, Colla G (2009) Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J Sci Food Agric 89:1682–1689
Franco-Nava MA, Blancheton JP, Deviller G, Charrier A, Le-Gall JY (2004) Effect of fish size and hydraulic regime on particulate organic matter dynamics in a recirculating aquaculture system: elemental carbon and nitrogen approach. Aquaculture 239(1–4):179–198
Frantz JM, Ritchie G, Cometti NN, Robinson J, Bugbee B (2004) Exploring the limits of crop productivity: beyond the limits of tipburn in lettuce. J Am Soc Hortic Sci 129:331–338
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Eitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vörösmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Herbeck LS, Unger D, Wub Ying, Jennerjahn TC (2013) Effluent, nutrient and organic matter export from shrimp and fish ponds causing eutrophication in coastal and back-reefwaters of NE Hainan, tropical China. Cont Shelf Res 57:92–104
Incrocci L, Malorgio F, Della Bartola A, Pardossi A (2006) The influence of drip irrigation or subirrigation on tomato grown in closed-loop substrate culture with saline water. Sci Hortic 107:365–372
Islam MS (2005) Nitrogen and phosphorus budget in coastal and marine cage aquaculture and impacts of effluent loading on ecosystem: review and analysis towards model development. Mar Pollut Bull 50:48–61
Jegatheesan V, Shu L, Visvanathan C (2011) Aquaculture Effluent: impacts and remedies for protecting the environment and human health. reference module in earth systems and environmental sciences. Encycl Environ Health 2011:123–135
Kitano M, Inoue Y, Yamazaki Y, Hayashi F, Kanbara S, Matsuishi S, Yokoyama T, Kim SW, Hara M, Hosono H (2012) Ammonia synthesis using a stable electrode as an electron donor and reversible hydrogen store. Nat Chem 4:934–940
Kroeze C, Hofstra N, Ivens W, Löhr A, Strokal A, Jvan Wijnen J (2013) The links between global carbon, water and nutrient cycles in an urbanizing world—the case of coastal eutrophication. Curr Opin Environ Sustain 5(6):566–572
Lennard WA, Leonard BV (2006) A comparison of three different hydroponic sub-systems (gravel bed floating and nutrient film technique) in an aquaponic test system. Aquac Int 14:539–550
Lewis WM, Yopp JH, Schramm HL, Brandenburg AM (1978) Use of hydroponics to maintain quality of recirculated water in a fish culture system. Trans Am Fish Soc 107:92–99
Lichtenthaler HK (1987) Chlorophylls and carotenoids, the pigments of photosynthetic membranes. Methods Enzymol 148:350–382
Martins CIM, Eding EH, Verdegem MCJ, Heinsbroek LTN, Schneider O, Blancheton JP, Roque d’Orbcastel E, Verreth JAJ (2010) New developments in recirculating aquaculture systems in Europe: a perspective on environmental sustainability. Aquac Eng 43:83–93
Orellana J, Waller U, Wecker B (2014) Culture of yellowtail kingfish (Seriola lalandi) in a marine recirculating aquaculture system (RAS) with artificial seawater. Aquac Eng 58:20–28
Park JBK, Craggs RJ, Sukias JPS (2009) Removal of nitrate and phosphorus from hydroponic wastewater using a hybrid denitrification filter (HDF). Bioresour Technol 100:3175–3179
Poulet L, Massa GD, Morrow RC, Bourget CM, Wheeler RM, Mitchell CA (2014) Significant reduction in energy for plant-growth lighting in space using targeted LED lighting and spectral manipulation. Life Sci Space Res 2:43–53
Randall DJ, Tsui TKN (2002) Ammonia toxicity in fish. Mar Pollut Bull 45:17–23
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Environment Canada Bulletin 191, Ottawa
Robaina L, Corraze G, Aguirre P, Blanc D, Melcion JP, Kaushik S (1999) Digestibility, postprandial ammonia excretion and selected plasma metabolites in European sea bass (Dicentrarchus labrax) fed pelleted or extruded diets with or without wheat gluten. Aquaculture 179:45–56
Rouphael Y, Colla G (2005) Growth yield, fruit quality, and nutrient uptake of hydroponically cultivated zucchini squash as affected by irrigation systems and growing seasons. Sci Hortic 105:177–195
Schroeder JP, Croot PL, Von Dewitz B, Waller U, Hanel R (2011) Potential and limitations of ozone for the removal of ammonia, nitrite, and yellow substances in marine recirculating aquaculture systems. Aquac Eng 45:35–41
Shpigel M, Ben-Ezra D, Shauli L, Sagi M, Ventura Y, Samocha T, Lee YY (2013) Constructed wetland with Salicornia as a biofilter for mariculture effluents. Aquaculture 412–413:52–63
Sikawa DC, Yakupitiyage A (2010) The hydroponic production of lettuce (Lactuca sativa L.) by using hybrid catfish (Clarias macrocephalus × C. gariepinus) pond water: potentials and constraints. Agric Water Manag 97:1317–1325
Tal Y, Schreier HJ, Sowers KR, Stubblefield JD, Place AR, Zohar Y (2009) Environmentally sustainable land-based marine aquaculture. Aquaculture 286:28–35
Trang NTD, Schierup HH, Brix H (2010) Leaf vegetables for use in integrated hydroponics and aquaculture systems: effects of root flooding on growth, mineral composition, and nutrient uptake. Afr J Biotechnol 27:4186–4196
Turekian KK (1968) Oceans. Prentice Hall, NJ
Van Bussel CGJ, Schroeder JP, Wuertz S, Schulz C (2012) The chronic effect of nitrate on production, performance, and health status of juvenile turbot (Psetta maxima). Aquaculture 326–329:163–167
Van Rijn J, Tal Y, Schreier HJ (2006) Denitrification in recirculating systems: theory and applications. Aquac Eng 34:364–376
Ventura Y, Wuddineh WA, Shpigel M, Samocha TM, Klim BC, Cohen S, Shemer Z, Santos R, Sagi M (2011) Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Sci Hortic 135:510–516
Ventura Y, Myrzabayeva M, Alikulov Z, Cohen S, Shemer Z, Sagi M (2013) The importance of iron supply during repetitive harvesting of Aster tripolium. Funct Plant Biol 40:968–976
Watten BJ, Busch RL (1984) Tropical production of tilapia (Sarotherodon aurea) and tomatoes (Lycopersicon esculentum) in a small-scale recirculating water system. Aquaculture 41:271–283
Webb JM, Quinta R, Papadimitriou S, Norman L, Rigby M, Thomas DN, Le Vay L (2012) Halophyte filter beds for treatment of saline wastewater from aquaculture. Water Res 46:512–514
Yao F, Sun J, Tang C, Ni W (2011) Kinetics of ammonium, nitrate and phosphate uptake by candidate plants used in constructed wetlands. Procedia Environ Sci 10:1854–1861
Yorio NC, Goins G, Kagie HR, Wheeler RM, Sager JC (2001) Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. Hortic Sci 36:380–383
Acknowledgments
The authors thank the gardeners, Yvonne Leye and Lutz Krüger, for taking care of the seedlings and young plants, Rebecca Hosang, Pamela von Trzebiatowski, and Julia Volker for technical assistance, and Christian Boestfleisch for support in harvesting the plants. The project was financially supported by the Deutsche Bundesstiftung Umwelt (DBU) (AZ27708/1-3) and the Erwin Sander Elektroapparatebau GmbH Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waller, U., Buhmann, A.K., Ernst, A. et al. Integrated multi-trophic aquaculture in a zero-exchange recirculation aquaculture system for marine fish and hydroponic halophyte production. Aquacult Int 23, 1473–1489 (2015). https://doi.org/10.1007/s10499-015-9898-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-015-9898-3